Abstract
Introduction
There are conflicting results whether elevated hematocrit is associated with an increased risk of thromboembolic events in individuals without polycythemia vera. To assess the risk of vascular events in relation to hemoglobin concentration, we conducted a large population-based cohort study based on Scandinavian health registers.
Materials and Methods
We included 1,538,019 Swedish and Danish blood donors between 1987 and 2012. Hazard ratios (HRs) of arterial and venous thrombosis were estimated using Cox regression. Additionally, we fitted person-stratified models where each donor was compared only to him-/herself.
Results
The risk of myocardial infarction and ischemic stroke increased with higher hemoglobin concentration in both men and women. The HRs for myocardial infarction and ischemic stroke in men with hemoglobin concentration ≥17.5g/dL were 3.52 (95% confidence interval [CI], 2.85–4.36) and 2.36 (95% CI, 1.63–3.43), respectively, compared to the reference group. The corresponding HRs for women with hemoglobin concentration ≥16.0 g/dL were 3.22 (2.12–4.89) and 2.35 (1.37–4.02) for myocardial infarction and ischemic stroke, respectively. The risk of venous thrombosis was highest in men with subnormal hemoglobin concentration (<13.0g/dL), HR 1.69 (95% CI, 1.40–2.04). In the person-stratified model, the association between elevated hemoglobin concentration and risk of myocardial infarction was attenuated but remained significant.
Conclusions
In this large cohort of Scandinavian blood donors, elevated hemoglobin concentration was associated with an increased risk of vascular events, primarily arterial events. Even though associations were weakened when each person served as their own control, a high hemoglobin concentration may serve as a cardiovascular risk marker.
Keywords: Hemoglobin level, Erythrocytosis, Myocardial infarction, Ischemic stroke, Venous thrombosis
Introduction
In patients with polycythemia vera (PV), there is a clear relationship between elevated hemoglobin concentration or elevated hematocrit and risk of thromboembolic events.[1] In PV, the abundance of red cells, activated leukocytes and platelets, as well as a hypercoagulative state contribute to the increased risk of thrombosis.[1–3] Whether an elevated hemoglobin concentration in individuals without PV, and thus presumably without PV-related procoagulative changes, is associated with an increased risk of vascular events is less clear. Several authors have reported increased risks of both arterial thrombosis and venous thrombosis in individuals with elevated hematocrit in large prospective cohort studies.[4–11] For example, Wannamethee et al. reported that men with an elevated hematocrit >50% were at a 2.5–fold higher risk of ischemic stroke and Kunnas et al. reported that a hematocrit of ≥ 50% was an independent risk factor for cardiovascular disease and was also associated with a poorer overall survival. [5–12] Overall, results from previous studies are conflicting and elevated hematocrit has not always been found to be an independent risk factor for arterial or venous vascular events.[6, 7, 13] To elucidate whether an elevated hemoglobin concentration is associated with an increased risk of thrombosis also in relatively healthy individuals, we assessed thrombotic risks in relation to hemoglobin concentration in over 1.5 million Swedish and Danish blood donors.
Materials and Methods
Data sources and study design
The study is based on the Scandinavian donations and transfusions (SCANDAT2) database, which contains all electronically recorded data on blood donors, donations, blood transfusions, and transfused patients in Sweden and Denmark since 1968 and 1982, respectively.[14] Hemoglobin concentration is routinely measured at each donation and the database holds information on more than 25 million hemoglobin measurements for a total of 1.6 million blood donors. The database, and its contents, has been described in detail previously.[14] Using unique national registration numbers that are assigned to all inhabitants of both countries, the database has been linked with the respective country’s nationwide Patient Registers (inpatient care throughout follow-up and outpatient care since 2001 and 1995 in Sweden and Denmark, respectively), Cause of Death Registers, and Cancer Registers.[15–18]
We conducted a register-based cohort study among all blood donors who had performed at least one whole blood, plasma, or platelet donation in or after 1987. The analyses were restricted to this time period as the Swedish Patient Register reached nationwide coverage this year. Donors were followed for the occurrence of myocardial infarction (coded according to the international classification of disease [ICD] revision 8: 410; revision 9: 410 or revision 10: I21 or I22), ischemic stroke (ICD-8: 433 or 434; ICD-9: 434 or 435; and ICD-10: I63) or VTE (ICD-8: 450 or 451.00; ICD-9: 415.1 or 451.1; ICD-10: I26, I80.1, or I80.2), recorded in the Swedish and Danish Patient and Cause of Death Registers. Donors with a previous vascular event were excluded, as were donors with a previously known myeloproliferative neoplasm (MPNs, ICD-7: 207.9, 208 or 209; ICD-10: D45, D47.1, D47.3, D47.4), as ascertained using the respective country’s Cancer Register. To limit the influence of the known association between elevated hemoglobin concentration seen in MPN and risk of vascular events, we censored individuals with an incident MPN diagnosis. The MPN diagnosis date was antedated two years to account for possible diagnostic delay.
