Abstract
We examined the interactive influence of the muscle reflex (MR) and the chemoreflex (CR) on the ventilatory response to exercise. Eleven healthy subjects (5 women/6 men) completed three bouts of constant-load single-leg knee-extension exercise in a control trial and an identical trial conducted with lumbar intrathecal fentanyl to attenuate neural feedback from lower-limb group III/IV muscle afferents. The exercise during the two trials was performed while breathing ambient air ( ~97%, ~84 mmHg, ~32 mmHg, pH ~7.39), or under normocapnic hypoxia ( ~79%, ~43 mmHg, ~33 mmHg, pH ~7.39) or normoxic hypercapnia ( ~98%, ~105 mmHg, ~50 mmHg, pH ~7.26). During coactivation of the MR and the hypoxia-induced CR (O2-CR), minute ventilation (V̇e) and tidal volume (VT) were significantly greater compared with the sum of the responses to the activation of each reflex alone; there was no difference between the observed and summated responses in terms of breathing frequency (fB; P = 0.4). During coactivation of the MR and the hypercapnia-induced CR (CO2-CR), the observed ventilatory responses were similar to the summated responses of the reflexes (P ≥ 0.1). Therefore, the interaction between the MR and the O2-CR exerts a hyperadditive effect on V̇e and VT and an additive effect on fB, whereas the interaction between the MR and the CO2-CR is simply additive for all ventilatory parameters. These findings reveal that the MR:CR interaction further augments the ventilatory response to exercise in hypoxia.
NEW & NOTEWORTHY Although the muscle reflex and the chemoreflex are recognized as independent feedback mechanisms regulating breathing during exercise, the ventilatory implications resulting from their interaction remain unclear. We quantified the individual and interactive effects of these reflexes during exercise and revealed differential modes of interaction. Importantly, the reflex interaction further amplifies the ventilatory response to exercise under hypoxemic conditions, highlighting a potential mechanism for optimizing arterial oxygenation in physically active humans at high altitude.
Keywords: control of breathing, exercise hyperpnea, group III and IV muscle afferents, hypercapnia, hypoxia
INTRODUCTION
The ventilatory response to exercise is tightly regulated to optimize arterial oxygenation and acid-base balance and ensure, in conjunction with the circulatory response, that the metabolic demand of skeletal muscle is met during physical activity (23). Although central command, a feedforward mechanism, has traditionally been considered the primary driver of exercise hyperpnea (5, 21), the importance of the muscle reflex (MR) and the chemoreflex (CR) has increasingly been recognized in recent years (19, 23).
The muscle reflex is triggered by the excitation of mechano- and metabosensitive group III and IV muscle afferents during exercise (16, 34, 42, 45, 57). Both animal (6, 30, 52) and human (1, 27) studies have demonstrated a crucial role of the neural feedback from these muscle afferents in determining adequate ventilatory responses to exercise. The chemoreflex is predominantly triggered by the stimulation of O2-sensitive arterial chemoreceptors in the carotid and aortic bodies (i.e., the peripheral chemoreflex) and CO2-sensitive medullary chemoreceptors (i.e., the central chemoreflex). In contrast to maximal whole body exercise, arterial blood gases and other humoral chemoreceptor stimuli (e.g., plasma K+, norepinephrine) seem to only play a minor role in activating the chemoreflex during submaximal effort (18). Nevertheless, despite that the magnitude of the increase in ventilation with exercise intensity may not be directly dictated by the chemoreflex, a constant afferent discharge from the carotid chemoreceptors to medullary respiratory neurons is required to maintain eupneic breathing at rest and during exercise (8, 23, 24). Although various studies have attempted to delineate the individual significance of the muscle reflex and the chemoreflex for the control of breathing, the effect of their interaction for regulating the hyperpnea of voluntary exercise remains obscure (10).
Previous studies in humans, focusing on the interaction between the muscle reflex and the chemoreflex, have altered the concentration of blood gases (to stimulate chemoreceptors) during postexercise circulatory occlusion (to stimulate metabosensitive muscle afferents; 11, 20, 40), or during passive limb stretch/movement (to stimulate mechano-sensitive muscle afferents; 11, 53). However, because the reflexes were engaged at rest, excluding both the background influence of central command (10, 37) and the interplay between mechano- and metabosensitive afferents (13), the outcome of these studies may not fully represent the scenario during exercise. This issue was considered by Fregosi and Seals (26) who had subjects perform dynamic handgrip exercise with a hypoxic inspirate (and also in normoxia) while occluding forearm circulation to activate the muscle reflex and to prevent the confounding effect of systemic hypoxemia on the metabolic milieu in the active muscles. The authors found that the ventilatory response to hypoxic exercise exceeded the summation of the responses during normoxic exercise alone and during hypoxia alone (i.e., at rest). Hence, their findings implicated a hyperadditive interaction when the muscle reflex and the chemoreflex are activated concurrently during exercise (26). However, the ischemic exercise may have, inadvertently, stimulated the metabonociceptors, a subset of metabosensitive afferent fibers not activated by typical dynamic exercise (33, 38, 48). Thus, the exact nature of the MR:CR interaction and the associated consequence for the ventilatory response to exercise remain uncertain.
Therefore, by using pharmacological blockade of muscle afferent feedback to decrease the muscle reflex during leg exercise, while triggering the chemoreflex via hypoxia (O2-CR) and hypercapnia (CO2-CR), this study aimed to elucidate the effect of the MR:CR interaction on ventilation during exercise. Of note, despite a diminished effect of the muscle reflex in determining the ventilatory response to exercise when engaging only a small muscle mass, we chose moderate single-leg knee-extension exercise for a short duration to minimize neural feedback from other muscles (e.g., upper body), confounding limitations of the cardiopulmonary systems, and disparities in intramuscular metabolic perturbation often evident during whole body exercise in hypoxia or hypercapnia. We hypothesized that the MR:CR interaction would potentiate the exercise-induced ventilatory responses.
METHODS
Subjects.
All experimental protocols were approved by the Institutional Review Board of the University of Utah and by the Salt Lake City Veterans Affairs Medical Center. Following written and verbal explanations of all procedures and risks, written informed consent was obtained from each subject before enrollment in the study. Eleven recreationally active healthy volunteers participated in the study (5 females and 6 males; age: 26 ± 3 yr; body mass: 75 ± 15 kg; height: 176 ± 12 cm). The participants were nonsmokers, nonmedicated, and asymptomatic for cardiovascular or respiratory disorders. All participants refrained from exercise for 24 h and from caffeine and alcohol for 12 h before each study visit. Female participants were premenopausal and studied during the early follicular phase of the menstrual cycle, which was confirmed by their circulatory sex hormone levels obtained during each experimental trial (Table 1). Data collected in these subjects during this study, focused on the exercise pressor reflex and CR interaction and the cardiovascular implications, have been published previously (58).
Table 1.
