Abstract
Weight fluctuations are common among individuals with obesity and are associated with increased morbidity. We examined adipose tissue immune and inflammatory markers in mice following weight loss and partial weight regain. Male C57BL/6 mice were randomized into four groups (n = 8–10/group): low-fat diet for 32 wk (LFD), high-fat diet for 32 wk (HFD), LFD for 28 wk and then changed to a HFD for 4 wk (LFD→H), and HFD for 21 wk and then changed to LFD for 7 wk and then changed to HFD for 4 wk (HFD→L→H). LFD→H and HFD→L→H mice did not differ in body weight, fat mass, or fat percentage; however, these parameters were greater than in LFD (P < 0.05) but lower than in HFD (P < 0.05). HFD→L→H mice had smaller adipocytes than HFD and LFD→H (P < 0.05) but not LFD mice. Expressions of CD11c and CD8a genes were elevated in epididymal fat of HFD→L→H compared with LFD→H and LFD (P < 0.05)mice. However, CD11c was lower in HFD→L→H than in HFD mice (P < 0.05), but there was no difference in CD8a between these groups. TNFα and IFNγ expressions were increased in HFD→L→H compared with LFD and LFD→H mice (P < 0.05), although HFD→L→H had lower expression of these cytokines than HFD (P < 0.05). IL-1β was greater in HFD→L→H compared with LFD (P < 0.05) but was not different from LFD→H or HFD mice. Monocyte chemoattractant protein-1 was lower (P < 0.05) in HFD→L→H than in LFD→H. These data reinforce the importance of maintaining a body weight in the range that is recommended for optimal health to reduce immune and inflammatory perturbations associated with obesity.
NEW & NOTEWORTHY We examined the immune and inflammatory status of adipose tissue in mice after they underwent weight loss followed by partial weight regain. We show an increase in selected immune cells and inflammatory mediators, in high-fat diet-fed mice that had prior exposure to a high-fat diet. Although weight fluctuations appear to exacerbate immune cell abundance and inflammation in adipose tissue, severity is less than in mice that were exposed to sustained high-fat diet feedings.
Keywords: adipose tissue inflammation, immune markers, obesogenic programming, weight fluctuations
INTRODUCTION
The World Health Organization (WHO) has estimated that populations that are overweight and obese have reached 39 and 13%, respectively. These numbers are even higher in the United States; men’s obesity and obesity/overweight prevalence are currently at 38.0 and 74.7%, respectively, and women are similar, with obesity and obesity/overweight reaching 41.5 and 68.9%, respectively (46). Obesity is a major risk factor for many chronic diseases, including type 2 diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), atherosclerosis, cardiovascular disease, and cancer (3, 27, 49). Moreover, obesity has been reported to accelerate the aging process and the onset of age-related diseases leading to premature death (20, 21, 28, 51). Understanding the pathophysiological changes that occur with obesity is paramount to developing effective prevention/treatment interventions to combat the obesity epidemic.
Behavioral interventions targeting changes in diet are considered the safest and most cost-effective weight management strategies and thus have been at the cornerstone of interventions for populations that are overweight or obese (46). Unfortunately, ~80% of individuals who intentionally achieve weight loss by dietary restriction will regain that weight within one year (27, 41, 48). This process of continued unintentional weight gain followed by intentional weight loss has been termed weight cycling or the “yo-yo effect” (3, 27, 41). A number of reports have associated such weight fluctuations with increased morbidity; however, the majority of these studies have focused on outcomes related to cardiovascular disease risk factors, diabetes and/or insulin resistance, body composition, and cancer risk (3, 27, 41).
Chronic inflammation is a well-characterized hallmark of obesity and has been implicated as a driver of obesity-related chronic diseases (4, 11, 15). The proinflammatory environment associated with an obese phenotype is largely driven by macrophages. Macrophages in lean mice and humans make up ~5% of adipose tissue cells, whereas macrophages from mice and humans with obesity constitute up to 50% of adipose tissue cells (25, 47). In addition to increasing in number, macrophages change their localization and phenotype during obesity; in an obese state, adipose tissue macrophages polarize to an M1 proinflammatory phenotype and surround dead adipocytes, forming crown-like structures (25, 47). We and others (4, 6, 14, 15, 43) have shown that the increase in M1 macrophages is related to the severity of the obesity and that weight loss can mitigate this proinflammatory environment. Similarly, cytotoxic T cells increase in adipose tissue and have been associated with adipose tissue inflammation (18, 22, 29). However, there is limited evidence on the impact of weight fluctuations on immune and inflammatory markers in adipose tissue (2, 5, 8, 24, 33).
Considering that weight fluctuations are persistent in populations that are overweight or obese and have been linked with increased morbidity, it is important to investigate whether obesogenic programming (i.e., lasting effects due to prior obesity event) exists within the adipose tissue that may indicate negative consequences of weight fluctuations. Understanding this process could aid in the design of pharmacological and lifestyle interventions used to combat continuous weight fluctuations in patients. Therefore, the primary purpose of the present study was to investigate the immune and inflammatory status of adipose tissue in mice after undergoing weight loss and partial weight regain.
METHODOLOGY
Animals.
Male wild-type (WT) C57BL/6J mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and cared for at the Department of Laboratory Animal Resources (DLAR) facility at the University of South Carolina. Mice (n = 8–10/group) were housed five per cage, maintained on a 12:12-h light-dark cycle in a low-stress environment (22°C, 50% humidity, low noise), and given water ad libitum. Principles of laboratory animal care were followed, and all experiments were approved by the Institutional Animal Care and Usage Committee of the University of South Carolina.
Diets and weight cycling regimen.
At 6 wk of age, mice were randomly assigned to either control purified AIN-76A low-fat diet (LFD; 3.77 kcal/g, product no. F1515; BioServ, Frenchtown, NJ) or a purified high-fat died (HFD; 40% of total kcals from fat, 4.57 kcal/g) designed to mimic the standard American diet (product no. F6379, BioServ). Sucrose concentrations were 500 and 381.5 g/kg for LFD and HFD, respectively. We have used this HFD in several of our previous investigations (4, 11, 13, 14, 43, 44). At 21 wk of dietary intervention, a subset of mice on HFD were switched to LFD for 7 wk (this duration was based on the time required to reduce weight to that of the control LFD mice). After the 7 wk of weight loss, at week 28, mice were switched back to HFD (HFD→L→H) in tandem with another group of mice that were on LFD for 28 wk that were introduced to a HFD (LFD→H). Mice were maintained on HFD for 4 wk to sufficiently induce an obese phenotype and then euthanized, and tissues were collected. Control LFD and HFD groups also were maintained for 32 wk on their respective diets. The diet regimen for the four groups of mice is illustrated in Fig. 1A. During the last 4 wk (week 28 to week 32) of HFD feeding, we utilized pair feeding to control for caloric intake between LFD→H and HFD→L→H mice; this was done to eliminate any potential increase in food intake in mice that were previously exposed to a HFD that might confound our primary outcomes (i.e., immune and inflammatory markers). Pair feedings were determined by measuring the food consumption of LFD→H mice and averaging the food intake per cage each day before food was administered to the counterpart HFD→L→H group.