Follow-up was extended from the first donation after January 1st 1987 until date of first vascular event (of any type), death, emigration, or end of follow-up (December 31st, 2012). Because the relevance of a given hemoglobin measurement can be assumed to decrease with time since that measurement, follow-up was terminated after two years of no additional donation activity (i.e. with no additional hemoglobin measurements). For donors who began donating again after at least two years of no donations, follow-up was resumed.
Statistical analyses
First, a standard model was performed where the relative rates of vascular events – expressed as hazard ratios [HR] – comparing donors with different hemoglobin concentrations, were estimated using Cox proportional hazards regression, with time-dependent covariates. The standard model, which did not account for the fact that the repeated hemoglobin concentration measurements were from the same individuals, included parameters for most recent hemoglobin concentration, attained age, calendar period of observation, geographical region, and country of birth (born in Sweden/Denmark, or immigrated), with time since most recent hemoglobin measurement/donation as time scale. In separate analyses we also added binary variables for a series of comorbid diagnoses (type 2 diabetes mellitus, chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, atrial fibrillation, cancer, angina pectoris, or hypertension, as ascertained in the Patient Registers) to the model. Hemoglobin concentration, attained age, calendar period, and comorbidity were treated as time-dependent, allowing subjects to move between categories with time. Attained age, calendar period and time since most recent measurement were fitted as restricted cubic splines with five evenly placed knots. Separate analyses were performed for men and women. For men, hemoglobin concentration was categorized as 8.0–12.9, 13.0–14.4, 14.5–15.9, 16.0–17.4, or ≥17.5 g/dL and for women as 8.0–11.9, 12.0–13.4, 13.5–14.9, 15.0–15.9, or ≥16.0 g/dL. Men with a hemoglobin concentration of 13.0–14.4 g/dL and women with a hemoglobin concentration of 12.0–13.4 g/dL were used as reference. To depict the association between hemoglobin concentration and risk of vascular events, we also performed analyses where the former was fitted as a restricted cubic spline with 5 evenly spaced knots.
In addition to the standard model, a within-person model using a stratified Cox proportional hazards regression was also performed, where each individual comprised a separate stratum and was thus only compared to her-/himself. Although this analysis should effectively adjust for confounding factors that do not vary in a given individual (e.g. genetic factors and sex) or confounding factors that on average only change only slowly (e.g. smoking habits), it does not fully account for factors where within-subject variation is more dynamic (e.g. comorbidity). To allow adjustment also for factors that are either highly correlated in a given individual (age and calendar period), these models were conducted in two steps. We first fitted a non-stratified Cox proportional hazards model which included all factors that were included in the standard adjusted model, above, except hemoglobin concentration. We then extracted the log-linear predictor estimated from this model, and used this as an offset in a second model where we added hemoglobin concentration as a time-dependent factor and where we included donor identity as a stratifying factor.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided. P values <0.05 were considered statistically significant. The conduct of this study was approved by ethics committees in both countries.
Results
In total, 1,537,246 blood donors with a total of 20.2 million hemoglobin measurements were included. Fifty percent were men and 64% of all blood donors were from Sweden. The median age at first donation was 32 years in men and 29 years in women. Altogether, 190 donors were diagnosed with MPNs, including PV, during follow-up (Table 1).
