Abstract
Objective:
To examine the assocations of a clinical and public health systems-change intervention on the prevalence of excess gestational weight gain among high-risk, low-income women.
Methods:
In a quasi-experimental trial, we compared the prevalence of excess gestational weight gain among women prior to (N=643) and after (N=928) implementation of the First 1,000 Days program in two community health centers in Massachusetts. First 1,000 Days is a systematic program starting in early pregnancy lasting through the first 24 months of infancy to prevent obesity among mother–infant pairs. The program includes enhanced gestational weight gain tracking and counseling, screening for adverse health behaviors and socio-contextual factors, patient navigation and educational materials to support behavior change and social needs, and individualized health coaching for women at high risk of excess gestational weight gain based on their pre-pregnancy body mass index (BMI) or excess first trimester weight gain. The primary outcome was gestational weight gain above the 2009 National Academy of Medicine guidelines according to pre-pregnancy BMI.
Results:
Among 1,571 women in the analytic sample, mean (SD) age was 30.0 (5.9) years and pre-pregnancy BMI was 28.1 (6.1) kg/m2; 65.8% of women started pregnancy with BMI ≥ 25 kg/m2 and 53.2% were Hispanic. We observed a lower prevalence (55.8% to 46.4%; unadjusted OR=0.69; 95% CI: 0.49, 0.97), similar to results in a multivariable analysis (aOR=0.69; 95% CI: 0.49, 0.99) of excess gestational weight gain among women with pre-pregnancy BMI between 25-<30 kg/m2. Among women who were overweight at the start of pregnancy, the lowest odds of excess gestational weight gain was observed among those with the most interaction with the program’s components. Program enrollment was not associated with reduced excess gestational weight gain among women with pre-pregnancy BMI ≥ 30 kg/mm2.
Conclusions:
Implementation of a systems-change intervention was associated with modest reduction in excess gestational weight gain among women who were overweight, but not obese at the start of pregnancy.
Précis:
Implementation of a systems-change intervention was associated with modest reduction in excess gestational weight gain among women who were overweight at the start of pregnancy.
INTRODUCTION
Obesity represents a major threat to public health and places a significant burden on morbidity, quality of life, and health care costs.1,2 Despite evidence of recent progress, overall rates of obesity remain at historically high levels, and socio-economic disparities are marked and growing.3,4 The reproductive health origins of obesity and related disparities are well documented and affect both maternal and child health over the lifecourse.5–10 Excessive weight gain during pregnancy is a strong risk factor for postpartum weight retention and obesity.11,12 Yet, there have been relatively few obesity prevention trials in the antenatal and postpartum period; and to-date, most are narrowly targeted at individual-level behavior changes, and not the broader context of clinical and public health systems and policies.13
The First 1,000 Days Program was co-created by a diverse set of stakeholders to build an infrastructure for sustained, systems-wide changes for obesity prevention across antenatal and postpartum clinical and public health services – addressing clinical, behavioral, and socio-contextual factors that contribute to excess weight gain during pregnancy and to childhood obesity during the first two years of life. The First 1,000 Days program uses a Collective Impact approach,14 involving Obstetrics, Pediatrics, Adult Medicine, Behavioral Health, Nutrition, and the Women, Infants and Children (WIC), Home Visiting and Fatherhood Programs at academically affiliated community health centers. Collective Impact has been defined as “the commitment of a group of important actors from different sectors to a common agenda for solving a specific social problem”.15–17
This article reports pregnancy outcomes of the First 1,000 Days program on gestational weight gain among diverse, low-income women at high-risk for obesity. We hypothesized that women receiving care after implementation of the program would have lower prevalence of excess gestational weight gain as compared to women who received care prior to the program’s implementation.
METHODS
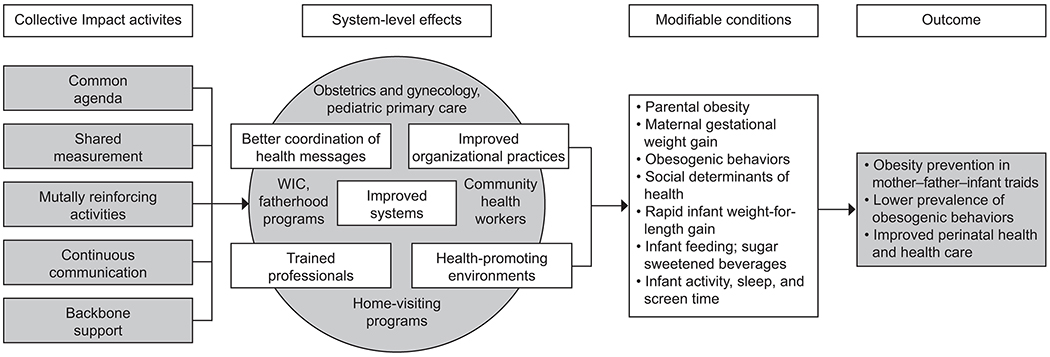
We conducted a quasi-experimental trial in two community health centers in Massachusetts with high prevalence of maternal and childhood obesity. The conceptual framework (Figure 1), intervention design and evaluation methods have been described in detail elsewhere.14 Briefly, the First 1,000 Days program is a systems-level initiative that engages stakeholders across clinical and public health sectors, using a Collective Impact model, to reduce the prevalence of obesity and obesity risk factors among low-income mother–infant pairs by addressing individual, family, and socio-contextual factors. The program’s systems-wide interventions start when women initiate prenatal care in their first trimester of pregnancy (“enrollment”) and supports the mothers, their partners, and the mother-partner or father-infant triads, throughout the first 24 months of age. Given implementation across the entire population of women receiving care at the health centers, the program uses a quasi-experimental, pre-post design to evaluate the extent to which the program is associated with lower prevalence of women gaining excess weight in pregnancy. The primary outcome of the current analysis was prevalence of excess gestational weight gain after the program’s implementation.
