Abstract
We developed a novel ex vivo mouse protocol to mimic in vivo human soleus muscle function predicted by musculoskeletal simulations to better understand eccentric contractions during gait and ultimately to better understand their effects in Duchenne muscular dystrophy (DMD) muscles. DMD muscles are susceptible to eccentric injury because the protein dystrophin is absent. The mdx mouse, a DMD model that also lacks dystrophin, is often subjected to ex vivo acute but nonphysiological eccentric injury protocols. It is possible these acute protocols either over- or underestimate eccentric stresses and strains compared with those from humans during gait. To explore this possibility, healthy human soleus excitation, force, and length change profiles during a single walking stride (gait cycle) were simulated using OpenSim and then scaled to an ex vivo mouse soleus preparation based on muscle architectural measurements. Aurora Scientific, Inc., software and a 701C electrical stimulator were modified to discretely modulate muscle stimulation voltage at constant frequency and finely control muscle length changes to produce a force pattern that correctly mimicked the gait cycle from simulations. In a proof-of-principle study, wild-type and mdx mice soleus muscles were subjected to 25 gait cycles. Modest fatigue was evident in the muscles at the 25th versus first gait cycle for both genotypes, but both rapidly recovered isometric force within 1 min of the last cycle. These data indicate that the ex vivo gait protocol was well tolerated. More important, this protocol provides a novel assessment tool to determine the effects of physiological eccentric contractions on dystrophic muscle.
NEW & NOTEWORTHY A novel ex vivo mouse soleus protocol that mimics scaled length change and excitation profiles predicted by a mathematical model of human soleus during gait is presented. A custom stimulator was developed that enabled an innovative muscle stimulation technique to modulate voltage to closely match the excitation pattern of human soleus during gait. This ex vivo protocol provides assessment of simulated human movement in mouse muscle, including components of eccentric contractions.
Keywords: dystrophy, eccentric, function, gait, skeletal muscle
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a fatal, x-linked disease characterized by progressive muscle weakness that ultimately results in cardiorespiratory failure and death. At present, there is no effective cure, although there are several possibilities (50, 59). DMD is caused by mutations in the gene encoding the protein dystrophin. Mutations yield a disrupted reading frame that results in a nonfunctional dystrophin protein and subsequent loss of the dystrophin glycoprotein complex (DGC) from the sarcolemma (36, 39). In the absence of the DGC, mechanical and signaling functions are lost, and the muscle fibers degenerate, followed by incomplete regeneration (36, 55).
Mouse models of DMD such as the mdx mouse (26) have been widely used to study dystrophic pathophysiology and to assess potential treatments for DMD. In these models, ex vivo eccentric injury protocols have been be used to assess muscle contractility and membrane integrity (20, 42, 43, 63).
In typical eccentric injury protocols, muscles are lengthened at a given rate during a contraction set by a specific electrical stimulation frequency delivered at a constant voltage. Beyond these fundamental characteristics, protocols vary widely, including number of eccentric contractions, rest time between contractions, magnitude of length change, rate of length change, frequency and duration of stimulation, and initial starting length relative to the optimal length, i.e., the length at which muscle generates the maximum isometric force (5, 12, 30, 42). These methodological differences between protocols designed to acutely challenge a dystrophic muscle and the excitation, length, and force changes in a dystrophic muscle during a common movement such as gait potentially hinder better understanding of disease progression. For example, the stimulation characteristics of these protocols do not resemble the time-varying activations (i.e., action potentials) in human muscles during functional tasks such as walking. In addition, eccentric contractions during a movement such as gait begin below and finish above the optimal length (1, 9). Although some eccentric injury protocols use physiological strains, the stresses produced are typically supramaximal (12, 20, 43, 61), typically overestimating the eccentric forces during gait. To overcome this limitation, we developed a novel protocol to mimic human gait in mouse muscles ex vivo, including both the patterns and magnitudes of physiological strains and stresses. This approach provides greater insight into the effects of eccentric contractions on muscle function during gait. More importantly, in the context of DMD and other muscle diseases, this approach provides greater sensitivity for the assessment of disease progression and of treatments.
Musculoskeletal modeling and simulation provide detailed quantification of muscle function during movements in humans and other animals, such as mice. For example, three-dimensional musculoskeletal models of human lower limb, mouse hindlimb, and rat hindlimb have been developed based on a combination of extensive imaging and dissection measurements (2, 11, 28, 45). Studies using these models revealed the levels of muscle eccentric contractions during walking (24) and the differences in muscle stretch during walking between human and mouse (25). These insights provide a scientific basis that enables the development of a gait mimicking protocol for mouse muscle.
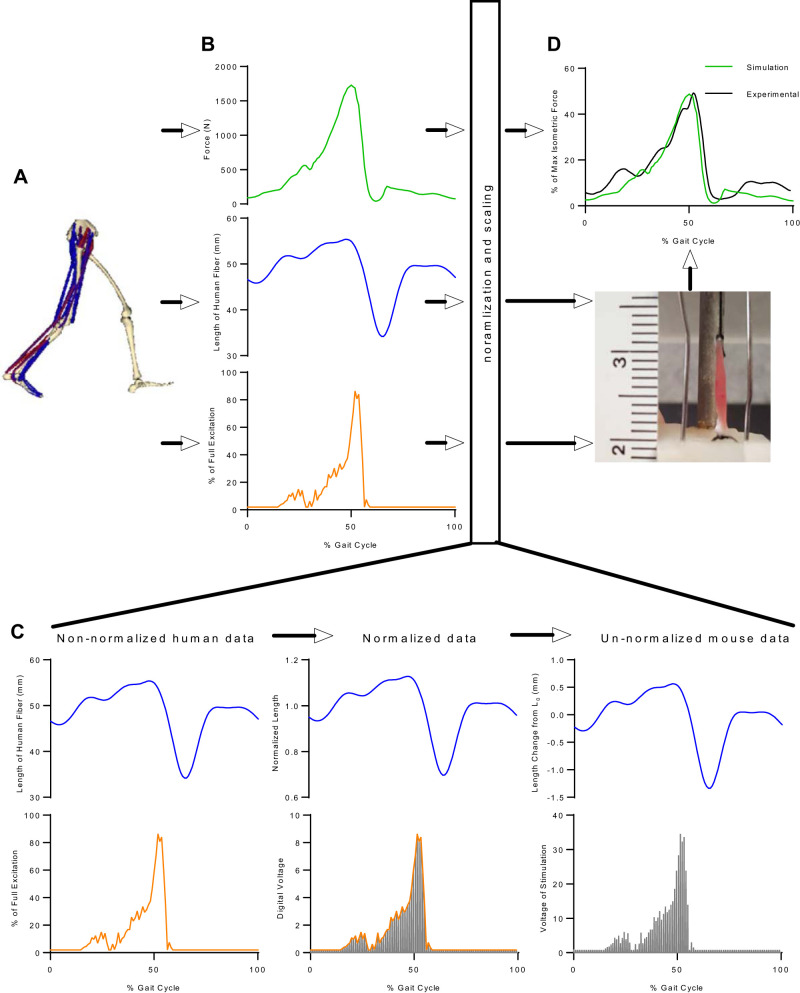
The purpose of this study was to develop a novel ex vivo mouse protocol to mimic in vivo human soleus muscle stresses and strains during walking (Fig. 1). This protocol incorporates the simulation-predicted nervous excitation pattern and length changes in human soleus responsible for the force changes during normal gait. Specifically, it scales length changes from human soleus to mouse soleus based on muscle architectural measurements and uses voltage modulation at a constant frequency to excite mouse soleus in a pattern close to human soleus. Force produced from the protocol is then compared with that from simulation to ensure comparable force levels with respect to their respective maximum isometric forces in the human and the mouse soleus (Fig. 1). We present herein the development of our ex vivo gait mimic protocol in mice.
Fig. 1.
Overview of ex vivo gait protocol development. A and B: dynamic musculoskeletal simulation of walking (A) was used to extract changes of force, length, and excitation of human soleus during walking (B). C and D: to mimic human gait in much smaller mice, the changes in force and length were normalized and/or scaled based on muscle architectural parameters (Lo and Fo), and the changes in excitation were adjusted based on the actual force of each individual mouse soleus (C); then, the processed changes in length and excitation were applied to mouse soleus in the ex vivo protocol, and the normalized forces from simulation were compared to ensure that comparable forces were produced in the ex vivo protocol (D).
