Abstract
Gastrin, secreted by stomach G cells in response to ingested sodium, stimulates the renal cholecystokinin B receptor (CCKBR) to increase renal sodium excretion. It is not known how dietary sodium, independent of food, can increase gastrin secretion in human G cells. However, fenofibrate (FFB), a peroxisome proliferator-activated receptor-α (PPAR-α) agonist, increases gastrin secretion in rodents and several human gastrin-secreting cells, via a gastrin transcriptional promoter. We tested the following hypotheses: (1.) the sodium sensor in G cells plays a critical role in the sodium-mediated increase in gastrin expression/secretion, and (2.) dopamine, via the D1R and PPAR-α, is involved. Intact human stomach antrum and G cells were compared with human gastrin-secreting gastric and ovarian adenocarcinoma cells. When extra- or intracellular sodium was increased in human antrum, human G cells, and adenocarcinoma cells, gastrin mRNA and protein expression/secretion were increased. In human G cells, the PPAR-α agonist FFB increased gastrin protein expression that was blocked by GW6471, a PPAR-α antagonist, and LE300, a D1-like receptor antagonist. LE300 prevented the ability of FFB to increase gastrin protein expression in human G cells via the D1R, because the D5R, the other D1-like receptor, is not expressed in human G cells. Human G cells also express tyrosine hydroxylase and DOPA decarboxylase, enzymes needed to synthesize dopamine. G cells in the stomach may be the sodium sensor that stimulates gastrin secretion, which enables the kidney to eliminate acutely an oral sodium load. Dopamine, via the D1R, by interacting with PPAR-α, is involved in this process.
Keywords: gastrin, dopamine D1 receptor, PPAR-α, sodium, fenofibrate, SW626
Introduction
Hypertension develops when renal, neural, and hormonal mechanisms fail to enable the body to excrete the ingested sodium, and the sodium that accumulates in the body can no longer be sequestered (for example, in interstitium/skin); the extracellular fluid volume becomes expanded in salt-sensitive states (Feng et al. 2017, Wiig et al. 2018). However, an increase in sodium intake in normotensive individuals may not always increase blood pressure or total body water but causes a shift of the interstitial fluid into the intravascular (Heer et al. 2000) or non-extracellular compartment (Palacios et al. 2004). The sodium load has been shown to accumulate in the skin (Nikpey et al. 2017, Selvarajah et al. 2017). The ability of the kidney to increase sodium excretion following an oral salt load is enhanced by the increase in blood pressure associated with the increase in sodium intake, a phenomenon called pressure natriuresis (Granger et al. 2002, Hall et al. 2012). However, orally ingested sodium can be excreted acutely without an increase in blood pressure (Andersen et al. 2000, Preston et al. 2012). The mechanism(s) by which orally ingested sodium is acutely excreted is not well understood, and the presence of a gastrorenal axis is disputed (Preston et al. 2012). We have reported that a gastrorenal pathway exists in which gastrin secreted into the blood from the stomach, in response to ingested sodium, stimulates the gastrin receptor, also known as cholecystokinin B receptor (CCKBR), in the renal proximal tubule; CCKBR inhibits renal sodium transport (Chen et al. 2013, Jiang et al. 2016, 2017, Jose et al. 2016). The gastrorenal axis is abetted by the renal dopaminergic pathway because both the dopamine D1-like receptors, D1R and D5R, physically interact with the renal CCKBR to inhibit sodium transport in renal proximal tubule (RPT) cells (Chen et al. 2013, Jiang et al. 2016). The natriuresis caused by gastrin or fenoldopam (agonist for both D1R and D5R) can be blocked by a CCKBR antagonist or a D1R/D5R antagonist in mice and rats (Chen et al. 2013, Jiang et al. 2016). Thus, gastrin, via the CCKBR, and D1-like receptors (D1R and D5R) synergize to increase urinary sodium by inhibiting renal sodium transport (von Schrenck et al. 2000, Chen et al. 2013, Liu & Jose 2013, Jiang et al. 2016, 2017, Jose et al. 2016, Liu et al. 2016). The stomach also synthesizes dopamine (Vieira-Coelho & Soares-da-Silva 1993, Eisenhofer et al. 1997, Eldrup & Richter 2000) where the D1R is also expressed (Vaughan et al. 2000, Wang et al. 2012, 2016), but whether dopamine receptors are expressed in G cells is not known.
Ciprofibrate, a peroxisome proliferator-activated receptor α (PPAR-α) agonist, given by oral gavage, increases gastrin secretion, associated with a doubling of G cell density in the stomach antrum of Sprague–Dawley rats (Martinsen et al. 2005). This did not occur in gastric-bypassed rats and PPAR-α−/− mice (Martinsen et al. 2005). PPAR-α agonists have been reported also to increase progastrin production in human colorectal carcinoma cells (Lachal et al. 2004), but this mechanism has not been studied in normal human stomach G cells. The involvement of the PPAR-α pathway in gastrin secretion is supported by the association of PPAR-α with the gastrin transcriptional promoter by CHIP-SEQ (ENCODE database). PPAR-α may also positively regulate the number of dopaminergic neurons in the substantia nigra (Gonzalez-Aparicio et al. 2011). However, it is not known if PPAR-α is involved in gastrin expression or secretion in humans. Therefore, the current study tested the novel hypothesis that D1-like receptors, via PPAR-α, increase gastrin production in gastrin-producing cells.
In order to investigate the role of the dopaminergic system and PPAR-α in gastrin expression/secretion in humans, we generated stable cell lines of normal stomach antrum gastrin-secreting cells, that is, G cells. For concurrent controls, we compared these new human G cell lines with established human gastrin-secreting cell lines, that is, human gastric adenocarcinoma (AGS) (Ofori-Darko et al. 2000, Datta De et al. 2011) and ovarian adenocarcinoma cells (SW626) (Kim et al. 1998).
Materials and methods
Cell culture of human gastrin-secreting cells
Human gastrin-secreting carcinoma cell lines (gastric adenocarcinoma AGS (Ofori-Darko et al. 2000, Datta De et al. 2011) and ovarian adenocarcinoma SW626 (Kim et al. 1998) were purchased from ATCC. They were cultured in DMEM (Dulbecco’s Modified Eagle’s medium) supplemented with 4.5 g/L glucose, 10% FBS, and 1× penicillin/streptomycin (Invitrogen). Human G cells were isolated from human stomach in our laboratory. All cells were grown in humidified conditions at 37°C, 95% air, and 5% CO2. The cells were grown in standard T flasks, split when 80% confluent, and passaged into T flasks or directly into microplates.
Preparation of human stomach antrum for immunohistochemistry
Human stomach tissue was obtained, after institutional review board-approved informed consent, from patients undergoing gastrectomy. The tissue was immediately placed on ice and transported to the laboratory. Some tissues were snap-frozen and sectioned (10 μm) on a cryostat for immunohistochemistry.
Culture of human stomach G cells
Human G cells were isolated using the protocol described by Lambrecht et al. (2005, 2006) and Kidd et al. (2009) with modifications. Fresh human stomach antrum was obtained from the Biorepository and Tissue Research Facility, The University of Virginia. The outside layer of the stomach wall (serosa) was removed. The remaining stomach wall was purse-string-sutured to make a bag, inside-out with the gastric rugae (mucosa) facing outside. The stomach bag was digested using a series of solutions, as reported by Lambrecht et al. (2005, 2006) and Kidd et al. (2009). Then, the cells were resuspended in 4 mL DMEM-F12, containing 10% FBS, 1× sodium pyruvate, 1× MEM NEAA, 10 μM SCH28080 to inhibit H+/K+ ATPase activity, and 100 mg/mL DTT and transferred into a 6-well dish with 2 mL/well. The medium was changed every other day by removing 1 mL of the medium and adding 1 mL of fresh medium. After 1 week, the cells started to attach to the bottom of the growth dish. The cells were then immortalized by human Tert for 18 h (Gildea et al. 2013). The immortalized G cells were cultured in the same medium with insulin, transferrin, and selenium (1×, ThermoFisher 41400045), in order to maintain normal growth rates.
Immunofluorescence
(a). Human stomach antrum:
The serosa of the stomach antrum was removed. The stomach antrum without the serosa was cut into small pieces and treated under different conditions, as indicated below. The pieces of tissue were then rinsed by PBS, fixed, and sectioned following a previously published method (Gildea et al. 2015).
(b). Human G, SW626, and AGS cells:
The cells were passaged onto a collagen-coated 96-well glass-bottom dish and cultured overnight. After different treatments, as indicated below, the cells were rinsed with PBS, fixed in 4% paraformaldehyde, and then treated with 0.2% Triton ×100 for 5 min. The cells were rinsed twice in PBS and incubated for 10 min with 100 mM Tris pH 7.4, followed by blocking with Odyssey Blocking buffer (Li-Cor, 927–40000) for 30 min. The cells were then stained with anti-goat gastrin antibody (Santa Cruz) or anti-rabbit antibodies to various targets (gastrin, DAKO 1:200), complex type carbohydrate residues marker Phaseolus vulgaris-leucoagglutinin (PHA-L, Vector Laboratories), D1R (generated in our laboratory) (Sanada et al. 1999), D5R (Santa Cruz, 1:100), tyrosine hydroxylase (TH, Cell Signaling, 1:200), and DOPA decarboxylase (DDC, Millipore, 1:200) for 2 h at room temperature. After incubation with the primary antibody, the cells were washed three times in PBST (PBS with Tween-20) and 30 min in TBST (TRIS-buffered saline with Tween-20) and then incubated for 1 h in secondary antibody (Alexa anti-rabbit 647). The cells were washed again in PBST and incubated in TBST containing Hoechst 33342 for 30 min. Finally, the cells were rinsed in PBS before imaging. The images were obtained using an Olympus IX81 automated multi-well spinning disk confocal microscope. D1R and D5R antibodies were also used in AGS (Ofori-Darko et al. 2000, Datta De et al. 2011) and SW626 (Kim et al. 1998) cells.
BMG PHERAstar FS was used to quantify (relative fluorescence units (RFU)) the immunofluorescent proteins in the cells. Gastrin RFU was normalized by DAPI RFU for each well. Six confocal images were taken for each intervention (different NaCl concentration) and normalized to control (no fluorescence). Then, the gastrin signal was measured by ImageJ and divided by the number of cells on each image. Finally, the fold-change of each condition, relative to 90 mM NaCl or VEH, was calculated.
Reverse transcription – polymerase chain reaction
mRNA was quantified using real-time quantitative reverse transcription-PCR (qRT-PCR) and the delta/delta method. The gene expression levels were normalized to the expression of α-actin and expressed as fold-change, relative to the expression in 90 mM NaCl- or VEH-treated cells. All PCR reactions were performed in 20 μL volume, using the iCycler CFX Connect (Biorad Laboratories, Inc, Berkeley, CA, USA). Each reaction contained 10 μL SYBR RT-PCR buffer, 10 μM of each primer, 40 μM iScript, and 200 nM cDNA. The amplification was initiated by 10-min incubation at 50°C, followed by two-step amplification of 5 min at 95°C and 30 s at 95°C for 40 cycles. RNA was extracted by the Zymo Quick-RNA mini-prep, and cDNA was prepared using SuperScript® III First Strand Synthesis Kit.