Figure 1.
Conceptual framework for the First 1,000 Days program. WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
The First 1,000 Days program was conducted in two community health centers serving predominantly low-income, racial and ethnic minority populations in Revere and Chelsea, MA. All women receiving care at the two community health centers and who delivered a live singleton infant at a Partners HealthCare-affiliated hospital were eligible for the longitudinal analyses of excess gestational weight gain. We received a waiver of informed consent to use longitudinal electronic health record data. The First 1,000 Days study protocol was approved by the Partners Human Research Committee, the Institutional Review Board of Partners HealthCare.
Evidence-informed program components were implemented across both health centers and aimed to improve primary and secondary prevention of obesity. Program components, previously described,14 included: (1) Staff and provider training to standardize obesity prevention efforts across health center staff including physicians, clinical staff such as nurses or medical assistants, administrative leaders, community health workers, and representatives from WIC and the Home Visiting programs; (2) Enhanced tracking of excess gestational weight gain through newly implemented clinical decision supports in the electronic health record and surveillance by health coaches; (3) Universal screening for health behaviors and socio-contextual factors at the first prenatal visit; (4) Patient navigation to support healthy behavior change, social needs, and strengthen integration of clinical and public health services (Appendix 1); and (5) Individual health coaching and care coordination for women at high risk of obesity. Women with a pre-pregnancy BMI ≥ 25 kg/mm2 or with first trimester weight gain ≥ 2 kgs were flagged as high-risk for excess gestational weight gain and received up to four individual health coaching telephone sessions during their second and third trimesters of pregnancy.
The First 1,000 Days Program focuses on five behavioral targets during pregnancy (Figure 2): (1) eating a balanced diet that is high in fruits, vegetables, and fiber; include protein in most meals; and reducing fast food consumption; (2) drinking mainly water and avoiding sugar-sweetened beverages; (3) being physically active most days; (4) getting recommended amounts of sleep; and (5) reducing stress via increased social support. Printed educational materials were created with the primary purpose of providing consistent messaging during pregnancy. Materials included posters throughout health center clinical and public health offices and individual booklets provided to patients – available in English, Spanish, Vietnamese, and Arabic with general health information relevant to distribution at first and third trimester prenatal visits (Figure 2) and customizable sections for individual weight gain recommendations and patient-created behavior change goal setting. We developed a text messaging program that sent 2-3 messages per week throughout pregnancy. Enrollment was offered during routing prenatal care visits. The text message program was intended to provide additional health education and social support services between regularly scheduled prenatal care visits. In addition, we created over 50 short informational videos (Vidscrips®) in English and Spanish to reinforce the behavioral and socio-contextual goals of the program. Weblinks to the videos were provided to women and their partners throughout pregnancy. The videos provided answers to common questions, such as how much weight to gain in pregnancy, recommendations for diet and exercise, and on other important topic areas including maternal depression, smoking, gestational diabetes, and places families can access resources, such as WIC, farmers markets, behavioral health, and support services for fathers. Clinical and public health program staff are featured in the videos.
Figure 2.
Selected First 1,000 Days pregnancy program materials. Images (photographs) used under license from Shutterstock.com.
The primary outcome of the current analysis was excess gestational weight gain. Medical assistants at each health center measured women’s height and weight according to the written standardized protocol of the health centers and entered the information into the electronic health record. From the electronic health record, we collected height and self-reported pre-pregnancy weight to calculate pre-pregnancy body mass index (BMI). Women with a BMI ≥ 18.5 to < 25 kg/mm2 were considered to be normal weight, women with a BMI ≥25 to <30 kg/mm2 were considered to have overweight, and women with a BMI ≥30 kg/mm2 were considered to have obesity. We also tracked weights from all clinical visits during pregnancy and defined gestational weight gain as the difference between the last documented weight prior to delivery (within 14 days of birth) and the first measured weight when prenatal care was initiated in the first trimester. If a measured weight in the first trimester was not available, self-reported weight was used, or last reported weight within the 9 months before pregnancy. In order to collect complete data from the electronic health record, women were required to deliver a singleton birth at a Partners HealthCare affiliated hospital between September 1, 2015 – May 31, 2018 to be included in analyses.