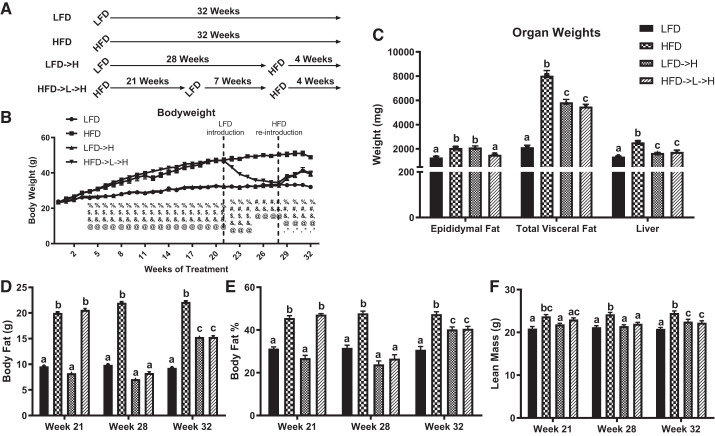
Fig. 1.
Body weight and composition of weight-cycling mice is comparable to mice not previously exposed to HFD. A: experimental design of weight cycling. B: body weight shows weight cycling causes reduction of body weight and then comparable regain of body weight to mice receiving HFD for first time. C: HFD→L→H mice have significantly lower epididymal fat mass but comparable total visceral fat and liver weights to LFD→H mice. DEXA was used to measure body fat in grams (g; D), body fat percentage (%; E), and lean mass (g; F) between groups. LFD, low-fat diet for 32 wk; HFD, high-fat diet for 32 wk; LFD→H, LFD for 28 wk and then changed to a HFD for 4 wk; HFD→L→H, HFD for 21 wk, then changed to LFD for 7 wk, and then changed to HFD for 4 wk. Data are shown as means ± SE (n = 8–10/group). Bar graphs not sharing a common letter are significantly different from one another according to one-way ANOVA (P < 0.05). Body weight measurements were tested against one another each week: %, HFD→L→H vs. LFD; #, HFD→L→H vs. HFD; $, HFD→L→H vs. LFD→H, &- HFD vs. LFD, @ - HFD vs. LFD→H; *, LFD→H vs. LFD indicate significance according to one-way ANOVA (P < 0.05).
Body weights and body composition.
Body weight was monitored on a weekly basis throughout the study. Body composition was assessed at every time point in which there was a change in diet and at the conclusion of the study (weeks 21, 28, and 32). For this procedure, mice were placed under brief anesthesia using isoflurane inhalation and were assessed for lean mass, fat mass, and body fat percentage via dual-energy X-ray absorptiometry (DEXA; Lunar PIXImus, Madison, WI).
Tissue collection.
At the conclusion of the study (32 wk), mice were euthanized via isoflurane inhalation overdose for tissue collection. Epididymal, kidney, and mesenteric fat pads as well as liver were removed, weighed, and immediately snap-frozen in liquid nitrogen and stored at −80°C or fixed overnight in 10% formalin and subsequently embedded in paraffin blocks via standard protocol.
Adipose and liver histopathology.
Formalin-fixed tissues were embedded in paraffin blocks and sectioned at 5 μm (8–10/group). The epididymal fat pads and livers were stained with hematoxylin and eosin (H&E) and trichrome according to standard protocols (44). Representative images were taken at ×10 and ×40 for epididymal fat tissue and at ×20 and ×40 for liver using a Nikon E600 microscope. Liver histopathology was assessed by a board-certified pathologist (I. Chatzistamou) blinded to the experimental conditions. Grading of the histological changes of the specimens was performed as described previously (43). Adipocyte size of epididymal fat tissue was measured using the Adiposoft plug-in to ImageJ (National Institutes of Health, Bethesda, MD). At least 150 adipocytes were assessed per sample. We performed histopathological quantification of adipose tissue fibrosis using trichrome staining of epididymal adipose tissue. The percent area fractions of fibrosis were measured using Material Image Processing and Automated Reconstruction (MIPAR) software, as previously described (37, 45). Five images were assessed for each mouse (n = 5 mice/group). All measurements were performed by an investigator blinded to the experimental conditions.
F4/80 (no. MCA497) (Bio-Rad, Hercules, CA) immunofluorescence (IF) was performed as previously described and detected with Alexa Fluor 594 goat anti-rat secondary antibody (Jackson ImmunoResearch no. 112-585-143) and counterstained with DAPI (4). Crown-like structures were identified and counted by an investigator blinded to the experimental conditions. Five images were assessed for each mouse (n = 3–5 mice/group). Total crown-like structures were counted and normalized to every 100 nuclei counted (26). Representative fluorescent images were taken at ×40 using a Leica DM2500 fluorescent microscope.
Quantitative real-time RT-PCR.
RNA isolation and RT-PCR were performed as previously described (38). Briefly, RNA was isolated from epidydimal adipose tissue (n = 8–10/group) using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). TaqMan reverse transcription reagents and gene expression assays (Applied Biosystems, Forster City, CA) were used to reverse transcribe and analyze the expressions of the following genes: monocyte chemoattractant protein-1 (MCP1), IL-6, IL-1β, TNFα, IFNγ, IL-10, EMR1 (EGF-like module containing, mucin-like, hormone receptor-like 1), CD11c, CD206, and CD8a. Reference genes HMBS, TBP, H2AFV, and HPRT were analyzed for stability using Qbase+ and utilized as the normalization factor. Gene expression quantification was calculated using the ΔΔCT method.
Western blotting.
Western blotting was performed as previously described (4). Briefly, liver tissue (n = 8–9/group) was homogenized in Mueller buffer containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Total protein concentration was determined by the Bradford method. Equal amounts of crude protein homogenates were fractioned on hand-casted 8–15% SDS-polyacrylamide gels and electrophoretically transferred to a PVDF membrane with a Genie Blotter (IDEA Scientific, Minneapolis, MN). Membranes were stained with Ponceau S solution to verify equal protein loading and transfer efficiency. Subsequently, membranes were blocked for 1 h in 5% nonfat milk in Tris-buffered saline-0.1% Tween-20 (TBST). Primary antibodies for total (no. 8242) and phosphorylated Ser536 (no. 3033) NF-κB (p65), total (no. 12640) and phosphorylated Tyr705 (no. 9145) STAT3, total (no. 8690) and phosphorylated Thr180/Tyr182 (no. 4511) p38 MAPK, and total (no. 9102) and phosphorylated Thr202/Tyr204 (no. 4370) ERK1/2 from Cell Signaling (Danvers, MA) were diluted 1:1,000 in 5% nonfat milk in TBST and incubated on membranes for 1 h at room temperature or overnight at 4°C. Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (Cell Signaling) was incubated with the membranes at 1:2,000 dilution in 5% nonfat milk in TBST for 1 h at room temperature. An enhanced chemiluminescent substrate for detection of horseradish peroxidase (Thermo Scientific, Waltham, MA) was used to visualize the antibody-antigen interaction. Autoradiography films were scanned, and blots were quantified using scientific imaging software (Image J). All samples were run on the same gel (n = 36) for each protein and scanned together; images were cropped (dotted line) for representation. After completion of the Western blot, all membranes were stained with Amido black solution, and the densitometry of each lane was calculated and utilized for total protein normalization. This method of normalization has been shown to be an acceptable alternative to typical loading controls (1).