Primers: Actin sense 5′-AGAAAATCTGGCACCACA CC-3′; Actin anti-sense 5′-CTCCTTAATGTCACGCAC GA-3′; Gastrin sense 5′-ATGCAGCGA CTATGTGTG-3′; Gastrin anti-sense 5′-GTTCTCATCCTCAGCACTG-3′; PPAR-α sense 5′-CTATCATTTGCTGTGGAGATCG-3′; and PPAR-α sense 5′-AAGATATCGTCCGGGTGGTT-3′ (Chandran et al. 2016).
The human stomach antrum, isolated human G cells, AGS (Ofori-Darko et al. 2000, Datta De et al. 2011), and SW626 (Kim et al. 1998) were treated with the media containing different sodium concentrations for 4 h and then fixed for immunoblotting and gastrin immunofluorescence staining. For the qRT-PCR studies of the isolated human G cells cultured in the 96-well plates, the duration of the treatment with the different sodium concentrations was for 30 min.
Effect of increased extracellular sodium concentration on gastrin expression and secretion
Preparation of incubation media with different sodium concentrations
Since ingestion of a meal with high NaCl concentration increases sodium excretion that is related to gastrin (Tarasova et al. 1996, Wang et al. 2017), we determined if increasing the sodium concentration in the incubation medium of human G cells would increase gastrin expression. Low and high sodium solutions were prepared according to Quadri’s method (Quadri & Siragy 2016), with some modification. We added 35 mM NaCl to DMEM/F12 (Invitrogen, 135 mM, sodium) to increase the sodium concentration to 170 mM, and 8 or 10 mM NaCl was added to DMEM/F12 to make a 143 or 145 mM sodium buffer and the osmolality was adjusted to be the same as the 170 mM sodium buffer by adding 0.5 M mannitol. The osmolality of the low salt buffer (90 mM Na+) was also adjusted with mannitol to the same osmolality as the 170 mM sodium buffer. The G cells were plated on 96-well plates and incubated for 4 h or 30 min, as indicated, with media containing varying sodium concentrations.
Measurement of the effect of by the PPAR-α agonist FFB on gastrin expression or secretion in AGS, SW626, and human G cells and stomach antrum
AGS (Ofori-Darko et al. 2000, Datta De et al. 2011) and SW626 (Kim et al. 1998) cells secrete progastrin/gastrin, but it is not known if their ability to express or secrete gastrin can be regulated by PPAR-α. However, PPAR-α has been reported to regulate the secretion of gastrin in colorectal carcinoma cells (Lachal et al. 2004) and mouse and rat stomach (Martinsen et al. 2005). Therefore, in SW626 (Kim et al. 1998), stomach antrum, and isolated human G cells, we studied the effect of the PPAR-α agonist FFB (5 μM/30 min, Sigma-Aldrich) (Lachal et al. 2004, Martinsen et al. 2005, Gonzalez-Aparicio et al. 2011), and the PPAR-α antagonist GW6471 (GW) (5 μM/30 min, Sigma-Aldrich) (Florio et al. 2017) alone, or in combination. All cells were washed twice with PBS prior to stimulation. Some of these cells were used to measure the level of gastrin transcription by RT-PCR. Some cells were grown in 96-well glass bottom plates to measure gastrin protein levels.
Role of D1-like receptors in the regulation of gastrin expression/secretion
To determine the role of D1-like receptors on gastrin secretion, we studied the effect of the D1-like receptor antagonist LE300 (Sigma-Aldrich) (Kassack et al. 2002) on FFB-stimulated gastrin secretion in SW626 and isolated human G cells. The cells were treated with FFB (5 μM) alone, LE300 (10 μM) alone, or in combination for 30 min. The interaction between FFB and LE300 was also tested in isolated human gastrin antrum but with the incubation period increased to 1 h. The cells and tissues were then fixed, 10 μm-frozen sections were cut, and staining was performed, as described earlier.
In additional studies, the role of D1-like receptors in the sodium-mediated increase in gastrin mRNA was studied in SW626 cells. The cells were treated with the D1-like receptor agonist, SKF38393 (10 μM/24 h, Sigma-Aldrich) (Glavin & Hall 1995, Kassack et al. 2002), monensin (1 μM/24 h, Sigma-Aldrich) (Aowicki & Huczyński 2013), and the combination of monensin and the D1-like receptor antagonist LE300 (10 μM/24 h) (Kassack et al. 2002). Gastrin mRNA was measured by qRT-PCR, as described previously.
Statistical analysis
The data are expressed as mean ± s.e. Comparisons were made by Student’s t-test for a two-group comparison or one-way ANOVA for multiple comparisons (groups >2), followed by Tukey post-hoc test. P < 0.05 was considered significant.
Results
Human stomach antrum and G cell immunochemistry for gastrin and PHA-L
Gastrin (red) and phytohemagglutinin-leucoagglutinin (PHA-L) (Lueth et al. 2005) (specific for complex-type carbohydrate residues which are expressed in gastric parietal cells, green) are observed in fresh human stomach (antrum section) (Fig. 1A). Gastrin and PHA-L colocalize in some cells, indicating that antrum parietal cells produce gastrin as expected (Martinsen et al. 2005). However, the green and red fluorescence do not completely overlap (yellow) in most cells but appear polarized on opposite sides of the G cells. The merged image shows that there are cells within the antrum crypts that do not stain for either PHA-L or gastrin.
Figure 1.
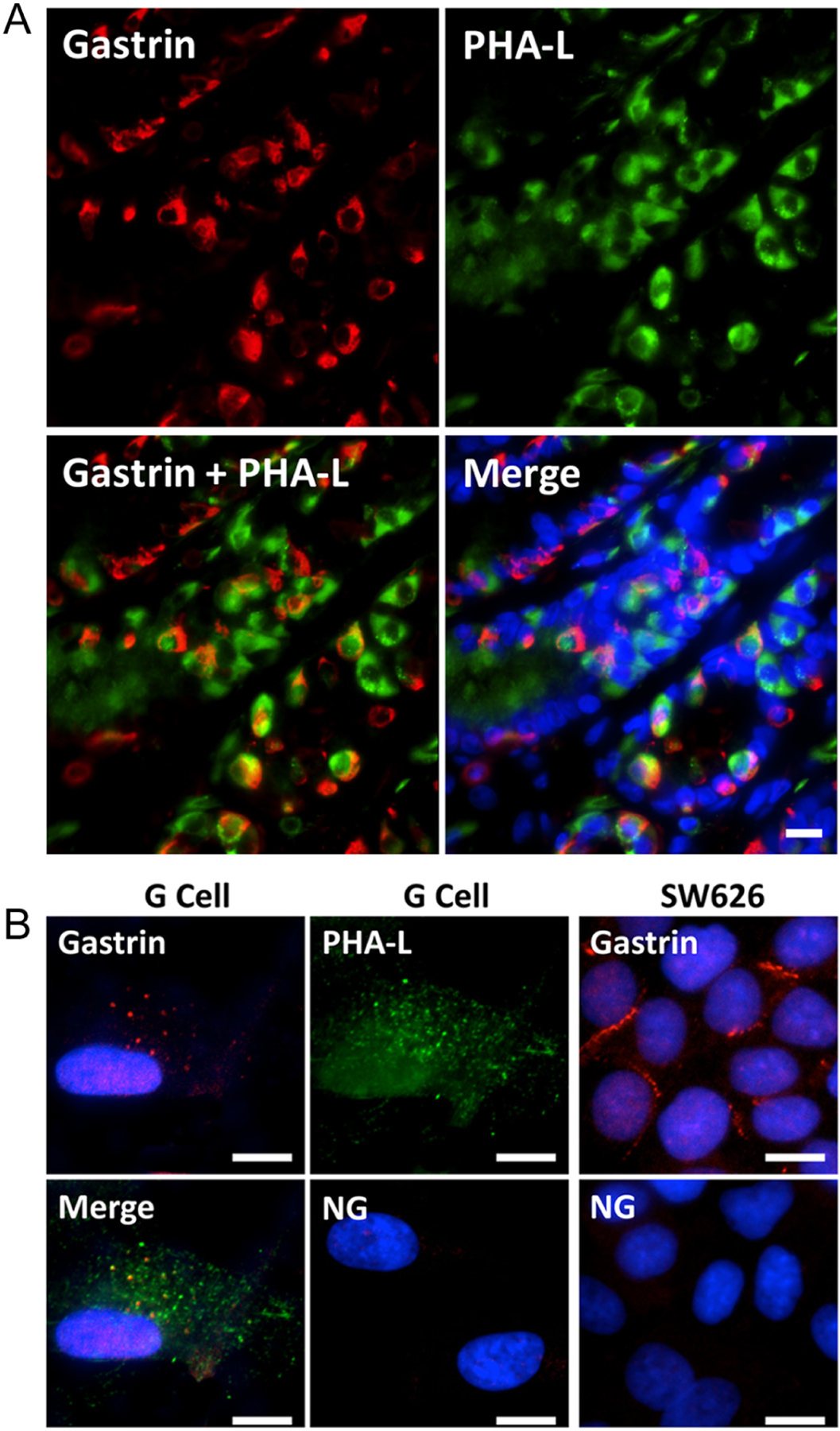
Human stomach antrum and G cell immunochemistry for gastrin and PHA-L. (A) Gastrin, phytohemagglutinin-leucoagglutinin (PHA-L, marker of complex type carbohydrate residues which are expressed in gastric parietal cells), and nuclei (blue) in the antrum of fresh human stomach. Scale bar = 10 μm. (B) Gastrin and PHA-L staining in G cells isolated from human stomach and gastrin staining in SW626 cells (ovarian adenocarcinoma cells). G cell- and SW626 cell-control using non-specific rabbit IgG antibody show almost no staining. NG = negative control, non-specific rabbit IgG antibody. Scale bar = 10 μm.
Human G cell and ovarian adenocarcinoma SW626 cell immunochemistry for gastrin and PHA-L
Immunohistochemistry for gastrin (red) and PHA-L (green) was performed in a G cell line derived from human stomach, as well as a cell line that is known to secrete gastrin (ovarian adenocarcinoma SW626) (Kim et al. 1998) (Fig. 1B). In stomach G cells, both gastrin and PHA-L show a punctate staining pattern. Merging the gastrin and PHA-L images shows partial colocalization of gastrin and PHA-L (yellow punctate pattern). The SW626 cells also show positive gastrin staining (red). The negative control, using non-specific rabbit IgG antibody, shows almost no staining in G cells and SW626 cells. Cell nuclei are stained blue with DAPI, except in the PHA-L images.
Gastrin mRNA in G cells and ovarian adenocarcinoma SW626 cells
RT-PCR demonstrated the presence of gastrin mRNA (actin served as a control) in human stomach G cells and gastrin-secreting ovarian adenocarcinoma SW626 cells (Kim et al. 1998). Human G and SW626 cells express the appropriate molecular size of gastrin mRNA, ~300 base pairs expected from the primers used (Supplementary Fig. 1, see section on supplementary materials given at the end of this article).