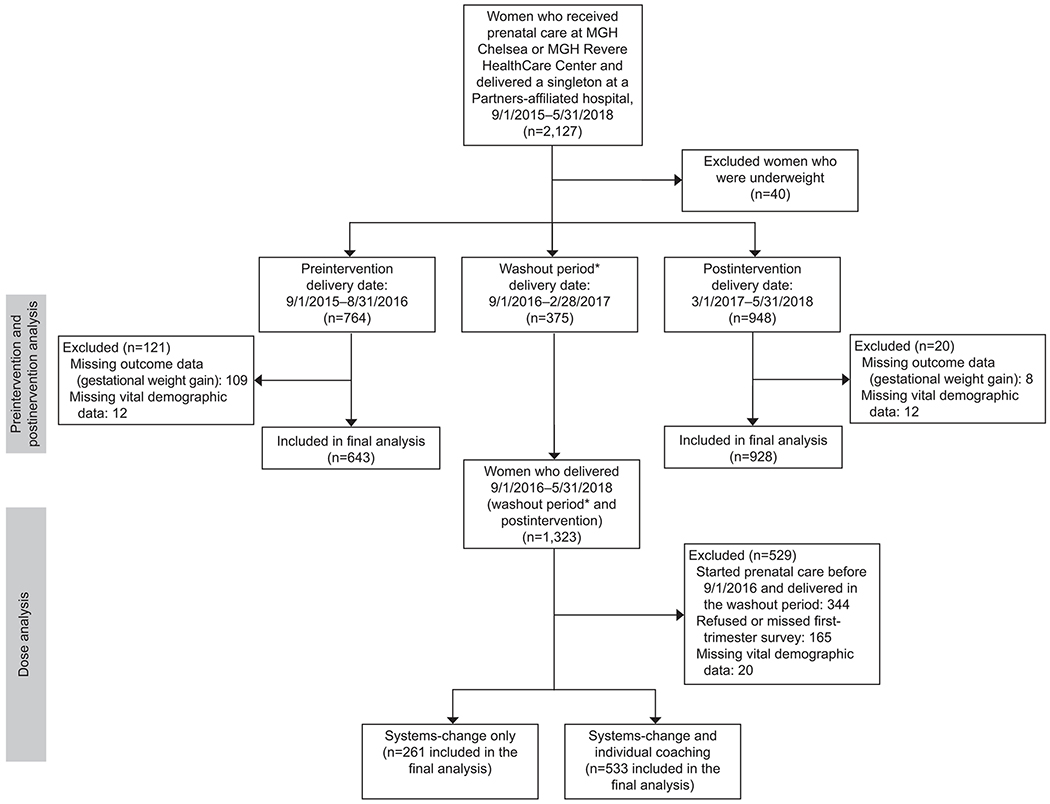
The primary analysis contrasts gestational weight gain collected before and after implementation of the First 1,000 Days program. Delivery data were collected before the intervention began (9/1/2015-8/31/2016) and after implementation (3/1/2017-5/31/2018). For the present study, our primary outcome of interest was the percentage of women with excess gestational weight gain, defined as weight gain above the 2009 National Academy of Medicine guidelines according to pre-pregnancy BMI.18 To create this measure, we extracted longitudinal data from the electronic health records for 2,127 women, who received care at either of the health centers, had a singleton birth, and delivered at a Partners HealthCare-affiliated hospital between September 2015 (approximately one year prior to the implementation of the program) through May 2018 (approximately 21 months after program implementation and the date of our final electronic health record data pull). To be included in the analyses, women needed to have received prenatal care at either health center, have vital demographic and anthropometric information available (e.g. age, sex, race and ethnicity, height, weights), have a pre-pregnancy or first trimester weight documented, and have a weight measurement within 14 days of delivery. We excluded from analyses women with pre-pregnancy BMI in the underweight category due to small sample sizes but included all other BMI categories. The sample size for our analyses included 1,571 women, of whom 643 were in the pre-implementation group and 928 were in the post-implementation group. A post-hoc analysis showed that a sample size > 191 women per group (pre- and post-implementation; total N > 382) would have the power of 0.8 at a significance level of .05 to detect a 13.2% reduction in the prevalence of excess gestational weight gain. Figure 3 shows the study participant flow.
Figure 3.
Intervention flowchart for First 1,000 Days prenatal program. *Washout period includes deliveries from 9/1/2016 to 2/28/2017. Assumes women who started prenatal care in the first trimester and delivered at 39 weeks of gestation are in the postintervention group. MGH, Massachusetts General Hospital.
To assess potential unintended consequences of the program, we also examined birth weight, birth weight for gestational age z-score, pre-term birth (<37 weeks), macrosomia, large-for-gestational age, small-for-gestational age, and cesarean delivery as secondary outcomes. In a subset of women in the post-implementation phase only (N=264), we also conducted surveys in the first and third trimesters of pregnancy to assess implementation process outcomes, e.g. awareness and satisfaction with the program and exposure to various components of the program (Appendix 2). We were unable to evaluate changes in prevalence of gestational diabetes due to incomplete data in the electronic health record for the pre-implementation sample.
We summarize descriptive measures as mean (SD) or percentage, as noted, both for the entire sample and by study period (e.g., comparing women before and after implementation of the First 1,000 Days program). Pairwise Wilcoxon comparisons and chi-square tests were used to compare differences between pre-implementation and post-implementation measures.