Statistical analysis.
Data were analyzed using the commercially available statistical software SigmaStat v.3.5 (Systat Software, San Jose, CA). A one-way ANOVA followed by a Newman–Keuls post hoc analysis was used to determine differences between groups for normally distributed data. A Kruskal–Wallis test was used for nonparametric data (i.e., NASH and fibrosis scores). Any statistical test that did not pass normality or the equal variance test (Bartlett’s test for equal variances) was log transformed and then reanalyzed. Data are represented as means ± SE, and the level of significance was set at P < 0.05. Figures were generated using GraphPad Prism (San Diego, CA).
RESULTS
Weight cycling impacts fat mass and body composition.
As expected, all mice exposed to HFD treatment had increased body weight at the conclusion of the study (i.e., 32 wk; P < 0.05). The HFD group presented with the greatest body weight compared with HFD→L→H, LFD→H, and LFD (P < 0.05). There was no difference in body weight between HFD→L→H and LFD→H groups at the end of the study (as they were pair-fed) but both were elevated compared with LFD (Fig. 1B; P < 0.05). Total visceral fat (epididymal, kidney, and mesenteric) was significantly higher in HFD mice compared with LFD controls (Fig. 1C; P < 0.0001). Both LFD→H and HFD→L→H mice had lower total visceral fat compared with HFD mice (Fig. 1C; P < 0.05) but had higher total visceral fat compared with LFD controls (Fig. 1C; P < 0.05). There was no difference in total visceral fat between LFD→H and HFD→L→H groups (Fig. 1C). Mesenteric fat and kidney fat results were similar to total visceral fat (data not shown). However, epididymal fat mass was significantly higher in HFD and LFD→H mice Fig. 1C; (P < 0.05) compared with HFD→L→H and LFD, which were not different from each other (Fig. 1C). Liver weight was significantly higher in all groups exposed to HFD (Fig. 1C: P < 0.05) compared with LFD mice. Specifically, the HFD group presented with the greatest liver weight compared with HFD→L→H and LFD→H (Fig. 1C; P < 0.05). There was no difference in liver weight between HFD→L→H and LFD→H groups at the conclusion of the study (Fig. 1C).
Using DEXA, we determined the body composition of mice at all intervention time points (i.e., 21, 28, and 32 wk). Calculated body fat mass and percentage were significantly greater at the 21-wk time point in HFD and HFD→L→H mice compared with LFD→H and LFD controls (Fig. 1, D and E; P < 0.05). This difference was eliminated in HFD→L→H mice at the 28-wk time point, as expected, after 7 wk on a LFD. At the conclusion of the study, HFD mice had greater body fat mass and percentage compared with all groups (Fig. 1, D and E; P < 0.05). LFD→H and HFD→L→H mice had significantly greater body fat mass and percentage compared with LFD controls (P < 0.05) but did not show between-group differences (Fig. 1, D and E). At the 21-wk time point, lean mass was elevated in HFD compared with LFD and LFD→H (Fig. 1F; P < 0.05) but not HFD→L→H mice. Similarly, lean mass was greater in HFD→L→H compared with LFD mice (Fig. 1F; P < 0.05). At 28 wk, lean mass was greater in HFD mice compared with all other groups (Fig. 1F; P < 0.05). At the conclusion of the study, lean mass was elevated in HFD mice compared with all other groups (Fig. 1F; P < 0.05). Furthermore, LFD→H and HFD→L→H mice had elevated lean mass compared with LFD mice (Fig. 1F; P < 0.05) but were not different from each other.
Weight cycling leads to differences in epididymal adipocyte area and frequency distribution.
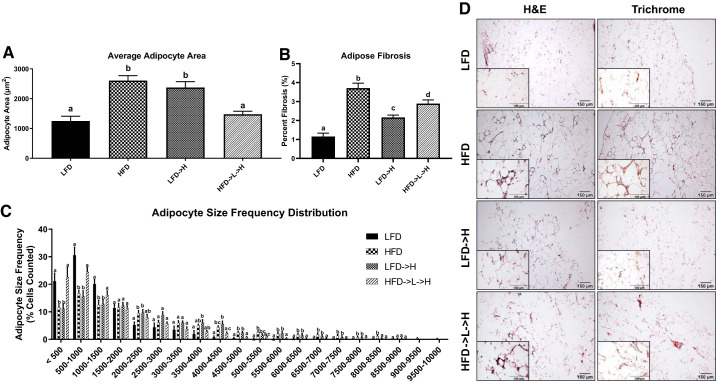
At the conclusion of the study (i.e., 32 wk) HFD and LFD→H mice showed greater average adipocyte area compared with LFD controls (Fig. 2A; P < 0.05). Interestingly, HFD→L→H showed lower average adipocyte area compared with HFD and LFD→H mice (P < 0.05) and was not significantly different from LFD (Fig. 2A). An adipocyte size frequency distribution was applied, which separated the adipocyte sizes into 500-μm2 increments. This analysis shows that HFD and LFD→H groups, in general, had greater numbers of larger adipocytes (e.g., 5,000 μm2 and above) compared with LFD and HFD→L→H groups, whereas LFD and HFD→L→H had significantly greater numbers of smaller adipocytes (500, 500–1,000, and 1,000–1 500 μm2; P < 0.05) compared with HFD and LFD→H groups (Fig. 2C). Trichrome staining was paired with H&E staining to observe the presence of fibrosis in the adipose tissue (Fig. 2D). As expected, we observed an increase in fibrosis in all groups exposed to HFD (Fig. 2, B and D). To confirm this finding, we measured the level of adipose tissue fibrosis on epididymal fat pad specimens stained with trichrome staining and then used MIPAR image analysis software to quantify the percent collagen deposition. This analysis revealed that mice in the HFD group had the greatest amount of collagen deposition compared with LFD, LFD→H, and HFD→L→H groups (Fig. 2, B and D; P < 0.05). LFD→H and HFD→L→H groups had significantly greater collagen deposition compared with LFD (P < 0.05) but remained lower than the HFD group (Fig. 2, B and D; P < 0.05). Finally, there was a significantly greater percentage of collagen deposition in HFD→L→H mice compared with LFD→H mice (Fig. 2, B and D; P < 0.05).
Fig. 2.
