Abstract
Objective
The aim of this study was to compare the functional results of 2 different procedure types, medical or surgical used in treating native joint septic arthritis.
Methods
In this cohort study, we reviewed the clinical registries of patients admitted to a single third-level hospital with the diagnosis of septic arthritis during the period of January 1, 2008, to January 31, 2016.
Results
A total of 63 cases of septic arthritis were identified in which the initial approach for 49 patients was medical (arthrocentesis), whereas the initial approach for 14 patients was surgical (arthroscopy or arthrotomy). Of the 49 patients who received initial medical treatment (IMT), 15 patients (30%) later required surgical treatment because of poor progress. The median age of the patients was 60 (SD, 18) years. The group who received IMT were older than those who received initial surgical treatment (median, 64 years [interquartile range {IQR}, 54–76 years], vs. 48 years [IQR, 30–60 years]). There was a larger percentage of male patients in the surgical group (78% vs. 42% [p = 0.018]). Thirty percent of the medical group had been receiving corticosteroid treatment (p = 0.018). Results of complete recovery of joint functionality showed no significant differences after 1 year (68% with MT vs. 67% with ST, p = 0.91). Both groups had similar symptom duration until diagnosis, duration of antibiotic therapy (median, 30 days [IQR, 28–49 days], vs. 29.5 days [IQR, 27–49] days), and mortality rate (3 in the medical group).
Conclusions
The results of the study show that initial surgical treatment in patients with native joint septic arthritis is not superior to IMT. However, half of the patients with shoulder and hip infections treated with IMT eventually required surgical intervention, suggesting that perhaps this should be the preferred initial approach in these cases.
Key Words: arthritis, septic arthritis
Septic arthritis is an arthropathy caused by the invasion of microorganisms, (commonly bacteria) into the synovial membranes, resulting in purulent effusion within the joint capsule, by direct inoculation or secondary hematogenous dissemination, with the consequent destruction of the synovial membranes.1,2 Clinical characteristics include pain, erythema, and swelling with reduced range of articular movement. The reported incidence is 7.8 cases per 100,000 persons per year, with a mortality rate of approximately 10%.3 Delayed diagnosis and treatment may result in irreversible joint damage and permanent disability and/or death.4 The standard therapeutic modality includes intravenous administration of broad-spectrum antibiotics and drainage of the affected joint by daily needle aspirations or by surgical procedures such as arthroscopy or arthrotomy. The selection of the type of drainage is generally based on the experience of the treating physician. Data that describe the efficacy of each type of intervention are based on small studies and systemic literature reviews, which show that an initial surgical approach is not superior to serial needle aspirations.5–8
The objective of the present study was to compare the functional results of patients diagnosed as having septic arthritis and treated with initial medical treatment (IMT) with those treated with initial surgical treatment (IST). Both groups received antibiotic therapy according to hospital protocol.
METHODS
Patients
A medical records review study was done on patients with septic arthritis in a single third-level hospital (Hospital Universitario Puerta de Hierro Majadahonda) during the period of January 1, 2008 to January 31, 2016. Sample selection was done by accessing all case files with the diagnostic code, septic arthritis (711.0), according to the International Disease Classification, through the hospital's database (SELENE). A total of 163 cases were identified and reviewed using the following inclusion criteria:
the pathogen was isolated and identified in a synovial fluid culture,
the pathogen was isolated and identified in a blood culture or other sample, and/or
purulent joint material had a sterile culture due to previous administration of antibiotics with negative study results for microcrystals.
The following exclusion criteria were applied:
patients younger than 18 years,
infection of a prosthetic joint, and
arthritis due to mycobacteria, fungi, or parasites.
Ethical Guidelines
Approval was received by the hospital's clinical research ethics committee, and informed consent from patients was deemed unnecessary for this retrospective, observational study.
Protocol for Antibiotic Therapy
After obtaining specimen samples of synovial fluid and/or blood cultures, empirical intravenous therapy was initiated with 2 g cloxacillin every 4 hours plus 2 g ceftriaxone every 24 hours in most patients. Upon receiving culture results, treatment was adjusted according to the sensitivity of each microorganism. The selection of antibiotics and duration of treatment were determined by the type of bacteria and each patient's individual profile.