To evaluate the associations of the First 1,000 Days program with our main outcome, we used a quasi-experimental, pre-post design to examine differences in the proportion of women with excess gestational weight gain before and after program implementation. We decided a priori to stratify models by pre-pregnancy BMI category. Additional outcomes included infant birthweight (in kilograms), birthweight for gestational age z-score, pre-term birth (<37 weeks), macrosomia, large-for-gestational age, small-for-gestational age, caesarean delivery, and implementation process outcomes. As a secondary analysis, we also examined outcomes according to degree of exposure to the programs’ components, e.g. among women exposed to trained providers and patient education materials only (“systems-change only” group) and the group of women who additionally received at least one (and up to four) health coaching phone call from a First 1,000 Days health coach during pregnancy (“systems-change plus coaching” group). We hypothesized that women exposed to more components of the intervention would have better outcomes. We used SAS PROC LOGSITC to calculate odds ratios for associations between variables and excessive gestational weight gain and PROC GLM for analyses of mean differences in infant birthweight and birthweight for gestational age z-scores. We ran models unadjusted and models controlling for infant gestational age at delivery, maternal race and ethnicity, and public insurance status.
RESULTS
Data were collected on 2,127 women receiving prenatal care at the two intervention health centers and delivering at an affiliated hospital from 9/1/2015 to 5/31/2018. Figure 3 shows the distribution of women between the pre- and post-implementation groups, as well as those in the intervention group who were exposed to the systems-level intervention components only and those who additionally received individual health coaching.
There were 375 women who delivered in the 6-month period after the start of program implementation and were excluded from pre- and post-intervention analyses given that the intervention was not in place from the start of their prenatal care (washout period). The post-implementation group included 928 women who were exposed to the First 1,000 Days program from the initiation of prenatal care, and who had complete outcome and vital demographic data. Six hundred forty-three women who delivered prior to start of the First 1,000 Days program and had complete outcome and vital demographic data comprised the pre-implementation group. Women excluded for missing data did not differ from women included in the study in terms of age, parity, race/ethnicity, public insurance status, or pre-pregnancy BMI.
Table 1 shows demographic and clinical characteristics of women in the pre- and post-implementation groups. Across both groups, women averaged approximately 30 years of age and a majority identified as Hispanic. A greater proportion of women in the post-implementation group (61% vs. 44%, unadjusted OR: 0.51 [95% CI: 0.42, 0.63]). were publicly insured. This was likely due to inconsistency of reporting insurance type in the electronic medical record system used in the pre-implementation period rather than a true temporal trend; however final analyses are adjusted for this characteristic. Average pre-pregnancy BMI was 28.1 kg/mm2, with roughly one-third of women in each of the normal, overweight, and obese BMI categories, across both the pre- and post-implementation periods.
Table 1.
Characteristics of Women Receiving Care in the Participating Community Health Centers, Prior to and Following Implementation of the First 1,000 Days Program.
| Participant Characteristics | Overall N= 1571 | Pre-Implementation N=643 | Post-Implementation N=928 | P-value |
|---|---|---|---|---|
| Mean±SD or N (%) | ||||
| Age, years | 30.0±5.9 | 29.9±5.9 | 30.0±5.9 | 0.63 |
| Parity | 1.2±1.1 | 1.2±1.1 | 1.2±1.2 | 0.81 |
| Race and ethnicity, n (%) | ||||
| White, non-Hispanic | 420 (26.7) | 159 (24.7) | 261 (28.1) | 0.36 |
| Hispanic or Latino | 835 (53.2) | 353 (54.9) | 482 (51.9) | |
| Black, non-Hispanic | 123 (7.8) | 55 (8.6) | 68 (7.3) | |
| Asian or Other, non-Hispanic | 193 (12.3) | 76 (11.8) | 117 (12.6) | |
| Public Insurance, n (%) | 847 (53.9) | 284 (44.2) | 563 (60.7) | <.001 |
| Pre-Pregnancy BMI, kg/mm2 | 28.1±6.1 | 28.1±6.0 | 28.2±6.1 | 0.77 |
| Pre-Pregnancy BMI Category*, n (%) | ||||
| Normal Weight | 538 (34.3) | 223 (34.7) | 315 (33.9) | 0.59 |
| Overweight | 532 (33.9) | 224 (34.8) | 308 (33.2) | |
| Obesity | 501 (31.9) | 196 (30.4) | 305 (32.9) | |
| GWG by pre-pregnancy BMI Category | ||||
| Normal Weight | 30.7±11.4 | 30.7±12.0 | 30.6±11.0 | 0.92 |
| Overweight | 25.9±11.8 | 27.1±11.6 | 25.1±11.8 | 0.06 |
| Obesity | 20.1±14.2 | 18.9±13.4 | 20.8±14.6 | 0.15 |
| Gestational age at delivery, weeks | 39.2±1.8 | 39.3±1.8 | 39.2±1.8 | 0.66 |
| C-section, n (%) | 356 (22.7) | 156 (24.3) | 200 (21.6) | 0.21 |
| Preterm birth, n (%) | 103 (6.6) | 39 (6.1) | 64 (6.9) | 0.51 |
| Infant birth weight, kgs | 3.4±0.5 | 3.4±0.6 | 3.4±0.5 | 0.96 |
| Birth weight for gestational age z-score | −0.03±0.94 | −0.04±0.97 | −0.03±0.91 | 0.83 |
| Infant large for gestational age, n (%) | 129 (8.2) | 63 (9.8) | 66 (7.1) | 0.06 |
| Macrosomia, n (%) | 163 (10.4) | 75 (11.7) | 88 (9.5) | 0.16 |
| Infant small for gestational age , n (%) | 183 (11.7) | 80 (12.5) | 103 (11.1) | 0.41 |
Women who started pregnancy with a BMI < 18.5 (underweight) were excluded from the analysis due to a very small number.