Table 1.
Characteristics of the 1,538,019 included Swedish and Danish blood donors. IQR=interquartile range.
| Men | Women | |||
|---|---|---|---|---|
| Total, N (%) | 767,468 | (49.9) | 769,778 | (50.1) |
| Country, N (%) | ||||
| Denmark | 264,515 | (34.5) | 285,088 | (37.0) |
| Sweden | 502,953 | (65.5) | 484,690 | (63.0) |
| Median age at first donation, years (IQR) | 32 | (24–43) | 29 | (22–40) |
| Median duration of follow-up, years (IQR) | 6.3 | (2.7–12.6) | 4.9 | (2.3–10.3) |
| Median hemoglobin concentration at first presentation, g/dL (IQR) | 15.0 | (14.3–15.6) | 13.2 | (12.7–13.9) |
| Number of donations, N (%) | ||||
| 1–5 | 295,453 | (38.5) | 381,544 | (49.6) |
| 6–10 | 126,094 | (16.4) | 141,492 | (18.4) |
| 10–20 | 139,395 | (18.2) | 131,043 | (17.0) |
| >20 | 206,526 | (26.9) | 115,699 | (15.0) |
| Median (IQR) | 9 | (3–22) | 6 | (2–14) |
| Total number of donations | 12,148,651 | 8,013,173 | ||
| Diagnosis of comorbidity during follow-up, N (%) | ||||
| Angina pectoris | 2,778 | (0.4) | 1,254 | (0.2) |
| Atrial fibrillation | 3,446 | (0.4) | 1,439 | (0.2) |
| Cancer | 4,129 | (0.5) | 9,732 | (1.3) |
| Chronic obstructive pulmonary disease | 7,249 | (0.9) | 6,010 | (0.8) |
| Diabetes mellitus | 1,018 | (0.1) | 1,118 | (0.1) |
| Hypertension | 4,031 | (0.5) | 3,141 | (0.4) |
| Obstructive sleep apnea syndrome | 6,603 | (0.9) | 1,301 | (0.2) |
| Polycythemia Vera | 64 | (0.01) | 29 | (0.00) |
| Other Myeloproliferative Neoplasm | 44 | (0.01) | 53 | (0.01) |
The risk of myocardial infarction was significantly increased in both men and women with elevated hemoglobin concentration. In men with hemoglobin concentration of ≥17.5 g/dL and 16.0–17.4 g/dL, the HRs of myocardial infarction were 3.52 (95% CI, 2.85–4.36) and 2.15 (95% CI 1.99–2.31), respectively, compared to men with hemoglobin concentration of 13.0–14.9 g/dL (Table 2, Figure 1). Also, men with a hemoglobin concentration of 14.5–15.9g/dL, thus within the normal reference range, had a significantly increased risk of myocardial infarction, HR=1.37, 95% CI 1.29–1.45, compared to the reference group. Similarly, there was a three-fold risk increase of myocardial infarction in women with hemoglobin concentration ≥16.0 g/dL, HR=3.22; 95% CI 2.12–4.89, and women with hemoglobin concentration of 15.0–15.9 g/dL had a HR of 2.73, 95% CI 2.19–3.38, compared to those with hemoglobin concentration of 12.0–13.4g/dL. In the person-stratified model, men with hemoglobin >17.5 g/dL and women with hemoglobin of ≥16.0 g/dL had HRs of 1.72 (95% CI 1.24–2.38) and 1.78 (95% CI 0.94–3.38), respectively, compared to the corresponding reference groups (Table 2).
Table 2.
Relative risks of myocardial infarction in relation to hemoglobin concentration
| Hemoglobin concentration (g/dL) | Number of events | Standard adjusted model, HR** (95% CI) | Standard model HR* (95% CI) | Person-stratified adjusted model HR** (95% CI) |
|---|---|---|---|---|
| Men | ||||
| ≥17.5 | 91 | 3.52 (2.85–4.36) | 3.54 (2.86–4.38) | 1.72 (1.24–2.38) |
| 16.0–17.4 | 1,142 | 2.15 (1.99–2.31) | 2.15 (2.00–2.32) | 1.14 (1.00–1.30) |
| 14.5–15.9 | 3,321 | 1.37 (1.29–1.45) | 1.37 (1.29–1.45) | 1.01 (0.92–1.10) |
| 13.0–14.4 | 1,752 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8.0–12.9 | 133 | 0.95 (0.80–1.13) | 0.95 (0.80–1.14) | 1.19 (0.92–1.53) |
| Women | ||||
| ≥16.0 | 25 | 3.22 (2.12–4.89) | 3.30 (2.17–5.00) | 1.78 (0.94–3.38) |
| 15.0–15.9 | 109 | 2.73 (2.19–3.38) | 2.76 (2.22–3.43) | 1.70 (1.17–2.46) |
| 13.5–14.9 | 404 | 1.25 (1.09–1.44) | 1.26 (1.09–1.44) | 1.03 (0.84–1.28) |
| 12.0–13.4 | 413 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8.0–11.9 | 63 | 1.13 (0.86–1.47) | 1.13 (0.87–1.48) | 1.25 (0.85–1.84) |
HR=Hazard ratio, CI=confidence interval
Adjusted for country, attained age, calendar year, time since most recent blood donation.