Epididymal adipose tissue of weight-cycling mice is smaller in area and contains a smaller adipocyte size frequency distribution. A: average adipocyte area measured from hematoxylin-eosin (H&E)-stained tissue sections. B: quantitative analysis of collagen deposition in epididymal fat pad. C: frequency distribution analysis of adipocytes measured in 500-μm increments indicating weight-cycling mice have different adipocyte sizes compared with HFD and LFD→H mice. D: representative ×10 H&E and Trichrome stains of epididymal adipose tissue; insets are ×40. LFD, low-fat diet for 32 wk; HFD, high-fat diet for 32 wk; LFD→H, LFD for 28 wk and then changed to a HFD for 4 wk; HFD→L→H, HFD for 21 wk, then changed to LFD for 7 wk, and then changed to HFD for 4 wk. Data are shown as means ± SE (n = 8–10/group). Bar graphs not sharing a common letter are significantly different from one another according to one-way ANOVA (P < 0.05).
Weight cycling leads to greater expression of immune cell and inflammatory markers compared with mice that were not previously exposed to HFD.
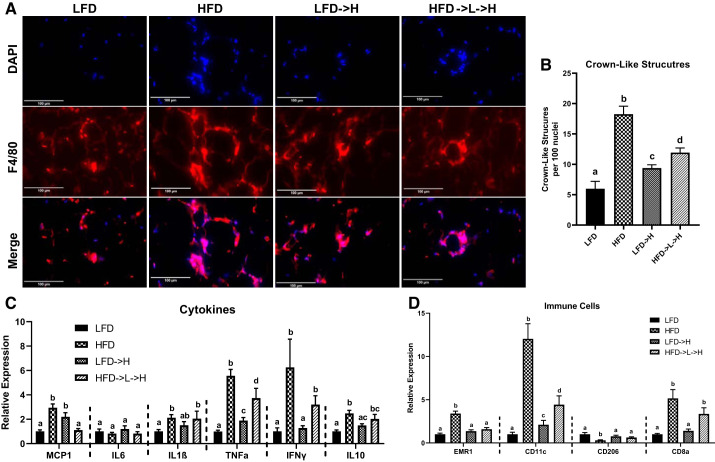
We utilized RT-PCR analysis to investigate the inflammatory status of the epididymal adipose tissue. HFD mice showed significantly greater MCP1, IL-1β, TNFα, IFNγ, and IL-10 (P < 0.05) compared with LFD controls (Fig. 3C). LFD→H mice exhibited increased MCP1 and TNFα (P < 0.05) compared with LFD controls (Fig. 3C), but there was no difference in IL-1β, IL-6, IFNγ, or IL-10. On the contrary, HFD→L→H mice did have increased IL-1β, IFNγ, and IL-10, as well as TNFα (P < 0.05), compared with LFD controls (Fig. 3C), but MCP1 was not significantly elevated. When HFD→L→H and LFD→H mice were compared, it was found that HFD→L→H mice had increased TNFα and IFNγ (P < 0.05) and decreased MCP1 (P < 0.05), but IL-1β and IL-10 were not different (Fig. 3C). When HFD to LFD→H were compared, we report that TNFα, IFNγ, and IL-10 were greater in HFD (P < 0.05) but not MCP1 or IL-1β (Fig. 3C). For the comparison between HFD and HFD→L→H, we found that MCP1 and TNFα were greater in HFD mice (P < 0.05) but there was no detectable difference for IL-1β, IFNγ, and IL-10 (Fig. 3C). There was no difference in IL-6 between any of the groups (Fig. 3C).
Fig. 3.
Epididymal fat gene expression indicates inflammatory memory may exist in weight-cycling mice. A: representative ×40immunofluorescence staining images of macrophage marker F4/80 in epididymal adipose tissue shows significant crowning in HFD and HFD→L→H mice. B: quantification of crown-like structures per 100 nuclei. C: gene expression of proinflammatory cytokines MCP1, IL-6, IL-1β, TNFα, IFNγ, and anti-inflammatory cytokine IL-10. D: gene expression of total macrophage marker EMR1, M1 macrophage marker CD11c, M2 macrophage marker CD206, and cytotoxic T cell marker CD8a. LFD, low-fat diet for 32 wk; HFD, high-fat diet for 32 wk; LFD→H, LFD for 28 wk and then changed to a HFD for 4 wk; HFD→L→H, HFD for 21 wk, then changed to LFD for 7 wk, and then changed to HFD for 4 wk. Data are shown as means ± SE (n = 8–10/group). Bar graphs not sharing a common letter are significantly different from one another according to one-way ANOVA (P < 0.05).
Mice exposed to HFD for 32 wk (i.e., HFD) showed increased expression of EMR1 (total macrophages), CD11c (M1 macrophages), and CD8a (cytotoxic T cells) (P < 0.05) but lower CD206 (M2 macrophages) (P < 0.05) compared with LFD controls (Fig. 3D). LFD→H mice did not show any difference in expression of EMR1, CD206, or CD8a compared with LFD controls but did show increased expression of CD11c (Fig. 3D; P < 0.05). Interestingly, HFD→L→H showed increased CD11c and CD8a compared with LFD and LFD→H mice (Fig. 3B; P < 0.05), but EMR1 and CD206 were not different across these groups (Fig. 3D). We utilized F4/80 immunofluorescence staining to observe any increase in macrophages in the adipose tissue. Increased crown-like structures could be observed in the adipose tissue of HFD, LFD→H, and HFD→L→H mice compared with LFD controls (Fig. 3A). We confirmed this finding by counting total crown-like structures observed. Using this method, we established that HFD mice had nearly four times the abundance of crown-like structures compared with LFD controls (Fig. 3B; P < 0.05). LFD→H mice showed about twice the abundance of crown-like structures compared with LFD controls (Fig. 3B; P < 0.05). Interestingly, HFD→L→H mice had significantly greater abundance of crown-like structures compared with LFD→H mice and LFD controls but remained lower compared with HFD (Fig. 3B; P < 0.05).
Weight cycling effects on nonalcoholic steatohepatitis score and liver fibrosis.
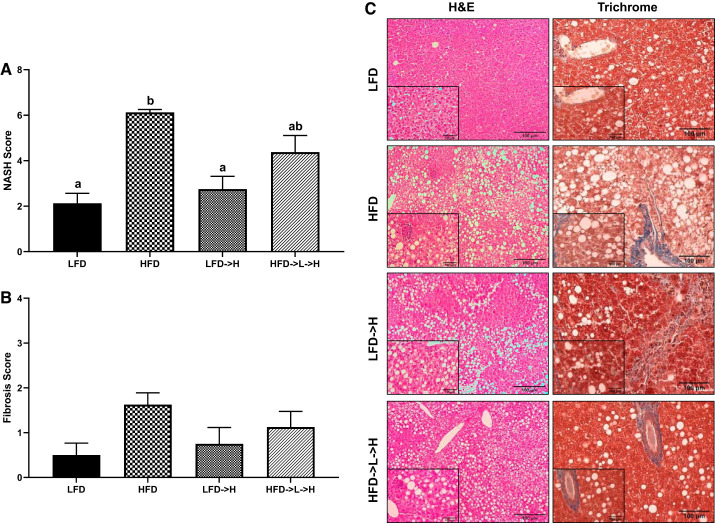
Activity score of nonalcoholic steatohepatitis (NASH) was increased in the HFD group compared with LFD→H and LFD mice (Fig. 4, A and C; P < 0.05). However, there was no difference between HFD and HFD→L→H groups. Furthermore, HFD→L→H mice were not statistically different from LFD→H and LFD groups (Fig. 4, A and C). There were no significant differences in liver fibrosis scores across all groups (Fig. 4, B and C).