Abbreviations: BMI (Body Mass Index); GWG (Gestational Weight Gain)
Note: Percentages in the table may not sum to 100 due to rounding.
Table 2 presents the unadjusted prevalence and adjusted odds of excess gestational weight gain for women in the pre and post implementation groups. Among women with pre-pregnancy BMI in the overweight range, enrollment in the program was associated with lower prevalence (46.4% vs. 55.8%); and unadjusted odds (OR=0.69, 95% CI: 0.49, 0.97) and adjusted odds (OR = 0.69, 95% CI: 0.49, 0.99) of excess gestational weight gain. Models were adjusted for gestational age at delivery, race and ethnicity, and public insurance. The prevalence of excess gestational weight gain did not differ for women who started pregnancy with a BMI < 25 kg/mm2 or ≥ 30 kg/mm2. Results of the multivariable analyses after Bonferroni adjustment for multiple comparisons are available as Appendix 3 (α level of adjustment was P < . 0166).
Table 2.
Associations of the First 1,000 Days program implementation on excess gestational weight gain and secondary birth outcomes.
| Primary & Secondary Outcomes | Pre-Implementation N=643 | Post-Implementation N=928 | Unadjusted odds of excess GWG after implementation | Adjusted * odds of excess GWG after implementation |
|---|---|---|---|---|
| Primary Outcomes | N (%) | N (%) | OR (95% CI) | OR (95% CI) |
| Excess GWG | ||||
| Pre-pregnancy Normal Weight | 66 (29.6%) | 94 (29.8%) | 1.01 (0.70-1.47) | 1.04 (0.70-1.52) |
| Pre-pregnancy Overweight | 125 (55.8%) | 143 (46.4%) | 0.69 (0.49-0.97) | 0.69 (0.49-0.99) |
| Pre-pregnancy Obesity | 81 (41.3%) | 140 (45.9%) | 1.21 (0.84-1.73) | 1.20 (0.83-1.75) |
| Mean GWG | ||||
| Pre-pregnancy Normal Weight | 30.7±12.0 | 30.6±11.0 | −0.10 (−2.06-1.87) | 0.08 (−1.89-2.04) |
| Pre-pregnancy Overweight | 27.1±11.6 | 25.1±11.8 | −1.93 (−3.95-0.09) | −1.78 (−3.80-0.24) |
| Pre-pregnancy Obesity | 18.9±13.4 | 20.8±14.6 | 1.86 (−0.68-4.4) | 1.85 (−0.75-4.45) |
| Secondary Outcomes | N (%) or Mean±SD | N (%) or Mean±SD | OR (95% CI) or β (95% CI) | OR (95% CI) or β (95% CI) |
| Infant birth weight, kgs | 3.4±0.6 | 3.4±0.5 | 0.001 (−0.05-0.06) | 0.01 (−0.03-0.06) |
| Birth weight for gestational age z-score | −0.04±0.97 | −0.03±0.91 | 0.01 (−0.08- 0.10) | 0.02 (−0.07-0.12) |
| Cesarean birth | 156 (24.3) | 200 (21.6) | 0.86 (0.68-1.09) | 0.80 (0.63-1.03) |
| Preterm birth <37 weeks | 39 (6.1) | 64 (6.9) | 1.15 (0.76-1.73) | 1.15 (0.76-1.75) |
| Macrosomia | 75 (11.7) | 88 (9.5) | 0.79 (0.57-1.10) | 0.83 (0.59-1.15) |
| Infant large for gestational age | 63 (9.8) | 66 (7.1) | 0.71 (0.49-1.01) | 0.74 (0.51-1.07) |
| Infant small for gestational age | 80 (12.5) | 103 (11.1) | 0.88 (0.64-1.20) | 0.87 (0.63-1.19) |
Boldface type indicates statistical significance at the p=0.05 level.
Models adjusted for gestational age at delivery, race and ethnicity, and public insurance.
Abbreviations: GWG (Gestational Weight Gain)
In secondary analyses, we found that among women who began their pregnancy in the overweight range, the lowest prevalence of excess gestational weight gain was observed among those whose program exposure included individual-level health coaching (“systems-change plus coaching”) in addition to the systems-level intervention components (Table 3).
Table 3.
Secondary Gestational weight gain outcomes among women exposed the First 1000 Days program, by level of exposure.
| Secondary Gestational Weight Gain Outcomes | Pre-Implementation N=643 | Prevalence and adjusted odds of excess GWG among women exposed to Systems-Change only N=261 | Prevalence and adjusted* odds of excess GWG among women exposed to Systems-Change plus Coaching N=533 | P† for trend |
|---|---|---|---|---|
| Excess GWG | N (%) | N (%) | N (%) | |
| Pre-pregnancy Normal Weight | 66 (29.6) | 29 (31.5) | 50 (28.4) | 0.87 |
| Pre-pregnancy Overweight | 125 (55.8) | 44 (50.0) | 82 (44.8) | 0.09 |
| Pre-pregnancy Obesity | 81 (41.3) | 41 (50.6) | 73 (42.0) | 0.33 |
| Excess GWG | OR (95% CI)* | OR (95% CI)* | ||
| Pre-pregnancy Normal Weight | 1.00 (Reference) | 1.16 (0.68-1.99) | 0.96 (0.61-1.50) | 0.79 |
| Pre-pregnancy Overweight | 1.00 (Reference) | 0.83 (0.50-1.39) | 0.64 (0.43-0.96) | 0.09 |
| Pre-pregnancy Obesity | 1.00 (Reference) | 1.40 (0.82-2.39) | 1.00 (0.66-1.53) | 0.40 |
Models adjusted for gestational age at delivery, race and ethnicity, and public insurance.