Circulating levels of sex hormones in female subjects during the study trials
| Estrogen, pg/mL | Progesterone, ng/mL | FSH, mIU/mL | LH, mIU/mL | |
|---|---|---|---|---|
| Control | 133 ± 73 | 0.1 ± 0.1 | 4.7 ± 2.5 | 5.3 ± 5.9 |
| Fentanyl | 107 ± 53 | 0.8 ± 1.2 | 3.0 ± 3.0 | 5.2 ± 6.6 |
Values are means ± SD. Data were assessed from arterial blood samples; n = 5. Control, trial with intact leg muscle afferent feedback; fentanyl, trial with attenuated leg muscle afferent feedback using fentanyl blockade; FSH, follicle-stimulating hormone; LH, luteinizing hormone. No differences between the trials, P ≥ 0.31.
Experimental design.
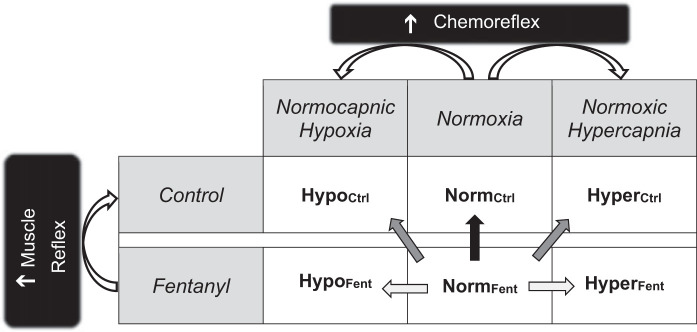
Participants initially performed an incremental single-leg knee-extensor test (10 + 10 W/min) to task failure to determine their peak work rate (Wpeak, 55 ± 15 W) and thereafter were familiarized with all noninvasive study procedures during an independent practice session. Subsequently, on two separate occasions, participants completed a control trial (Ctrl) and an identical trial during which feedback from µ-opioid receptor-sensitive muscle afferents was blocked via lumbar intrathecal fentanyl (Fent). All visits were separated by ≥48 h and conducted at the same time of day. The participants performed three bouts [4 min each, 60 revolutions/min (rpm)] of dynamic knee-extension exercise with the right leg at 60% of their Wpeak (33 ± 9 W). The three bouts of exercise in each of the two experimental trials (Ctrl and Fent) were conducted in ambient room air, i.e., normoxia (NormCtrl and NormFent), normocapnic hypoxia (HypoCtrl and HypoFent), and normoxic hypercapnia (HyperCtrl and HyperFent) conditions (Fig. 1). The exercise bouts were separated by a 6-min break breathing room air. The experimental trials and conditions were initially randomized and then counterbalanced.
Fig. 1.
Schematic illustration of the individual and the concurrent activation of the muscle reflex (MR) and the chemoreflex (CR) during exercise. Each of the two experimental trials (control and fentanyl) included 3 conditions (normoxia, normocapnic hypoxia, and normoxic hypercapnia). This design resulted in 6 bouts of single-leg knee-extension exercise, performed at a constant workload. NormCtrl and NormFent, control and fentanyl trials conducted in ambient room air, i.e., normoxia; HypoCtrl and HypoFent, control and fentanyl trials conducted under normocapnic hypoxia conditions; HyperCtrl and HyperFent, control and fentanyl trials conducted under normoxic hypercapnia conditions. The straight arrows denote the comparisons used to estimate the ventilatory effects of activation of the MR (black arrow), activation of the CR (light gray arrow), and coactivation of the MR and CR (dark gray arrow).
Intrathecal fentanyl injection.
Participants were seated in a flexed position, and 0.5 mL of an anesthetic solution containing 0.05 mg/mL fentanyl was delivered intrathecally at the vertebral interspace between L3 and L4 (3). To assess whether fentanyl migrated beyond the cervical level, the cardiorespiratory response to an arm-cranking test (15 and 30 W, 3 min each, 60 rpm; Monark-Crescent, Varberg, Sweden) was measured before the fentanyl administration and again after the exercise bouts were completed (1). Heart rate (HR) was monitored using a three-lead electrocardiogram (CardioCard; Nasiff Associates, Central Square, NY). The arm-cranking test was performed in both Ctrl and Fent to ensure a similar protocol across study trials. The time between fentanyl injection and the first exercise bout was between 15 and 25 min, and all participants completed the entire protocol within 75 min after the injection of fentanyl.
Ventilatory responses to exercise in normoxia, normocapnic hypoxia, and normoxic hypercapnia.
Ventilatory responses, i.e., minute ventilation (V̇e), breathing frequency (fB) and tidal volume (VT), and pulmonary gas exchange, i.e., end-tidal partial pressure of O2 () and CO2 (), O2 consumption, and CO2 production, were measured breath-by-breath using an open-circuit calorimetry system (Innocor; Innovision, Glamsbjerg, Denmark). Oxyhemoglobin saturation () was estimated using pulse oximetry (OxiMax N-600x; Nellcor, Minneapolis, MN) with a forehead sensor. To induce normocapnic hypoxia, participants inspired from a Douglas bag containing 8% O2, 3%–4% CO2, and balance N2 to achieve a decrease in to ~80%. and were then held constant by titrating pure O2 or CO2 in the inspirate. For the normoxic hypercapnia conditions, an increase in to ~50 mmHg was induced by inspiring a gas mixture of 6%–7% CO2, 21% O2, and balance N2; was maintained by titrating pure CO2 in the inspirate when needed. After the target level for hypoxemia or hypercapnia was reached (<45 s), participants sat quietly during a 2-min lead-in phase preceding the exercise bout. During each exercise bout, arterial blood samples were collected anaerobically via a radial artery catheter and assessed in a cooximeter and blood gas analyzer (GEM 4000; Instrumentation Laboratory, Bedford, MA).
Data processing.
For all respiratory responses, averages were calculated from every minute during each exercise bout. Based on the assumption that normoxic exercise performed with fentanyl blockade is characterized by minimal MR and CR activity, the ventilatory response during NormFent was considered as the baseline. The individual and interactive effects of the reflexes on the ventilatory response during the final minute of exercise were defined as follows (Fig. 1): 1) the individual effects of the MR, the O2-CR, and the CO2-CR were estimated by the differences between NormCtrl and NormFent (△NormCtrl-NormFent), between HypoFent and NormFent (△HypoFent-NormFent), and between HyperFent and NormFent (△HyperFent-NormFent), respectively, where a difference = 0 indicates no effect resulting from the reflex activation; 2) the interactive effects of the MR and O2-CR and of the MR and CO2-CR during the reflex coactivation were calculated as the differences between HypoCtrl and NormFent (△HypoCtrl-NormFent) and between HyperCtrl and NormFent (△HyperCtrl-NormFent), respectively, where a difference = 0 indicates no effect resulting from the reflex coactivation; 3) to investigate the mode of interaction between the reflexes, the observed ventilatory changes during the coactivation of the MR and the CR (O2-CR or CO2-CR) were compared with the arithmetic sum of the changes elicited by each reflex alone (△HypoCtrl-NormFent versus △NormCtrl-NormFent + △HypoFent-NormFent or △HyperCtrl-NormFent versus △NormCtrl-NormFent + △HyperFent-NormFent). The definition of the interaction mode has been previously described (53, 58). Briefly, the interaction was deemed hyperadditive if the response observed during the coactivation of the reflexes was greater than the summation of the responses evoked by each reflex alone, reflecting that the interactive effect was synergistic, while the interaction was deemed additive if the observed response was equal to the summated response, indicating that the interactive effect was a simple addition of the individual effects of the reflexes.