Fig. 4.
Weight cycling results in similar nonalcoholic steatohepatitis (NASH) score as mice on a sustained high-fat diet. A: NASH score. B: fibrosis score. C: representative ×20 hematoxylin-eosin (H&E) and Trichrome stains of liver tissue; insets are ×40. LFD, low-fat diet for 32 wk; HFD, high-fat diet for 32 wk; LFD→H, LFD for 28 wk and then changed to a HFD for 4 wk; HFD→L→H, HFD for 21 wk, then changed to LFD for 7 wk, and then changed to HFD for 4 wk. Data are shown as means ± SE (n = 8–10/group). Bar graphs not sharing a common letter are significantly different from one another according to one-way ANOVA (P < 0.05).
Weight cycling effects on inflammatory liver protein expression.
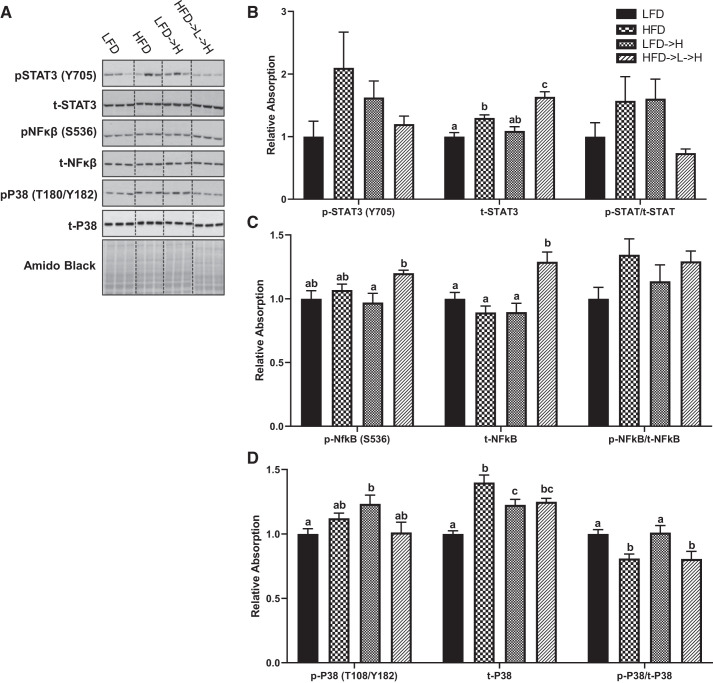
There were no observed statistical differences in phospho-STAT3 (Tyr705) across all groups (Fig. 5, A and B). However, an increase in total STAT3 was found in the HFD compared with the LFD mice (Fig. 5, A and B; P < 0.01). Total STAT3 was further increased in the HFD→L→H compared with all groups (Fig. 5, A and B; P < 0.01). There were no observed statistical differences in STAT3 phospho/total ratio across all groups (Fig. 5, A and B). We found a significant increase in phospho-NF-κB (Ser536) in the HFD→L→H compared with the HFD→L groups (Fig. 5, A and C; P < 0.05), and there was a trend for phospho-NF-κB (Ser536) to be increased in the HFD→L→H compared with LFD controls, but this did not reach statistical significance (P = 0.08). There was, however, an increase in total NF-κB in the HFD→L→H compared with all groups (Fig. 5, A and C; P < 0.01). There were no observed statistical differences in NF-κB phospho/total ratio across all groups (Fig. 5, A and C). For phospho-p38 (Thr180/Tyr182), we found an increase in HFD→L compared with the LFD group (Fig. 5, A and D; P < 0.05). There was an increase in total p38 in HFD compared with LFDmice (Fig. 5, A and D; P < 0.0001) and in LFD→H compared with LFD mice (Fig. 5, A and D; P < 0.01). We detected an increase in total p38 in HFD→L→H compared with LFD (Fig. 5, A and D; P < 0.001) and in total p38 in HFD compared with LFD→H mice (Fig. 5, A and D; P < 0.05). There was a significant reduction in p38 phospho/total ratio in both the HFD and HFD→L→H compared with both LFD and LFD→H groups (Fig. 5, A and D; P < 0.05).
Fig. 5.
Weight cycling increases total STAT3 and NF-κB pools within the liver tissue. A: representative immunoblots of phosphorylated (p-)STAT (Y705), total (t-)STAT3, p-NF-κB (S536), t-NF-κB, p-p38 (T180/Y182), t-p38, and amido black stain. Relative densitometry of p-STAT3 (Y705), t-STAT3, and p-STAT3/t-STAT3 (B), p-NF-κB (S536), t-NF-κB, and p-NF-κB/t-NF-κB ratio (C), p-p38 (T180/Y182), t-p38, and p-p38/t-p38 ratio (D). LFD, low-fat diet for 32 wk; HFD, high-fat diet for 32 wk; LFD→H, LFD for 28 wk and then changed to a HFD for 4 wk; HFD→L→H, HFD for 21 wk, then changed to LFD for 7 wk, and then changed to HFD for 4 wk. All data were normalized to average densities of the LFD groups. All samples were run on the same gel (n = 36) for each protein and scanned together; images were cropped (dotted line) for representation. Data are shown as means ± SE (n = 8–9/group). Bar graphs not sharing a common letter are significantly different from one another according to one-way ANOVA (P < 0.05).
DISCUSSION
Weight fluctuations are persistent among populations that are overweight or obese (27, 41, 48). This process of continued unintentional weight gain followed by intentional weight loss has been associated with increased morbidity in some, yet not all, studies (3, 27, 41). Specifically, studies have linked weight fluctuations to outcomes related to cardiovascular disease risk factors, diabetes and/or insulin resistance, body composition, and cancer risk (3, 27, 41). However, there is limited evidence on the impact of weight fluctuations on adipose tissue immune cell infiltration and subsequent inflammation, a process that is central to the pathophysiology associated with obesity. Moreover, there are some inconsistencies in the findings of the available reports (2, 5, 8, 24, 33). Using a mouse model of diet-induced obesity, we show that weight loss followed by partial weight regain leads to exacerbation of selected immune cell markers in the adipose tissue (epididymal fat pad), which is complemented by an increased production of proinflammatory cytokines, compared with high-fat diet-fed mice that did not undergo prior exposure to a high-fat diet. However, it is important to note that these parameters were lower in the weight cycling group compared with chronically fed high-fat diet mice.