MATERIALS AND METHODS
Dynamic Simulation of Human Gait
The dynamic musculoskeletal simulation of walking of a healthy human subject developed by John et al. (27) (publicly available at https://simtk.org/projects/muscleprops) was used to extract the necessary data describing muscle function in human walking gait. This set of dynamic gait simulation parameters represents a healthy male subject (height: 1.83 m; weight: 65.9 kg) walking at a self-selected speed on an instrumented treadmill (cadence: 109 steps/min; stride length: 1.45 m; speed: 1.36 m/s). The simulation was created using a three-dimensional (3D) musculoskeletal model (14, 57) consisting of 12 body segments, 19 degrees of freedom, and 92 muscle-tendon units in OpenSim (publicly available at https://simtk.org/projects/opensim) (13). Each muscle tendon unit is described by a Hill-type muscle model (56). Each lower-limb muscle has muscle-specific architectural and geometric parameters to characterize their contraction dynamics and force-generating capacity. Specifically, time-varying values of soleus excitation, fiber length, and force in one gait cycle were extracted from the results of the simulation.
Definitions
Definitions are as follows: 1) step length, from heel contact of one foot to heel contact of the contralateral foot; 2) stride length, from heel contact of one foot to heel contact of the same foot (i.e., 2 steps); and 3) gait cycle, the duration of a stride, which can also be expressed as time or as percent of support and swing for each lower limb to complete the stride (41).
In this study, we used a gait cycle of 60 cycles/min (1 Hz) close to the gait cycle of 55 cycles/min (0.92 Hz) of the simulation step rate described above (e.g., 109 steps/min divided by 2 steps per gait cycle, ∼55 cycles/min).
Scaling Data of Human Muscle in Walking to Mouse Muscle
To mimic function of human muscle in mouse muscle that is much smaller in size, scaling based on muscle architectural parameters was applied. Specifically, optimal fiber length of human soleus ( = 49.1 mm) from the lower-limb musculoskeletal model (14, 57) was used to calculate the normalized length of soleus in one gait cycle (Fig. 1C):
| (1) |
Where Lhuman(t) is the simulation-predicted length of a human soleus fiber at time instant t during one gait cycle. Then, the length of a mouse soleus fiber in one gait cycle [Lmouse(t)] corresponding to that of human soleus fiber was calculated by scaling the normalized fiber length by the optimal fiber length of mouse soleus ( = 4.4 mm) (10):
| (2) |
Finally, the length changes with respect to the optimal fiber length in mouse soleus ΔLmouse(t) were calculated as
| (3) |
The resulting data reported the predicted scaled change in length from the optimal fiber length (Fig. 1C). Scaling of the forces produced during gait was performed similarly to that for length. Therefore, the simulation-predicted forces [Fhuman(t)] of human soleus were normalized to the maximum isometric force of human soleus ( = 3549 N) (14, 57), and those of mouse soleus muscle [Fmouse(t)] to the measured maximum isometric force of mouse soleus muscle () from each individual mouse:
| (4) |
where species can be either human or mouse. Normalized forces from human [] and mouse [] soleus muscles were compared to ensure that comparable forces were generated in mouse soleus in the ex vivo protocol (Fig. 1D).
Equipment
Equipment used was as follows, from Aurora Scientific, Inc. (ASI) (https://aurorascientific.com/products/muscle-physiology):
-
•
Two 300C-LR Dual-Mode lever systems (servo motors); 1 N Force, 10 mm excursion
-
•
Two 603C: 16-bit Data Acquisition Card
-
•
Two 604A: Signal Interface
-
•
Two 615A: Dynamic Muscle Data Acquisition and Analysis software.
These programs are also known as dynamic muscle control (DMC) and dynamic muscle analysis (DMA) software (modified as noted below):
-
•
Two 701C: High Power, Bi-Phase Stimulators (modified as noted below).
Additional equipment consisted of a custom-built metal frame to hold two 25-mL Radnoti Jacketed water baths (no. 158326) (https://radnoti.com/d_product/components/tissue-organ-baths/1583-series-tissue-organ-baths) and a heat therapy pump (HTP)-1500 (http://adroitmedical.com/htp1500/).
Prescribe Soleus Fiber Length Change in Ex Vivo Experimental Protocol (No Soleus Muscle Required)
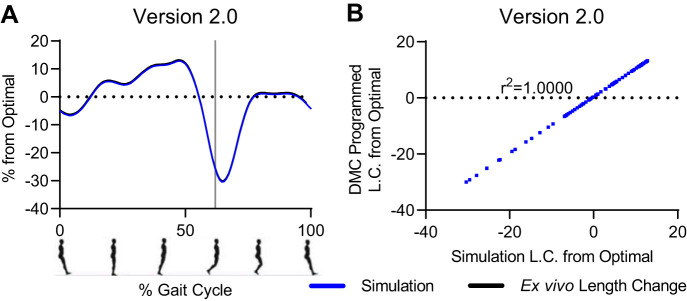
The first objective to create the ex vivo protocol to mimic in vivo human gait was to match the fiber length changes of human soleus predicted by the dynamic simulation of one gait cycle (i.e., 1 stride) to the length changes of mouse soleus fibers using Aurora Scientific’s DMC software. Note that we did not need a muscle to program DMC to mimic the length change profile; the movement of the servo motor lever arm was enough. The Aurora dual mode servo motor accepts both position and force commands written as a protocol in DMC and provides both position change and force responses of the lever arm in response to the protocol back to the DMC software. To develop the length change protocol, we compared the predicted length change profile from simulation with the position changes of the lever arm of the servo motor in response to the DMC protocol. Initially, the position of the lever arm was controlled by a written protocol within DMC consisting of sinewaves and ramps; this approach was effective on its own but ultimately not compatible with the stimulation protocol described later. The best approach to drive the lever arm position was to directly input the predicted scaled changes in length from Lo (Eq. 3). Predicted length changes were scaled and listed in digital format in a .txt file. Aurora Scientific modified the DMC software so that this .txt file holding the column of digital values could be uploaded into a DMC protocol. The digital values were translated to voltages by the digital-to-analog (D/A) converter to drive the lever arm directly. As expected, in the final ex vivo length change protocol, version 2.0, the position profiles of the lever arm and from gait simulation matched exactly, point for point (r2 = 1.0; Fig. 2).
Fig. 2.
After multiple iterations, the ex vivo length change protocol matched exactly the normalized, scaled length change predicted by OpenSim. A: overlay of length change profiles from OpenSim and length .txt file uploaded into dynamic muscle control (DMC); x-axis reported as %gait cycle that begins and ends with toe-off and heal strike at ∼62%. B: comparison of the value of points predicted in OpenSim gait simulation to values from length .txt file with r2 value. See text for additional details. Positive length changes indicate muscle stretch, and negative length changes indicate muscle shortening.
Animals and Soleus Muscles
All mouse protocols were approved by the Institutional Animal Care and Use Committee at Virginia Tech. Soleus muscles were obtained from male C57BL/10 mice at age 7–9 wk purchased from Jackson Laboratories. Mice were fed standard chow, provided water ad libitum, and maintained on a 12-h light-dark cycle. Mice were deeply anesthetized and soleus muscles dissected as described (52). Soleus muscles were subjected to contractile assays using an ASI Dual-Mode Lever System (servo motor) and were electrically stimulated by an ASI High Power Bi-Phase stimulator as described (52) and as modified (described below).