Adjusted for country, attained age, calendar year, time since most recent blood donation, and comorbidity (chronic obstructive pulmonary disease, cancer, diabetes mellitus, atrial fibrillation, obstructive sleep apnea syndrome, hypertension, and angina pectoris)
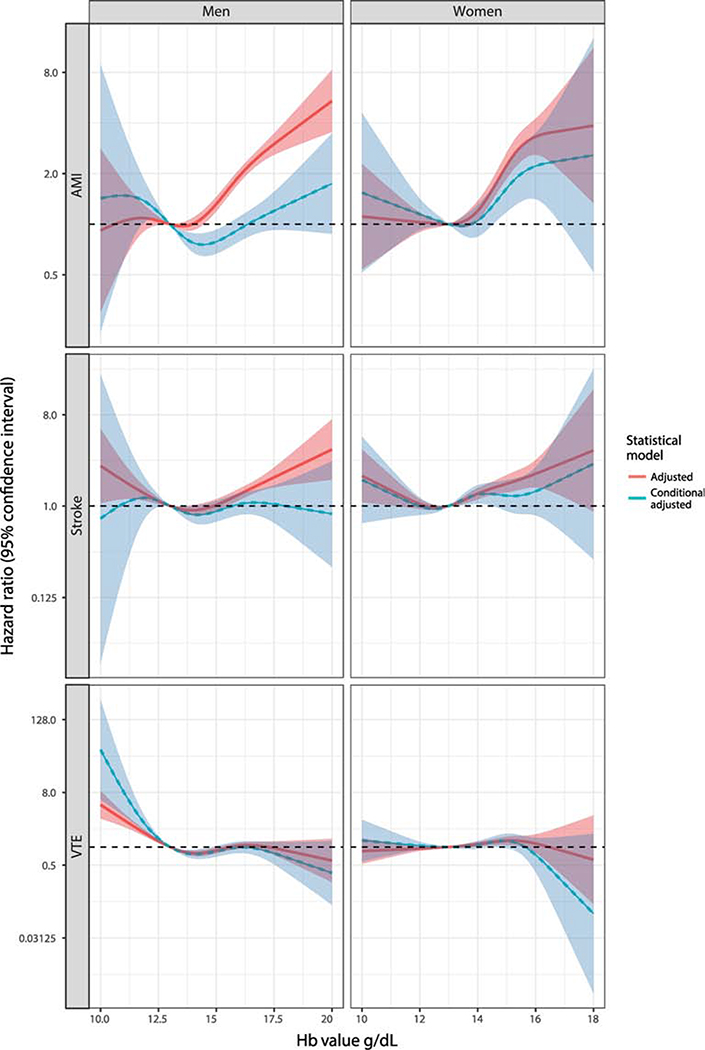
Figure 1.
Hazard of myocardial infarction (AMI), ischemic stroke and venous thromboembolism (VTE) in relation to hemoglobin concentration in male and female blood donors. All hazard ratios are adjusted for country, attained age, calendar year, time since most recent blood donation, and comorbidities (chronic obstructive pulmonary disease, cancer, diabetes mellitus, atrial fibrillation, obstructive sleep apnea syndrome, hypertension, and angina pectoris).
A similar pattern with an increased risk of ischemic stroke was observed in individuals with elevated hemoglobin concentration (Table 3, Figure 2). The adjusted HRs for ischemic stroke were 2.36 (95% CI 1.63–3.43) and 1.55 (95% CI 1.38–1.75) in men with hemoglobin concentration of >17.5g/L and 16.0–17.4 g/dL, respectively, compared to the reference group. Comparable effects were observed in women where females with hemoglobin concentration ≥16.0 g/dL and 15.0–15.9 g/dL had adjusted HRs for ischemic stroke of 2.35 (95% CI 1.37–4.02) and 1.66 (95% CI 1.27–2.16), respectively. In the person-stratified models, the dose response effect was largely removed in both men and women, with HRs for ischemic stroke of 1.08 (95% CI, 0.57–2.03) in men with a hemoglobin concentration of >17.5g/L, and 1.14 (95% CI, 0.51–2.55) in women with hemoglobin concentrations of ≥16.0 g/dL as compared to the respective reference group.
Table 3.
Relative risks of ischemic stroke in relation to hemoglobin concentration
| Hemoglobin concentration (g/dL) | Number of events | Standard adjusted model, HR** (95% CI) | Standard model HR* (95% CI) | Person-stratified adjusted model HR** (95% CI) |
|---|---|---|---|---|
| Men | ||||
| ≥17.5 | 29 | 2.36 (1.63–3.43) | 2.40 (1.65–3.48) | 1.08 (0.57–2.03) |
| 16.0–17.4 | 406 | 1.55 (1.38–1.75) | 1.56 (1.38–1.76) | 1.19 (0.97–1.45) |
| 14.5–15.9 | 1,392 | 1.16 (1.07–1.27) | 1.17 (1.07–1.27) | 1.02 (0.90–1.16) |
| 13.0–14.4 | 842 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8.0–12.9 | 88 | 1.32 (1.06–1.64) | 1.33 (1.07–1.65) | 1.26 (0.91–1.72) |
| Women | ||||
| ≥16.0 | 14 | 2.35 (1.37–4.02) | 2.37 (1.38–4.06) | 1.14 (0.51–2.55) |
| 15.0–15.9 | 63 | 1.66 (1.27–2.16) | 1.67 (1.28–2.18) | 1.09 (0.71–1.68) |
| 13.5–14.9 | 437 | 1.30 (1.14–1.48) | 1.30 (1.14–1.49) | 1.20 (0.97–1.47) |
| 12.0–13.4 | 459 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8.0–11.9 | 72 | 1.07 (0.84–1.38) | 1.08 (0.84–1.38) | 0.85 (0.58–1.25) |
HR=Hazard ratio, CI=confidence interval
Adjusted for country, attained age, calendar year, time since most recent blood donation.
Adjusted for country, attained age, calendar year, time since most recent blood donation, and comorbidity (chronic obstructive pulmonary disease, cancer, diabetes mellitus, atrial fibrillation, obstructive sleep apnea syndrome, hypertension, and angina pectoris).
Trend tests were performed by fitting hemoglobin concentration as a linear term.