Other investigations have shown evidence of obesogenic programming during various weight cycling paradigms (7, 9, 27, 33, 34, 41). However, reports on the impact of weight fluctuations on adipose tissue immune cell infiltration and inflammation are sparse, and findings are inconsistent (2, 5, 8, 24, 33). In the current study, we show the immune cell and inflammatory consequences of weight loss followed by partial weight regain within the adipose tissue (Fig. 3), which may have implications for individuals with obesity, given the well-documented association between adipose tissue immune cells and the metabolic syndrome (12, 16, 29, 32, 36, 43, 44). Unique to our study is the inclusion of a group that was fed a low-fat diet for 28 wk and then introduced to a high-fat diet (LFD→H) for 4 wk in tandem with the reintroduction of the high-fat diet to the weight loss followed by partial weight regain group (Fig. 1A), allowing for comparison of weight loss followed by partial weight regain versus a naive high-fat diet exposure. Specifically, our data support evidence of an increase in an M1 proinflammatory macrophage marker (CD11c; Fig. 3D), cytotoxic T cell marker (CD8; Fig. 3D), and select inflammatory mediators (TNFα and IFNγ; Fig. 3C), in high-fat diet-fed mice that had prior exposure to a high-fat diet (HFD→L→H) compared with mice that had no prior exposure (LFD→H). This was supported by an increased presence of crown-like structures in HFD→L→H mice (Fig. 3, A and B). Thus, despite the weight loss followed by partial weight regain mice reaching the body weight of low-fat diet-fed mice before being reintroduced to a high-fat diet, it is speculated that 1) M1 proinflammatory macrophages and cytotoxic T cells persist in the adipose tissue or 2) the adipose tissue is more sensitive to recruitment of M1 macrophages and cytotoxic T cells. We did not assess the adipose tissue inflammatory status before reintroduction of the high-fat diet (i.e., at 28 wk), and therefore are not able to make this determination. However, it is interesting to note that MCP1, an important chemokine for macrophage recruitment, was lower in the HFD→L→H group compared with LFD→H mice despite this group having elevated expression of the M1 marker CD11c (Fig. 3A). Thus, it is possible that fluctuations in weight may reduce the propensity for macrophage recruitment, suggesting that the documented increase in M1 macrophages in the HFD→L→H group may reflect macrophages that persist in the adipose tissue during the period of low-fat diet feeding. In either case, accumulation of M1 macrophages in the adipose tissue would be suggestive of more inflammatory adipose tissue and consequently poorer health.
Our data support previous studies documenting a relationship between CD8+ T cells and macrophages in obesity (22, 29, 40, 50). For example, an elegant study by Nishimura et al. (29) documented that infiltration by CD8+ T cells preceded the accumulation of macrophages and that depletion of CD8+ T cells lowered macrophage infiltration and adipose tissue inflammation. Conversely, adoptive transfer of CD8+ T cells to mice deficient in CD8 aggravated adipose inflammation (29). Similarly, others have reported a positive relationship between CD8+ T cells and macrophages in adipose tissue of rodents (22, 40, 50). However, there are some discrepancies in the literature regarding the order of immune cell infiltration. The majority of studies report that CD8+ T cells infiltrate the adipose tissue before macrophages, but some report the opposite order (22, 29, 40, 50). In our study, CD8a expression followed a similar trend as the M1 macrophage marker CD11c, confirming the relationship between these immune cells (Fig. 3D). However, the order of immune cell infiltration was not assessed. To our knowledge, only one other study examined CD8+ T cells following a weight cycling paradigm and reported an increase in CD8+ T cells, but adipose tissue macrophages were not increased (2).
It is no surprise that IL-1β, TNFα, IFNγ, and IL-10 expressions followed a similar trend as the M1 macrophage and CD8+ T cell findings, as it is well documented that these cytokines increase in adipose tissue with obesity, given their association with the aforementioned immune cells (4). However, there was no change in IL-6 expression in the adipose tissue of any of the groups (Fig. 3C); this is not surprising, given that we (4) have previously documented no change in this cytokine in response to chronic high-fat diet feedings. Our findings are largely supported by a study by Schmitz et al. (33) that reported indications of lasting effects of high-fat diet exposure, or obesogenic programming, on adipose tissue inflammation in obese mice and bariatric surgery patients after weight loss; however, reintroduction of a high fat diet was not completed in that study. Given that diet paradigms are inconsistent across studies, it makes it difficult to directly compare findings.
Although our findings appear to be consistent with the dogma that weight fluctuations may lead to unfavorable outcomes, it is important to note that expression of the M1 macrophage marker CD11c was actually lower in the HFD→L→H group compared with the chronically fed high-fat diet (HFD) group, suggesting that partial weight loss maintenance may have some benefits in reducing M1 macrophages and subsequent inflammation in populations with obesity (Fig. 3D). This is inconsistent with a previous report documenting an increase in adipose tissue inflammatory cytokines in a weight-cycling group above that of a chronically fed high-fat diet group (24). However, in the study by Li et al. (24), body weight was not different between the weight-cycling group and the chronically fed high-fat diet group, whereas our weight loss and partial weight regain group had a lower body weight than the chronically fed high-fat diet group. Thus, fat mass, prior exposure to high-fat diet, and duration of high-fat diet feedings clearly influence the expression of inflammatory mediators in the adipose tissue.
Our findings on the immune cell status in adipose tissue cannot be explained by alterations in body weight or body composition, as there was no difference in body weight, fat mass, or percent body fat between HFD→L→H and LFD→H groups (Fig. 1, B, D, and E). These groups were pair fed to prevent any potential increase in food consumption in mice that had prior exposure to a high-fat diet. Interestingly, epididymal fat was actually lower in HFD→L→H mice (Fig. 1C), the group with elevated expression of CD11c, CD8a, and proinflammatory cytokines, compared with the LFD→H group. In fact, the average adipocyte size of the epididymal fat pad in HFD→L→H mice was significantly lower compared with LFD→H mice (Fig. 2A). A size frequency analysis showed that weight-cycling mice (HFD→L→H) had more small adipocytes compared with an acute exposure to HFD (LFD→H; Fig. 2C). Indeed, weight loss decreases the amount of fat stored in adipocytes without changing the number, which ultimately results in an increased capacity to store fat. Furthermore, there is now evidence to suggest that smaller adipocytes may be associated with metabolic dysfunction including insulin resistance, suggesting that the proportion of various fat cell sizes should be considered in relation to metabolic health (39). Interestingly, collagen deposition (Fig. 2D) followed a similar trend as the M1 macrophage marker and crown-like structure data, suggestive of a relationship between these factors. Although more experiments are needed, our results provide evidence to suggest that prior exposure to a high-fat diet may create obesogenic programming in adipocytes, which promotes hyperplasia of adipocytes rather than hypertrophy (17, 19, 30). Both hypertrophy and hyperplasia contribute to increased adipose tissue mass. However, whether the increased hyperplasia in the HFD→L→H group had negative consequences related to weight gain could not be determined from the current study, given that there was no difference in body weight between HFD→L→H and LFD→H mice at the 32-wk time point. Longer exposure to high-fat diet, or ad libitum feeding, may tease out any potential consequences of the increased hyperplasia documented in the weight loss and partial weight regain group. Contrary to our findings, a study by Schofield et al. (34) reported that having multiple cycling events leads to a significant increase in internal fat deposition even when compared with animals on a sustained high-fat diet; however, it is important to note that, unlike in the current study, weight-cycling mice and mice on a sustained high-fat diet were of the same body weight, which likely contributed to the disparate findings. As such, different rodent diets, inconsistencies between the weight fluctuation paradigm, and whether pair feeding was included in the design likely contributed to the discrepancies between the studies.