Statistical analysis.
Data were analyzed using statistical analysis software (SPSS 22; IBM, Armonk, NY). Descriptive statistics were used for the description of subject characteristics. The Mauchly’s test was used to determine sphericity, and the Geiser-Greenhouse correction was applied when sphericity was violated. The Student’s paired t-test was used to detect the difference in female sex hormone levels between the two experimental trials as well as the effect of fentanyl blockade on cardiorespiratory responses during the arm-cranking test. Two-way ANOVA (trial × time) with repeated measures was used to examine temporal changes in the gas exchange and ventilatory responses across the 4 min of exercise under each condition: normoxia, normocapnic hypoxia, and normoxic hypercapnia. Because this study aimed to activate the CR with hypoxia or hypercapnia and to investigate its interaction with the MR, data obtained from the hypoxic and hypercapnic conditions were also separately compared with those from the normoxic conditions. Thus, two-way ANOVA (trial × condition) with repeated measures was used to test alterations in arterial blood chemistry and ventilatory responses during the final minute of exercise. If the ANOVA detected a significant main effect or interaction, the Tukey’s post hoc analysis was performed to identify the differences. To directly test our hypotheses, a priori planned comparisons were made to determine the individual/interactive effects and the interaction mode of the reflexes on the ventilatory changes during exercise, using the Holm-Bonferroni method to correct for familywise error of multiple comparisons. The 95% confidence interval (95% CI) was also calculated for the difference between the observed and summated responses. All data are presented as means ± SD, and statistical significance was set at P < 0.05.
RESULTS
Cardiorespiratory responses to arm exercise.
Lumbar intrathecal fentanyl did not affect the cardiorespiratory responses to arm cranking (pre- vs. postinjection at 15 W/30 W; HR: 110 ± 20 versus 111 ± 20/121 ± 22 versus 122 ± 23 beats/min; fB: 23 ± 5 versus 22 ± 5/25 ± 4 versus 24 ± 6 breaths/min; VT: 1.1 ± 0.2 versus 1.1 ± 0.2/1.3 ± 0.3 versus 1.3 ± 0.2 L; ventilatory equivalent for CO2: 35 ± 3 versus 37 ± 3/34 ± 3 versus 36 ± 4; all P ≥ 0.13). This reflects the lack of significant cephalad migration and direct binding of fentanyl to cerebral µ-opioid receptors (36).
Gas exchange and ventilatory responses during exercise.
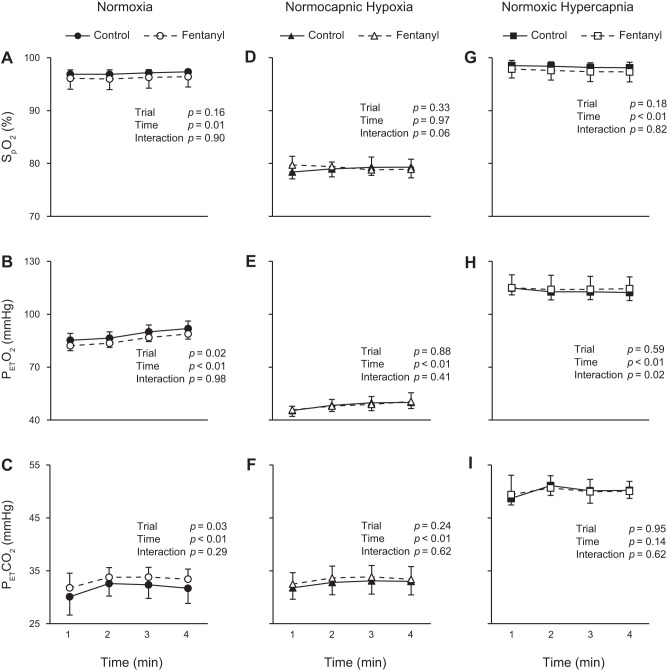
During the 4 min of exercise in normoxia, was not different between Ctrl and Fent (Fig. 2A) while was significantly higher and significantly lower in Ctrl than in Fent (Fig. 2, B and C; significant main effects of trial). Hypoxia and hypercapnia altered blood gases during exercise under each condition, with no significant differences between Ctrl and Fent in terms of , , and (Fig. 2, D–I).
Fig. 2.
Oxyhemoglobin saturation and gas exchange during exercise. Exercise was performed with intact (control) and blocked (fentanyl) leg muscle afferent feedback in nomoxia, normocapnic hypoxia, and normoxic hypercapnia conditions. , estimated oxyhemoglobin saturation; , end-tidal partial pressure of O2; , end-tidal partial pressure of CO2.
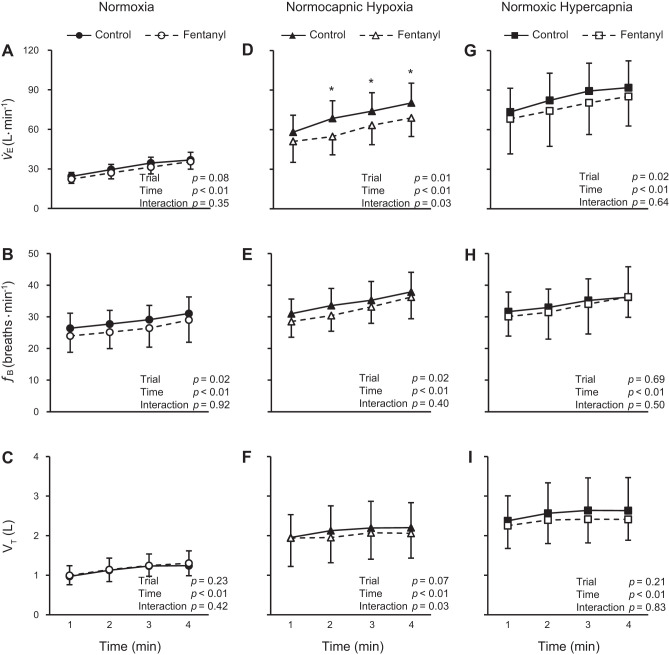
The ventilatory changes in response to the 4 min of exercise are illustrated in Fig. 3. During the normoxic exercise, fB was significantly decreased in Fent compared with Ctrl (Fig. 3B; significant main effect of trial) while V̇e and VT were similar between the trials (Fig. 3, A and C). During the hypoxic exercise, V̇e and fB were significantly lower in Fent compared with Ctrl (Fig. 3, D and E; significant main effects of trial) while VT was not different between the trials (Fig. 3F). Moreover, there was a significant interaction (trial × time) in terms of both V̇e and VT during the hypoxic exercise. During the hypercapnic exercise, V̇e was significantly lower in Fent compared with Ctrl (Fig. 3G; significant main effect of trial) while fB and VT were not different between the trials (Fig. 3, H and I).
Fig. 3.