Description of Medical Treatment (Arthrocentesis)
Daily needle aspirations were done percutaneously following aseptic technique and washing the joint with physiological serum. Samples were obtained for culture and microscope slide preparation for Gram staining, cell count, and microcrystal detection.
Data Obtained From Case Files
The following parameters were taken into account: sex, age, site of service of hospital admission, risk factors (rheumatoid arthritis, arthrosis, diabetes mellitus, chemotherapy, previous surgeries, recent trauma, cancer, respiratory infections, other septic foci, and other existing arthropathies), anatomic location, use of immunosuppressants, previous use and dosage of corticosteroids, symptom duration until diagnosis, number of arthrocenteses, number of arthroscopies or arthrotomies, initial medical or surgical therapeutic approach, types of isolated microorganisms, types of specimens collected for microbiological culture isolates (synovial fluid, blood, etc.), synovial fluid leukocyte count, polymerase chain reaction upon admission and polymerase chain reaction upon discharge, duration of intravenous and oral antibiotic therapy, previous emergency room consultations, related deaths or complications derived from the course of the disease, treatment or management, and lastly functional recovery at 3, 6, and 12 months. The 2 groups were divided according to whether the initial admission was in a surgical specialty (traumatology and orthopedics) or a medical specialty (rheumatology, internal medicine).
Functional Recovery
Patients with full functional recovery were defined as those who were able to return to doing daily activities without pain or limitations. Patients with partial recovery were defined as those who continued to have range-of-movement limitations and/or pain after eradicating the joint infection. Data were gathered by accessing rehabilitation reports as well as follow-up reports from each site of service.
Statistical Analysis
A descriptive analysis of the categorical variables was done by using absolute and relative frequencies; numerical variables were analyzed by using the mean, SD or median, and 25th and 75th percentiles in accordance with the normal distribution. Univariate analysis was done by the Mann-Whitney U test to contrast numerical variables, whereas the χ2 test or Fisher exact test was used to contrast the hypothesis of categorical variables. The significance level was set to 0.05. Being an exploratory analysis, there was no application of correction for multiple comparisons. The statistical software used was Stata/IC v.14.1 (2015 Stata Statistical Software: Release 14; Stata Corp LP, College Station, TX).
RESULTS
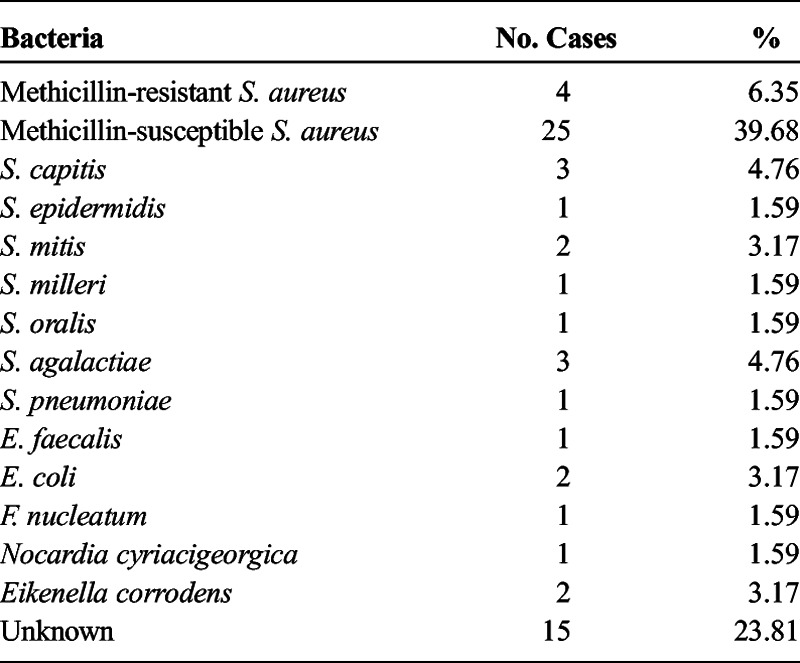
A total of 63 cases of septic arthritis that fulfilled study criteria were reviewed and analyzed. Sixty-two cases were monoarticular; 32 cases involved men (51%), and 31 cases involved women (49%); the median age was 60 (SD, 18) years (interval, 18–93 years), and the mean duration of symptoms until diagnosis was 11.8 days (interquartile range [IQR], 2–15 days). The site of service of each patient's admission was distributed accordingly: 36 patients from rheumatology, 14 from orthopedics, 10 from internal medicine, 