Effect of NaCl on gastrin protein expression in gastric carcinoma AGS and ovarian adenocarcinoma SW626 cells
In addition to SW626 cells (Kim et al. 1998), there are other commercially available cell lines of intestinal origin that secrete gastrin, for example, gastric carcinoma (AGS) and human colon cancer cells (Carroll et al. 2000, Ofori-Darko et al. 2000, Datta De et al. 2011). The effect of two different extracellular concentrations of sodium as NaCl (135 mM and 170 mM/4 h, osmolality kept at 340 mosolm/L with mannitol as needed) on gastrin immunofluorescence was studied in AGS and SW626 cells (Supplementary Fig. 2). Gastrin protein expression is increased in both AGS and SW626 cells incubated with 170 mM sodium, relative to 135 mM sodium (as NaCl) (P < 0.01, n = 6, one-way ANOVA, Tukey test).
Effect of NaCl on gastrin expression in isolated human G cells
We also examined the effect of increasing extracellular sodium concentration on gastrin expression in normal human stomach G cells (Fig. 2). The G cells were plated on 96-well plates and incubated for 4 h with media containing varying sodium concentrations, with the osmolality kept at 340 mosmol/L, using mannitol as needed. The highest sodium concentration (170 mM) increases gastrin protein expression in G cells (Fig. 2A). The individual data points from four independent G cell lines, each studied in triplicate, are shown in Fig. 2B. An increase in extracellular sodium also increases gastrin mRNA, but the increase occurs in both 145 mM and 170 mM NaCl (Fig. 2C). The highest sodium concentration, 170 mM, also increases PPAR-α mRNA expression (Fig. 2C). Similar to the increase in gastrin protein expression in G cells caused by 170 mM sodium, this sodium concentration also increases the secretion of gastrin into the incubation medium (Fig. 2D). G cells were also grown on alginate microcarrier beads, which enabled them to grow as an ion-transporting monolayer, in the polarized state (Global Cell Solutions, Inc.) (Justice et al. 2009, Gildea et al. 2015). The G cells in 3D culture (Supplementary Fig. 3A) have an increase in gastrin immunofluorescence when exposed for 4 h to 170 mM sodium (as NaCl), relative to 143 mM sodium (as NaCl) (Supplementary Fig. 3B and C), similar to G cells cultured in plates. We also found that gastrin mRNA is increased (P < 0.05, n = 4) in G cells incubated in media with 143 mM sodium (as NaCl, 286 mosmol/L), containing the ionophore monensin (MON, 1 μM/24 h) (Fig. 2E) (Efendiev et al. 2003, Gildea et al. 2015).
Figure 2.
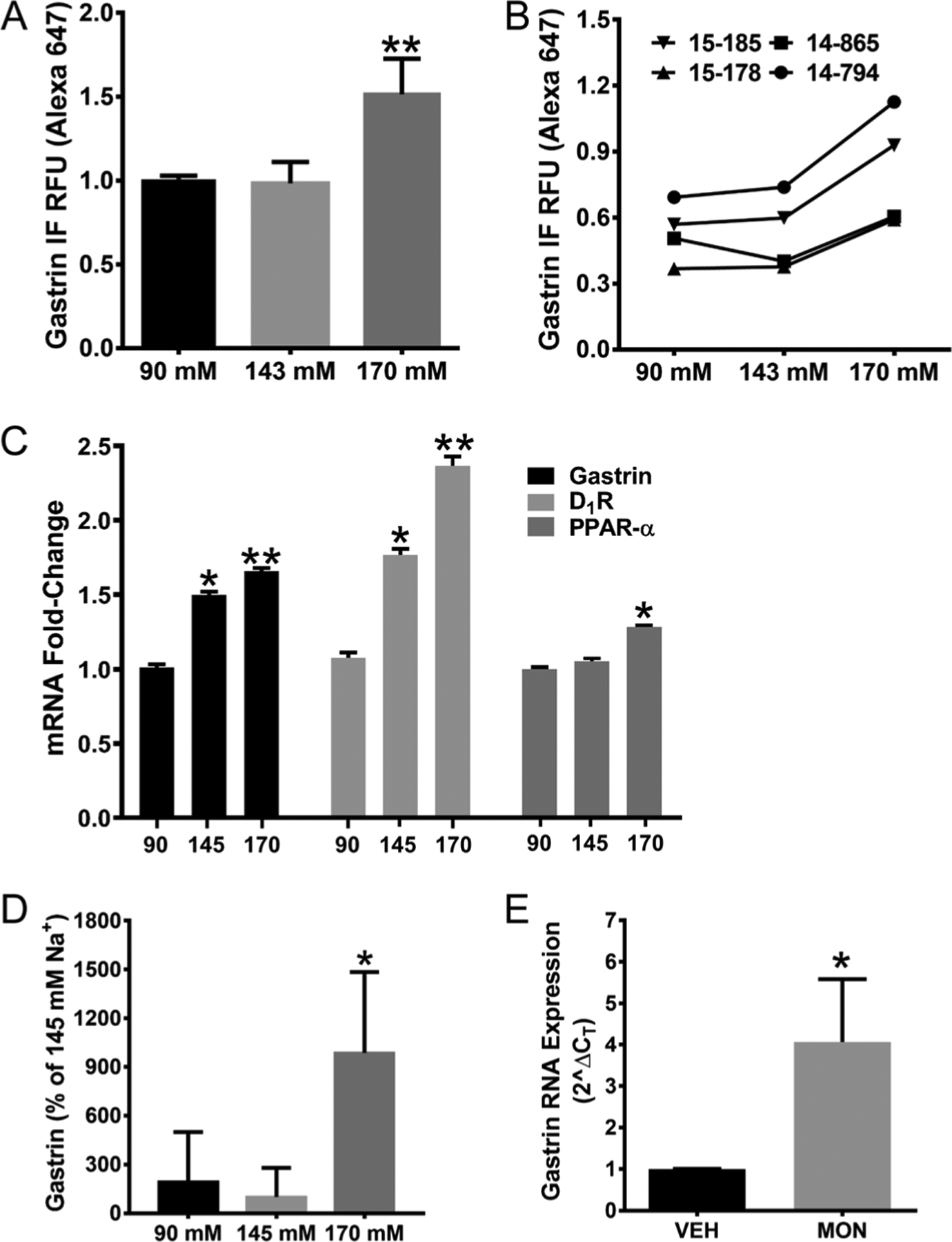
Effect of NaCl on gastrin, D1R, and PPAR-α expression in isolated human G cells. Isolated human G cells, cultured in 96-well cells, were incubated in media with sodium concentrations ranging from 90 to 170 mM NaCl/4 h; osmolality was kept at 340 mosmol/L with mannitol. (A and B) Group data and individual data points show an increase in gastrin expression in isolated human G cells incubated with 170 mM NaCl (**P < 0.01, vs 143 mM and others, one-way ANOVA, Tukey test, n = 4/group). BMG PHERAstar FS was used to quantify the immunofluorescence (IF) in G cells, expressed as relative fluorescence units (RFU). (C) A 30-min incubation of another set of isolated human G cells in varying amounts of sodium concentration also increases Gastrin, D1R, and PPAR-α mRNA, quantified by qRT-PCR (*, ** P < 0.01, vs others in a particular group, one-way ANOVA, Tukey test, n = 3/group for Gastrin, n = 4/group for D1R and PPAR-α). (D) Gastrin secreted by human G cells into the incubation medium is increased by the highest concentration of NaCl (170 mM/4 h) (*P < 0.01, vs others, one-way ANOVA, Tukey test, n = 4/group). (E) Increasing intracellular sodium with the ionophore monensin (MON, 1 μM/24 h) increases gastrin mRNA expression in human G cells (*P < 0.05, t-test, n = 4/group). All data (except the individual data points) are expressed as M ± s.e.
Gastrin and D1R expression in human stomach antrum tissue and G cells
Dopamine, via D1-like but not D2-like receptors, can stimulate gastric acid secretion in the stomach (Tsai & Cheng 1995). The stomach and other segments of the gastrointestinal tract synthesize dopamine (Vieira-Coelho & Soares-da-Silva 1993, Eisenhofer et al. 1997, Mezey et al. 1999, Eldrup & Richter 2000). The D1R and D5R are expressed in the stomach and other segments of the gastrointestinal tract (Vaughan et al. 2000, Wang et al. 2012). We now show that D1R mRNA (Fig. 2C) is expressed in G cells which is increased by 145 and 170 mM NaCl, relative to 90 mM NaCl, similar to the effect on gastrin mRNA. D1R protein (Fig. 3) is also expressed in human G cells in human stomach antrum. There is no D5R expressed in human G cells or any cell in the human stomach antrum (Supplementary Fig. 4). Therefore, we determined if D1R colocalizes with gastrin in human stomach antrum (Fig. 3A, B and C). As shown previously in Fig. 1A and B and in this other set of studies (Fig. 3A, red), gastrin is expressed in many, but not all, cells within the crypt of the stomach antrum. By contrast, D1R (Fig. 3B, green) is expressed in all cells lining the lumen of the crypt. The merged image shows no overlap between gastrin and D1R staining (Fig. 3C). D1R is at the apical membrane (Fig. 3B and C) while gastrin is in the cytoplasm of G cells in the stomach antrum (Fig. 3A and C). The isolated human G cells demonstrate similar gastrin and D1R staining patterns as in fresh stomach antrum, that is, punctate gastrin staining (Fig. 3D) in the cytoplasm and D1R staining throughout the membrane (Fig. 3E). There is minimal colocalization of gastrin and D1R staining (Fig. 3F). This could be taken to indicate that a cellular messenger produced in response to D1R stimulation is needed to enable the D1R to induce gastrin production. In contrast to the absence of D5R expression in human G cells (Supplementary Fig. 4), both D1R and D5R are expressed in both AGS and SW626 cells (Supplementary Fig. 5). These data indicate the importance of using normal human G cells to study human G cell function – gastrin secretion in this instance.
Figure 3.