There was a moderate increase in the risk of VTE in men with elevated hemoglobin concentrations, however, associations were not consistently significant across all groups. The adjusted HRs for VTE in men with hemoglobin concentrations of >17.5, 16.0–17.4, and 14.5–15.9 g/dL were 1.51 (95% CI 0.97–2.35), 1.23 (95% CI 1.09–1.38), and 1.12 (95% CI 1.03–1.21), respectively, compared to the reference group. Moreover, men with a subnormal hemoglobin concentration were at an increased risk of VTE; the HR was 1.69 (95% CI 1.40–2.04, Table 4) in men with hemoglobin concentration of 8.0–12.9 g/dL compared to men with a hemoglobin concentration of 13.0–14.4 g/dL. In women, there was no clear pattern in the association between hemoglobin concentration and risk of VTE. The adjusted HRs for VTE in women with hemoglobin concentrations of ≥16.0, 15.0–15.9, 13.5–14.9, and 8.0–11.9 g/dL were 0.97 (95% CI 0.50–1.88), 1.26 (95% CI 1.01–1.58), 1.13 (95% CI 1.02–1.24), and 0.90 (95% CI 0.75–1.08), respectively, compared to females with a hemoglobin of 12.0–13.4 g/dL (Table 4). As in the analyses of myocardial infarction and ischemic stroke, the person-stratified analyses led to attenuated HRs for men with elevated hemoglobin concentrations. However, we also observed that the HR in men with a hemoglobin concentration of 8.0–12.9 g/dL was strengthened (HR, 2.00; 95% CI 1.51–2.66). In women, results from the person-stratified model were very similar to the results from the standard models.
Table 4.
Relative risks of venous thromboembolism (lower extremity deep venous thrombosis and pulmonary embolism) in relation to hemoglobin concentration
| Hemoglobin concentration (g/dL) | Number of events | Standard adjusted model, HR** (95% CI) | Standard model HR* (95% CI) | Person-stratified adjusted model HR** (95% CI) |
|---|---|---|---|---|
| Men | ||||
| >17.5 | 20 | 1.51 (0.97–2.35) | 1.51 (0.97–2.36) | 1.15 (0.61–2.18) |
| 16.0–17.4 | 393 | 1.23 (1.09–1.38) | 1.23 (1.09–1.38) | 1.11 (0.92–1.35) |
| 14.5–15.9 | 1,703 | 1.12 (1.03–1.21) | 1.12 (1.03–1.21) | 1.06 (0.94–1.19) |
| 13.0–14.4 | 1,024 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8.0–12.9 | 124 | 1.69 (1.40–2.04) | 1.70 (1.41–2.05) | 2.00 (1.51–2.66) |
| Women | ||||
| ≥16.0 | 9 | 0.97 (0.50–1.88) | 0.97 (0.50–1.89) | 0.74 (0.29–1.90) |
| 15.0–15.9 | 85 | 1.26 (1.01–1.58) | 1.27 (1.01–1.59) | 1.10 (0.77–1.57) |
| 13.5–14.9 | 760 | 1.13 (1.02–1.24) | 1.13 (1.03–1.24) | 1.13 (0.97–1.32) |
| 12.0–13.4 | 991 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 8.0–11.9 | 136 | 0.90 (0.75–1.08) | 0.90 (0.75–1.08) | 1.02 (0.77–1.35) |
HR=Hazard ratio, CI=confidence interval
Adjusted for country, attained age, calendar year, time since most recent blood donation.
Adjusted for country, attained age, calendar year, time since most recent blood donation, and comorbidity (chronic obstructive pulmonary disease, cancer, diabetes mellitus, atrial fibrillation, obstructive sleep apnea syndrome, hypertension, and angina pectoris).
Trend tests were performed by fitting hemoglobin concentration as a linear term.
Discussion
In this large study including 1.5 million blood donors with over 20 million hemoglobin concentration measurements over a period of 26 years, we found a distinct association between elevated hemoglobin concentration and increased risk of arterial vascular events. A threefold increased risk of myocardial infarction was observed in individuals with the highest hemoglobin concentrations and the risk was also significantly elevated in individuals with moderately increased hemoglobin concentrations. Conversely, for VTE, the highest risks were seen in men with subnormal hemoglobin concentrations. With the exception of VTE, the person-stratified models, where individuals served as their own controls, generally returned attenuated risk estimates. Irrespectively, our findings indicate that an elevated hemoglobin concentration can be used as a marker for identifying individuals with an increased risk of vascular events.