Ventilatory responses during exercise. Exercise was performed with intact (control) and blocked (fentanyl) leg muscle afferent feedback in nomoxia, normocapnic hypoxia, and normoxic hypercapnia conditions. V̇e, minute ventilation; fB, breathing frequency; VT, tidal volume. *Significantly different from the fentanyl trial, P < 0.05.
Individual and interactive ventilatory effects of the MR, the O2-CR, and the CO2-CR.
The arterial blood chemistry and ventilatory data collected during the final minute of exercise in normoxia, hypoxia, and hypercapnia are documented in Tables 2 and 3. Figure 4 presents the separate and combined ventilatory effects triggered by the activation of the MR and the O2-CR during exercise. Individual activation of the MR (i.e., △NormCtrl-NormFent) had no significant effect on V̇e, fB, and VT. Individual activation of the O2-CR (i.e., △HypoFent-NormFent) significantly increased V̇e, fB, and VT during exercise. When both reflexes were activated concurrently (i.e., △HypoCtrl-NormFent), V̇e, fB, and VT significantly increased. As for the mode of interaction between the MR and the O2-CR (i.e., △HypoCtrl-NormFent versus △NormCtrl-NormFent + △HypoFent-NormFent), the V̇e and VT responses were significantly greater during the coactivation of the two reflexes compared with the summation of the responses elicited by each reflex alone (95% CI: V̇e = 10.3 ± 6.2 L/min; VT = 0.3 ± 0.2 L), reflecting a hyperadditive interaction. By contrast, the fB response to the coactivation of the MR and the O2-CR was not different from the summated response (P = 0.37; 95% CI = −0.5 ± 2.8 breaths/min), indicating an additive interaction.
Table 2.
Arterial blood chemistry and ventilatory responses during the final minute of normoxic and hypoxic exercise
| Normoxia |
Normocapnic Hypoxia |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Control | Fentanyl | Control | Fentanyl | Main effect of fentanyl | Main effect of hypoxia | Interaction effect | |
| , % | 97 ± 0 | 96 ± 2 | 79 ± 2† | 79 ± 1† | 0.12 | <0.01 | 0.23 |
| , mmHg | 84 ± 4 | 81 ± 3* | 43 ± 3† | 44 ± 5† | 0.03 | <0.01 | <0.01 |
| , mmHg | 32 ± 3 | 33 ± 2* | 33 ± 3† | 33 ± 2 | 0.05 | 0.23 | 0.02 |
| Arterial pH | 7.39 ± 0.03 | 7.37 ± 0.04 | 7.39 ± 0.03 | 7.37 ± 0.04 | 0.03 | 0.49 | 0.43 |
| Arterial K+, mmol/L | 3.9 ± 0.3 | 4.0 ± 0.3 | 3.9 ± 0.2 | 3.9 ± 0.2 | 0.46 | 0.26 | 0.23 |
| Arterial lactate, mmol/L | 3.0 ± 1.2 | 3.3 ± 1.1 | 3.0 ± 1.0 | 3.3 ± 0.8 | 0.04 | 1.00 | 0.63 |
| V̇e, L/min | 37 ± 6 | 35 ± 6 | 80 ± 15† | 69 ± 14*† | 0.02 | <0.01 | <0.01 |
| fB, breaths/min | 31 ± 5 | 29 ± 7 | 38 ± 6† | 36 ± 7† | 0.09 | <0.01 | 0.75 |
| VT, L | 1.2 ± 0.3 | 1.3 ± 0.3 | 2.2 ± 0.6† | 2.0 ± 0.6*† | 0.09 | <0.01 | <0.01 |
| V̇o2, L/min | 1.2 ± 0.2 | 1.3 ± 0.2 | |||||
| V̇co2, L/min | 1.1 ± 0.2 | 1.2 ± 0.2 | |||||
| V̇e/V̇o2 | 30 ± 4 | 27 ± 2* | |||||
| V̇e/V̇co2 | 32 ± 3 | 31 ± 2* | |||||
Values are means ± SD. Exercise was performed at 60% of peak work rate (33 ± 9 W); n = 11. Control, exercise performed with intact leg muscle afferent feedback; fentanyl, exercise performed with attenuated leg muscle afferent feedback using fentanyl blockade; , arterial oxyhemoglobin saturation; , arterial partial pressure of O2; , arterial partial pressure of CO2; V̇e, minute ventilation; fB, breathing frequency; VT, tidal volume; V̇o2, O2 consumption; V̇co2, CO2 production; V̇e/V̇o2, ventilatory equivalent for O2; V̇e/V̇co2, ventilatory equivalent for CO2.
P < 0.05 vs. control.
P < 0.05 vs. normoxia. Note, V̇o2 and V̇co2 were unable to be obtained in the hypoxic conditions because of gas titration and were analyzed using paired t-test; otherwise, all data were analyzed using 2 × 2 repeated-measure ANOVA and Tukey’s post hoc test.
Table 3.
Arterial blood chemistry and ventilatory responses during the final minute of normoxic and hypercapnic exercise
| Normoxia |
Normoxic Hypercapnia |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Control | Fentanyl | Control | Fentanyl | Main effect of fentanyl | Main effect of hypercapnia | Interaction effect | |
| , % | 97 ± 0 | 96 ± 2 | 98 ± 1† | 97 ± 2* | 0.05 | 0.04 | 0.78 |
| , mmHg | 84 ± 4 | 81 ± 3* | 105 ± 4† | 104 ± 7† | 0.01 | <0.01 | 0.01 |
| , mmHg | 32 ± 3 | 33 ± 2* | 50 ± 2† | 50 ± 1† | 0.01 | <0.01 | 0.02 |
| Arterial p | 7.39 ± 0.03 | 7.37 ± 0.04 | 7.26 ± 0.03† | 7.26 ± 0.03† | 0.37 | <0.01 | 0.06 |
| Arterial K+, mmol/L | 3.9 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.4 | 3.8 ± 0.4† | 0.61 | 0.18 | 0.02 |
| Arterial lactate, mmol/L | 3.0 ± 1.2 | 3.3 ± 1.1 | 2.4 ± 0.6 | 2.8 ± 0.9 | 0.14 | 0.10 | 0.96 |
| V̇e, L/min | 37 ± 6 | 35 ± 6 | 91 ± 20† | 85 ± 22*† | 0.05 | <0.01 | 0.13 |
| fB, breaths/min | 31 ± 5 | 29 ± 7 | 36 ± 7 | 36 ± 10† | 0.55 | 0.03 | 0.57 |
| VT, L | 1.2 ± 0.3 | 1.3 ± 0.3 | 2.6 ± 0.8† | 2.4 ± 0.5† | 0.44 | <0.01 | 0.26 |
| V̇o2, L/min | 1.2 ± 0.2 | 1.3 ± 0.2 | |||||
| V̇co2, L/min | 1.1 ± 0.2 | 1.2 ± 0.2 | |||||
| V̇e/V̇o2 | 30 ± 4 | 27 ± 2* | |||||
| V̇e/V̇co2 | 32 ± 3 | 31 ± 2* | |||||
Values are means ± SD. Exercise was performed at 60% of peak work rate (33 ± 9 W); n = 11. Control, exercise performed with intact leg muscle afferent feedback; fentanyl, exercise performed with attenuated leg muscle afferent feedback using fentanyl blockade; , arterial oxyhemoglobin saturation; , arterial partial pressure of O2; , arterial partial pressure of CO2; V̇e, minute ventilation; fB, breathing frequency; VT, tidal volume; V̇o2, O2 consumption; V̇co2, CO2 production; V̇e/V̇o2, ventilatory equivalent for O2; V̇e/V̇co2, ventilatory equivalent for CO2.