2 from oncology, and 1 from nephrology. Three of 7 patients with RA were on anti–tumor necrosis factor α treatment. Of the 63 cases, 14 (22.22%) received IST, and 49 (77.78%) received IMT. Fifteen (30.6%) of the 49 patients receiving IMT later required at least 1 arthroscopy or arthrotomy during the course of the disease because of poor progress, of which 7 had an affected knee (21%), 4 an affected shoulder (50%), and 3 an affected hip (43%). Globally, the most affected joint was the knee, involving 34 cases (53.97%), followed by the shoulder, which involved 8 cases (12.7%), and hip, which involved 7 cases (11.11%). Other infected joints included hands, feet, elbows, and sternoclavicular joint. Methicillin-susceptible Staphylococcus aureus was the most frequent causative microorganism yielded, found in 25 (40%) of 63 cases, followed by methicillin-resistant S. aureus, which was isolated in 4 cases (6.35%). Other isolated microorganisms include Staphylococcus capitis (3 cases), Enterococcus faecalis (1 case), Escherichia coli (2 cases), Streptococcus mitis (2 cases), Streptococcus agalactiae (3 cases), Streptococcus oralis (1 case), Streptococcus pneumoniae (1 case), Fusobacterium nucleatum (1 case), Staphylococcus epidermidis (1 case), Nocardia (1 case), Eikenella corrodens (2 cases), and Streptococcus milleri (1 case). Fifteen of the 63 cases (23.8%) did not yield positive microbiological cultures; nevertheless, all patients presented purulent synovial fluid without the presence of microcrystals as seen by polarized light microscopy or other rheumatic diseases that may have presented a similar clinical profile, and all patients were treated with antibiotics. Fifteen patients (23.8%) had consulted in an urgent care center at least once before asserting the diagnosis (p = 0.682).
Comparison of Both Groups
When comparing both groups, it was noted that 11 of 14 patients receiving IST and 21 of 49 patients receiving IMT were men (78% vs. 43%; p = 0.018). The mean age of the IMT group was 64 years (IQR, 54–76 years), and the mean age of the IST group was 48 years (IQR, 30–60 years). The mean duration of antibiotic treatment for the IMT group was 30 days (IQR, 28–49 days) and 29.5 days (IQR, 20–59.5 days) for the IST group. In reference to risk factors for the development of septic arthritis, 39 of 49 patients of the IMT group presented 1 or more risk factors, whereas 12 of 14 patients of the IST group presented 1 or more risk factors (80% vs. 85%; p = 0.607). At the time of diagnosis of septic arthritis, 15 of 39 patients of the IMT group were taking corticosteroid treatment (range, 2.5–15 mg/d of prednisone prescribed for rheumatic diseases); none of the patients of the IST group were taking corticosteroids (24% vs. 0%; p = 0.018). Fifteen of the 63 patients were taking immunosuppressive treatment other than corticosteroids when diagnosed with septic arthritis, of which 14 of 15 of these patients were in the IMT group (p = 0.097). The origin of the infection was determined to be hematogenous in 37 (58%) of 63 cases, whereas 7 (11.11%) of 63 cases were subsequent to corticosteroid infiltration. Other determined infection sources were peripheral catheters, central catheters, human bite, and adjacent posttraumatic wounds.