We found a significant association between elevated hemoglobin concentration and myocardial infarction and ischemic stroke in a dose-dependent relationship as risks increased with higher hemoglobin concentrations. These findings are in line with several previous studies where hematocrit >50% has been associated with an increased risk of cardiovascular and cerebrovascular events.[4–6, 12, 19] The risks remained significantly elevated also when adjusting for co-morbidities in most but not all of studies.[5–7, 12, 13] In the present study, an elevated hemoglobin concentration remained an independent risk factor for myocardial infarction and ischemic stroke also after adjustments for a number of comorbidities such as hypertension, obstructive sleep apnea syndrome, atrial fibrillation, and chronic obstructive pulmonary disease, which was used as a proxy for smoking. In the person-stratified model, where the effect of constant and slowly changing confounding factors should be removed entirely, there was an attenuated but still significantly elevated risk of myocardial infarction while the risk of stroke was no longer significant. This indicated that even though residual effects of comorbidities or smoking cannot fully be ruled out, elevated hemoglobin concentration is associated with an increased risk of arterial thrombosis also in individuals without PV.
For VTE, risks were not consistently elevated in individuals with high hemoglobin concentrations. The risk of VTE in patients with non-PV erythrocytosis is not very well studied and also in the literature, the association between elevated hemoglobin level and VTE is less pronounced in relation to arterial events.[8, 9, 11, 20] While we found a trend towards an elevated risk of VTE in individuals with increased hemoglobin concentrations, these risks disappeared in the person-stratified models. Thus, elevated hemoglobin concentration should not be regarded as a strong risk factor for VTE.
The highest risk of VTE was instead observed in men with subnormal hemoglobin concentrations. Although the estimates were adjusted for a number of comorbidities, we believe that this observation is driven mostly by undiagnosed systemic conditions which affect both the hemoglobin concentration and the risk of VTE. Supporting this theory, a decline in hemoglobin concentration has been observed prior to cancer diagnoses in Swedish and Danish blood donors.[21] We therefore emphasize that individuals with unexplained anemia should be investigated not only for deficiencies but should also be liberally assessed for underlying systemic conditions including malignancies.
Several mechanisms behind the hemoglobin-associated thrombotic risks have been proposed, including reduced coronary and cerebral blood flow as well as enhanced exposure of platelets and coagulation factors to the vascular endothelium resulting in increased platelet-vessel wall adhesion.[5, 12, 22–24] Whether these changes can be reversed by lowering the hemoglobin concentration through phlebotomy remains to be elucidated. Individuals included in our study donated blood in volumes equivalent to phlebotomies, however, as blood donors are only allowed to donate whole blood a maximum of three and four times per year for men and women, respectively, and in reality only donated an average of 1.6 and 1.9 times per year, we argue that their donation habits are unlikely to have had large therapeutic effects. Nevertheless, our results from the person-stratified models indicate that a given individual’s risk becomes higher with an elevated hemoglobin concentration, there could be a possible beneficial effect of phlebotomy. However, due to the observational nature of this study, it is not possible to draw confident conclusions on whether phlebotomy is favorable in individuals with high hemoglobin level. Furthermore, there is limited evidence for phlebotomies in non-PV erythrocytosis and we do not have enough support for recommending phlebotomy in individuals with elevated hemoglobin concentration.[25]
Blood donors are in general healthier than the general population and both Sweden and Denmark have strict criteria for health clearance of blood donors.[14] In a detailed assessment of blood donors with elevated hemoglobin concentration in Denmark, 85% were smokers but only a minority of individuals had comorbidities such as hypertension and diabetes mellitus.[26] PV as well JAK2V617F positivity have been reported in blood donors with high hemoglobin concentrations but overall, blood donors do not have an increased incidence of PV.[26–29] We censored patients with PV two years prior to diagnosis and, we therefore do not believe that possible undiagnosed PV had a large effect on our risk estimates. On the other hand, we cannot exclude residual confounding due to smoking despite our adjustment for chronic obstructive pulmonary disease as a proxy. However, the fact that associations mostly persisted in the person-stratified models, where most of the confounding effects from factors such as smoking should have been removed, indicates that smoking is unlikely the sole explanation behind the elevated risks of arterial thrombosis in blood donors with elevated hemoglobin concentrations.
The strengths of our study are the large sample size with over 1.5 million blood donors included and followed over a time-period of 26 years. The study is based on high-quality Swedish and Danish registers which in validations have a high coverage and accuracy,[15, 30] The study population included an even distribution of male and female blood donors with a low median age at first blood donation and of general good health.[31] Limitations of our study include restrictions in the available data which does not include information on smoking, additional blood counts such as white blood cell or platelet counts, as well as lack of information on the hemoglobin concentration at the time of the vascular events. The SCANDAT2 database does not hold information on hematocrit but since there is a strong correlation between hemoglobin concentration and hematocrit (r=0.93),[5] we expect that the results would have been very similar if hematocrit had been used instead. Also, despite including over 20 million hemoglobin measures, there were generally few events among individuals with the highest hemoglobin concentrations.