Prescribe Soleus Muscle Stimulation to Mimic Forces During Human Gait (Soleus Muscle Required)
The next objective was to determine how to control the muscle stimulator to mimic muscle forces during human gait. Most ex vivo stimulation protocols involve determination and maintenance of optimal voltage while the muscle is held at optimal length, then manipulating frequency of electrical stimulation to modulate the muscle’s force response. Optimal voltage is established to ensure that the ex vivo muscle is producing the maximum force at its optimal length and is typically established for the twitch or the tetanus (52). Thereafter, traditionally, the optimal voltage is used over a range of stimulation frequencies (e.g., 1–180 Hz). The largest predicted force produced during gait from simulation occurred at the eccentric peak (i.e., longest muscle length) and was 49% of isometric maximum force for the soleus (green lines in Fig. 1D). Additionally, the soleus muscle fiber length change during gait exceeded optimal length (maximum length of 1.13 Lo). Taken together, the soleus would be stretched beyond optimal length (i.e., eccentric peak) but needed to produce forces up to and less than 49% of maximum isometric force.
We explored three different strategies to control electrical stimulation and compared the forces that resulted from each strategy, with the force profile predicted by the model during the gait cycle. Throughout all subsequent stimulation development experiments, the muscles were subjected to the same length change protocol as described above that matched the predicted simulated length changes. Any differences and changes in the experimental force profiles were due to changes in the stimulation protocol. First, we used typical stimulation modulation techniques by altering frequency at a given voltage with the standard ASI 701C stimulator. Frequency from 1 to 200 Hz can be set by digital commands in the DMC software. Decreasing frequency low enough to hit the target force peak (49% of maximal isometric force) resulted in an unfused contraction. The force response to each stimulation pulse was visible (i.e., unfused) rather than the desired smooth, fused force response predicted by the gait simulation. This approach resulted in measured force exceeding predicted force (Supplemental Fig. S1; all supplemental material for this article can be found online at https://doi.org/10.6084/m9.figshare.12854966). For this reason, modulating frequency did not work.
Second, we modulated voltage in addition to frequency, which was promising but ultimately led to the development of a modified ASI 701C stimulator (described below). Pilot experiments indicated that stimulation at voltages below optimal but applied at a specific frequency could decrease force and produce a smooth, fused force profile, so we tested this approach. Although modulating voltage seemed a viable approach, the standard ASI 701C stimulator could not deliver individual stimulation pulses at different voltages; rather, a voltage set to 40 V meant every pulse of stimulation (e.g., 100 Hz) delivered to the muscle was 40 V (Supplemental Fig. S1). For these reasons, this strategy also did not work.
Third, we hypothesized that, with a constant frequency high enough to ensure fused contractions, continuously modulating voltage over time throughout the entire gait cycle to mimic the excitation pattern of the human soleus would produce the desired force profile in the mouse soleus.
Modified DMC software/hardware to accommodate modified 701C stimulator.
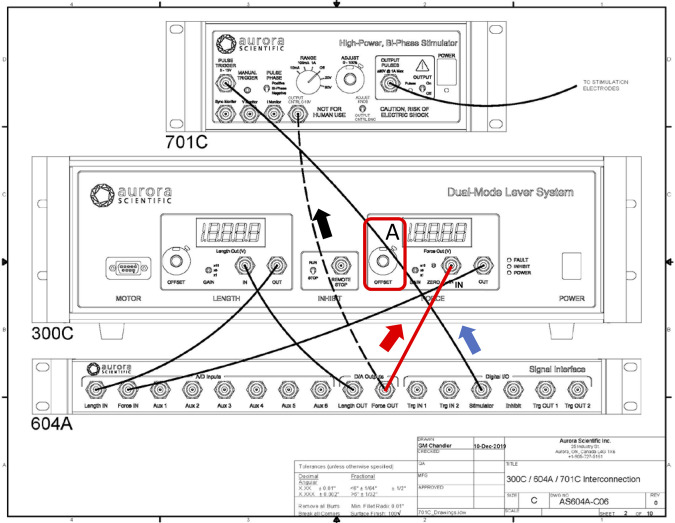
In the DMC software/hardware channel setup, there are four basic channels: length in, length out, force in, and force out. There is a fifth channel, stimulation trigger, which originates from the DMC software that connects via the 604A signal interface to the pulse trigger of the 701C stimulator (Fig. 3). “In” and “out” refer to the direction of the signal to (in) or from (out) the software via the 604A. For example, a command from DMC sending +4V of length out via the 604A to the length in connector of the 300C corresponds to +2 mm movement of the servo motor lever arm, whereas a command from DMC sending 3V of Force “Out” via the 604A to the 301C Force in connector corresponds to a force load on the arm of 300 mN. Conversely, DMC receiving 3V of force in via the 604A from the 301C force out connector (Fig. 3) corresponds to 300 mN of force measured by the servo motor lever arm. It was necessary that one of the two output channels be repurposed to deliver commands to control stimulator voltage. Four channels were essential for the gait cycle: 1) stimulation trigger, 2) length out, 3) length in, and 4) force in. These were required to 1) trigger the 701C stimulator to control stimulation frequency, 2) control and 3) record the position of the lever arm, and 4) record the muscle’s force production from the lever arm, respectively. Therefore, the only available channel to repurpose was force out (Fig. 3). This channel was previously used by the DMC software to set offset force on the lever arm. The offset force determines the maximum force the lever arm can hold to maintain an isometric muscle length; if the contractile force of the muscle exceeds this load, the lever arm moves down. However, there is also the option to set the offset force on the servo motor hardware with the force offset potentiometer (Fig. 3). Therefore, the force out channel (Fig. 3) was used to control the modified 701C stimulator to deliver the appropriate voltage of each stimulation pulse.
Fig. 3.
Interconnections diagram of Aurora Scientific, Inc., devices. Top: 701C High Power, Bi-Phase Stimulator. Middle: 300C Dual-Mode Lever System (servo motor). Bottom: 604A Signal Interface. The 604A provides the connections to and from the digital to analog card in the computer to the 701C and to/from the 300C. The connectors on the 604A are labeled “IN” (e.g., Force IN) and “OUT” (e.g., Force OUT), which refer to the direction of the signals to (IN) or from (OUT) the software via the 604A (see arrows for examples). The solid black lines and 1 red line indicate the standard connections between the devices. The force offset potentiometer (range: 0–10; A) is typically maintained at a setting of 0 so that the servo motor can be controlled by the dynamic muscle control (DMC) software via the 604A (i.e., 604A Force OUT to 300C Force IN; red line). To repurpose the force OUT channel in the DMC software to modulate voltage for each stimulation pulse, force offset (A) was set to 10 for maximum load on the servo motor lever arm, and the connection between 604A force OUT and the modified 701C stimulator connector established (black dashed line; see materials and methods and Fig. 5 for details), whereas the connection shown by the solid red line is removed. For use of the 701C to vary voltage of each stimulation pulse, all other connections remain intact.
ASI custom 701C stimulator to modulate voltage.