In regard to functional recovery, data were available for only 51 of 63 patients at 3 months and 50 of 63 patients at 12 months. Of the 51 patients, 39 were in the IMT group, and 12 were in the IST group. At 3 months, 22 of 39 patients in the IMT group had made a full recovery, whereas 6 of 12 patients in the IST group had made a full recovery (56% vs. 50%; p = 0.696). At 12 months, full recovery was achieved in 26 of 38 patients in the IMT group and 8 of 12 patients in the IST group (68% vs. 66.66%; p = 0.91). Derived complications and/or death from the infection or treatment occurred in 5 of 63 cases, 3 in the IMT group and 2 in the IST group. No statistical differences were determined when comparing the following laboratory data: C-reactive protein upon admission and discharge, synovial fluid leukocyte count, and type of bacterial isolate. Also, significant differences were not found when studying the rest of the variables Tables 1–3.
TABLE 1.
Anatomic Distribution of Affected Joints in 63 Cases of Septic Arthritis
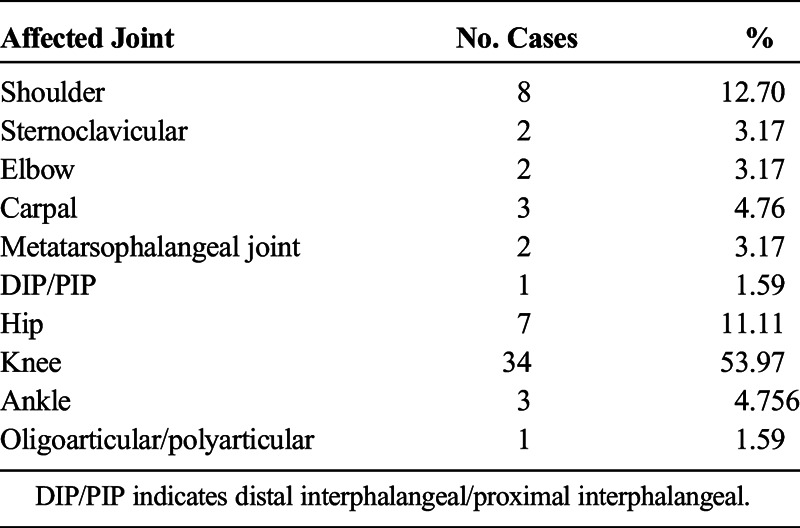
TABLE 3.
Comparison of Patients With Septic Arthritis Receiving IMT and IST
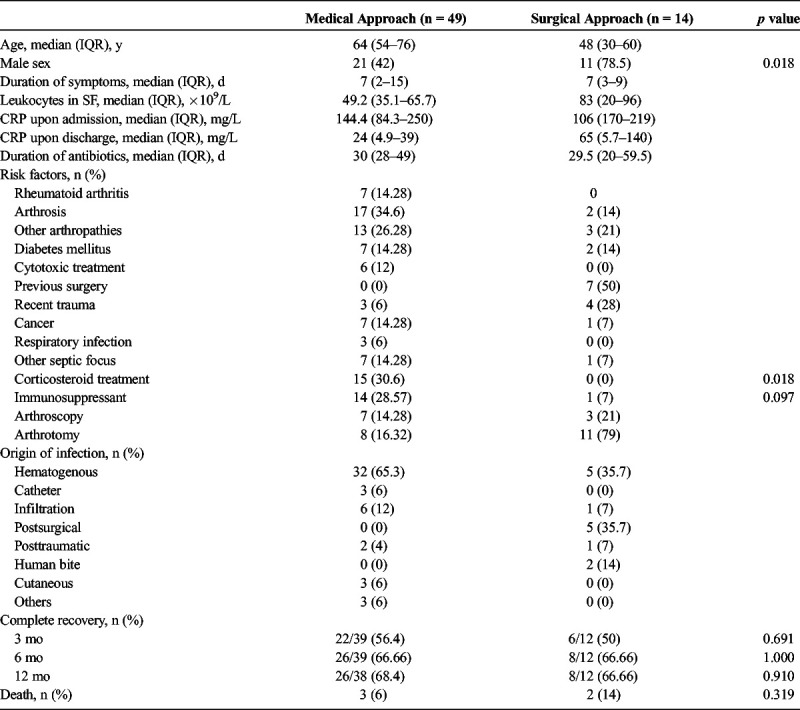
TABLE 2.