P-value of Wald chi-square test assessing the association between exposure and outcomes
Boldface type indicates statistical significance of the point estimate at the p=0.05 level.
Abbreviations: GWG (Gestational Weight Gain)
We did not observe any unintended or adverse associations of program implementation on birth weight, birth weight for gestational age z-score, pre-term birth (<37 weeks), macrosomia, large-for-gestational age, small-for-gestational age, and cesarean delivery (Table 2). For example, infant birth weight was 3.4 kgs in the pre- and post-implementation groups (unadjusted OR: 0.001; 95% CI: −0.05, 0.06 and adjusted OR: 0.01; 95% CI: −0.03, 0.06), with no change in birthweight for gestational age z-score (−0.04 pre-implementation and −0.03 post-implementation). Pre-term birth rates were similar with 6.1% of women delivering <37 weeks in the pre-implementation period and 6.9% of women in the post-implementation period. Prevalence of cesarean birth was 24.3% pre-implementation and 21.6% post-implementation (unadjusted OR: 0.86, 95% CI: 0.68, 1.09 and adjusted OR: 0.80, 0.63, 1.03). Infant large for gestational age rates also did not change (9.8% pre-implementation and 7.1% post-implementation, unadjusted OR: 0.71; 95% CI: 0.49, 1.01, and adjusted OR: 0.74; 95% CI: 0.51, 1.07).
Among 286 women who responded to a 3rd trimester survey (out of 293 women eligible, 98% response rate), 87% reported hearing of the First 1,000 Days program with 89% of those reporting they were satisfied or very satisfied with their experience in the program. (Appendix 2). Additionally, 73% of women reported they believed the program would improve theirs and their family’s health and well-being. When asked about the components of the First 1,000 Days program, 77% of women reported seeing a poster, 68% saw booklet, 63% received text messages, 15% watched videos, 74% received a call from a First 1,000 days coordinator, and 53% received a list of community and health center resources (Appendix 2).
DISCUSSION
In this systems-level intervention, we found that >65% of women entered pregnancy with a BMI in the overweight or obese range and approximately half of these women gained excessive weight than recommended by National Academy of Medicine guidelines - a risk factor for adverse pregnancy, birth, and neonatal outcomes.7,19,20 After program implementation, we observed a 31% reduction in odds and nearly 10% reduction in prevalence of excess gestational weight gain among women who began their pregnancy with an overweight BMI, and within this group there were stronger associations for those with greater program exposure. However, similar associations were not observed among women entering pregnancy with a BMI ≥ 30 kg/m2. or < 25 kg/mm2, suggesting that the program did not sufficiently influence changes to daily energy intake and expenditures necessary for pregnant women of either normal weight or obesity to achieve optimal gestational weight gain. Overall, the intervention components were feasible to deliver, acceptable to women, and did not have adverse associations with maternal or infant birth outcomes.
The primary strengths of the First 1,000 Days program lie in its systems-level coordination between clinical and public health programs co-located within community health care centers to concurrently target individual health behaviors, health systems and technologies, and socio-contextual risk factors for excess weight gain. Socio-contextual factors,21 such as depression, stress,22 lack of social support, and limited access to affordable healthy foods and exercise opportunities, also influence risk for excess gestational weight gain, particularly among low-income and minority women23 and can negate the positive associations of individual-level counseling.24 To address this, First 1,000 Days program implemented universal screening for social needs (e.g. food and housing security), stress and social support, and used patient navigators to connect women with identified needs to public health resources (e.g. WIC program), local community programs (e.g. food banks, low-cost gyms), and health center-based social services. (Appendix 4). Prior data also indicate that prenatal care providers often do not feel adequately prepared to provide diet and physical activity counseling,25 however such counseling has been associated with an increase in appropriate gestational weight gain.26 As such, we included universal trainings and support materials for prenatal clinical staff to promote standardized gestational weight gain goal-setting and counseling.