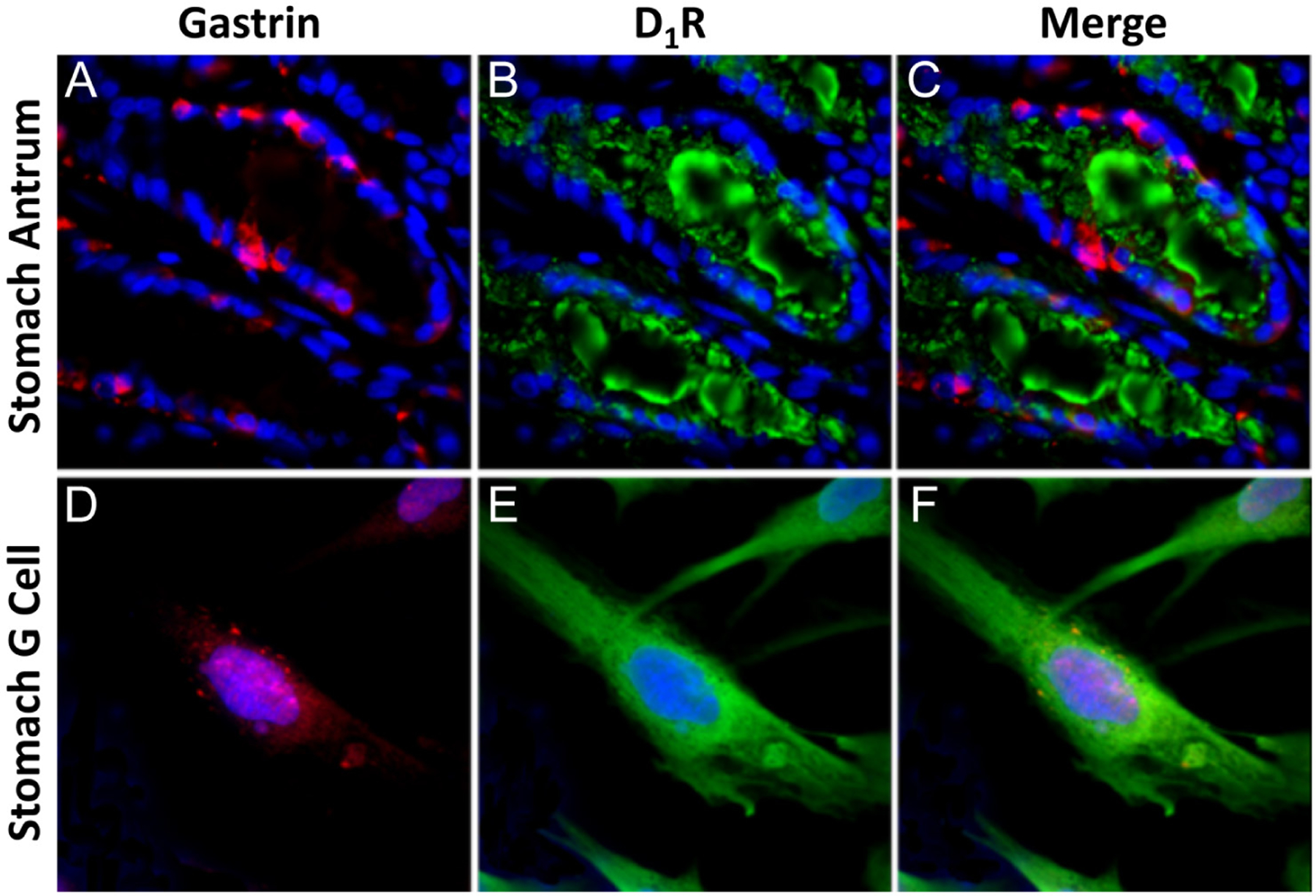
Gastrin and D1R expression in human stomach antrum and isolated human G cells. (A) Gastrin (red) is found in the cytoplasm of some cells within the crypts of the stomach antrum. (B) D1R in green is expressed in all cells lining the lumen of the crypts. (C) Merged images of both gastrin and D1R in stomach antrum. (D) Gastrin, stained red, is mainly in the cytoplasm of an isolated G cell. (E) D1R green staining is throughout the cell membrane of isolated G cells. (F) Merged images of gastrin and D1R in isolated G cells. Nucleus is stained with DAPI (blue). Scale bar = 10 μm.
Effect of PPAR-α agonist, fenofibrate (FFB), D1-like receptor antagonist, LE300, and high NaCl concentration on gastrin expression in human stomach antrum
As aforementioned, PPAR-α associates with the gastrin promoter (CHIP-SEQ) and increases progastrin production in gastrin-secreting carcinoma cells (Lachal et al. 2004) and gastric antrum (Martinsen et al. 2005). In isolated human G cells, when PPAR-α expression is decreased by about 70% using PPAR-α-specific siRNA (Supplementary Fig. 6A), there is about a 40% decrease in gastrin mRNA expression (P < 0.05, n = 4) (Supplementary Fig. 6B). In the human stomach antrum, the D1R-mediated gastrin synthesis may occur through PPAR-α, because the stimulatory effects of FFB (PPAR-α agonist, 5 μM/1 h) (P < 0.05, n = 6) (Fig. 4A) and high extracellular sodium concentration (170 mM/4 h) (P < 0.05, n = 4) (Fig. 4B) on gastrin protein tend to be decreased by the addition of LE300 (D1R/D5R receptor antagonist) (Kassack et al. 2002) (Fig. 4A and B). The immunohistochemistry (Fig. 4C), corresponding to the numerical data in Fig. 4A and B, shows that LE300 (10 μM/4 h) tends to block the gastrin immunofluorescence induced by FFB (5 μM/1 h) and 170 mM NaCl (4 h). Both FFB and 170 mM sodium also increase gastrin mRNA measured by real time RT-PCR. LE300 (10 μM/4 h) impairs the ability of 170 mM sodium (4 h) to increase gastrin mRNA in stomach antrum (Fig. 4D and E). However, as in the immunofluorescence data (Fig. 4A and B), LE300 (10 μM/4 h) (Fig. 4D and E), tends to, but does not, significantly impair the ability of the PPAR-α agonist FFB to increase gastrin mRNA. It must be noted that PPARα gene silencing decreases PPARα mRNA expression to a much greater extent than the decrease in gastrin mRNA expression (Supplementary Fig. 6). The D1-like receptor antagonist LE300 only partially blocks the 170 mM sodium-induced gastrin mRNA (Fig. 4D and E) and only tends to block gastrin protein expression (Fig. 4B). These findings indicate that sodium increases gastrin secretion by pathways in addition to the D1R and PPARα. Preliminary studies suggest the involvement of D1R-stimulated cAMP production in the D1R-mediated stimulation of gastrin expression (Xu et al. 2019). However, it has to be kept in mind that these studies were performed in the stomach antrum in which counter regulatory pathways in cells other than G cells could have occurred (Tsai & Cheng 1995, Li et al. 2006, Eliassi et al. 2008). For example, the D2R, in gastric myenteric neurons, can inhibit gastrin secretion (Li et al. 2006, Eliassi et al. 2008), thus the need to study D1R and PPARα in isolated normal human G cells (see below).
Figure 4.
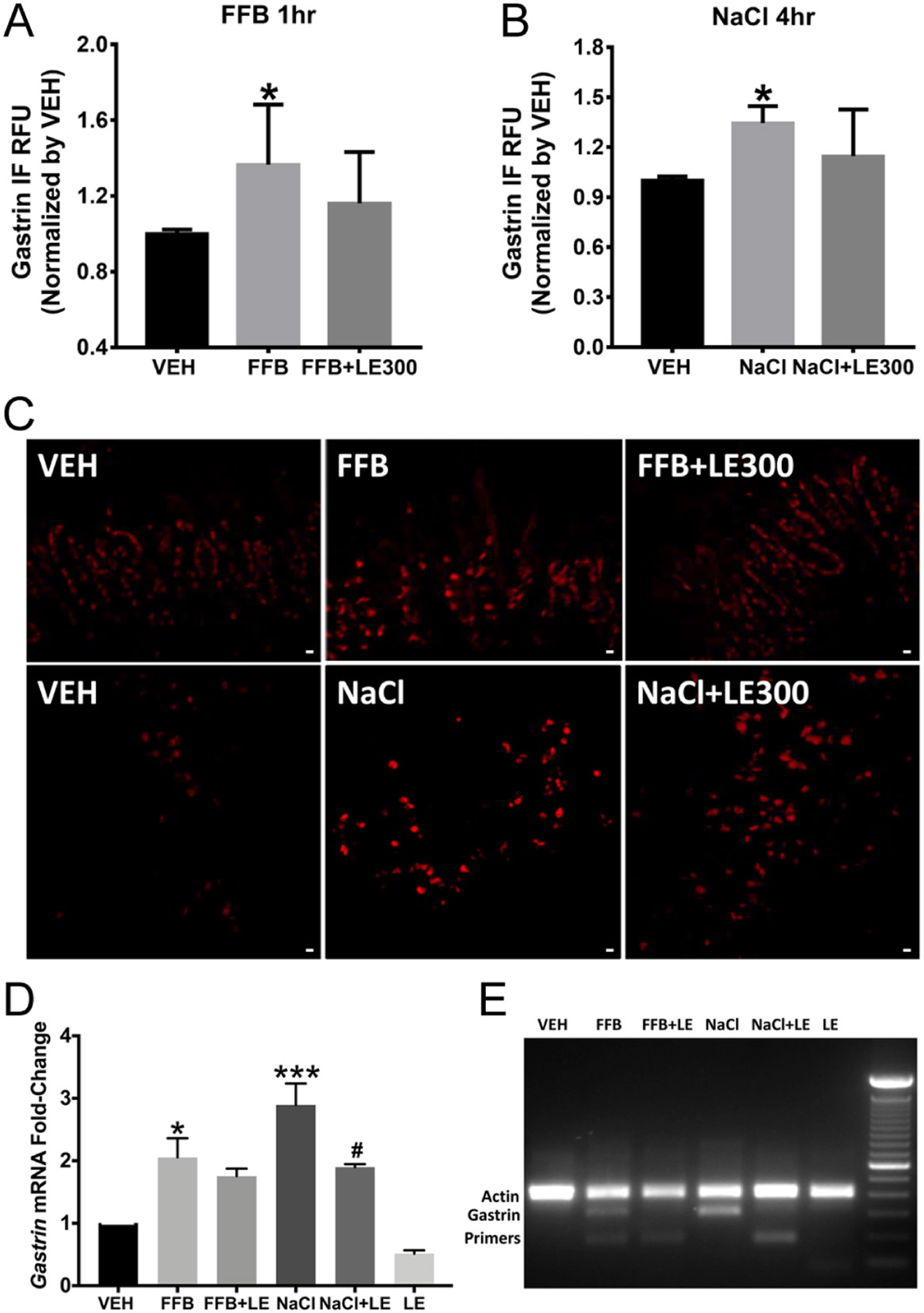
Effect of the PPAR-α agonist, fenofibrate (FFB), D1-like receptor antagonist, LE300, and high NaCl concentration on gastrin expression in human stomach antrum. (A) FFB (5 μM/1 h) stimulates gastrin expression in human stomach antrum (P < 0.05, vs VEH, one-way ANOVA, n = 6, Tukey test). (B) 170 mM (4 h) sodium increases gastrin expression in intact human stomach G cells (P < 0.05, vs. VEH, one-way ANOVA, n = 4, Tukey test). The stimulatory effects of FFB and 170 mM NaCl tend to be blocked by the D1-like receptor antagonist LE300 but do not achieve statistical significance. (C) Immunostained human stomach antrum shows an increase in gastrin expression with fenofibrate (FFB, upper center image), relative to vehicle (VEH, upper left image). The D1R/D5R antagonist LE300 (10 μM/1 h) tends to block the stimulatory effect of FFB (upper right image). High extracellular NaCl (170 mM) (lower center image) also increases antrum gastrin staining, relative to VEH (lower left image). The D1R/D5R antagonist LE300 (10 μM/4 h) tends to block the stimulatory effect of 170 mM NaCl on gastrin expression (lower right image). (D) RT-PCR of gastrin in stomach antrum treated with FFB (5 μM/1 h) and 170 mM NaCl (4 h); LE300 blocks the stimulatory effect of 170 mM NaCl on gastrin mRNA but not the stimulatory effect of FFB (* and *** P < 0.05 vs VEH; #P < 0.05, NaCl + LE vs NaCl, one-way ANOVA, Tukey test). (E) Representative DNA gel image of gastrin and actin mRNA. All data are expressed as M ± s.e.