Isolated Bacteria in Culture Medium of 63 Patients With Septic Arthritis
DISCUSSION
Septic arthritis is a medical emergency with a mortality rate of approximately 10% despite adequate treatment.6 Currently, the initial treatment of native joint infection with a surgical approach is controversial.9 Nevertheless, the standard therapy is joint drainage (by needle aspiration or surgically) and systemic antibiotics, which decrease the risks of irreversible damage and impaired joint functionality. According to existing literature, it is known that the medical approach by daily arthrocenteses has similar results when compared with patients treated by surgical lavage fundamentally in acute cases.2,5,10,11 In the present study, we observed that there were no significant differences between initial approaches, whether daily arthrocenteses or arthroscopy/arthrotomy. Initial management should be selected according to case specifics such as the patient's personal history and the type of affected joint; for example, it would be reasonable to consider IST when treating a deep joint infection such as a hip.
Currently S. aureus is the causative agent most frequently involved in septic arthritis; a nationwide study done in Iceland showed that 42.6% of all cases were caused by this microorganism.12 Our study showed that 47.03% of cases were caused by S. aureus, a similar result found in other studies. In our study, no infectious agent was isolated in 23% of cases; this figure is similar to other previously reported studies in which the percentage of total isolated microorganisms varies between 62% and 100%.12 According to the British Society for Rheumatology guidelines, an infectious process cannot be ruled out by using microbiological culture as the only criterion, and patients should be treated with the same regimen if clinical suspicion is high.13,14
In almost all of our cases, the patients presented monoarticular infections (with the exception of 1 patient who presented simultaneously septic arthritis of the shoulder and the metatarsophalangeal joint). The knee was the joint most commonly involved, affecting 54% of cases; this percentage is greater than that in other studies we reviewed in which the knee was involved in approximately 30% of cases.15,16 In deep joints infections, 5 of 7 patients with hip infections required surgical treatment, as well as 5 of 8 patients with shoulder infections. Studies on infected deep joints suggest that the initial treatment be surgical because it was observed to prevent long-term sequelae such as serious avascular necrosis.17–19
When comparing both study groups (IMT vs. IST), the patients treated with serial arthrocenteses were older (64 vs. 48 years) and presented with a higher number of comorbidities, but both groups obtained the same results in respect to progress and functional recovery. Therefore, the medical treatment approach reached similar results, although the patients are older and presented more comorbidities. The proportion of male patients in the IST group was larger than that in the IMT group (p = 0.018), probably because of the fact that many of the patients in this group were admitted from the orthopedics/traumatology service, where experience using arthroscopy/arthrotomy as an initial approach is more established; nevertheless, the fact that male sex is a risk factor for the development of septic arthritis is controversial. Fifteen (30%) of 49 patients who received IMT later required surgical intervention by arthroscopy or arthrotomy because of poor clinical progress. It is important to note that 50% of these cases presented deep joint (hip or shoulder) infections, and the analysis of this subgroup did not find any statistically significant data in any of the variables. The risk factors for the development of septic arthritis are contiguous cutaneous lesions,4,15 arthrosis,15 diabetes mellitus,20 rheumatoid arthritis,4,15 previous surgeries,4,20 human immunodeficiency virus,21 and immunosuppressant therapy,22 among others. Although significant statistical differences were not found, 30% of patients in the IMT group and none of the patients in the IST group were on some type of immunosuppressant treatment. In a like manner, 30% of patients in the IMT group were on corticosteroid treatment (p = 0.018). Most of the patients in the IMT group were established rheumatology patients with medical histories of preexisting comorbidities, which may have required immunosuppressant and/or corticosteroid treatment prior to the acute onset of septic arthritis.