In recent years, an increasing number of prenatal interventions have employed mobile technologies to help women achieve appropriate gestational weight gain.27–29 The First 1,000 Days program implemented a text messaging campaign and informational videos to provide counseling. A previous trial to decrease excess gestational weight gain among women with overweight and obesity was similarly effective when delivered by mobile phone compared to usual prenatal care.30 Additionally, in a pilot feasibility study of a health coaching intervention that used phone calls, text messages, and emails to provide behavioral support during pregnancy, women reported high satisfaction with health coaching services delivered virtually.31
Inherent limitations of our multi-component, systems-level intervention include inability to differentiate which program components have the greatest magnitude of association for specific women and the natural variability in exposure to the various program components. Most gestational weight gain interventions to date have targeted individual-level behaviors, with a 2015 Cochrane review showing an average 20% reduced odds of excess gestational weight gain across the trials.32 A recent meta-analysis of coordinated clinical trials that implemented a variety of lifestyle interventions to decrease excess gestational weight gain showed a 14% lower prevalence of excess gestational weight gain among the composite 1,150 women randomized across all sites, when compared to usual care in racially and socioeconomically diverse prenatal populations.33 Further, a 2017 meta-analysis of diet and physical activity interventions demonstrated lower gestational weight gain in intervention groups, and did not find variation of associations by age, parity, BMI, ethnicity, or pre-pregnancy medical conditions.34 As such, it may be that interventions implemented at an individual level have higher effectiveness in preventing excess gestational weight gain compared to more modest effects of system-wide changes. This could help explain why we did not see differences among women in the normal weight category who were only exposed to the systematic intervention components but were not eligible for the individualized coaching and patient navigation. Thus, it is possible that the “dose” of intervention received by these normal weight women was insufficient to prevent excess gestational weight gain. Similarly, we observed that women with overweight pre-pregnancy BMI who enrolled in our program had the greatest reduction in excess gestational weight gain when they received individualized health coaching in addition to the systematic components. However, many individual-level lifestyle interventions would ultimately need to be scaled to a systems-level when applied outside of randomized trials, which may weaken their effects.
Another limitation of the First 1,000 Days program is the inability for individual-level randomization given implementation across the entire prenatal care system. Lack of randomization can increase susceptibility to confounding by baseline differences between the intervention and control groups, thus we included these characteristics in our adjusted models. In addition, our pre-post quasi-experimental study design is susceptible to confounding by temporal trends in gestational weight gain, however there were no known contextual differences at the health centers between the pre- and post-intervention time periods. Despite its limitations, the quasi-experimental study design demonstrates “real-world” applicability and the efficacy of a systems-wide intervention.
The First 1,000 Days program demonstrates that excess gestational weight gain may be targeted during the prenatal period for women who are overweight at the start of pregnancy using a multi-component, systems-level intervention. However, our findings also suggest the need for more intensive, evidence-based gestational weight management approaches for women entering pregnancy with obesity. Application of systems-wide changes to address excess gestational weight gain in additional prenatal care centers with a variety of population characteristics and clinical formats will improve understanding of the reproducibility of our findings. Ultimately, systems-level implementation of effective interventions to reduce excess gestational weight gain in routine prenatal clinical care and public health programs holds the potential to improve population-wide pregnancy outcomes and the long-term health of mothers and their children.
Supplementary Material
Acknowledgments
This research was funded by The Boston Foundation (G2015-0007), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K24DK105989), and Massachusetts General Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The sponsors had no role in the study design; collection, analysis and interpretation of data; writing of report; or decision to submit for publication.
The authors thank Monica Gerber, MPH (Fred Hutchison Cancer Research Center, Seattle, WA) and Man Luo, MPH (Massachusetts General Hospital, Boston, MA) for their expertise in pulling and organizing the electronic health record data, and the staff of the MGH Chelsea and Revere HealthCare Centers for their work implementing the program.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Authors’ Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? Not applicable.
What other documents will be available? Not applicable.
When will data be available (start and end dates)? Not applicable.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
References
- 1.Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, et al. The state of US health, 1990-2016: Burden of diseases, injuries, and risk factors among US states. JAMA 2018;319(14):1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massetti GM, Dietz WH, Richardson LC. Excessive weight gain, obesity, and cancer: Opportunities for clinical intervention. JAMA 2017;318(20):1975–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossen LM, Schoendorf KC. Measuring health disparities: Trends in racial-ethnic and socioeconomic disparities in obesity among 2- to 18-year old youth in the United States, 2001-2010. Ann Epidemiol 2012;22(10):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olds T, Maher C, Zumin S, Péneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: Data from nine countries. Int J Pediatr Obes 2011;6(5-6):342–360. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 2017;5(1):53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias-adjusted meta-analysis. Obes Rev 2014;15(4):338–347. [DOI] [PubMed] [Google Scholar]
- 7.Gilmore LA, Klempel-Donchenko M, Redman LM. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Semin Perinatol 2015;39(4):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the First 1,000 Days: A systematic review. Am J Prev Med 2016;50(6):761–779. [DOI] [PubMed] [Google Scholar]