Gastrin synthesis, D1R, and the PPAR-α pathway in ovarian adenocarcinoma SW626 cells
Gastrin expression in SW626 cells was studied in the presence of a D1-like receptor agonist (SKF-38393 (SKF)) (Kassack et al. 2002). SKF (10 μM/24 h) markedly increases the transcription of gastrin, relative to vehicle (VEH) treatment (Supplementary Fig. 7). Monensin (1 μM/24 h) (Efendiev et al. 2003, Gildea et al. 2015), an ionophore that increases intracellular sodium, also increases gastrin mRNA expression in SW626 cells that is completely blocked by LE300 (10 μM/24 h), a D1-like receptor antagonist (Supplementary Fig. 7). It should be noted that monensin also increases gastrin RNA in isolated human G cells (Fig. 2E). We also determined if the monensin-induced increase in gastrin expression in SW626 cells could be related to PPAR-α. GW6471, a PPAR-α antagonist, by itself, reduces the expression of gastrin suggesting that PPAR-α constitutively stimulates basal gastrin synthesis in SW626 cells (Fig. 5A). The PPAR-α agonist FFB-mediated increase in gastrin expression is also blocked by the PPAR-α antagonist GW6741 (Fig. 5A). Monensin also slightly increases gastrin protein but is not blocked by GW6741 (Fig. 5A), which could be taken to indicate that intracellular sodium can increase gastrin secretion independent of PPAR-α, in gastrin tumor-secreting cells. As aforementioned, preliminary studies suggest the involvement of D1R-stimulated cAMP production in the D1R-mediated stimulation of gastrin expression (Xu et al. 2019).
Figure 5.
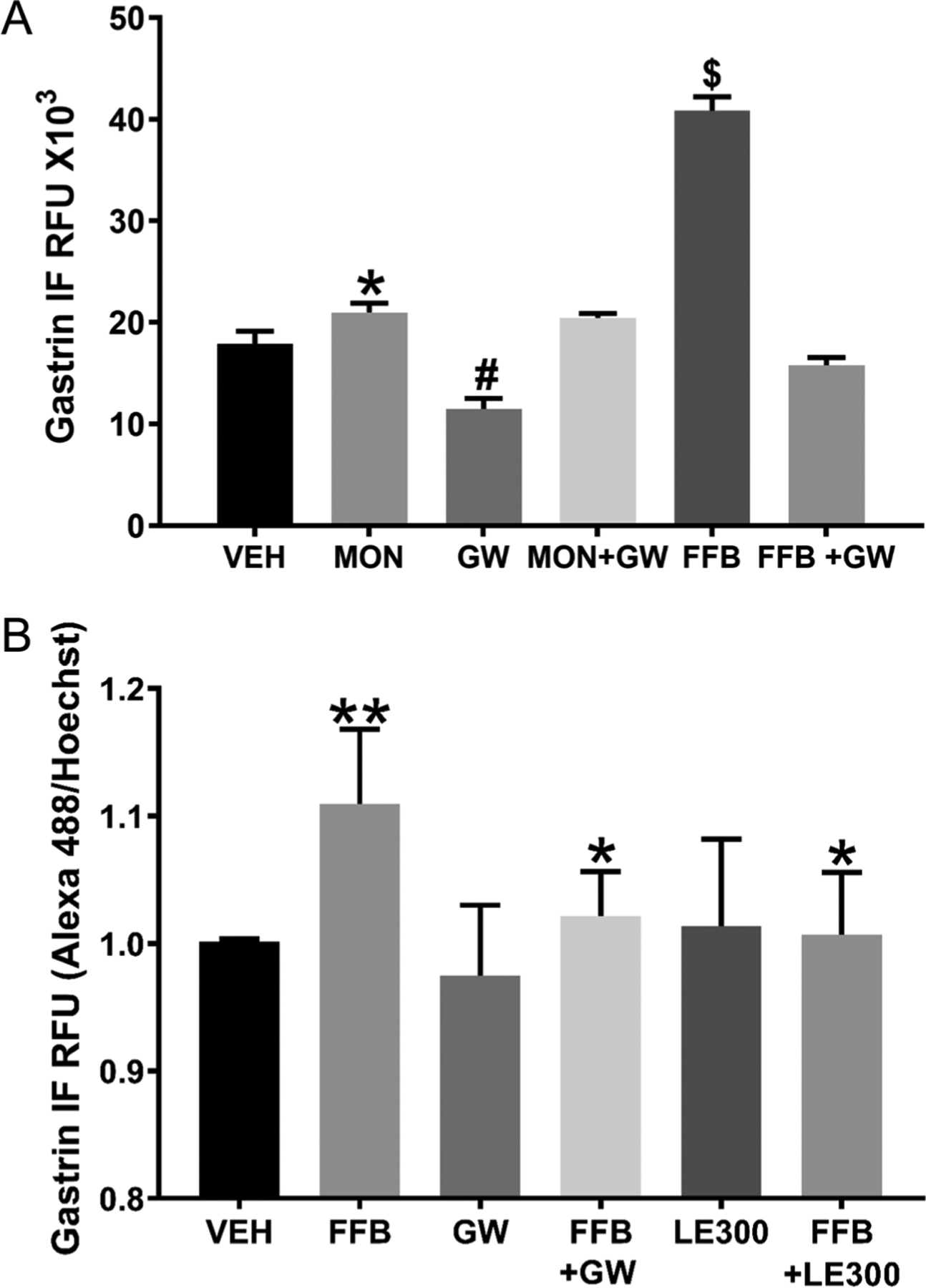
Gastrin synthesis, D1R, and PPAR-α in SW626 and human G cells. (A) Monensin (MON, 1 μM/24 h) or fenofibrate (FFB, PPAR-α agonist, 5 μM/30 min) increases gastrin protein in SW626 cells (IF, immunofluorescence; RFU, relative fluorescence units) (*P < 0.05 vs vehicle (VEH/24 h), one-way ANOVA, Tukey test). GW6471 (GW, PPAR-α antagonist, 5 μM/30 min) decreases gastrin protein (#P < 0.05, vs VEH, FFB, one-way ANOVA, Tukey test) and prevents the stimulatory effect of FFB but not MON on gastrin protein ($P < 0.001 vs others, one-way ANOVA, Tukey test). n = 10/group. (B) FFB (5 μM/30 min) increases gastrin protein in isolated human G cells (relative fluorescence units, RFU) (**P < 0.01, vs others, one-way ANOVA, Tukey test, n = 6/group). GW6471 (GW, PPAR-α antagonist, 5 μM/30 min) and LE300 (D1-like receptor antagonist, 10 μM/30 min), by themselves, do not affect gastrin protein expression but prevent the stimulatory effect of FFB on gastrin protein expression (*P < 0.05, vs FFB, one-way ANOVA, Tukey test, n = 6/group).
Gastrin synthesis and the PPAR-α pathway in human G cells
We next determined the role of the PPAR-α pathway in the synthesis of gastrin in isolated normal human G cells by treating them with the PPAR-α agonist FFB, PPAR-α antagonist GW6471, or D1-like receptor antagonist LE300 for 30 min (Fig. 5B). FFB (5 μM/30 min) increases gastrin protein (P < 0.01, n = 6) in isolated human G cells, similar to that observed in human stomach antrum (Fig. 4A) and ovarian adenocarcinoma SW626 cells (Fig. 5A). The PPAR-α antagonist GW6471 (5 μM/30 min) by itself has no effect, unlike that observed in ovarian adenocarcinoma SW626 cells. However, similar to those observed in ovarian adenocarcinoma SW626 cells, in isolated human G cells, GW6471 also completely blocks the increase in gastrin protein expression stimulated by FFB (P < 0.05, n = 6) (Fig. 5B). We also tested the effect of blocking the D1R (D5R is not expressed in G cells) with LE300 and found that LE300 (10 μM/30 min) by itself has no effect but completely blocks the FFB-induced stimulation of gastrin protein expression (P < 0.05, n = 6). These studies show some minor differences in gastrin secretion among intact G cells in human antrum, isolated human G cells, and gastrin-secreting carcinoma SW626 cells, bearing in mind that SW626 cells constitutively secrete gastrin while G cells need to be stimulated to secrete gastrin. There is also a minor difference between isolated G cells and intact G cells in the human stomach antrum; in the human stomach antrum, LE300, the D1-like receptor antagonist tends to block the stimulatory effect of FFB, the PPAR-α agonist, on gastrin protein expression but in isolated human G cells, LE300 completely blocks the stimulatory effect of FFB on gastrin secretion. As aforementioned, this could be related to counter regulatory mechanisms in intact human stomach antrum not found in isolated G cells; the D2R, expressed in gastric myenteric neurons, can inhibit gastrin secretion (Li et al. 2006, Eliassi et al. 2008).
Expression of dopamine biosynthetic enzymes in G cells
As aforementioned, the stomach and other segments of the gastrointestinal tract can synthesize dopamine (Vieira-Coelho & Soares-da-Silva 1993, Eisenhofer et al. 1997, Mezey et al. 1999, Eldrup & Richter 2000). However, it is not known if G cells, per se, can synthesize dopamine. Therefore, we studied the expression of dopamine biosynthetic enzymes in human stomach antrum (Supplementary Fig. 8A) and cultured human G cells (Supplementary Fig. 8B). There is widespread tyrosine hydroxylase (TH) red fluorescent staining (Supplementary Fig. 8A) throughout the crypt cells in the stomach antrum. Gastrin and TH colocalize in G cells but not always in the same intracellular domain (Supplementary Fig. 8A, merge). These data prove the expression of the first enzyme, TH, in the synthesis of dopamine in human G cells. The gastrin and TH distribution in isolated G cells in culture (Supplementary Fig. 8B) is similar to that found in the stomach antrum (Supplementary Fig. 8A), that is, only in some cells do gastrin and TH colocalize.
We next examined the location of the enzyme responsible for the catalysis of dopamine from L-DOPA (aromatic L-amino acid decarboxylase (DOPA decarboxylase, DDC)) in human stomach antrum (Supplementary Fig. 9A) and isolated human G cells in culture (Supplementary Fig. 9B). As with TH, there is widespread DDC red fluorescent staining (Supplementary Fig. 9A) in most cells in the stomach antrum. Gastrin green fluorescent staining is similar to that in Supplementary Figs 8A and 9A. The merged image (Supplementary Fig. 9A) demonstrates that there is colocalization of gastrin and DDC in human stomach G cells but not always in the same intracellular domain, as is the case for TH (Supplementary Fig. 8A). Similar to those observed regarding the colocalization of gastrin and TH in G cells (Supplementary Fig. 8B), gastrin and DDC colocalization is also seen in some but not all G cells (Supplementary Fig. 9B).
Discussion
Sensing the amount of ingested sodium by the stomach and other segments of the gastrointestinal tract may be an important mechanism by which sodium balance is regulated (Michell et al. 2008, Haid et al. 2012, Furness et al. 2013, Jose et al. 2016, Yang et al. 2017). However, previous reports on gastrorenal communication have not dealt with the sodium sensor in the stomach. Therefore, we isolated G cells from the antrum of the human stomach (Kasacka & Majewski 2007, Takaishi et al. 2011, Haid et al. 2012) in order to determine the effect of sodium, independent of food, on gastrin secretion. Similar to rodents, we found that gastrin secretion, by human G cells, can be increased by sodium, independent of food (Survé & Håkanson 1998). Moreover, we found that increasing the intracellular sodium concentration, using the non-selective ionophore monensin, increases gastrin mRNA and protein in isolated human G cells. We also found that an increase in extracellular or intracellular sodium concentration increases gastrin expression in human G cells and other gastrin-secreting cells, such as AGS (Ofori-Darko et al. 2000, Datta De et al. 2011) and SW626 (Kim et al. 1998) cells.