In reference to the results of recovered functionality when comparing both groups, no significant differences were found, being that 56% of patients in the IMT group and 50% of patients in the IST group had recovered full range of motion of the affected joint. Follow-up after 1 year showed that 68% of patients in the IMT group and 67% of patients in the IST group had recovered full joint functionality (p = 0.91); these figures are comparable with other reported series.23 Regarding mortality, 5 (8%) of 63 patients died of disease or treatment complications, data that are also comparable with other studies.6,24
The advantage in offering daily needle aspirations is that patients are not submitted to the risks derived from surgery and obtain the same result in functional recovery.2,5,10,11 Advantage of arthroscopy over the IMT approach is that it grants better accessibility to deep joints, better visualization of the infected joint, and direct access for synovial fluid sampling for bacterial cultures and germ detection in patients with negative synovial fluid culture. Arthroscopy compared with arthrotomy has shown greater long-term functional results with a rate of success of 79% to 100%.22,25,26
Currently, with the absence of prospective randomized studies, evidence suggests that functional results at 1 year are similar and that initial approach depends on the type of joint and duration of symptoms before diagnosis, as well as habitual practice of the treating physician and available resources. Independent of the chosen initial approach, the administration of empirical broad-spectrum antibiotic therapy is imperative, which may later be adapted according to microbiological studies.27
Initial surgical treatment is recommended in complicated cases that may include contiguous soft tissue infection, infection involving a prosthesis, poor clinical progress after using serial aspirations, and deep joint infections such as those in the hip and shoulder.28–30 In our study, 30% of patients initially treated medically had to have surgical drainage performed. Of these, half were on hip and shoulder joints. It may be therefore advisable to initially approach these deep joints surgically.
Limitations of this study include that it is retrospective, and the conclusions could be affected by unregistered factors of confusion. The study was done in a single health center with a small sample size, which affects the statistical power in detecting differences between the groups. Other limitations include the inability to isolate the causative agent in 23% of cases, and the number of patients treated with IST was significantly fewer than patients with IMT. However, few recent studies exist that compare both approaches,9,24 and this is the first study of its kind done in Spain.
CONCLUSIONS
Significant differences in functional recovery were not demonstrated with respect to initial treatment with daily needle aspirations or arthroscopy/arthrotomy. Similar results were obtained, even though the patients in the IMT group were older and have a higher number of comorbidities. In health centers where resources for an IST approach are not available, daily drainage of the affected joint has been shown to be as effective. However, our results also suggested that in cases of hip and shoulder infections a surgical approach initially when available may be more appropriate because half of these cases eventually required surgery.
Footnotes
The authors declare no conflict of interest.
B.J.F.-R. and M.J.P. had access to all study data and assumed responsibility for data integrity and accuracy of data analysis. Study supervision: J.M.M. and J.L.A.S.
REFERENCES
- 1.Pioro M, Mandell B. Septic artritis. Rheum Dis Clin North Am. 1997;23:239–258. [DOI] [PubMed] [Google Scholar]
- 2.Manadan AM, Block JA. Daily needle aspiration versus surgical lavage for the treatment of bacterial septic arthritis in adults. Am J Ther. 2004;11:412–415. [DOI] [PubMed] [Google Scholar]
- 3.Kaandorp CJ, Dinanat HJ, van de Laar MA, et al. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaandorp CJ, van Schaardenburg D, Krijnen P, et al. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 1997;38:1819–1925. [DOI] [PubMed] [Google Scholar]