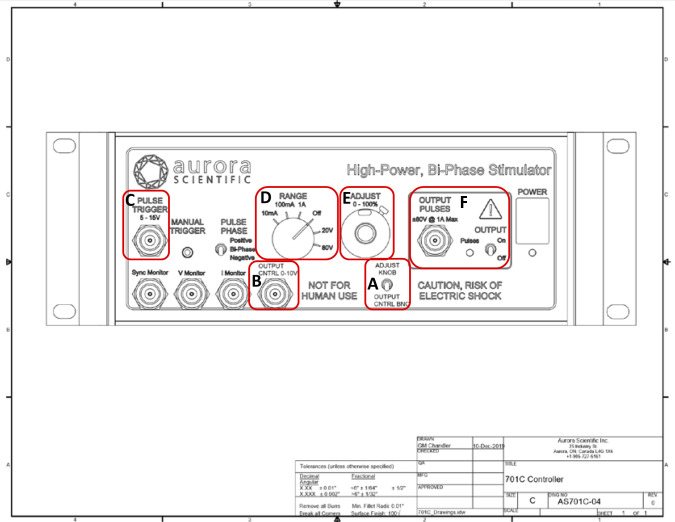
ASI modified their standard 701C stimulator so that voltage could be modulated using customized DMC software and hardware (Figs. 3 and 4). This approach was thought to better mimic the predicted excitation pattern for human soleus and, therefore, represent in vivo recruitment to regulate muscle force. A switch on the modified 701C stimulator (Fig. 4A) could be toggled “up” for standard DMC software control or toggled “down” for DMC voltage modulation control (Fig. 4A). For both the standard and modified stimulator, input to the pulse trigger (Fig. 4C) from the 604A (Fig. 3) controlled stimulator frequency. Voltage for the standard stimulator was set as follows: 1) range was set at 0–80 V (Fig. 4D), and 2) the Adjust potentiometer (Fig. 4E) was used to select the percentage of the range from 0 to 100% (e.g., a setting of 5.0 is 50% of 80 V = 40V output). For the modified stimulator, voltage was set as follows. As with the length .txt file to control the lever arm position, the modified DMC software could also upload an excitation .txt file to control 701C stimulator output (Fig. 4F). The voltage pattern delivered to the muscle with each stimulation pulse mimicked the pulse-by-pulse pattern of excitation determined by the gait simulation. The values in the excitation .txt file initially ranged from 0 (no excitation or rest) to 1 (full excitation). Each value in this file was multiplied by 10 to match the 0- to 10-V range of the DMC D/A converter. The ×10 multiplied digital values in the excitation .txt file were delivered to the modified stimulator from the 604A force out channel (Fig. 3A) to the modified 701C stimulator (Fig. 4B). Each digital value in the excitation .txt file produced a proportional output voltage from the 701C stimulator to the muscle, which was dependent on 1) the ratio of the maximum D/A converter 10-V output represented by each digital value and 2) the voltage range selected on the stimulator (i.e., either 0–20 V or 0–80 V; Fig. 4D). Note that the Adjust potentiometer (Fig. 4E) was not used to set the percent voltage range; rather, the voltage value per pulse was controlled by the excitation .txt file. For example, a 4-V value in the excitation .txt file input to the stimulator would result in a 32-V stimulator output to the muscle with the voltage range set to 0–80 V [i.e., 4 V/10 V (D/A ratio) × 80 V (range set on stimulator) = 32 V].
Fig. 4.
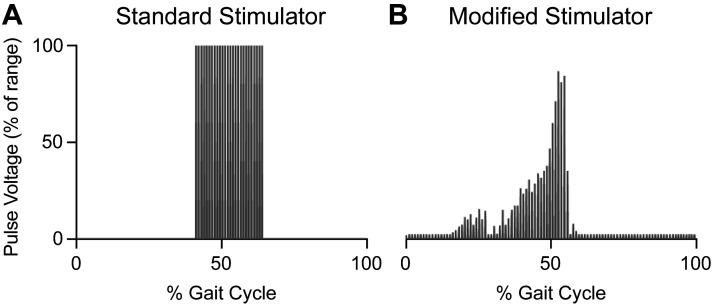
Aurora Scientific, Inc., modified 701C High Power, Bi-Phase Stimulator. A: toggle switch for standard stimulator output at a set voltage driven by standard dynamic muscle control (DMC) software commands (up position) or to accept the voltage signal sent by DMC .txt file via 604A force out channel to modulate voltage for each stimulation pulse (down position). B: connector that receives the signals from DMC software .txt file via 604A force out channel. C: pulse trigger input to control stimulator frequency. D: set voltage range knob. E: adjust potentiometer to set %voltage range (e.g., 5.0 is 50% of 80 V = 40 V). F: voltage delivered to the muscle baths (i.e., Output Pulses). Note: C, D, and F were unchanged for either standard or modified stimulation; E was not used with the modified stimulation setting.
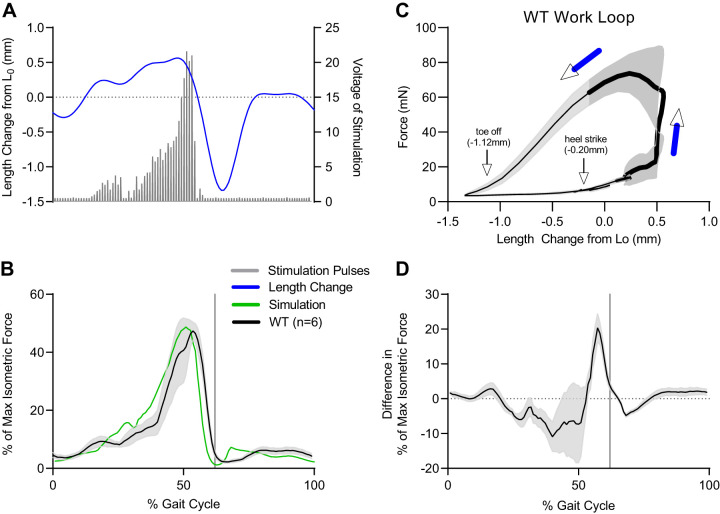
There was a clear difference in voltage pattern delivered by the standard (Fig. 5A) compared with modified 701C stimulator (Fig. 5B). The total number of digital values in the excitation .txt file was 222; therefore, the digital file was delivered at a frequency of 222 Hz, which corresponds to one gait cycle per second. The soleus force response using this stimulation method during the length change protocol reasonably matched the predicted force profile (Fig. 6, B and D). The error between the ex vivo force response and the simulation-predicted force was ±10% of maximum isometric force for most of the gait cycle (Fig. 6D). Compared with the simulation force, ex vivo forces were lower during muscle lengthening (0–50%) and higher during muscle shortening (50–60%). The largest error occurred at ∼57% of the gait cycle right before toe off, when the muscle was rapidly shortening. Note, the standard deviation of the ex vivo force response was minimal during this shortening phase, suggesting that all muscles responded consistently. The difference between simulated and ex vivo forces during shortening could be due to a modest discrepancy between the muscle force-velocity relationship used in the simulation compared with the actual relationship for mouse soleus. Nevertheless, the shape and peak of the ex vivo force responses still closely mimicked the simulation-predicted force (Fig. 6D). A counterclockwise work loop revealed that the greatest force was generated during muscle shortening, indicating that the muscles produced positive work (Fig. 6C).
Fig. 5.
Voltage of stimulation pulses delivered by the standard vs. modified ASI 701C stimulator. A: with the standard stimulator, a given frequency was delivered at a constant voltage. The magnitude of voltage was determined by both the range knob (0–20 V or 0–80 V) and the Adjust potentiometer (0–100%). With the standard 701C stimulator, the voltage delivered would be 100% of the voltage determined by these 2 settings. B: with the modified 701C stimulator, the selected frequency was delivered, but the voltage was modulated for each stimulation pulse. The voltage delivered was determined by the range knob (0–20 V or 0–80 V) and digitally by the excitation .txt file. This approach best mimicked the predicted excitation pattern from the human soleus simulation.
Fig. 6.
Force response to stimulation following the simulated, predicted excitation pattern. A: pattern (frequency and magnitude in volts) of stimulation pulses is shown as gray bars. With the modified 701C stimulator, the selected frequency was delivered, but the voltage was modulated for each stimulation pulse (gray bars). B and C: the voltage delivered was determined by the range knob (0–20 V or 0–80 V) and digitally by the excitation .txt file. This approach best mimicked the predicted excitation pattern from the human soleus simulation. The ex vivo force response (black line, n = 6; gray shaded area is SD) to this dynamic stimulation matched the predicted force profile (green line; B). The corresponding work loop shows where stimulation occurred (bold black line) and with black arrows, heel strike where the protocol began and ended, and toe-off at ∼62% of the gait cycle (C). The average error between the ex vivo force and predicted force profiles shown in B is represented by the black line and the SD of this error by the shaded area. D: the greatest error occurred at 57% of gait cycle, just before toe-off (62% of gait cycle; vertical gray line). WT, wild type.
Additional excitation voltage modulation.
Recall that the percent maximum isometric force predicted by the simulation was 49%. The voltage range needed to achieve up to 49% of maximal isometric force was unique for each muscle, so additional voltage modulation was required. The modified 701C stimulator only had ranges of 0–20 V (range often too small) or 0–80 V (range often too large), so to finely modulate the voltage output, the digital values in the excitation .txt file (0–10 V) were adjusted by a scale factor. For example, the values in the excitation .txt file (0–10) could be multiplied by 0.375, which would correspond to 37.5% of the stimulator voltage range set at 0–80 V, i.e., 0–30 V.