P < 0.05 vs. control.
P < 0.05 vs. normoxia. Note, V̇o2 and V̇co2 were unable to be obtained in the hypercapnic conditions because of gas titration and were analyzed using paired t-test; otherwise, all data were analyzed using 2 × 2 repeated-measure ANOVA and Tukey’s post hoc test.
Fig. 4.
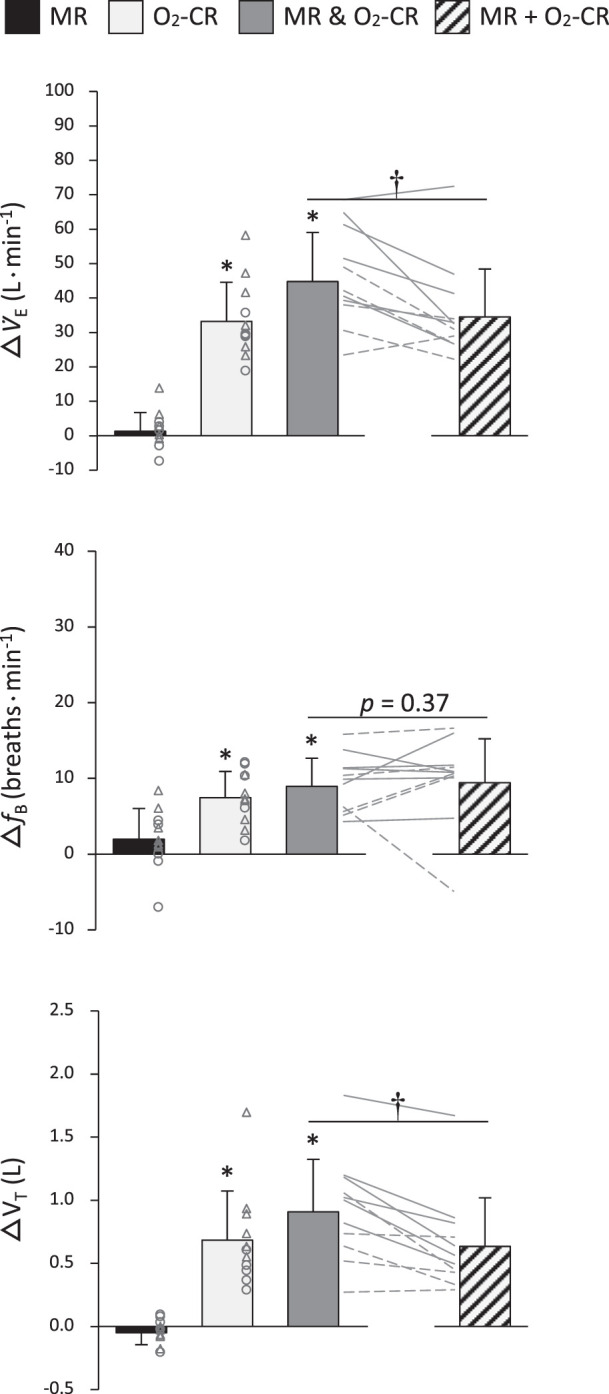
Ventilatory changes during individual and concurrent activation of the muscle reflex (MR) and the hypoxia-induced chemoreflex (O2-CR). MR & O2-CR, observed changes during coactivation of the MR and O2-CR; MR + O2-CR, sum of the changes induced by each reflex alone; V̇e, minute ventilation; fB, breathing frequency; VT, tidal volume. Individual subject data are shown for females (○ and broken lines) and males (△ and solid lines). *Significantly different from zero, P < 0.05; †significantly different between MR & O2-CR and MR + O2-CR, P < 0.05.
Figure 5 exhibits the separate and combined ventilatory effects triggered by the activation of the MR and the CO2-CR during exercise. Individual activation of the CO2-CR (i.e., △HyperFent-NormFent) significantly increased V̇e and VT during exercise while fB was unaffected. When the MR and the CO2-CR were activated simultaneously (i.e., △HyperCtrl-NormFent), V̇e, fB, and VT significantly increased. The mode of interaction between the MR and the CO2-CR (i.e., △HyperCtrl-NormFent versus △NormCtrl-NormFent + △HyperFent-NormFent) was additive for all variables, since the observed and the summated changes were similar (all P ≥ 0.13; 95% CI: V̇e = 5.5 ± 6.4 L/min; fB = −1.9 ± 6.3 breaths/min; VT = 0.3 ± 0.4 L).
Fig. 5.
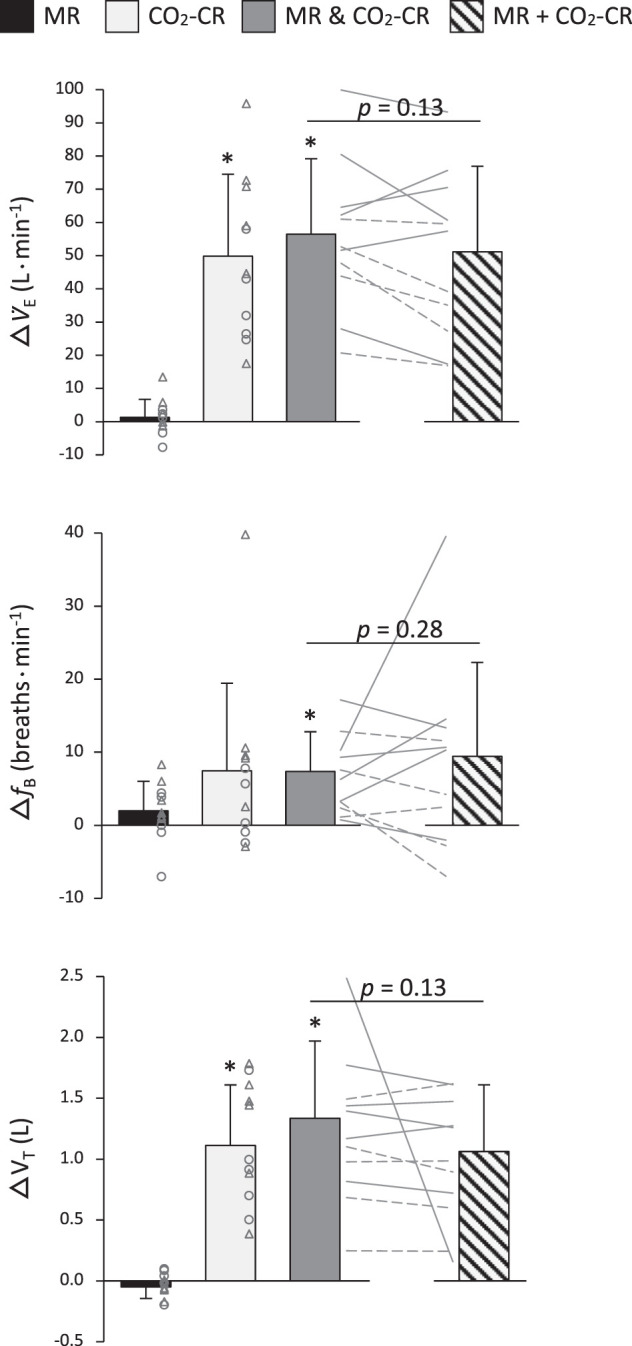
Ventilatory changes during individual and concurrent activation of the muscle reflex (MR) and the hypercapnia-induced chemoreflex (CO2-CR). MR & CO2-CR, observed changes during coactivation of the MR and CO2-CR; MR + CO2-CR, sum of the changes induced by each reflex alone; V̇e, minute ventilation; fB, breathing frequency; VT, tidal volume. Individual subject data are shown for females (○ and broken lines) and males (△ and solid lines). *Significantly different from zero, P < 0.05. No differences between MR & CO2-CR and MR + CO2-CR.