PPAR-α activation increases gastrin secretion, independent of gastric pH (Lachal et al. 2004, Martinsen et al. 2005). The incubation of human stomach antrum, isolated human G cells, and gastrin-secreting SW626 adenocarcinoma cells (Kim et al. 1998) with the PPAR-α agonist FFB increases gastrin expression. This effect can be blocked by the PPAR-α antagonist GW6471. Because the D1R is expressed in G cells and its plasma membrane expression can be increased by an increase in intracellular sodium, at least in renal epithelial cells (Efendiev et al. 2003), we examined the interaction between the gastrin and dopaminergic systems. We found that SKF38393 (a D1R/D5R agonist) (Glavin & Hall 1995, Kassack et al. 2002) increases gastrin mRNA expression in human SW626 gastrin-producing carcinoma cells. The increase in gastrin mRNA expression caused by monensin, presumably due to an increase in intracellular sodium, in SW626 cells can be blocked by the D1R/D5R antagonist LE300 (Kassack et al. 2002). LE300 also completely blocks the ability of FFB to stimulate gastrin protein expression in isolated normal human G cells. Which D1-like receptor, D1R or D5R, is involved in the dopaminergic stimulation of gastrin secretion was not determined in SW626 cells, but these cells express both D1R and D5R. By contrast, D5R is not expressed in human G cells. Therefore, the dopaminergic stimulation of gastrin expression/secretion in human G cells is mediated by the D1R, not the D5R. Preliminary studies suggest that cAMP produced by D1R may stimulate PPARα expression (Xu et al. 2019).
In the normal human stomach antrum, the D1-like receptor antagonist LE300 tends to, but does not, significantly block the ability of high NaCl concentration or the PPAR-α agonist FFB to increase gastrin protein expression. This is in contrast to the ability of LE300 to block the stimulatory effect of high intracellular NaCl, induced by monensin in SW626 cells, or the PPAR-α agonist FFB in isolated human G cells. As aforementioned, in the stomach antrum, counter regulatory pathways in cells other than G cells could have occurred (Tsai & Cheng 1995, Li et al. 2006, Eliassi et al. 2008). For example, the D2R, in gastric myenteric neurons, can inhibit gastrin secretion (Li et al. 2006, Eliassi et al. 2008).
Our present study suggests that dopamine, via D1-like receptors, can acutely (30 min) stimulate gastrin secretion from gastrin-secreting cells. Dopamine can be synthesized by neuronal and non-neuronal cells in the gastrointestinal tract, including the stomach (Vieira-Coelho & Soaresda-Silva 1993, Eisenhofer et al. 1997, Mezey et al. 1999, Eldrup & Richter 2000, Zheng et al. 2014). However, the enzymes that are involved in dopamine synthesis have not been studied in G cells. We now report that human G cells express tyrosine hydroxylase, which converts tyrosine to L-DOPA and DOPA decarboxylase which converts L-DOPA to dopamine. An increase in salt intake increases jejunal dopamine (Lucas-Teixeira et al. 2000). However, it remains to be determined if an increase in ingested sodium increases dopamine synthesis in G cells.
In summary, exposure of human G cells to increased extracellular sodium concentration or monensin that presumably leads to an increase in intracellular sodium concentration stimulates gastrin expression/secretion. The presence of dopamine-synthesizing enzymes in G cells suggest that G cells can increase dopamine synthesis. Dopamine, via the D5R, stimulates gastrin expression, in part, by interacting with PPAR-α (Fig. 6).
Figure 6.
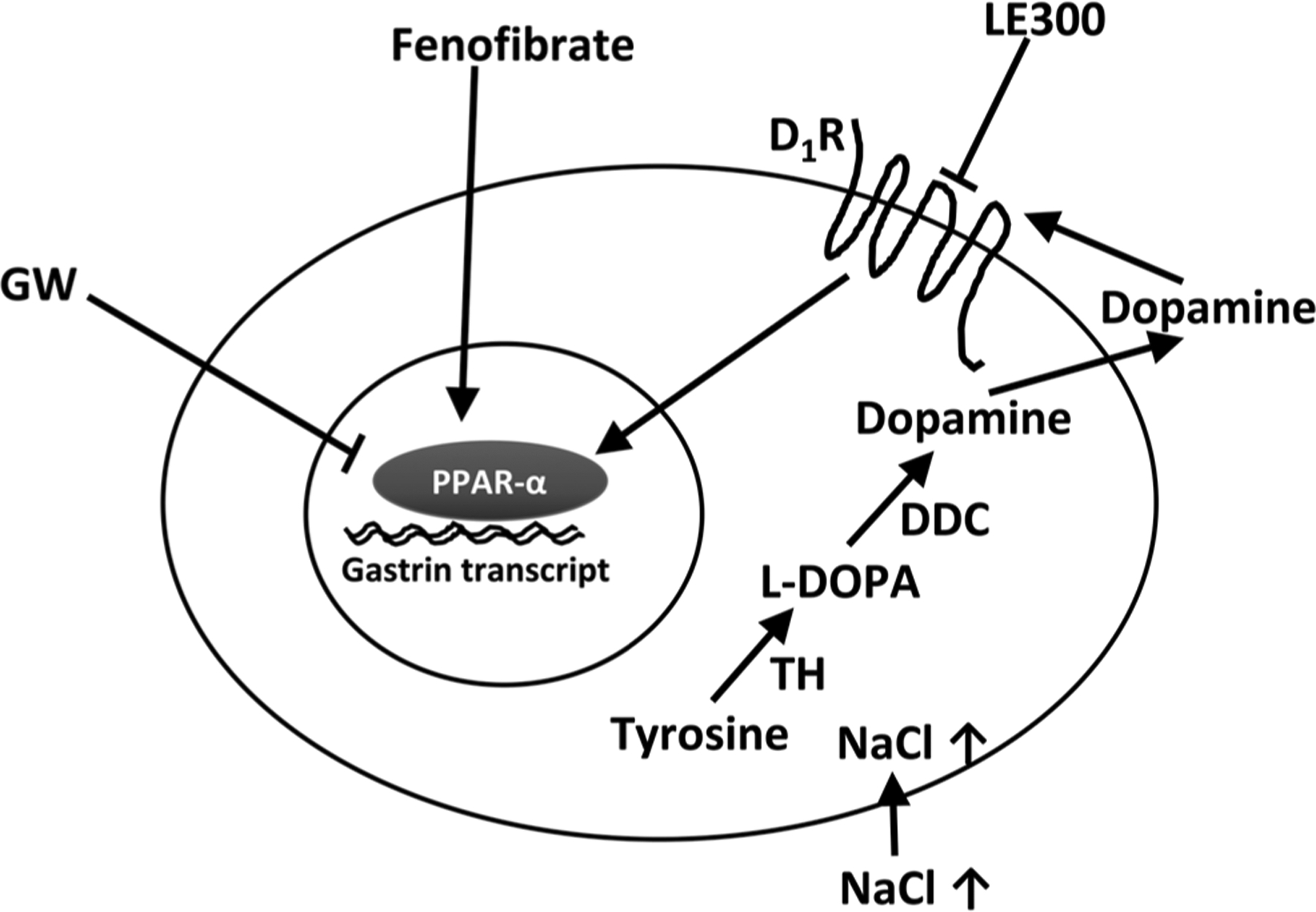
Putative mechanism by which sodium induces gastrin expression in human stomach G cell. Sodium stimulates the production of dopamine. Dopamine, via the D1R, increases gastrin secretion that involves the PPAR-α pathway, TH, tyrosine hydroxylase; L-DOPA, L-3,4-dihydroxyphenylalanine; DDC, DOPA decarboxylase.
Supplementary Material
Supplemental Figure 1. Human G and ovarian adenocarcinoma SW626 cells express the appropriate molecular (Mol) size of gastrin mRNA, ~300 base pairs (bp), expected from the primers used. Actin is used for control.
Supplemental Figure 2. Effect of NaCl concentration in the incubation medium on gastrin expression in gastrin-secreting tumor cells, SW626 and AGS. Increasing the sodium concentration in the buffer from 135 to 170 mM NaCl (4 hr incubation) increases gastrin protein expression in both SW626 and AGS cells (*, #, P<0.01 vs 135 mM NaCl, n=6/group, one-way ANOVA, Holm-Sidak test). IF = immunofluorescence, RFU = relative fluorescence unit
Supplemental Figure 3. (A) Human G cells were grown on alginate microspheres (Global Cell Solutions). Nuclei were stained with DAPI (green) and F-actin stained with phalloidin (red). This 3D model system enables the study of polarized G cells in monolayer. (B) G cells, grown as a transporting monolayer in a 3D system, have increased gastrin protein expression under high extracellular sodium concentration (170 mM, 4 hr) (P<0.05, paired t-test, n=4), but not substantially different from conventional cell culture demonstrating that Petri dish cultures achieve optimal results. (C) Individual data points for 4 independent cell lines measured in 3 independent assays are shown. The numbers in the graph indicate the assigned cell identification numbers. IF = immunofluorescence, RFU = relative fluorescence unit
Supplemental Figure 4. There is no D5R expression in human stomach antrum. Gastrin is stained red and nucleus stained blue with DAPI.
Supplemental Figure 5. Both D1R and D5R are expressed in AGS and SW626 cells.
Supplemental Figure 6. PPAR-α silencing in G cells. The transcriptions of intracellular PPAR-α and gastrin mRNAs were measured using qRT-PCR in G cells. The G cells were incubated with PPAR-α siRNA (100 nM/3 d). PPAR-α silencing RNA (siRNA) decreases PPAR-α mRNA to 0.28±0.02-fold and gastrin mRNA to 0.63±0.08-fold, relative to scrambled RNA, set at 1.0 (t-test, p<0.05, n=4).
Supplemental Figure 7. Gastrin synthesis, D1-like receptor, and the PPAR-α pathway in SW626 cells. The transcription of intracellular gastrin mRNA was measured using RT-PCR in SW626 gastric carcinoma cells. Monensin (MON, 1 μM/24 hr), which increases intracellular Na+ (* P<0.001, one-way ANOVA, Tukey test, n=3), and SKF (D1-like receptor agonist, 10 μM/24 hr), #P<0.001, one-way ANOVA, Tukey test, n=3) increase intracellular gastrin transcription. However, the D1-like receptor antagonist, LE300 (10 μM/24 hr), completely blocks the MON-induced increase in gastrin RNA level ($P<0.001, one-way ANOVA, Tukey test, n=3).