While the primary focus of our study was assessment of immune cells and inflammation in the adipose tissue, we also assessed liver pathology, given its central role in metabolic processes (32, 35). Prolonged exposure to high-fat diet has consistently been shown to induce steatosis and NASH associated with NAFLD and obesity (43). The findings in the liver largely mirrored those of the adipose tissue. Consistent with the literature, HFD for 32 wk increased the NASH score compared with LFD and LFD→H (Fig. 4A). However, the weight loss and partial weight regain group (HFD→L→H) was not significantly different from any of the other groups. There was no difference in fibrosis scores across groups (Fig. 4B), suggesting that a longer period of high-fat diet feedings may be needed to observe significant fibrosis in the liver. Perturbations to liver inflammatory signaling with high-fat diet have been associated with NAFLD (15) and our results demonstrate subtle changes to liver STAT3 and p38 with high-fat diet (Fig. 5); however, weight loss and partial weight regain appeared to only increase total STAT3. Although we do not show increased activated or phosphorylated STAT3, the increase in total STAT3 is of interest to the potential mechanism of obesogenic programming. The modest changes in liver inflammatory signaling with the varying HFD groups are consistent with our NASH and NAFLD results.
Our findings contribute to the literature on the perturbations associated with weight fluctuations. A major strength of our study is the inclusion of a group that was fed a low-fat diet for 28 wk and then introduced to a high-fat diet (LFD→H) for 4 wk in tandem with the reintroduction of the high-fat diet to the weight loss and partial weight regain group. These groups that were pair fed allowed for a comparison of weight loss and partial weight regain versus a naive high-fat diet exposure that was not confounded by food intake. However, it also should be noted that there are several limitations to our study. Our major outcomes were assessed only at the study end point (i.e., 32 wk), precluding a complete assessment of immune cells and inflammatory mediators at the points at which diets were switched (i.e., 21 and 28 wk). In the adipose tissue, our assessment of immune cells and inflammatory mediators was limited to RT-PCR and immunohistochemistry (IHC); use of flow cytometry to assess immune cells in the adipose tissue would have complemented the RT-PCR and IHC work, providing a more comprehensive assessment of immune status. This is particularly true for the CD11c marker that has been widely used as an M1 macrophage marker in adipose tissue (25, 47) but also has been reported to be present on adipose tissue dendritic cells (42). Related to this, it should be noted that the M1/M2 dichotomous classification of macrophages does not properly reflect the true diversity and nature of resident/infiltrating macrophages in adipose tissue and should be interpreted cautiously. Extending the period for which the high-fat diet was reintroduced (i.e., beyond 4 wk) might have further contributed to our interpretations of the long-term consequences of weight cycling. Additionally, this study was not intended to look at sex differences; therefore, only males were included in the study, which limits translational relevance. Indeed, metabolic derangements in adipose tissue are known to be influenced by sex. For example, obese men, compared with obese women, have lower insulin sensitivity and elevated glucose levels promoting insulin resistance (10). In rodent models, male mice exhibit greater infiltration of proinflammatory macrophages in adipose tissue compared with females (31).
In summary, this study examined changes in adipose tissue immune cells and inflammatory markers in the context of weight fluctuations. Our data provide evidence of an increase in the expression of markers for M1 proinflammatory macrophages, cytotoxic T cells, and selected inflammatory mediators (TNFα and IFNγ), in high-fat diet-fed mice that had prior exposure to a high-fat diet compared with mice that had no prior exposure. While weight loss plus partial weight regain appears to exacerbate immune cell abundance and inflammation in adipose tissue compared with mice that were not previously exposed to high-fat diet, the severity is less than in mice that were subjected to sustained high-fat diet feedings. These data reinforce the importance of maintaining a body weight in the range that is recommended for optimal health to reduce immune and inflammatory perturbations associated with obesity.
GRANTS
This work was funded by the following grants: American Institute of Cancer Research 359566 (E. A. Murphy) and the National Institutes of Health 1R21 CA-191966 (E. A. Murphy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.T.S., B.N.V., T.L.C., R.T.E., K.T.V., J.E.B., and E.A.M. conceived and designed research; A.T.S., B.N.V., T.L.C., R.T.E., K.T.V., and J.E.B. performed experiments; A.T.S., B.N.V., T.L.C., R.T.E., K.T.V., S.M., and I.C. analyzed data; A.T.S., B.N.V., T.L.C., R.T.E., K.T.V., S.M., I.C., and E.A.M. interpreted results of experiments; A.T.S., B.N.V., T.L.C., and I.C. prepared figures; A.T.S., B.N.V., and E.A.M. drafted manuscript; A.T.S., B.N.V., T.L.C., R.T.E., K.T.V., S.M., J.E.B., I.C., and E.A.M. edited and revised manuscript; A.T.S., B.N.V., T.L.C., R.T.E., K.T.V., S.M., J.E.B., I.C., and E.A.M. approved final version of manuscript.