DISCUSSION
The goal of this study was to examine the individual and interactive influences of the muscle reflex and the chemoreflex on the ventilatory response to exercise, and to evaluate the mode of interaction between these reflexes. Muscle afferent blockade and hypoxia/hypercapnia were employed to manipulate the reflexes individually and in parallel. The results of these experiments suggest that the MR:O2-CR interaction is hyperadditive, meaning that, mainly through augmenting tidal volume, the ventilatory responses to exercise were larger than expected based on the summation of each reflex alone. When the chemoreflex is activated via hypercapnia, the interactive effect of the muscle reflex and CO2-CR was simply additive, that is, equaling the sum of the effects of each reflex alone. Taken together, this study documents that the interaction effect and associated ventilatory consequences of MR:CR coactivation are distinct when chemoreflex activation is achieved via hypoxia versus hypercapnia, revealing that the MR:CR interaction further amplifies the ventilatory response to exercise in hypoxia. Because the MR:CR coactivation in hypoxia has a synergistic effect on the ventilatory response to exercise, it is likely of particular importance for attempting to maintain arterial oxygenation in the physically active human at high altitude.
Ventilatory effects of the individual activation of the muscle reflex and the chemoreflex.
To assess the effect of individual activation of the muscle reflex, the ventilatory responses to exercise in the normoxic control and fentanyl trials were compared. Although the suppressing effect of the fentanyl blockade did not attain statistical significance in terms of minute ventilation (P = 0.08, Fig. 3A), the attenuation of the muscle reflex significantly compromised the ventilatory equivalents for O2 and CO2 (Table 2), and this alveolar hypoventilation resulted in consistent CO2 retention and reductions in arterial and end-tidal Po2 during exercise (Table 2 and Fig. 2, B and C). While these findings corroborate previous studies using the same exercise modality (4) and highlight the role of the muscle reflex in regulating the ventilatory response to single-leg knee-extension exercise, the observed ventilatory effect of the fentanyl blockade is of a much smaller magnitude than that documented during cycling exercise (1–3, 27, 47). The less significant impact of the fentanyl blockade on the ventilatory response to knee-extensor, compared with cycling, exercise is likely attributed to the smaller active leg muscle mass recruited during this exercise modality and, in turn, the lower group III/IV-mediated afferent feedback from the leg (22, 32, 43, 50).
It should be noted that intrathecal fentanyl exclusively affects μ-opioid receptor-sensitive muscle afferents and only blocks ~60% of group III/IV-mediated feedback (31). Although both group III and IV muscle afferents contain μ-opioid receptors, the exact distribution remains unknown, despite some findings indirectly suggesting predominance of δ-opioid receptors on the spinal endings of thinly myelinated pain fibers (51). Regardless, δ-opioid receptor-sensitive group III/IV muscle afferent neurons were unaffected in the present study and continued to discharge in response to muscle contractions (35), which caused an underestimation of the ventilatory impact of the muscle reflex. In addition, the ventilatory effect of the fentanyl blockade might also be underrated, due in part to the influence of attenuating leg muscle afferent feedback on the blood pressure response to exercise. Specifically, decreases in arterial blood pressure can increase minute ventilation (while increases in pressure decrease ventilation), a phenomenon known as the “ventilatory baroreflex” (12, 44, 56). Because the fentanyl blockade indeed effectively curtailed arterial blood pressure during the current knee-extension exercise (i.e., from ~108 to ~99 mmHg; data shown in Ref. 58), the ventilatory component of the arterial baroreflex could have elevated breathing, and this might have partially masked the fentanyl-induced reduction in minute ventilation during exercise. However, this influence was presumably small, since the impact of the ventilatory baroreflex is limited at rest (12) and becomes negligible above a mean arterial blood pressure of ~90 mmHg (56).
Finally, to assess the individual activation of the chemoreflex, hypoxia or hypercapnia was imposed during exercise with attenuated muscle afferent activity (i.e., little muscle reflex activation). As expected (11, 20, 26, 40, 53), the ventilatory responses increased remarkably (light gray bars in Figs. 4 and 5).
Ventilatory effect of the MR:CR coactivation.
To evaluate the effect of the MR:CR coactivation, the ventilatory response during exercise performed with high muscle reflex and chemoreflex activity (i.e., hypoxic/hypercapnic exercise with intact muscle afferent feedback) was compared with the ventilatory response during exercise performed with low activity of both reflexes (i.e., normoxic exercise with attenuated muscle afferent feedback). The differences in the ventilatory response between the two conditions were profound and indicate that the MR:CR coactivation further increases minute ventilation, breathing frequency, and tidal volume during exercise (dark gray bars in Figs. 4 and 5). In effect, the observed ventilatory changes are not surprising and attributable to the combined afferent inputs from both group III/IV muscle afferents and chemoreceptors to the pontomedullary respiratory center and the resultant augmentation in ventilatory neural drive (29, 39, 49). However, accumulating evidence has pointed to a potential interaction between the neural mechanisms (10), and it has yet to be determined whether these ventilatory changes are a simple addition of the effects of the muscle reflex activation and an increased chemoreflex, or are a consequence of the interactive effect of coactivating the two reflexes. To address this issue, as discussed below, we attempted to unravel the muscle reflex and the chemoreflex interaction by comparing the ventilatory response during the coactivation of these reflexes with the mathematical sum of the discrete responses evoked by the stimulation of each reflex.
Mode of the MR:CR interaction.
The coactivation of the muscle reflex and the O2-CR resulted in various modes of interaction with corresponding consequences for the ventilatory response to exercise. Specifically, the MR:O2-CR coactivation evoked a hyperadditive effect upon minute ventilation and tidal volume and an additive effect upon breathing frequency (Fig. 4). These findings corroborate an earlier investigation using circulatory occlusion during handgrip exercise in normoxia and hypoxia (26), a strategy used to establish similar metabolic milieus in the working muscle and assure a comparable engagement of central command and the muscle reflex in both gas conditions. The current observations are also in agreement with a study using passive exercise, an approach that does not engage central command and the metaboreflex. These authors found that the increase in minute ventilation during passive leg movement in hypoxia was greater than the summation of the minute ventilation increases elicited by leg movement and hypoxia alone and concluded that the interaction between the muscle mechanoreflex and the O2-CR was hyperadditive (53).