In summary, in this large study based on Swedish and Danish blood donors, we found a significant association between an elevated hemoglobin concentration and increased risks of myocardial infarction and ischemic stroke. There was also an increased risk of VTE in individuals with subnormal hemoglobin concentrations likely related to underlying systemic conditions including malignancies. These associations were largely unaffected by adjusting for a number of cardiovascular comorbidities. However, associations were weakened but remained significant when each person served as his/her own control, indicating that residual effects of comorbidities or smoking cannot entirely be ruled out but does not fully explain the increased risk. Nevertheless, we conclude that hemoglobin concentration may serve as a marker for elevated risk of vascular events and abnormal hemoglobin concentrations motivates thorough assessment of cardiovascular risk factors and underlying comorbidities.
Highlights.
An elevated hemoglobin level was associated with an increased risk of myocardial infarction and ischemic stroke
Subnormal hemoglobin levels were associated with an increased risk of venous thrombosis in men
In person-stratified models, where individuals served as their own controls, the association between elevated hemoglobin concentration and risk of myocardial infarction was attenuated but remained significant
Acknowledgements
We are greatly indebted to all the blood banks in Sweden and Denmark for both collecting and contributing data to this study.
Sources of Funding
The creation of the SCANDAT2 database was funded by Swedish Research Council (2017-01954, 2011-30405, 2007-7469), the Swedish Heart-Lung Foundation (20090710), the Swedish Society for Medical Research and the Strategic research program in Epidemiology at Karolinska Institutet (Edgren). We also thank the Cancer Research Foundations of Radiumhemmet, Blodcancerfonden, the Swedish Research Council (2015-00564), the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (Hultcrantz) and the Memorial Sloan Kettering Core Grant (P30 CA008748) (Hultcrantz, Landgren) for grant support of this work.
The funding agencies had no role in the design, analysis, or interpretation of the analysis; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
No relevant conflict of interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, De Stefano V, Elli E, Iurlo A, Latagliata R, Lunghi F, Lunghi M, Marfisi RM, Musto P, Masciulli A, Musolino C, Cascavilla N, Quarta G, Randi ML, Rapezzi D, Ruggeri M, Rumi E, Scortechini AR, Santini S, Scarano M, Siragusa S, Spadea A, Tieghi A, Angelucci E, Visani G, Vannucchi AM, Barbui T, C.-P.C. Group, Cardiovascular events and intensity of treatment in polycythemia vera, N Engl J Med 368(1) (2013) 22–33. [DOI] [PubMed] [Google Scholar]
- [2].Barbui T, Finazzi G, Falanga A, Myeloproliferative neoplasms and thrombosis, Blood 122(13) (2013) 2176–84. [DOI] [PubMed] [Google Scholar]
- [3].Hultcrantz M, Bjorkholm M, Dickman PW, Landgren O, Derolf AR, Kristinsson SY, Andersson TML, Risk for Arterial and Venous Thrombosis in Patients With Myeloproliferative Neoplasms: A Population-Based Cohort Study, Ann Intern Med 168(5) (2018) 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gagnon DR, Zhang TJ, Brand FN, Kannel WB, Hematocrit and the risk of cardiovascular disease--the Framingham study: a 34-year follow-up, Am Heart J 127(3) (1994) 674–82. [DOI] [PubMed] [Google Scholar]
- [5].Wannamethee G, Perry IJ, Shaper AG, Haematocrit, hypertension and risk of stroke, J Intern Med 235(2) (1994) 163–8. [DOI] [PubMed] [Google Scholar]
- [6].Kannel WB, Gordon T, Wolf PA, McNamara P, Hemoglobin and the risk of cerebral infarction: the Framingham Study, Stroke 3(4) (1972) 409–20. [DOI] [PubMed] [Google Scholar]
- [7].Brown DW, Giles WH, Croft JB, Hematocrit and the risk of coronary heart disease mortality, Am Heart J 142(4) (2001) 657–63. [DOI] [PubMed] [Google Scholar]
- [8].Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB, Hematocrit and risk of venous thromboembolism in a general population. The Tromso study, Haematologica 95(2) (2010) 270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eischer L, Tscholl V, Heinze G, Traby L, Kyrle PA, Eichinger S, Hematocrit and the risk of recurrent venous thrombosis: a prospective cohort study, PLoS One 7(6) (2012) e38705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rezende SM, Lijfering WM, Rosendaal FR, Cannegieter SC, Hematologic variables and venous thrombosis: red cell distribution width and blood monocyte count are associated with an increased risk, Haematologica 99(1) (2014) 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR, Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology, Arch Intern Med 162(10) (2002) 1182–9. [DOI] [PubMed] [Google Scholar]