Proof-of-Principle Experiments
In a brief proof-of-principle pilot study, soleus muscles from male C57BL/10ScSn-Dmdmdx/J (mdx), stock no. 001801, and C57BL/10ScSnJ (WT), stock no. 000476 mice (Jackson Laboratories), were subjected to an ex vivo protocol that included 25 gait cycles. Mice were deeply anesthetized by an intraperitoneal injection of ketamine-xylazine (2 mg of ketamine-20 mg xylazine/100 g body mass), and once a deep plane of anesthesia was achieved, dissection began. The right and left soleus muscles were carefully dissected with 4-O suture tied to both myotendinous junctions (52). The muscles were immersed in a jacketed water bath of physiological saline solution (PSS) maintained at 30°C. At the completion of dissection, the mouse was euthanized with an intracardiac injection of ketamine-xylazine, followed by cervical dislocation.
The muscles were hung by clamping the Achilles tendon and distal suture at the bottom of the bath and the proximal suture tied to the arm of the dual-mode servomotor system (Aurora 300C-LR). Muscles were set and maintained at 10 mN resting tension (i.e., ) while they equilibrated in the bath.
After equilibration, the ex vivo protocol included 1) pretwitches and tetani (150 Hz), 2) pre-force frequency 3) voltage optimization for gait cycling, 4) gait mimicking, and 5) post-force frequency. In step 1, muscles underwent three twitch and three tetanic stimulations spaced 1 min apart. After 10 min, the in millimeters was measured, and the pre-force-frequency stimulations were initiated. Stimulation frequencies varied from 1 Hz (twitch) to 180 Hz, with 1 min between each. From step 2, the maximal isometric force () was recorded. Then, to determine the voltage needed to produce 49% of in the stimulated gait cycle, the initial voltage range was set to 0–20 V with a scale factor of 1. If the 49% force value could not be achieved with this range, the voltage range was set to 0–80 V, and a scale factor was determined as described above (see Additional excitation voltage modulation) until the force response was close to 49% of from step 2. With experience, the scale factor was determined within three to four attempts. Once the voltage range needed to produce 49% of was established, and after another 5 min of rest, the muscle underwent 25 consecutive gait cycles at a rate of 1 cycle/s using the voltage range setting and scale factor determined in step 3 (Fig. 7A). One minute following the 25th gait cycle, a 120-Hz tetani was delivered. Muscles then rested for 10 min, and the force-frequency protocol from step 2 was repeated.
Fig. 7.
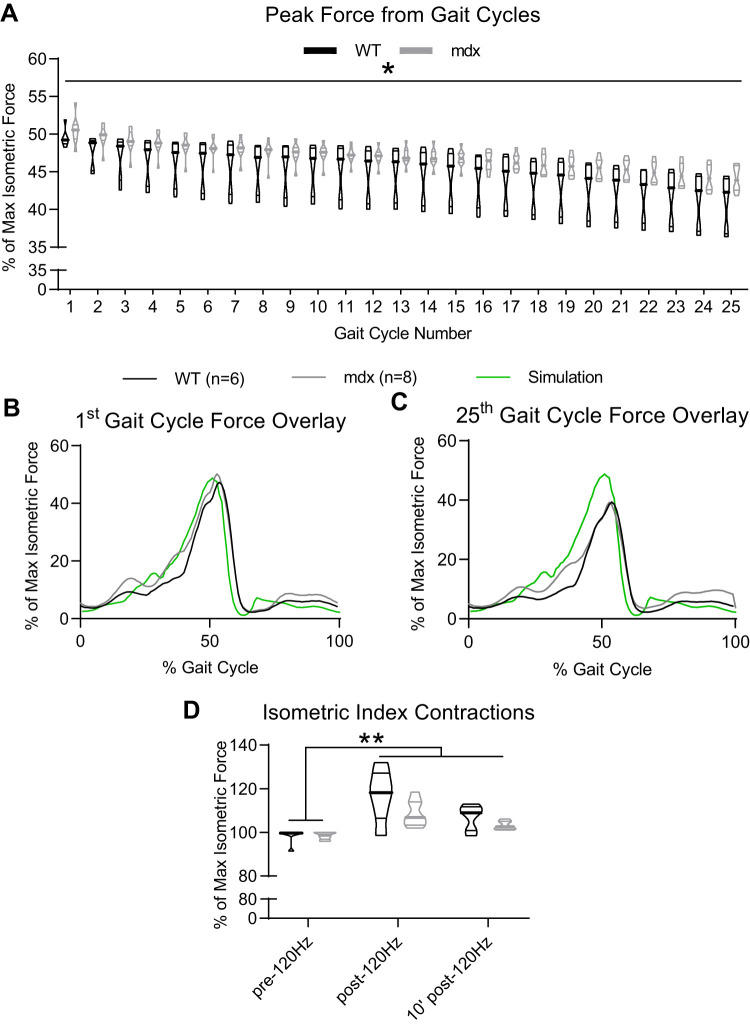
Application of the ex vivo gait protocol at a rate of 1 cycle/s for 25 consecutive cycles to both wild-type (WT; n = 6) and C57BL/10ScSn-Dmdmdx/J (mdx; n = 8) solei did not result in significant loss of isometric force. A: *there was some fatigue experienced by both muscles regardless of genotype (P < 0.05). B and C: despite a loss in peak force between the 1st and 25th gait cycle, the shape of the force response was maintained for both genotypes. D: additionally, neither WT nor mdx showed a loss of isometric force 10 min following the 25 gait cycles. **The post-gait cycling isometric forces for both genotypes were greater than the pre-gait cycling values (P < 0.05).
RESULTS
Morphological Data
The mean (±SD) ages for the two genotypes were slightly different: wild type (WT), 59.5 ± 2.9 days (n = 6); and mdx, 54.4 ± 2.7 days (n = 8) (P = 0.01). The WT and mdx mice body masses were 28.0 ± 2.0 g and 24.3 ± 4.1 g, respectively. The average soleus muscle length was 9.7 ± 0.4 mm for WT and 9.6 ± 0.5 mm for mdx. The soleus masses were also not different between the WT (8.0 ± 0.9 mg) and mdx (8.3 ± 1.6 mg) mice.
Contractile Data
The mdx and WT soleus muscles produced similar maximum isometric forces during the pre-force frequency assay (mdx: 129.6 ± 23.2 mN; WT: 157.0 ± 32.6 mN). There was a unique scale factor and, therefore, voltage range required for each muscle to reach 49% of isometric max. The average range of stimulation voltage after a unique scale factor was applied for mdx soleus muscles was 0–24.75 ± 3.33 V and 0–23.50 ± 5.17 V for WT; there was no difference in stimulation voltage range between genotypes. Both mdx and WT soleus muscles experienced peak force loss from the first to the 25th gait cycle (mdx and WT P < 0.01; Fig. 7A). Peak force was defined as the maximum force achieved during a given gait cycle. As a percent of initial force (i.e., %1st gait cycle peak force), there was no difference in the force lost between mdx (12.7 ± 3.6%) and WT (17.1 ± 6.6%; P = 0.13). For mdx, peak force for the third through 25th gait cycles was less than for the first (P < 0.05). For WT, peak force for the 18th through 25th gait cycles was less than for the first (P < 0.05). Despite the decrease in peak force, both mdx and WT muscles maintained a consistent force profile (Fig. 7, B and C).
To quantify muscle work, we plotted force versus cyclic length change and calculated the area inside the loop. There was no significant decrease in work output from the first to the 25th gait cycle for mdx or WT. The average work output for the first gait cycle in WT soleus muscles was greater than the average work output for the first and the 25th gait cycles in mdx soleus muscles (Supplemental Fig. S2E). Because the gait cycle rate (1 Hz) and the strain on the muscle (length change during gait protocol) were maintained throughout the experiment, differences in work were due to differences in force. Peak force occurred during the first gait cycle and then decreased with each subsequent cycle. As the muscles fatigued, force decreased and the work loops became smaller (i.e., less work). (Supplemental Fig. S2).