By contrast, a study using postexercise circulatory occlusion following handgrip exercise in hypoxia, an approach that does not engage central command and the mechanoreflex, demonstrated that the interaction effect resulting from the coactivation of the muscle metaboreflex and the O2-CR on minute ventilation was simply additive (20). Because the muscle mechanoreflex and the muscle metaboreflex are two major components of the muscle reflex, it is tempting to combine the outcome of those studies and postulate that the hyperadditive effect of MR:O2-CR coactivation in the present study is primarily driven by the activation of the muscle mechanoreflex. Nevertheless, a direct comparison between the current findings and those previous observations is rather problematic, since the current experiments, utilizing voluntary exercise, included central command, whereas the aforementioned studies were conducted in the absence of central command. This poses a critical discrepancy, since it has recently been documented that the ventilatory effect of the muscle metaboreflex could only be revealed in the presence of central command (37). Thus, it is plausible that the muscle mechanoreflex, the muscle metaboreflex, or both may have contributed to the hyperadditive effect of the MR:O2-CR coactivation observed in the present study.
In contrast to the findings from the MR:O2-CR trial (i.e., hyperadditive interaction), the coactivation of the muscle reflex and the CO2-CR resulted in an additive interaction for all ventilatory variables (Fig. 5). This difference is in correspondence with prior investigations which consistently documented that the gain of the hypoxic ventilatory response increases from mild to moderate exercise, i.e., enhanced chemosensitivity (17, 41, 59), while the gain of the hypercapnic ventilatory response remains unchanged with increases in exercise intensity (14, 15, 59). The current data suggest that the distinction of the relationship between minute ventilation and exercise intensity in hypoxia and hypercapnia may, at least in part, be a consequence of the different modes of MR:CR interaction.
The specificity of the gases used to activate the chemoreceptors in the present study likely contributed to the different interaction modes in MR:O2-CR compared with MR:CO2-CR. In particular, whereas normocapnic hypoxia predominately activates peripheral chemoreceptors, normoxic hypercapnia stimulates both central and, albeit to a lesser extent, peripheral chemoreceptors (25). Therefore, the interaction between the peripheral chemoreflex and the central chemoreflex may have further modulated the ventilatory response during the hypercapnic exercise (9, 28, 46, 54, 55) and, thus, may have led to the difference in the interaction mode between MR:O2-CR and MR:CO2-CR. Second, the excitatory inputs from peripheral and central chemoreceptors to the respiratory center in the brain stem are conveyed through divergent neuronal circuits and can be modified by other influences via synaptic interconnectivities (28, 29, 39). Third, the ventilatory effect of O2-CR can be potentiated by a muscle reflex-mediated increase in sympathetic outflow to the carotid bodies (7, 17) while the impact of an increase in sympathetic outflow to the central chemoreceptors is not clear. Consequently, chemoreflex-related neural inputs to the medulla may have been quite different in hypoxia compared with hypercapnia, potentially altering the central integration of the feedback from the muscle afferents and chemoreceptors, and, ultimately, resulting in differential modes of interaction.
Conclusion and significance.
This study documents that the interaction effect and associated ventilatory consequences of MR:CR coactivation are distinct when chemoreflex activation is achieved via hypoxia versus hypercapnia, revealing that the MR:CR interaction further amplifies the ventilatory response to exercise in hypoxia. Because the MR:CR coactivation in hypoxia has a synergistic effect on the ventilatory response to exercise, it is, therefore, likely of particular importance for attempting to maintain arterial oxygenation in the physically active human at high altitude. The ramifications of this reflex synergy are also relevant for clinical populations characterized by abnormal muscle reflexes and chemoreflexes (e.g., hypertension, heart failure) and offer a potential explanation for the patients’ aberrant ventilatory control and heightened exertional dyspnea.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute (HL-116579, HL-139451, and HL-091830) and the U.S. Department of Veterans Affairs (E6910-R, E1697-R, E1433-P, E9275-L, and E1572-P).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.-Y.W. and M.A. conceived and designed research; H.-Y.W., J.C.W., T.S.T., V.P.G., A.D.B., J.E.J., M.J.B., R.S.R., and M.A. performed experiments; H.-Y.W. analyzed data; H.-Y.W. interpreted results of experiments; H.-Y.W. prepared figures; H.-Y.W. drafted manuscript; H.-Y.W. and M.A. edited and revised manuscript; H.-Y.W., J.C.W., T.S.T., V.P.G., A.D.B., J.E.J., M.J.B., R.S.R., and M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate our participants’ enthusiasm and willingness to participate in this study.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol 589: 5299–5309, 2011. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asmussen E, Johansen SH, Jorgensen M, Nielsen M. On the nervous factors controlling respiration and circulation during exercise. Experiments with curarization. Acta Physiol Scand 63: 343–350, 1965. doi: 10.1111/j.1748-1716.1965.tb04073.x. [DOI] [PubMed] [Google Scholar]
- 6.Barron W, Coote JH. The contribution of articular receptors to cardiovascular reflexes elicited by passive limb movement. J Physiol 235: 423–436, 1973. doi: 10.1113/jphysiol.1973.sp010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biscoe TJ, Purves MJ. Factors affecting the cat carotid chemoreceptor and cervical sympathetic activity with special reference to passive hind-limb movements. J Physiol 190: 425–441, 1967. doi: 10.1113/jphysiol.1967.sp008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol (1985) 106: 1564–1573, 2009. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2). J Physiol 588: 2455–2471, 2010. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce RM, Jolley C, White MJ. Control of exercise hyperpnoea: Contributions from thin-fibre skeletal muscle afferents. Exp Physiol 104: 1605–1621, 2019. doi: 10.1113/EP087649. [DOI] [PubMed] [Google Scholar]
- 11.Bruce RM, White MJ. Muscle afferent activation causes ventilatory and cardiovascular responses during concurrent hypercapnia in humans. Exp Physiol 97: 208–218, 2012. doi: 10.1113/expphysiol.2011.061606. [DOI] [PubMed] [Google Scholar]
- 12.Brunner MJ, Sussman MS, Greene AS, Kallman CH, Shoukas AA. Carotid sinus baroreceptor reflex control of respiration. Circ Res 51: 624–636, 1982. doi: 10.1161/01.RES.51.5.624. [DOI] [PubMed] [Google Scholar]
- 13.Carrington CA, Ubolsakka C, White MJ. Interaction between muscle metaboreflex and mechanoreflex modulation of arterial baroreflex sensitivity in exercise. J Appl Physiol (1985) 95: 43–48, 2003. doi: 10.1152/japplphysiol.00895.2002. [DOI] [PubMed] [Google Scholar]
- 14.Casey K, Duffin J, McAvoy GV. The effect of exercise on the central-chemoreceptor threshold in man. J Physiol 383: 9–18, 1987. doi: 10.1113/jphysiol.1987.sp016392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JM, Sinclair RD, Lenox JB. Chemical and nonchemical components of ventilation during hypercapnic exercise in man. J Appl Physiol 48: 1065–1076, 1980. doi: 10.1152/jappl.1980.48.6.1065. [DOI] [PubMed] [Google Scholar]