- 5.Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72:296–310. [DOI] [PubMed] [Google Scholar]
- 6.Broy SB, Stulberg SD, Schmid FR. The role of arthroscopy in the diagnosis and management of the septic joint. Clin Rheum Dis. 1986;12:489–500. [PubMed] [Google Scholar]
- 7.Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;656:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews CJ, Coakley G. Septic arthritis: current diagnostic and therapeutic algorithm. Curr Opin Rheumatol. 2008;20:457–462. [DOI] [PubMed] [Google Scholar]
- 9.Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48:1320–1322. [DOI] [PubMed] [Google Scholar]
- 10.Bynum DK, Jr, Nunley JA, Goldner JL, et al. Pyogenic arthritis: emphasis on the need for surgical drainage of the infected joint. South Med J. 1982;75:1232–1235. [PubMed] [Google Scholar]
- 11.Maneiro JR, Souto A, Cervantes EC, et al. Predictors of treatment failure and mortality in native septic arthritis. Clin Rheumatol. 2015;34:1961–1967. [DOI] [PubMed] [Google Scholar]
- 12.Geirsson AJ, Statkevicius S, Vikingsson A. Septic arthritis in Iceland 1990–2002: increasing incidence due to iatrogenic infections. Ann Rheum Dis. 2008;67:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coakley G, Mathews C, Field M, et al. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford). 2006;45:1039–1041. [DOI] [PubMed] [Google Scholar]
- 14.Kodumuri P, Geutjens G, Kerr HL. Time delay between diagnosis and arthroscopic lavage in septic arthritis. Does it matter? Int Orthop. 2012;36:1727–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weston VC, Jones N, Bradbury N, et al. Clinical features and outcomes of septic arthritis in a single UK Heath District 1982–1991. Ann Rheum Dis. 1999;58:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolla JM, Lora Tamayo J, Gómez Vaquero C, et al. Pyogenic arthritis of native joints in non-intravenous drug users: a detailed analysis of 268 cases attended in a tertiary hospital over a 22-year period. Semin Arthritis Rheum. 2015;45:94–102. [DOI] [PubMed] [Google Scholar]
- 17.Nusem I, Jabur MK, Playford EG. Arthroscopic treatment of septic arthritis of the hip. Arthroscopy. 2006;8:902. [DOI] [PubMed] [Google Scholar]
- 18.Aïm F, Delambre J, Bauer T, et al. Efficacy of arthroscopic treatment for resolving infection in septic arthritis of native joints. Orthop Traumatol Surg Res. 2015;101:61–64. [DOI] [PubMed] [Google Scholar]
- 19.Stutz G, Kuster MS, Kleinstück F, et al. Arthroscopic management of septic arthritis: stages of infection and results. Knee Surg Sports Traumatol Arthrosc. 2000;8:270–274. [DOI] [PubMed] [Google Scholar]
- 20.Le Dantec L, Maury F, Flipo RM, et al. Peripheral pyogenic arthritis. A study of one hundred seventy-nine cases. Rev Rhum Engl Ed. 1996;63:103–110. [PubMed] [Google Scholar]
- 21.Ross JJ, Shamsuddin H. Sternoclavicular septic arthritis: review of 180 cases. Medicine (Baltimore). 2004;89:139–148. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2001;84:653–660. [PubMed] [Google Scholar]
- 23.Balabaud L, Gaudias J, Boeri C, et al. Results of septic knee arthritis: a retrospective series of 40 cases. Knee Surg Sports Traumatol Arthrosc. 2007;15:387–392. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg DL, Brandt KD, Cohen AS, et al. Treatment of septic arthritis: comparison of needle aspiration and surgery as initial modes of joint drainage. Arthritis Rheum. 1975;18:83–90. [DOI] [PubMed] [Google Scholar]
- 25.Parisien JS, Shaffer B. Arthroscopic management of pyoarthrosis. Clin Orthop. 1992;275:243–247. [PubMed] [Google Scholar]
- 26.Thiery JA. Arthroscopic drainage in septic arthritides of the knee: a multicenter study. Arhroscopy. 1989;5:65–69. [DOI] [PubMed] [Google Scholar]
- 27.Ohl CA. Infectious arthritis of native joints. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious diseases. 7th ed Philadelphia, PA: Churchill Livingstone; 2010. [Google Scholar]
- 28.Blizer CM. Arthroscopic management of septic arthritis of the hip. Arthroscopy. 1993;9:414–416. [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Choi NH, Ko SH, et al. Arthroscopic treatment of septic arthritis of the hip. Clin Orthop. 2003;407:211–214. [DOI] [PubMed] [Google Scholar]
- 30.Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1996;12:264–267. [DOI] [PubMed] [Google Scholar]