- [12].Kunnas T, Solakivi T, Huuskonen K, Kalela A, Renko J, Nikkari ST, Hematocrit and the risk of coronary heart disease mortality in the TAMRISK study, a 28-year follow-up, Preventive medicine 49(1) (2009) 45–7. [DOI] [PubMed] [Google Scholar]
- [13].Carter C, McGee D, Reed D, Yano K, Stemmermann G, Hematocrit and the risk of coronary heart disease: the Honolulu Heart Program, Am Heart J 105(4) (1983) 674–9. [DOI] [PubMed] [Google Scholar]
- [14].Edgren G, Rostgaard K, Vasan SK, Wikman A, Norda R, Pedersen OB, Erikstrup C, Nielsen KR, Titlestad K, Ullum H, Melbye M, Nyren O, Hjalgrim H, The new Scandinavian Donations and Transfusions database (SCANDAT2): a blood safety resource with added versatility, Transfusion 55(7) (2015) 1600–6. [DOI] [PubMed] [Google Scholar]
- [15].Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO, External review and validation of the Swedish national inpatient register, BMC Public Health 11 (2011) 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lynge E, Sandegaard JL, Rebolj M, The Danish National Patient Register, Scand J Public Health 39(7 Suppl) (2011) 30–3. [DOI] [PubMed] [Google Scholar]
- [17].Sverige. Socialstyrelsen, Cancerincidens i Sverige 2013 : nya diagnosticerade cancerfall år 2013, Socialstyrelsen, Stockholm, 2014. [Google Scholar]
- [18].Socialstyrelsen S, Dödsorsaksstatistik historik, produktionsmetoder och tillförlitlighet, in: Socialstyrelsen (Ed.) 2010. [Google Scholar]
- [19].Sorlie PD, Garcia-Palmieri MR, Costas R Jr., Havlik RJ, Hematocrit and risk of coronary heart disease: the Puerto Rico Health Program, Am Heart J 101(4) (1981) 456–61. [DOI] [PubMed] [Google Scholar]
- [20].Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW, Cardiovascular risk factors and venous thromboembolism: a meta-analysis, Circulation 117(1) (2008) 93–102. [DOI] [PubMed] [Google Scholar]
- [21].Edgren G, Bagnardi V, Bellocco R, Hjalgrim H, Rostgaard K, Melbye M, Reilly M, Adami HO, Hall P, Nyren O, Pattern of declining hemoglobin concentration before cancer diagnosis, Int J Cancer 127(6) (2010) 1429–36. [DOI] [PubMed] [Google Scholar]
- [22].Gori T, Damaske A, Muxel S, Radmacher MC, Fasola F, Schaefer S, Fineschi M, Forconi S, Jung F, Munzel T, Parker JD, Endothelial function and hemorheological parameters modulate coronary blood flow in patients without significant coronary artery disease, Clinical hemorheology and microcirculation 52(2–4) (2012) 255–66. [DOI] [PubMed] [Google Scholar]
- [23].Vaya A, Suescun M, Hemorheological parameters as independent predictors of venous thromboembolism, Clinical hemorheology and microcirculation 53(1–2) (2013) 131–41. [DOI] [PubMed] [Google Scholar]
- [24].Wang S, Zhang Y, Cheng Y, Huang J, Sun T, Liu T, Wang Q, Yin C, Tao Y, Que B, Zhang J, Li Z, Zhou Y, Correlation between the hematocrit and slow coronary flow, Clinical hemorheology and microcirculation 58(2) (2014) 297–305. [DOI] [PubMed] [Google Scholar]
- [25].Kim KH, Oh KY, Clinical applications of therapeutic phlebotomy, J Blood Med 7 (2016) 139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Magnussen K, Hasselbalch HC, Ullum H, Bjerrum OW, Characterization of blood donors with high haemoglobin concentration, Vox sanguinis 104(2) (2013) 110–4. [DOI] [PubMed] [Google Scholar]
- [27].Edgren G, Nyren O, Hultcrantz M, Nielsen KR, Pedersen OB, Bjorkholm M, Rostgaard K, Hjalgrim H, Blood donation and risk of polycythemia vera, Transfusion 56(6 Pt 2) (2016) 1622–7. [DOI] [PubMed] [Google Scholar]
- [28].Merk K, Mattsson B, Mattsson A, Holm G, Gullbring B, Bjorkholm M, The incidence of cancer among blood donors, Int J Epidemiol 19(3) (1990) 505–9. [DOI] [PubMed] [Google Scholar]
- [29].Tagariello G, Di Gaetano R, Sartori R, Zanotto D, Belvini D, Radossi P, Risato R, Roveroni G, Salviato R, Tassinari C, Toffano N, The JAK2(V617F) tyrosine kinase mutation in blood donors with upper-limit haematocrit levels, Blood transfusion = Trasfusione del sangue 7(2) (2009) 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Turesson I, Linet MS, Bjorkholm M, Kristinsson SY, Goldin LR, Caporaso NE, Landgren O, Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003, Int J Cancer 121(10) (2007) 2260–6. [DOI] [PubMed] [Google Scholar]
- [31].Edgren G, Tran TN, Hjalgrim H, Rostgaard K, Shanwell A, Titlestad K, Wikman A, Norda R, Jersild C, Wideroff L, Gridley G, Adami J, Melbye M, Nyren O, Reilly M, Improving health profile of blood donors as a consequence of transfusion safety efforts, Transfusion 47(11) (2007) 2017–24. [DOI] [PubMed] [Google Scholar]