We used 120-Hz isometric contractions as an index of muscle viability before the gait cycles (pre-120 Hz) and immediately post-120 Hz, and the 120-Hz value from the force-frequency assay was conducted 10 min after the gait cycles (10′ post-120Hz). Both mdx and WT muscles maintained their viability. The 120-Hz tetanic forces were greater after compared with before the gait cycles (mdx and WT P < 0.05; Fig. 7D). Additionally, post-gait cycling isometric maximum forces from the post-force frequency (mdx: 165.8 mN; WT: 223.1 mN) were greater than the max values in the pre-force-frequency assay (P < 0.05). There was still no difference between mdx and WT soleus maximum isometric forces following gait cycling. Note that the pre- and post- force-frequency plots are not shown.
DISCUSSION
The main goal of our study was to develop a novel mouse muscle ex vivo protocol to closely mimic human in vivo muscle function during gait. Our results demonstrated that the developed ex vivo gait protocol closely mimicked human soleus length changes and muscle forces. Accomplishing this goal required incorporating insights from dynamic simulations of human movement into ex vivo experiments on mouse muscles as well as development of a modified stimulator and customization of DMC software from Aurora Scientific, Inc.
The fiber stretch induced in our ex vivo gait protocol was in a similar range compared with typical acute eccentric injury protocols in the literature. Furthermore, our ex vivo protocol closely resembled the time-varying activation pattern and the resultant force that human soleus muscles experience in walking at a self-selected pace of ∼55–60 gait cycles/min for a healthy male. The simulation of human gait and, therefore, our ex vivo gait cycle protocol accounted for true timing between contractions, magnitude and rate of length change, pattern and duration of stimulation, and initial starting length relative to the optimal length. During the eccentric phase of the ex vivo gait cycle, mouse soleus muscles were stretched from 0.93 to 1.13 , an overall stretch of 0.2 at an average rate of 0.45 /s. Ex vivo acute eccentric protocols published in the literature typically start the stretch at 0.95 to 1 , with an overall stretch varying from 0.1 to 0.3 (4, 20, 42, 43, 63). Additionally, the rates of stretch in these acute eccentric protocols can vary, including 0.5 /s (4, 20, 42), 1.5 /s (12), and 3.0 /s (63). The gait protocol used a magnitude of length change within the range of acute ex vivo eccentric protocols and a rate of stretch close to the low end of those reported in these protocols.
Notably, the main difference between acute eccentric protocols and our gait cycle protocol is the stimulation strategy used to modulate force production. In acute eccentric protocols, the stimulation voltage is optimized and delivered at frequencies that can vary from 70Hz at supramaximal voltages (43) to 300Hz at 2 times the maximal voltage (12), which produce eccentric peak forces greater than isometric maximum tetanic force. This stimulation strategy is not physiologically representative of the eccentric component of gait because according to the simulation, eccentric peak force does not exceed 49% of maximum tetanic force). Therefore, the acute protocols overestimate the eccentric peak force. We overcame this overestimation by using a modified stimulator that varied voltage at a constant stimulation frequency to closely mimic the simulated human soleus excitation pattern and thereby the simulated force. We recognize that one important limitation of external electrical stimulation is that orderly recruitment of fibers typically associated with voluntary muscle activation does not occur (23). It has been shown that electrical stimulation directly through nerve cuff on motor nerves often leads to the reversal of an orderly recruitment pattern (15) and that electrical stimulation using intramuscular or surface electrodes tends to recruit muscle fibers in a nonselective pattern influenced by electrode placement and nerve branching (21, 51). Because the human soleus is predominantly composed of slow fibers, voluntary contraction of human soleus muscle largely relies on progressive recruitment of fibers to adjust force up to nearly the maximum level (40). In contrast, the mouse soleus is ∼60% slow type I and 40% fast type IIa fibers but has no fast type IIb fibers (7). It is unclear how orderly recruitment of this fiber type distribution would impact our results. To address this question, one possibility is to use optogenetically modified mice in which muscles stimulated by light do follow orderly recruitment (33).
To develop and test the ex vivo gait cycle model, we selected the soleus as a synergistic muscle that contributes to the high load toe-off action during gait (i.e., plantarflexion). The proof-of-principle study demonstrated that the mdx and WT soleus muscles tolerated 25 gait cycles at a rate of 60 gait cycles/min. Although fatigue was evident during the protocol, maximal isometric force production rapidly recovered within 1 min after the last gait cycle. As mentioned above and according to the gait simulation, the soleus eccentrically contracts between heel strike and toe-off (Fig. 2). To assess the potential injury induced by eccentric contractions, force loss can be used (62), e.g., the loss in isometric force between the last and first eccentric contractions. Between the first and last gait cycles, both mdx and WT soleus muscle peak forces decreased similarly (∼13–17% of initial gait cycle peak force). We interpret these force losses as fatigue rather than injury because isometric force (i.e., at 120 Hz) recovered at 1 min after the gait cycle for both the mdx and WT muscles.
The area of the work loops from the first to the 25th gait cycle decreased, most likely due to decreased force during cycling (i.e., modest fatigue), but the shape of the work loops remained similar (Supplemental Fig. S2). Between the first and the 25th work loops for both genotypes, the slope of force loss during shortening (relaxation of force response) was similar, and the baseline force at heel strike returned to the same value. These characteristics suggest that the rate of relaxation did not change and, therefore, did not contribute to decreased area of the work loops. Other studies that tested concentric and eccentric fatigue protocols (sinusoidal strains) used cycle rates from 1 to 10 Hz and a maximum cycle number of 300 (3). Looking forward, this suggests that many more gait cycles may be needed to produce changes in the shape of the work loops indicative of permanent force loss (injury) in dystrophic soleus muscle. The outcome of this initial proof-of-principle study suggests that the 25 gait cycles were well tolerated by both genotypes.
Although the dystrophic soleus muscle tolerated the novel gait-mimicking protocol, other dystrophic muscles may not tolerate the protocol as effectively. For example, the dystrophic soleus is thought to be less susceptible to eccentric contractions because of its slow fiber composition compared with the EDL (37), which is composed of only fast-twitch fibers (7). The low-load fast-fiber EDL that is active primarily during the swing phase of gait may be at greater risk of injury during eccentric contractions compared with the high-load slow-fiber soleus. Future studies should test both the soleus and EDL at increasing numbers of gait cycles to identify the injury threshold for each. A membrane impermeable dye such as procion orange (20, 42), which only enters fibers when the membrane is leaky, could be used as an index of injury threshold.
Because our protocol goes from humans to mice, potential differences in geometry and architecture of muscles between two species were carefully considered. Specifically, comparable length changes and forces of soleus fibers in humans and mice were achieved by scaling absolute length changes and forces with optimal fiber lengths and maximum isometric forces of each species. The activation and deactivation time constants in the simulations used in the protocol are 10 ms and 40 ms, respectively, which are typical values used in musculoskeletal simulation of human walking (27, 64). These values are within the ranges of activation (WT mice: 8.9 ± 0.7 ms; mdx mice: 10.2 ± 1.3 ms) and deactivation (WT mice: 46.0 ± 10.9 ms; mdx mice: 53.8 ± 17.9 ms) time constants estimated from fitting the model of activation dynamics (nonlinear 1st-order differential equations) used in walking simulations (27) to the twitch responses of soleus in WT and mdx mice in our protocol. Although it is possible that the time constants used in simulations slightly underestimated true time constants in humans (46, 56), it has been shown that the results of walking simulations are not sensitive to these two parameters (49). Therefore, activation and deactivation constant are unlikely an issue in our protocol. In addition, the reported pennation angle of the human soleus is ∼28° (60), which was accounted for in the musculoskeletal simulations used in our protocol (27). We did not consider pennation angle in mouse soleus in our experiments because the pennation angle of soleus of wild-type mice is only ∼11° (11), and that of mdx mice may only increase by a few degrees (34). These small pennation angles in mice (cos11° = 0.982) were unlikely to influence the validity of the protocol.