Supplemental Figure 8. Expression of tyrosine hydroxylase in G cells in stomach antrum and isolated G cells in culture. The D1R participates in the increase in gastrin expression caused by an increase in extracellular and intracellular sodium. G cells in the human stomach antrum (Supplemental Figure 8A) and isolated human G cells in culture (Supplemental Figure 8B) express tyrosine hydroxylase (TH) which converts tyrosine to L-DOPA. Gastrin is stained green, TH is stained red, and nucleus is stained blue with DAPI. There is widespread TH red fluorescent staining throughout the crypt cells. The merged images show minimal colocalization of gastrin and TH-staining G cells and not always in the same intracellular site. Nevertheless, these images indicate the presence of the first enzyme (TH) in the synthesis of dopamine in human G cells. Scale bar = 10 μm
Supplemental Figure 9. Expression of DOPA decarboxylase in G cells in stomach antrum and isolated G cells in culture. G cells in the human stomach antrum (Supplemental Figure 9A) and isolated human G cells in culture (Supplemental Figure 9B) express aromatic L-amino acid decarboxylase (DDC) which converts L-DOPA to dopamine. Gastrin is stained green, DDC is stained red, and nucleus is stained blue with DAPI. There is widespread DDC red fluorescent staining throughout the crypt cells. The merged images show minimal colocalization of gastrin and DDC-staining G cells and not always in the same intracellular site. Nevertheless, these images indicate the presence of the second enzyme (DDC) in the synthesis of dopamine in human G cells. Scale bar = 10 μm
Funding
The study was supported, in part, by grants from the National Institutes of Health (R01DK039308 and P01HL074940).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supplementary materials
This is linked to the online version of the paper at https://doi.org/10.1530/JME-19-0053.
References
- Andersen LJ, Jensen TU, Bestle MH & Bie P 2000. Gastrointestinal osmoreceptors and renal sodium excretion in humans. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 278 R287–R294. ( 10.1152/ajpregu.2000.278.2.R287) [DOI] [PubMed] [Google Scholar]
- Aowicki D & Huczyński A 2013. Structure and antimicrobial properties of monensin A and its derivatives: summary of the achievements. BioMed Research International 2013 742149 ( 10.1155/2013/742149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RE, Ostrovskiy D, Lee S, Danilkovich A & Benya RV 2000. Characterization of gastrin-releasing peptide and its receptor aberrantly expressed by human colon cancer cell lines. Molecular Pharmacology 58 601–607. ( 10.1124/mol.58.3.601) [DOI] [PubMed] [Google Scholar]
- Chandran K, Goswami S & Sharma-Walia N 2016. Implications of a peroxisome proliferator-activated receptor alpha (PPARα) ligand clofibrate in breast cancer. Oncotarget 7 15577–15599. ( 10.18632/oncotarget.6402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Asico LD, Zheng S, Villar VA, He D, Zhou L, Zeng C & Jose PA 2013. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension 62 927–933. ( 10.1161/HYPERTENSIONAHA.113.01094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta De D, Bhattacharjya S, Maitra M, Datta A, Choudhury A, Dhali GK & Roychoudhury S 2011. IL1β induced Smad 7 negatively regulates gastrin expression. PLoS ONE 6 e14775 ( 10.1371/journal.pone.0014775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efendiev R, Budu CE, Cinelli AR, Bertorello AM & Pedemonte CH 2003. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. Journal of Biological Chemistry 278 28719–28726. ( 10.1074/jbc.M303741200) [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B & Mezey E 1997. Substantial production of dopamine in the human gastrointestinal tract. Journal of Clinical Endocrinology and Metabolism 82 3864–3871. ( 10.1210/jcem.82.11.4339) [DOI] [PubMed] [Google Scholar]
- Eldrup E & Richter EA 2000. DOPA, dopamine, and DOPAC concentrations in the rat gastrointestinal tract decrease during fasting. American Journal of Physiology: Endocrinology and Metabolism 279 E815–E822. ( 10.1152/ajpendo.2000.279.4.E815) [DOI] [PubMed] [Google Scholar]
- Eliassi A, Aleali F & Ghasemi T 2008. Peripheral dopamine D2-like receptors have a regulatory effect on carbachol-, histamine- and pentagastrin-stimulated gastric acid secretion. Clinical and Experimental Pharmacology and Physiology 35 1065–1070. ( 10.1111/j.1440-1681.2008.04961.x) [DOI] [PubMed] [Google Scholar]
- Feng W, Dell’Italia LJ & Sanders PW 2017. Novel paradigms of salt and hypertension. Journal of the American Society of Nephrology 28 1362–1369. ( 10.1681/ASN.2016080927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio R, De Lellis L, di Giacomo V, Di Marcantonio MC, Cristiano L, Basile M, Verginelli F, Verzilli D, Ammazzalorso A, Prasad SC, et al. 2017. Effects of PPARα inhibition in head and neck paraganglioma cells. PLoS ONE 12 e0178995 ( 10.1371/journal.pone.0178995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Rivera LR, Cho HJ, Bravo DM & Callaghan B 2013. The gut as a sensory organ. Nature Reviews: Gastroenterology and Hepatology 10 729–740. ( 10.1038/nrgastro.2013.180) [DOI] [PubMed] [Google Scholar]
- Gildea JJ, McGrath HE, Van Sciver RE, Wang DB & Felder RA 2013. Isolation, growth, and characterization of human renal epithelial cells using traditional and 3D methods. Methods in Molecular Biology 945 329–345. ( 10.1007/978-1-62703-125-7_20) [DOI] [PubMed] [Google Scholar]
- Gildea JJ, Xu P, Carlson JM, Gaglione RT, Bigler Wang D, Kemp BA, Reyes CM, McGrath HE, Carey RM, Jose PA, et al. 2015. The sodium-bicarbonate cotransporter NBCe2 (slc4a5) expressed in human renal proximal tubules shows increased apical expression under high-salt conditions. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 309 R1447–R1459. ( 10.1152/ajpregu.00150.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin GB & Hall AM 1995. Central and peripheral dopamine D1/DA1 receptor modulation of gastric secretion and experimental gastric mucosal injury. General Pharmacology 26 1277–1279. ( 10.1016/0306-3623(95)00009-p) [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Flores JA, Tasset I, Tunez I & Fernandez-Espejo E 2011. Mice lacking the peroxisome proliferator-activated receptor α gene present reduced number of dopamine neurons in the substantia nigra without altering motor behavior or dopamine neuron decline over life. Neuroscience 186 161–169. ( 10.1016/j.neuroscience.2011.03.062) [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT & Llinas M 2002. Mechanisms of pressure natriuresis. Current Hypertension Reports 4 152–159. ( 10.1007/s11906-002-0040-3) [DOI] [PubMed] [Google Scholar]
- Haid DC, Jordan-Biegger C, Widmayer P & Breer H 2012. Receptors responsive to protein breakdown products in G-cells and D-cells of mouse, swine and human. Frontiers in Physiology 3 65 ( 10.3389/fphys.2012.00065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J & Hall ME 2012. Hypertension: physiology and pathophysiology. Comprehensive Physiology 2 2393–2442. ( 10.1002/cphy.c110058) [DOI] [PubMed] [Google Scholar]
- Heer M, Baisch F, Kropp J, Gerzer R & Drummer C 2000. High dietary sodium chloride consumption may not induce body fluid retention in humans. American Journal of Physiology: Renal Physiology 278 F585–F595. ( 10.1152/ajprenal.2000.278.4.F585) [DOI] [PubMed] [Google Scholar]
- Jiang X, Chen W, Liu X, Wang Z, Liu Y, Felder RA, Gildea JJ, Jose PA, Qin C & Yang Z 2016. The synergistic roles of cholecystokinin B and dopamine D5 receptors on the regulation of renal sodium excretion. PLoS ONE 11 e0146641 ( 10.1371/journal.pone.0146641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang Y, Yang Y, Yang J, Asico LD, Chen W, Felder RA, Armando I, Jose PA & Yang Z 2017. Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of L-dopa. American Journal of Physiology: Endocrinology and Metabolism 312 E1–E10. ( 10.1152/ajpendo.00116.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose PA, Felder RA, Yang Z, Zeng C & Eisner GM 2016. Gastrorenal axis. Hypertension 67 1056–1063. ( 10.1161/HYPERTENSIONAHA.115.06424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice BA, Badr NA & Felder RA 2009. 3D cell culture opens new dimensions in cell-based assays. Drug Discovery Today 14 102–107. ( 10.1016/j.drudis.2008.11.006) [DOI] [PubMed] [Google Scholar]
- Kasacka I & Majewski M 2007. An immunohistochemical study of endocrine cells in the stomach of hypertensive rats. Journal of Physiology and Pharmacology 58 469–478. [PubMed] [Google Scholar]
- Kassack MU, Höfgen B, Decker M, Eckstein N & Lehmann J 2002. Pharmacological characterization of the benz[d]indolo[2,3-g]azecine LE300, a novel type of a nanomolar dopamine receptor antagonist. Naunyn-Schmiedeberg’s Archives of Pharmacology 366 543–550. ( 10.1007/s00210-002-0641-z) [DOI] [PubMed] [Google Scholar]
- Kidd M, Hauso Ø, Drozdov I, Gustafsson BI & Modlin IM 2009. Delineation of the chemomechanosensory regulation of gastrin secretion using pure rodent G cells. Gastroenterology 137 231–241, 241.e1. ( 10.1053/j.gastro.2009.01.005) [DOI] [PubMed] [Google Scholar]
- Kim HJ, Evers BM, Banker NA, Greeley GH Jr, Hellmich MR, Thompson JC & Townsend CM Jr 1998. Novel expression and regulation of gastrin gene in human ovarian cancer cell line, sw626. Digestive Diseases and Sciences 43 1465–1473. ( 10.1023/a:1018846311239) [DOI] [PubMed] [Google Scholar]
- Lachal S, Ford J, Shulkes A & Baldwin GS 2004. PPARalpha agonists stimulate progastrin production in human colorectal carcinoma cells. Regulatory Peptides 120 243–251. ( 10.1016/j.regpep.2004.03.015) [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Scott D & Sachs G 2005. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiological Genomics 21 81–91. ( 10.1152/physiolgenomics.00212.2004) [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Zer C & Sachs G 2006. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiological Genomics 25 153–165. ( 10.1152/physiolgenomics.00271.2005) [DOI] [PubMed] [Google Scholar]
- Li ZS, Schmauss C, Cuenca A, Ratcliffe E & Gershon MD 2006. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. Journal of Neuroscience 26 2798–2807. ( 10.1523/JNEUROSCI.4720-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T & Jose PA 2013. Gastrin induces sodium-hydrogen exchanger 3 phosphorylation and mtor activation via a phosphoinositide 3-kinase-/protein kinase c-dependent but Akt-independent pathway in renal proximal tubule cells derived from a normotensive male human. Endocrinology 154 865–875. ( 10.1210/en.2012-1813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Konkalmatt PR, Yang Y & Jose PA 2016. Gastrin decreases Na+, K+-ATPase activity via a PI3 kinase- and pkc-dependent pathway in human renal proximal tubule cells. American Journal of Physiology: Endocrinology and Metabolism 310 E565–E571. ( 10.1152/ajpendo.00360.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Teixeira V, Serrão MP & Soares-Da-Silva P 2000. Effect of salt intake on jejunal dopamine, Na+,K+-atpase activity and electrolyte transport. Acta Physiologica Scandinavica 168 225–231. ( 10.1046/j.1365-201x.2000.00656.x) [DOI] [PubMed] [Google Scholar]