- 16.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempsey JA, Gledhill N, Reddan WG, Forster HV, Hanson PG, Claremont AD. Pulmonary adaptation to exercise: effects of exercise type and duration, chronic hypoxia and physical training. 1 The marathon. Ann N Y Acad Sci 301: 243–261, 1977. doi: 10.1111/j.1749-6632.1977.tb38203.x. [DOI] [PubMed] [Google Scholar]
- 18.Dempsey JA, Rankin J. Physiologic adaptations of gas transport systems to muscular work in health and disease. Am J Phys Med 46: 582–647, 1967. [PubMed] [Google Scholar]
- 19.Dempsey JA, Smith CA. Pathophysiology of human ventilatory control. Eur Respir J 44: 495–512, 2014. doi: 10.1183/09031936.00048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgell H, Stickland MK. Activation of the carotid chemoreflex secondary to muscle metaboreflex stimulation in men. Am J Physiol Regul Integr Comp Physiol 306: R693–R700, 2014. doi: 10.1152/ajpregu.00472.2013. [DOI] [PubMed] [Google Scholar]
- 21.Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211: 844–846, 1981. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- 22.Estrada JA, Ducrocq GP, Kaufman MP. The magnitude of the exercise pressor reflex is influenced by the active skeletal muscle mass in the decerebrate rat. Am J Physiol Regul Integr Comp Physiol 318: R30–R37, 2020. doi: 10.1152/ajpregu.00263.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol 2: 743–777, 2012. doi: 10.1002/cphy.c100045. [DOI] [PubMed] [Google Scholar]
- 24.Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119: 199–208, 2000. doi: 10.1016/S0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- 25.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+. J Appl Physiol (1985) 108: 989–994, 2010. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fregosi RF, Seals DR. Hypoxic potentiation of the ventilatory response to dynamic forearm exercise. J Appl Physiol (1985) 74: 2365–2372, 1993. doi: 10.1152/jappl.1993.74.5.2365. [DOI] [PubMed] [Google Scholar]
- 27.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186: 606–615, 2012. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 28.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol (1985) 68: 2466–2472, 1990. doi: 10.1152/jappl.1990.68.6.2466. [DOI] [PubMed] [Google Scholar]
- 31.Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW, Amann M. Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. J Physiol 596: 1373–1384, 2018. doi: 10.1113/JP275465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iellamo F, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Role of muscular factors in cardiorespiratory responses to static exercise: contribution of reflex mechanisms. J Appl Physiol (1985) 86: 174–180, 1999. doi: 10.1152/jappl.1999.86.1.174. [DOI] [PubMed] [Google Scholar]
- 33.Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109: 2374–2381, 2013. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Kaufman MP. Stimulation of spinal δ-opioid receptors attenuates the exercise pressor reflex in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 316: R727–R734, 2019. doi: 10.1152/ajpregu.00013.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalley PM. Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285: R1287–R1304, 2003. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- 37.Lam E, Greenhough E, Nazari P, White MJ, Bruce RM. Muscle metaboreflex activation increases ventilation and heart rate during dynamic exercise in humans. Exp Physiol 104: 1472–1481, 2019. doi: 10.1113/EP087726. [DOI] [PubMed] [Google Scholar]
- 38.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsey BG, Nuding SC, Segers LS, Morris KF. Carotid bodies and the integrated cardiorespiratory response to hypoxia. Physiology (Bethesda) 33: 281–297, 2018. doi: 10.1152/physiol.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lykidis CK, Kumar P, Vianna LC, White MJ, Balanos GM. A respiratory response to the activation of the muscle metaboreflex during concurrent hypercapnia in man. Exp Physiol 95: 194–201, 2010. doi: 10.1113/expphysiol.2009.049999. [DOI] [PubMed] [Google Scholar]
- 41.Martin BJ, Weil JV, Sparks KE, McCullough RE, Grover RF. Exercise ventilation correlates positively with ventilatory chemoresponsiveness. J Appl Physiol 45: 557–564, 1978. doi: 10.1152/jappl.1978.45.4.557. [DOI] [PubMed] [Google Scholar]
- 42.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCloskey DI, Streatfeild KA. Muscular reflex stimuli to the cardiovascular system during isometric contractions of muscle groups of different mass. J Physiol 250: 431–441, 1975. doi: 10.1113/jphysiol.1975.sp011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMullan S, Pilowsky PM. The effects of baroreceptor stimulation on central respiratory drive: a review. Respir Physiol Neurobiol 174: 37–42, 2010. doi: 10.1016/j.resp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol 342: 383–397, 1983. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol 2: 221–254, 2012. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol 99: 414–426, 2014. doi: 10.1113/expphysiol.2013.075937. [DOI] [PubMed] [Google Scholar]
- 48.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99: 368–380, 2014. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci 25: 1965–1978, 2005. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossman MJ, Garten RS, Venturelli M, Amann M, Richardson RS. The role of active muscle mass in determining the magnitude of peripheral fatigue during dynamic exercise. Am J Physiol Regul Integr Comp Physiol 306: R934–R940, 2014. doi: 10.1152/ajpregu.00043.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137: 1148–1159, 2009. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senapati JM. Effect of stimulation of muscle afferents on ventilation of dogs. J Appl Physiol 21: 242–246, 1966. doi: 10.1152/jappl.1966.21.1.242. [DOI] [PubMed] [Google Scholar]
- 53.Silva TM, Aranda LC, Paula-Ribeiro M, Oliveira DM, Medeiros WM, Vianna LC, Nery LE, Silva BM. Hyperadditive ventilatory response arising from interaction between the carotid chemoreflex and the muscle mechanoreflex in healthy humans. J Appl Physiol (1985) 125: 215–225, 2018. doi: 10.1152/japplphysiol.00009.2018. [DOI] [PubMed] [Google Scholar]
- 54.Smith CA, Blain GM, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2: role of carotid body CO2. J Physiol 593: 4225–4243, 2015. doi: 10.1113/JP270114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol 173: 288–297, 2010. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am J Physiol Heart Circ Physiol 300: H1492–H1500, 2011. doi: 10.1152/ajpheart.01217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles. Some evidence for involvement of group III or IV nerve fibers. Circ Res 41: 332–341, 1977. doi: 10.1161/01.RES.41.3.332. [DOI] [PubMed] [Google Scholar]
- 58.Wan HY, Weavil JC, Thurston TS, Georgescu VP, Hureau TJ, Bledsoe AD, Buys MJ, Jessop JE, Richardson RS, Amann M. The exercise pressor reflex and chemoreflex interaction: cardiovascular implications for the exercising human. J Physiol 598: 2311–2321, 2020. doi: 10.1113/JP279456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weil JV, Byrne-Quinn E, Sodal IE, Kline JS, McCullough RE, Filley GF. Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol 33: 813–819, 1972. doi: 10.1152/jappl.1972.33.6.813. [DOI] [PubMed] [Google Scholar]