The modeling and experimental parts of the developed protocol involved some simplifications. The dynamic human walking simulation used the Hill-type muscle model, which represents all fibers as one muscle fiber connected with tendon. This generally captures overall muscle force-length-velocity relationships and the pennation between muscle fiber and tendon. Although the model treats tendon and muscle fibers separately, we only focused on the fiber in simulation as the main damage site in DMD. In the ex vivo experiments, free tendon was essentially isolated from the muscle because suture was tied at the myotendinous junction. We predict that the external tendon had minimal impact on overall muscle fiber stretch. Besides external tendon, the aponeurosis may also affect the intended length changes of the soleus muscle fiber delivered at the myotendinous junction, because in humans and some animals the aponeurosis may be stretched during soleus contractions (16, 31, 32, 38, 48). However, the aponeurosis exhibits higher stiffness during active contractions than in passive stretches of muscle and undergoes less strain than the external tendon, when muscle is actively generating force (6, 35, 38, 44). These observations indicate the major role of the aponeurosis in transmitting forces as opposed to storing and releasing energy as seen in external tendon (6, 35). Because mouse soleus muscle was mostly active in the stance phase (0–60% of gait cycle) in our protocol, during which the muscle is most likely to be damaged, the strain of the aponeurosis may only minimally affect the length changes of fiber and the subsequent potential damage to the muscle. Future studies may employ high-speed videography to verify the length changes of soleus fibers in our protocol.
It is worth noting that the current protocol is based on the simulations results from walking of a healthy adult male, which may not precisely reflect possible differences in the gait patterns and muscle properties in boys with DMD. Previous studies have shown possible kinematic and kinetic differences during walking in boys with DMD versus boys without DMD versus adult males (17–19, 47, 54, 58), although to what extent they differ remains debatable. These possible differences in gait certainly could influence soleus fiber length changes. However, to the authors’ best knowledge, studies have not yet been conducted that systematically assessed differences in muscle fiber length changes between 1) boys and adult males and 2) boys with and without DMD. In addition, although dystrophic muscles are stiffer than healthy muscles due to fibrosis (22, 53), to what extent this elevated stiffness influences muscle fiber length changes in gait still remains unclear. Future experimental and simulation studies should address these knowledge gaps. When these data become available, our mathematical model can incorporate them to examine whether DMD-specific muscle properties and gait patterns may impose additional adverse effects compared with those of healthy muscle.
We propose that the ex vivo gait cycle protocol represents a physiologically relevant assessment tool. The protocol recapitulates both the patterns and magnitudes of physiological strains and stresses to provide greater insight into the effects of eccentric contractions on muscle function during gait. Uniquely, the protocol incorporates a modified stimulator to deliver a physiological stimulation pattern that matches muscle force output during gait, which at present cannot be duplicated in existing acute eccentric protocols.
This approach may provide useful insight into opposing relationships between disease and function. For example, human lower-limb muscles experience different levels of eccentric contractions during gait that may be related to the selective degeneration in DMD (24). Therefore, the ex vivo protocol could provide a means to selectively target and discriminate these differences in those muscles. Conversely, this approach may minimize the biomechanical differences in muscle function during gait between humans and mice that may hinder the translation of results from mice to human trials (25). In addition, the versatility of this protocol means that with minor modification it can directly take experimental measurements of fiber length changes in locomotion, such as those from ultrasound (29), as the input to prescribe fiber length changes in mice, which may lead to a series of exciting experiments that go from one specific muscle of humans directly to the corresponding homolog in mice. It also can be extended to develop ex vivo protocols to mimic muscle functions in other functional movements simulated with musculoskeletal models (e.g., jumping). Ultimately, we hope to better understand how eccentric contractions during functional movements may translate to muscle damage in DMD and then test the efficacy of potential therapies during the same movements to limit damage.
GRANTS
Funding was provided by NIH Grant UO1-1U01-AR-069393.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.E.B., X.H., S.S.B., and R.W.G. conceived and designed research; K.E.B. and R.W.G. performed experiments; K.E.B., X.H., and R.W.G. analyzed data; K.E.B., X.H., S.S.B., and R.W.G. interpreted results of experiments; K.E.B., X.H., S.S.B., and R.W.G. prepared figures; K.E.B., X.H., and R.W.G. drafted manuscript; K.E.B., X.H., M.B., D.J., S.S.B., and R.W.G. edited and revised manuscript; K.E.B., X.H., M.B., D.J., S.S.B., and R.W.G. approved final version of manuscript.
REFERENCES
- 1.Arnold EM, Delp SL. Fibre operating lengths of human lower limb muscles during walking. Philos Trans R Soc Lond B Biol Sci 366: 1530–1539, 2011. doi: 10.1098/rstb.2010.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Ann Biomed Eng 38: 269–279, 2010. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askew GN, Young IS, Altringham JD. Fatigue of mouse soleus muscle, using the work loop technique. J Exp Biol 200: 2907–2912, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, Jaeger MA, Fitzsimons DP, Thayer SA, Lowe DA, Ervasti JM. Transgenic overexpression of γ-cytoplasmic actin protects against eccentric contraction-induced force loss in mdx mice. Skelet Muscle 1: 32, 2011. doi: 10.1186/2044-5040-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltusnikas J, Kilikevicius A, Venckunas T, Fokin A, Lionikas A, Ratkevicius A. Regenerated soleus muscle shows reduced creatine kinase efflux after contractile activity in vitro. Appl Physiol Nutr Metab 40: 129–133, 2015. doi: 10.1139/apnm-2014-0274. [DOI] [PubMed] [Google Scholar]
- 6.Bojsen-Møller J, Magnusson SP. Mechanical properties, physiological behavior, and function of aponeurosis and tendon. J Appl Physiol (1985) 126: 1800–1807, 2019. doi: 10.1152/japplphysiol.00671.2018. [DOI] [PubMed] [Google Scholar]
- 7.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 9.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 10.Charles JP, Cappellari O, Spence AJ, Hutchinson JR, Wells DJ. Musculoskeletal Geometry, Muscle Architecture and Functional Specialisations of the Mouse Hindlimb. PLoS One 11: e0147669, 2016. doi: 10.1371/journal.pone.0147669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles JP, Cappellari O, Spence AJ, Wells DJ, Hutchinson JR. Muscle moment arms and sensitivity analysis of a mouse hindlimb musculoskeletal model. J Anat 229: 514–535, 2016. doi: 10.1111/joa.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corona BT, Ingalls CP. Immediate force loss after eccentric contractions is increased with L-NAME administration, a nitric oxide synthase inhibitor. Muscle Nerve 47: 271–273, 2013. doi: 10.1002/mus.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 54: 1940–1950, 2007. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- 14.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng 37: 757–767, 1990. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 15.Fang ZP, Mortimer JT. A method to effect physiological recruitment order in electrically activated muscle. IEEE Trans Biomed Eng 38: 175–179, 1991. doi: 10.1109/10.76384. [DOI] [PubMed] [Google Scholar]
- 16.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol (1985) 95: 829–837, 2003. doi: 10.1152/japplphysiol.00775.2002. [DOI] [PubMed] [Google Scholar]
- 17.Ganley KJ, Powers CM. Gait kinematics and kinetics of 7-year-old children: a comparison to adults using age-specific anthropometric data. Gait Posture 21: 141–145, 2005. doi: 10.1016/j.gaitpost.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Goudriaan M, Van den Hauwe M, Dekeerle J, Verhelst L, Molenaers G, Goemans N, Desloovere K. Gait deviations in Duchenne muscular dystrophy-Part 1. A systematic review. Gait Posture 62: 247–261, 2018. doi: 10.1016/j.gaitpost.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Goudriaan M, Van den Hauwe M, Simon-Martinez C, Huenaerts C, Molenaers G, Goemans N, Desloovere K. Gait deviations in Duchenne muscular dystrophy-Part 2. Statistical non-parametric mapping to analyze gait deviations in children with Duchenne muscular dystrophy. Gait Posture 63: 159–164, 2018. doi: 10.1016/j.gaitpost.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Grange RW, Gainer TG, Marschner KM, Talmadge RJ, Stull JT. Fast-twitch skeletal muscles of dystrophic mouse pups are resistant to injury from acute mechanical stress. Am J Physiol Cell Physiol 283: C1090–C1101, 2002. doi: 10.1152/ajpcell.00450.2001. [DOI] [PubMed] [Google Scholar]