- 9.Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MA, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019;321(17):1702–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weden MM, Brownell P, Rendall MS. Prenatal, perinatal, early life, and sociodemographic factors underlying racial differences in the likelihood of high body mass index in early childhood. Am J Public Health 2012;102(11):2057–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: Birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol 2009;201(4):339 e331–314. [DOI] [PubMed] [Google Scholar]
- 12.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am J Clin Nutr 2011;94(5):1225–1231. [DOI] [PubMed] [Google Scholar]
- 13.Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the First 1,000 Days a systematic review. Am J Prev Med 2016;50(6):780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake-Lamb T, Boudreau AA, Matathia S, Tiburcio E, Perkins ME, Roche B, et al. Strengthening integration of clinical and public health systems to prevent maternal-child obesity in the First 1,000Days: A collective impact approach. Contemp Clin Trials 2018;65:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kania J, Kramer M. Collective impact. Stanford Social Innovation Review 2011:36–41. [Google Scholar]
- 16.Hanleybrown F, Kania J, Kramer M. Channeling change: Making collective impact work. Stanford Social Innovation Review 2012:1–8. [Google Scholar]
- 17.Boyce B Collective impact: Aligning organizational efforts for broader social change. J Acad Nutr Diet 2013;113(4):495–497. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: Reexamining the guidelines. The National Academies Collection; 2009. [Google Scholar]
- 19.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017;317(21):2207–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol 2013;121(5):969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul KH, Graham ML, Olson CM. The web of risk factors for excessive gestational weight gain in low income women. Matern Child Health J 2013;17(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kominiarek MA, Grobman W, Adam E, Buss C, Culhane J, Entringer S, et al. Stress during pregnancy and gestational weight gain. J Perinatol 2018;38(5):462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDowell M, Cain MA, Brumley J. Excessive gestational weight gain. J Midwifery Womens Health 2019;64(1):46–54. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien EC, Alberdi G, Geraghty AA, McAuliffe FM. Lower education predicts poor response to dietary intervention in pregnancy, regardless of neighbourhood affluence: Secondary analysis from the ROLO randomised control trial. Public Health Nutr 2017;20(16):2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraro ZM, Boehm KS, Gaudet LM, Adamo KB. Counseling about gestational weight gain and healthy lifestyle during pregnancy: Canadian maternity care providers’ self-evaluation. Int J Womens Health 2013;5:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo S, Walker JS, Caughey MC, Ferraro AM, Asafu-Adjei JK. What characteristics of nutrition and physical activity interventions are key to effectively reducing weight gain in obese or overweight pregnant women? A systematic review and meta-analysis. Obes Rev 2017;18(4):385–399. [DOI] [PubMed] [Google Scholar]
- 27.Willcox JC, Campbell KJ, McCarthy EA, Wilkinson SA, Lappas M, Ball K, et al. Testing the feasibility of a mobile technology intervention promoting healthy gestational weight gain in pregnant women (txt4two) - Study protocol for a randomised controlled trial. Trials 2015;16:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Horn L, Peaceman A, Kwasny M, Vincent E, Fought A, Josefson J, et al. Dietary approaches to stop hypertension diet and activity to limit gestational weight: Maternal offspring metabolics family intervention trial, a technology enhanced randomized trial. Am J Prev Med 2018;55(5):603–614. [DOI] [PubMed] [Google Scholar]
- 29.Chao AM, Srinivas SK, Studt SK, Diewald LK, Sarwer DB, Allison KC. A pilot randomized controlled trial of a technology-based approach for preventing excess weight gain during pregnancy among women with overweight. Front Nutr 2017;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redman LM, Gilmore LA, Breaux J, Thomas DM, Elkind-Hirsch K, Stewart T, et al. Effectiveness of SmartMoms, a novel eHealth intervention for management of gestational weight gain: Randomized controlled pilot trial. JMIR Mhealth Uhealth 2017;5(9):e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seward MW, Simon D, Richardson M, Oken E, Gillman MW, Hivert MF. Supporting healthful lifestyles during pregnancy: A health coach intervention pilot study. BMC Pregnancy Childbirth 2018;18(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015(6):Cd007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peaceman AM, Clifton RG, Phelan S, Gallagher D, Evans M, Redman LM, et al. Lifestyle interventions limit gestational weight gain in women with overweight or obesity: LIFE-Moms prospective meta-analysis. Obesity (Silver Spring) 2018;26(9):1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, et al. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health.1999;3(3):167–172. [DOI] [PubMed] [Google Scholar]
- 36.Miller SA, Taveras EM, Rifas-Shiman SL, Gillman MW. Association between television viewing and poor diet quality in young children. Int J Pediatr Obes 2008;3(3):168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Youth Risk Behavior Survey (YRBS). Available at: http://www.cdc.gov/healthyyouth/yrbs/pdf/questionnaire/2009MiddleSchool.txt. Retrieved January 6, 2020.
- 38.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System (BFRSS). Available at: https://www.cdc.gov/sleep/surveillance.html. Retrieved January 6, 2020.
- 39.Centers for Disease Control and Prevention. Pregnancy Risk Assessment Monitoring System (PRAMS). Available at: https://www.cdc.gov/prams/. Retrieved January 6, 2020.
- 40.World Health Organization. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) and pilot brief intervention: A technical report of phase II findings of the WHO ASSIST Project. WHO Library Cataloguing-in-Publication Data; 2006:1–125. [Google Scholar]
- 41.Pantell M, Rehkopf D, Jutte D, Syme SL, Balmes J, Adler N. Social isolation: A predictor of mortality comparable to traditional clinical risk factors. Am J Public Health 2013;103(11):2056–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 43.Elo AL, Leppanen A, Jahkola A. Validity of a single-item measure of stress symptoms. Scand J Work Environ Health 2003;29(6):444–451. [DOI] [PubMed] [Google Scholar]
- 44.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol 1999;18(4):333–345. [DOI] [PubMed] [Google Scholar]
- 45.Urban Institute, and Child Trends. National Survey of America’s Families (NSAF), 1999. Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2007. [Google Scholar]
- 46.Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics 2010;126(1):e26–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.