REFERENCES
- 1.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172: 250–254, 2008. doi: 10.1016/j.jneumeth.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes 62: 3180–3188, 2013. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson RL; National Task Force on the Prevention and Treatment of Obesity . Weight cycling. JAMA 272: 1196–1202, 1994. doi: 10.1001/jama.1994.03520150064038. [DOI] [PubMed] [Google Scholar]
- 4.Bader JE, Enos RT, Velázquez KT, Carson MS, Sougiannis AT, McGuinness OP, Robinson CM, Murphy EA. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am J Physiol Endocrinol Metab 316: E358–E372, 2019. doi: 10.1152/ajpendo.00438.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One 7: e39837, 2012. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia 59: 879–894, 2016. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownell KD, Greenwood MR, Stellar E, Shrager EE. The effects of repeated cycles of weight loss and regain in rats. Physiol Behav 38: 459–464, 1986. doi: 10.1016/0031-9384(86)90411-7. [DOI] [PubMed] [Google Scholar]
- 8.Caria CREP, Gotardo EMF, Santos PS, Acedo SC, de Morais TR, Ribeiro ML, Gambero A. Extracellular matrix remodeling and matrix metalloproteinase inhibition in visceral adipose during weight cycling in mice. Exp Cell Res 359: 431–440, 2017. doi: 10.1016/j.yexcr.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Chikamoto K, Misu H, Takayama H, Kikuchi A, Ishii KA, Lan F, Takata N, Tajima-Shirasaki N, Takeshita Y, Tsugane H, Kaneko S, Matsugo S, Takamura T. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem Biophys Res Commun 478: 1310–1316, 2016. doi: 10.1016/j.bbrc.2016.08.117. [DOI] [PubMed] [Google Scholar]
- 10.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46: 459–469, 2003. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 11.Cranford TL, Enos RT, Velázquez KT, McClellan JL, Davis JM, Singh UP, Nagarkatti M, Nagarkatti PS, Robinson CM, Murphy EA. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int J Obes 40: 844–851, 2016. doi: 10.1038/ijo.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 127: 74–82, 2017. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enos RT, Velázquez KT, McClellan JL, Cranford TL, Nagarkatti M, Nagarkatti PS, Davis JM, Murphy EA. High-fat diets rich in saturated fat protect against azoxymethane/dextran sulfate sodium-induced colon cancer. Am J Physiol Gastrointest Liver Physiol 310: G906–G919, 2016. doi: 10.1152/ajpgi.00345.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enos RT, Velázquez KT, Murphy EA. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem 25: 600–612, 2014. doi: 10.1016/j.jnutbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 16.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E, Amit I. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178: 686–698.e14, 2019. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang H, Kim M, Lee S, Kim J, Woo D-C, Kim KW, Song K, Lee I. Adipose tissue hyperplasia with enhanced adipocyte-derived stem cell activity in Tc1(C8orf4)-deleted mice. Sci Rep 6: 35884, 2016. doi: 10.1038/srep35884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang E, Perrard XD, Yang D, Khan IM, Perrard JL, Smith CW, Ballantyne CM, Wu H. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb Vasc Biol 34: 34–43, 2014. doi: 10.1161/ATVBAHA.113.302077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5: e1000324, 2009. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Dordr) 38: 23, 2016. doi: 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy RL, Chokkalingham K, Srinivasan R. Obesity in the elderly: who should we be treating, and why, and how? Curr Opin Clin Nutr Metab Care 7: 3–9, 2004. doi: 10.1097/00075197-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Khan IM, Dai Perrard XY, Perrard JL, Mansoori A, Wayne Smith C, Wu H, Ballantyne CM. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis 233: 419–428, 2014. doi: 10.1016/j.atherosclerosis.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Jiang L, Yang M, Wu YW, Sun JZ. Impact of weight cycling on CTRP3 expression, adipose tissue inflammation and insulin sensitivity in C57BL/6J mice. Exp Ther Med 16: 2052–2059, 2018. doi: 10.3892/etm.2018.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maliniak ML, Cheriyan AM, Sherman ME, Liu Y, Gogineni K, Liu J, He J, Krishnamurti U, Miller-Kleinhenz J, Ashiqueali R, He J, Yacoub R, McCullough LE. Detection of crown-like structures in breast adipose tissue and clinical outcomes among African-American and White women with breast cancer. Breast Cancer Res 22: 65, 2020. doi: 10.1186/s13058-020-01308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta T, Smith DL, Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes Rev 15: 870–881, 2014. doi: 10.1111/obr.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalakis K, Goulis DG, Vazaiou A, Mintziori G, Polymeris A, Abrahamian-Michalakis A. Obesity in the ageing man. Metabolism 62: 1341–1349, 2013. doi: 10.1016/j.metabol.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 30.Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol 537: 93–122, 2014. doi: 10.1016/B978-0-12-411619-1.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers NH, Perfield JW II, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rui L. Energy metabolism in the liver. Compr Physiol 4: 177–197, 2014. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz J, Evers N, Awazawa M, Nicholls HT, Brönneke HS, Dietrich A, Mauer J, Blüher M, Brüning JC. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol Metab 5: 328–339, 2016. doi: 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schofield SE, Parkinson JR, Henley AB, Sahuri-Arisoylu M, Sanchez-Canon GJ, Bell JD. Metabolic dysfunction following weight cycling in male mice. Int J Obes (Lond) 41: 402–411, 2017. doi: 10.1038/ijo.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schofield Z, Reed MA, Newsome PN, Adams DH, Günther UL, Lalor PF. Changes in human hepatic metabolism in steatosis and cirrhosis. World J Gastroenterol 23: 2685–2695, 2017. doi: 10.3748/wjg.v23.i15.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol 8: 709–716, 2012. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 37.Sosa JM, Huber DE, Welk B, Fraser HL. Development and application of MIPAR™: a novelsoftware package for two- and three-dimensionalmicrostructural characterization. Integr Materials 3: 123–140, 2014. doi: 10.1186/2193-9772-3-10. [DOI] [Google Scholar]
- 38.Sougiannis AT, VanderVeen BN, Enos RT, Velazquez KT, Bader JE, Carson M, Chatzistamou I, Walla M, Pena MM, Kubinak JL, Nagarkatti M, Carson JA, Murphy EA. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav Immun 80: 44–55, 2019. doi: 10.1016/j.bbi.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenkula KG, Erlanson-Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol 315: R284–R295, 2018. doi: 10.1152/ajpregu.00257.2017. [DOI] [PubMed] [Google Scholar]
- 40.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 18: 1918–1925, 2010. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strohacker K, Carpenter KC, McFarlin BK. Consequences of weight cycling: an increase in disease risk? Int J Exerc Sci 2: 191–201, 2009. [PMC free article] [PubMed] [Google Scholar]
- 42.Sundara Rajan S, Longhi MP. Dendritic cells and adipose tissue. Immunology 149: 353–361, 2016. doi: 10.1111/imm.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velázquez KT, Enos RT, Bader JE, Sougiannis AT, Carson MS, Chatzistamou I, Carson JA, Nagarkatti PS, Nagarkatti M, Murphy EA. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J Hepatol 11: 619–637, 2019. doi: 10.4254/wjh.v11.i8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velázquez KT, Enos RT, Carson MS, Cranford TL, Bader JE, Sougiannis AT, Pritchett C, Fan D, Carson JA, Murphy EA. miR155 deficiency aggravates high-fat diet-induced adipose tissue fibrosis in male mice. Physiol Rep 5: e13412, 2017. doi: 10.14814/phy2.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F, Wen J, Guo B, Wu L, Liu Z, Zaijun Z. Behavioral, biochemical and pathological characterization of a new MDX mouse model of Duchenne muscular dystrophy. J Pharm Biomed Sci 10: 119–128, 2020. [Google Scholar]
- 46.Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol 49: 810–823, 2020. doi: 10.1093/ije/dyz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–808, 2003. doi: 10.1172/JCI19246112/12/1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 21: 323–341, 2001. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 49.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62: 1723–1730, 2015. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 50.Yuan Y, Li H, Liao Y, Feng C. CD8+ T cells are involved in early inflammation before macrophages in a rat adipose tissue engineering chamber model. J Tissue Eng Regen Med 13: 1499–1506, 2019. doi: 10.1002/term.2836. [DOI] [PubMed] [Google Scholar]
- 51.Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes 29: 1011–1029, 2005. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]