- 21.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 85: 358–364, 2005. doi: 10.1093/ptj/85.4.358. [DOI] [PubMed] [Google Scholar]
- 22.Hakim CH, Grange RW, Duan D. The passive mechanical properties of the extensor digitorum longus muscle are compromised in 2- to 20-mo-old mdx mice. J Appl Physiol (1985) 110: 1656–1663, 2011. doi: 10.1152/japplphysiol.01425.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Blemker SS. Musculoskeletal simulation can help explain selective muscle degeneration in Duchenne muscular dystrophy. Muscle Nerve 52: 174–182, 2015. doi: 10.1002/mus.24607. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Charles JP, Akay T, Hutchinson JR, Blemker SS. Are mice good models for human neuromuscular disease? Comparing muscle excursions in walking between mice and humans. Skelet Muscle 7: 26, 2017. doi: 10.1186/s13395-017-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGreevy JW, Hakim CH, McIntosh MA, Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech 8: 195–213, 2015. doi: 10.1242/dmm.018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John CT, Anderson FC, Higginson JS, Delp SL. Stabilisation of walking by intrinsic muscle properties revealed in a three-dimensional muscle-driven simulation. Comput Methods Biomech Biomed Engin 16: 451–462, 2013. doi: 10.1080/10255842.2011.627560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson WL, Jindrich DL, Roy RR, Reggie Edgerton V. A three-dimensional model of the rat hindlimb: musculoskeletal geometry and muscle moment arms. J Biomech 41: 610–619, 2008. doi: 10.1016/j.jbiomech.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai A, Lichtwark GA, Schache AG, Lin YC, Brown NA, Pandy MG. In vivo behavior of the human soleus muscle with increasing walking and running speeds. J Appl Physiol (1985) 118: 1266–1275, 2015. doi: 10.1152/japplphysiol.00128.2015. [DOI] [PubMed] [Google Scholar]
- 30.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 119: 624–635, 2009. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtwark GA, Barclay CJ. A compliant tendon increases fatigue resistance and net efficiency during fatiguing cyclic contractions of mouse soleus muscle. Acta Physiol (Oxf) 204: 533–543, 2012. doi: 10.1111/j.1748-1716.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 32.Lichtwark GA, Barclay CJ. The influence of tendon compliance on muscle power output and efficiency during cyclic contractions. J Exp Biol 213: 707–714, 2010. doi: 10.1242/jeb.038026. [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn ME, Thompson KR, Deisseroth K, Delp SL. Orderly recruitment of motor units under optical control in vivo. Nat Med 16: 1161–1165, 2010. doi: 10.1038/nm.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovering RM, Shah SB, Pratt SJP, Gong W, Chen Y. Architecture of healthy and dystrophic muscles detected by optical coherence tomography. Muscle Nerve 47: 588–590, 2013. doi: 10.1002/mus.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnusson SP, Hansen P, Aagaard P, Brønd J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- 36.Min YL, Bassel-Duby R, Olson EN. CRISPR Correction of Duchenne Muscular Dystrophy. Annu Rev Med 70: 239–255, 2019. doi: 10.1146/annurev-med-081117-010451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moens P, Baatsen PHWW, Maréchal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil 14: 446–451, 1993. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- 38.Monti RJ, Roy RR, Zhong H, Edgerton VR. Mechanical properties of rat soleus aponeurosis and tendon during variable recruitment in situ. J Exp Biol 206: 3437–3445, 2003. doi: 10.1242/jeb.00550. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura A. Mutation-based therapeutic strategies for duchenne muscular dystrophy: from genetic diagnosis to therapy. J Pers Med 9: 16, 2019. doi: 10.3390/jpm9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oya T, Riek S, Cresswell AG. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol 587: 4737–4748, 2009. doi: 10.1113/jphysiol.2009.175695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function (2nd ed.). Thorofare, NJ: SLACK, Inc., 2010. [Google Scholar]
- 42.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichavant C, Burkholder TJ, Pavlath GK. Decrease of myofiber branching via muscle-specific expression of the olfactory receptor mOR23 in dystrophic muscle leads to protection against mechanical stress. Skelet Muscle 6: 2, 2016. doi: 10.1186/s13395-016-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raiteri BJ, Cresswell AG, Lichtwark GA. Muscle-tendon length and force affect human tibialis anterior central aponeurosis stiffness in vivo. Proc Natl Acad Sci USA 115: E3097–E3105, 2018. doi: 10.1073/pnas.1712697115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajagopal A, Dembia CL, DeMers MS, Delp DD, Hicks JL, Delp SL. Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait. IEEE Trans Biomed Eng 63: 2068–2079, 2016. doi: 10.1109/TBME.2016.2586891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MH, Rozumalski A, Trost JP. The effect of walking speed on the gait of typically developing children. J Biomech 41: 1639–1650, 2008. doi: 10.1016/j.jbiomech.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Scott SH, Loeb GE. Mechanical properties of aponeurosis and tendon of the cat soleus muscle during whole-muscle isometric contractions. J Morphol 224: 73–86, 1995. doi: 10.1002/jmor.1052240109. [DOI] [PubMed] [Google Scholar]
- 49.Scovil CY, Ronsky JL. Sensitivity of a Hill-based muscle model to perturbations in model parameters. J Biomech 39: 2055–2063, 2006. doi: 10.1016/j.jbiomech.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Shieh PB. Emerging strategies in the treatment of duchenne muscular dystrophy. Neurotherapeutics 15: 840–848, 2018. doi: 10.1007/s13311-018-00687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh K, Richmond FJ, Loeb GE. Recruitment properties of intramuscular and nerve-trunk stimulating electrodes. IEEE Trans Rehabil Eng 8: 276–285, 2000. [PubMed] [Google Scholar]
- 52.Sperringer JE, Grange RW. In vitro assays to determine skeletal muscle physiologic function. In: Skeletal Muscle Regeneration in the Mouse: Methods and Protocols, edited by Kyba M. New York: Springer New York, 2016, p. 271–291. [DOI] [PubMed] [Google Scholar]
- 53.Stevens ED, Faulkner JA. The capacity of mdx mouse diaphragm muscle to do oscillatory work. J Physiol 522: 457–466, 2000. doi: 10.1111/j.1469-7793.2000.t01-3-00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutherland DH, Olshen R, Cooper L, Wyatt M, Leach J, Mubarak S, Schultz P. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol 23: 3–22, 1981. doi: 10.1111/j.1469-8749.1981.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 55.Meyers TA, Townsend D. Cardiac pathophysiology and the future of cardiac therapies in duchenne muscular dystrophy. Int J Mol Sci 20: 4098, 2019. doi: 10.3390/ijms20174098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thelen DG. Adjustment of muscle mechanics model parameters to simulate dynamic contractions in older adults. J Biomech Eng 125: 70–77, 2003. doi: 10.1115/1.1531112. [DOI] [PubMed] [Google Scholar]
- 57.Thelen DG, Anderson FC. Using computed muscle control to generate forward dynamic simulations of human walking from experimental data. J Biomech 39: 1107–1115, 2006. doi: 10.1016/j.jbiomech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Sienko Thomas S, Buckon CE, Nicorici A, Bagley A, McDonald CM, Sussman MD. Classification of the gait patterns of boys with Duchenne muscular dystrophy and their relationship to function. J Child Neurol 25: 1103–1109, 2010. doi: 10.1177/0883073810371002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verhaart IEC, Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol 15: 373–386, 2019. doi: 10.1038/s41582-019-0203-3. [DOI] [PubMed] [Google Scholar]
- 60.Ward SR, Eng CM, Smallwood LH, Lieber RL. Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res 467: 1074–1082, 2009. doi: 10.1007/s11999-008-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warren GL, Ingalls CP, Armstrong RB. Temperature dependency of force loss and Ca(2+) homeostasis in mouse EDL muscle after eccentric contractions. Am J Physiol Regul Integr Comp Physiol 282: R1122–R1132, 2002. doi: 10.1152/ajpregu.00671.2001. [DOI] [PubMed] [Google Scholar]
- 62.Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27: 43–59, 1999. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- 63.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord 16: 845–854, 2006. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 64.Winters JM, Stark L. Analysis of fundamental human movement patterns through the use of in-depth antagonistic muscle models. IEEE Trans Biomed Eng 32: 826–839, 1985. [DOI] [PubMed] [Google Scholar]