- Lueth M, Sturegård E, Sjunnesson H, Wadström T & Schumacher U 2005. Lectin histochemistry of the gastric mucosa in normal and Helicobacter pylori infected guinea-pigs. Journal of Molecular Histology 36 51–58. ( 10.1007/s10735-004-3838-2) [DOI] [PubMed] [Google Scholar]
- Martinsen TC, Bakke I, Chen D, Sandvik AK, Zahlsen K, Aamo T & Waldum HL 2005. Ciprofibrate stimulates the gastrin-producing cell by acting luminally on antral PPAR-alpha. American Journal of Physiology: Gastrointestinal and Liver Physiology 289 G1052–G1060. ( 10.1152/ajpgi.00268.2005) [DOI] [PubMed] [Google Scholar]
- Michell AR, Debnam ES & Unwin RJ 2008. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annual Review of Physiology 70 379–403. ( 10.1146/annurev.physiol.69.040705.141330) [DOI] [PubMed] [Google Scholar]
- Nikpey E, Karlsen TV, Rakova N, Titze JM, Tenstad O & Wiig H 2017. High-salt diet causes osmotic gradients and hyperosmolality in skin without affecting interstitial fluid and lymph. Hypertension 69 660–668. ( 10.1161/HYPERTENSIONAHA.116.08539) [DOI] [PubMed] [Google Scholar]
- Ofori-Darko E, Zavros Y, Rieder G, Tarlé SA, Van Antwerp M & Merchant JL 2000. An OmpA-like protein from Acinetobacter spp. stimulates gastrin and interleukin-8 promoters. Infection and Immunity 68 3657–3666. ( 10.1128/iai.68.6.3657-3666.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, McCabe G & Weaver CM 2004. Sodium retention in black and white female adolescents in response to salt intake. Journal of Clinical Endocrinology and Metabolism 89 1858–1863. ( 10.1210/jc.2003-031446) [DOI] [PubMed] [Google Scholar]
- Preston RA, Afshartous D, Forte LR, Rodco R, Alonso AB, Garg D & Raij L 2012. Sodium challenge does not support an acute gastrointestinal-renal natriuretic signaling axis in humans. Kidney International 82 1313–1320. ( 10.1038/ki.2012.269) [DOI] [PubMed] [Google Scholar]
- Quadri S & Siragy HM 2016. (Pro)renin receptor contributes to regulation of renal epithelial sodium channel. Journal of Hypertension 34 486–494; discussion 494. ( 10.1097/HJH.0000000000000825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM & Felder RA 1999Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension 33 1036–1042. ( 10.1161/01.hyp.33.4.1036) [DOI] [PubMed] [Google Scholar]
- Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM & Wilkinson IB 2017. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension 70 930–937. ( 10.1161/HYPERTENSIONAHA.117.10003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Survé VV & Håkanson R 1998. Evidence that peroral calcium does not activate the gastrin-ecl-cell axis in the rat. Regulatory Peptides 73 177–182. ( 10.1016/s0167-0115(98)00002-0) [DOI] [PubMed] [Google Scholar]
- Takaishi S, Shibata W, Tomita H, Jin G, Yang X, Ericksen R, Dubeykovskaya Z, Asfaha S, Quante M, Betz KS, et al. 2011. In vivo analysis of mouse gastrin gene regulation in enhanced GFP-BAC transgenic mice. American Journal of Physiology: Gastrointestinal and Liver Physiology 300 G334–G344. ( 10.1152/ajpgi.00134.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasova NI, Kopp JA, Jose P, Farnsworth DW, Michejda CJ & Wank SA 1996. Postprandial changes in renal function are mediated by elevated serum gastrin acting at cholecystokinin type B receptors (CCKB-R) in the kidney. Gastroenterology 110 A1106. [Google Scholar]
- Tsai LH & Cheng JT 1995. Stimulatory effect of dopamine on acid secretion from the isolated rat stomach. Neuroscience Research 21 235–240. ( 10.1016/0168-0102(94)00854-9) [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Aherne AM, Lane E, Power O, Carey RM & O’Connell DP 2000. Identification and regional distribution of the dopamine D(1A) receptor in the gastrointestinal tract. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 279 R599–R609. ( 10.1152/ajpregu.2000.279.2.R599) [DOI] [PubMed] [Google Scholar]
- Vieira-Coelho MA & Soares-da-Silva P 1993. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fundamental and Clinical Pharmacology 7 235–243. ( 10.1111/j.1472-8206.1993.tb00237.x) [DOI] [PubMed] [Google Scholar]
- von Schrenck T, Ahrens M, de Weerth A, Bobrowski C, Wolf G, Jonas L, Jocks T, Schulz M, Bläker M, Neumaier M, et al. 2000. CCKB/gastrin receptors mediate changes in sodium and potassium absorption in the isolated perfused rat kidney. Kidney International 58 995–1003. ( 10.1046/j.1523-1755.2000.00257.x) [DOI] [PubMed] [Google Scholar]
- Wang Q, Ji T, Zheng LF, Feng XY, Wang ZY, Lian H, Song J, Li XF, Zhang Y & Zhu JX 2012. Cellular localization of dopamine receptors in the gastric mucosa of rats. Biochemical and Biophysical Research Communications 417 197–203. ( 10.1016/j.bbrc.2011.11.084) [DOI] [PubMed] [Google Scholar]
- Wang ZY, Lian H, Zhou L, Zhang YM, Cai QQ, Zheng LF & Zhu JX 2016. Altered expression of D1 and D2 dopamine receptors in vagal neurons innervating the gastric muscularis externa in a Parkinson’s disease rat model. Journal of Parkinson’s Disease 6 317–323. ( 10.3233/JPD-160817) [DOI] [PubMed] [Google Scholar]
- Wang YY, He WW, Liu YC, Lin YF & Hong LF 2017. The effect of salt intake and potassium supplementation on serum gastrin levels in Chinese adults: a randomized trial. Nutrients 9 E389 ( 10.3390/nu9040389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Luft FC & Titze JM 2018. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiologica 222 e13006 ( 10.1111/apha.13006) [DOI] [PubMed] [Google Scholar]
- Xu P, Gildea JJ, Yue W, Jose PA & Felder RA 2019. Dopaminergic stimulation increases gastrin secretion via a PKA-PPAR-alpha pathway. Hypertension 74 (Supplement_1) AP146 ( 10.1161/hyp.74.suppl_1.P3033) [DOI] [Google Scholar]
- Yang J, Jose PA & Zeng C 2017. Gastrointestinal-renal axis: role in the regulation of blood pressure. Journal of the American Heart Association 6 e005536 ( 10.1161/JAHA.117.005536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LF, Song J, Fan RF, Chen CL, Ren QZ, Zhang XL, Feng XY, Zhang Y, Li LS & Zhu JX 2014. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiologica 211 434–446. ( 10.1111/apha.12229) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Human G and ovarian adenocarcinoma SW626 cells express the appropriate molecular (Mol) size of gastrin mRNA, ~300 base pairs (bp), expected from the primers used. Actin is used for control.
Supplemental Figure 2. Effect of NaCl concentration in the incubation medium on gastrin expression in gastrin-secreting tumor cells, SW626 and AGS. Increasing the sodium concentration in the buffer from 135 to 170 mM NaCl (4 hr incubation) increases gastrin protein expression in both SW626 and AGS cells (*, #, P<0.01 vs 135 mM NaCl, n=6/group, one-way ANOVA, Holm-Sidak test). IF = immunofluorescence, RFU = relative fluorescence unit
Supplemental Figure 3. (A) Human G cells were grown on alginate microspheres (Global Cell Solutions). Nuclei were stained with DAPI (green) and F-actin stained with phalloidin (red). This 3D model system enables the study of polarized G cells in monolayer. (B) G cells, grown as a transporting monolayer in a 3D system, have increased gastrin protein expression under high extracellular sodium concentration (170 mM, 4 hr) (P<0.05, paired t-test, n=4), but not substantially different from conventional cell culture demonstrating that Petri dish cultures achieve optimal results. (C) Individual data points for 4 independent cell lines measured in 3 independent assays are shown. The numbers in the graph indicate the assigned cell identification numbers. IF = immunofluorescence, RFU = relative fluorescence unit
Supplemental Figure 4. There is no D5R expression in human stomach antrum. Gastrin is stained red and nucleus stained blue with DAPI.
Supplemental Figure 5. Both D1R and D5R are expressed in AGS and SW626 cells.
Supplemental Figure 6. PPAR-α silencing in G cells. The transcriptions of intracellular PPAR-α and gastrin mRNAs were measured using qRT-PCR in G cells. The G cells were incubated with PPAR-α siRNA (100 nM/3 d). PPAR-α silencing RNA (siRNA) decreases PPAR-α mRNA to 0.28±0.02-fold and gastrin mRNA to 0.63±0.08-fold, relative to scrambled RNA, set at 1.0 (t-test, p<0.05, n=4).
Supplemental Figure 7. Gastrin synthesis, D1-like receptor, and the PPAR-α pathway in SW626 cells. The transcription of intracellular gastrin mRNA was measured using RT-PCR in SW626 gastric carcinoma cells. Monensin (MON, 1 μM/24 hr), which increases intracellular Na+ (* P<0.001, one-way ANOVA, Tukey test, n=3), and SKF (D1-like receptor agonist, 10 μM/24 hr), #P<0.001, one-way ANOVA, Tukey test, n=3) increase intracellular gastrin transcription. However, the D1-like receptor antagonist, LE300 (10 μM/24 hr), completely blocks the MON-induced increase in gastrin RNA level ($P<0.001, one-way ANOVA, Tukey test, n=3).
Supplemental Figure 8. Expression of tyrosine hydroxylase in G cells in stomach antrum and isolated G cells in culture. The D1R participates in the increase in gastrin expression caused by an increase in extracellular and intracellular sodium. G cells in the human stomach antrum (Supplemental Figure 8A) and isolated human G cells in culture (Supplemental Figure 8B) express tyrosine hydroxylase (TH) which converts tyrosine to L-DOPA. Gastrin is stained green, TH is stained red, and nucleus is stained blue with DAPI. There is widespread TH red fluorescent staining throughout the crypt cells. The merged images show minimal colocalization of gastrin and TH-staining G cells and not always in the same intracellular site. Nevertheless, these images indicate the presence of the first enzyme (TH) in the synthesis of dopamine in human G cells. Scale bar = 10 μm
Supplemental Figure 9. Expression of DOPA decarboxylase in G cells in stomach antrum and isolated G cells in culture. G cells in the human stomach antrum (Supplemental Figure 9A) and isolated human G cells in culture (Supplemental Figure 9B) express aromatic L-amino acid decarboxylase (DDC) which converts L-DOPA to dopamine. Gastrin is stained green, DDC is stained red, and nucleus is stained blue with DAPI. There is widespread DDC red fluorescent staining throughout the crypt cells. The merged images show minimal colocalization of gastrin and DDC-staining G cells and not always in the same intracellular site. Nevertheless, these images indicate the presence of the second enzyme (DDC) in the synthesis of dopamine in human G cells. Scale bar = 10 μm


