Abstract
Purpose:
To compare the diurnal intraocular pressure (IOP)-lowering effect of latanoprostene bunod (LBN) 0.024% with timolol maleate 0.5% in subjects with open-angle glaucoma (OAG) or ocular hypertension (OHT).
Patients and Methods:
Pooled analysis of two phase 3, randomized, multicenter, double-masked, parallel-group, noninferiority trials (APOLLO and LUNAR), each with open-label safety extension phases. Adults with OAG or OHT were randomized 2:1 to double-masked treatment with LBN once daily (qd) or timolol twice daily (bid) for 3 months followed by open-label LBN treatment for 3 (LUNAR) or 9 (APOLLO) months. IOP was measured at 8 am, 12 pm, and 4 pm at week 2, week 6, and months 3, 6, 9, and 12.
Results:
Of the 840 subjects randomized, 774 (LBN, n=523; timolol crossover to LBN, n=251) completed the efficacy phase, and 738 completed the safety extension phase. Mean IOP was significantly lower with LBN versus timolol at all 9 evaluation timepoints during the efficacy phase (P<0.001). A significantly greater proportion of LBN-treated subjects attained a mean IOP ≤18 mm Hg and IOP reduction ≥25% from baseline versus timolol-treated subjects (P<0.001). The IOP reduction with LBN was sustained through the safety phase; subjects crossed over from timolol to LBN experienced additional significant IOP lowering (P≤0.009). Both treatments were well tolerated, and there were no safety concerns with long-term LBN treatment.
Conclusions:
In this pooled analysis of subjects with OAG and OHT, LBN 0.024% qd provided greater IOP-lowering compared with timolol 0.5% bid and maintained lowered IOP through 12 months. LBN demonstrated a safety profile comparable to that of prostaglandin analogs.
Key Words: intraocular pressure, latanoprostene bunod, diurnal, open-angle glaucoma, ocular hypertension, prostaglandin analog, nitric oxide donor, safety
Lowering of intraocular pressure (IOP) slows the progression of visual field loss in patients with open-angle glaucoma (OAG) and reduces the risk of onset of OAG in patients with ocular hypertension (OHT).1–4 In patients with OAG, every 1 mm Hg of IOP-lowering results in an estimated 10% to 19% reduction in the risk of visual field progression.3,5,6 Further, patients with OAG who achieve target IOP lowering demonstrate a significantly lower risk of disease progression.2 Pharmacological lowering of IOP is the most common initial intervention in patients with OAG or with OHT at risk for OAG.7 While patients with elevated IOP often initiate treatment with monotherapy, many will require treatment with >1 IOP-lowering agent to achieve and maintain target IOP.1,8,9 For example, of patients with primary OAG (POAG) in a managed care population 30% who initiated therapy with a topical prostaglandin analog (PGA) were prescribed 1 or more adjunctive therapies within 1 year.8
Latanoprostene bunod (LBN) is a nitric oxide (NO)-donating prostaglandin F2α analog that, following administration, is rapidly metabolized in situ to latanoprost acid and an NO-donating moiety, butanediol mononitrate.10–12 Whereas latanoprost acid reduces IOP by increasing aqueous humor outflow primarily through the uveoscleral pathway (unconventional route),13–22 NO is thought to facilitate aqueous humor outflow through relaxation of the trabecular meshwork and Schlemm’s canal (conventional route).23,24 In the healthy eye, most of the outflow occurs via the trabecular meshwork and Schlemm’s canal.25 NO has a role in IOP homeostasis, and NO production has been shown to be reduced in POAG subjects as well as in preclinical animal models of glaucoma.24,26 In POAG eyes, activity of the enzyme family responsible for endogenous NO generation (NO synthase) is decreased in the trabecular meshwork, Schlemm’s canal, and ciliary muscle, suggesting that reduced NO production may contribute to IOP elevation.27
Latanoprostene bunod ophthalmic solution 0.024% (LBN 0.024%) was recently (November 2017) approved by the US Food and Drug Administration for the lowering of IOP in patients with OAG or OHT. In several preclinical models of OAG and/or OHT, LBN reduced IOP to a greater degree than equimolar concentrations of latanoprost.10 LBN also reduced IOP in FP receptor knock-out mice, a model insensitive to the actions of PGAs.28 Further, LBN 0.024% was shown to be more effective in reducing IOP compared with latanoprost 0.005% in a phase 2 dose-ranging study in patients with OAG or OHT (VOYAGER).29 Given that latanoprost reached its maximal IOP-lowering effect at a concentration of 0.005%,30 the additional IOP lowering activity with LBN 0.024% was attributed to the action of NO.29 A phase 2, open-label, randomized trial (CONSTELLATION) found that, compared with timolol maleate 0.5%, LBN 0.024% had similar efficacy in reducing diurnal IOP and greater efficacy in reducing nocturnal IOP in subjects with OAG or OHT.31 In addition, open-label studies in Japanese populations demonstrated that LBN 0.024% provided robust 24-hour IOP lowering in subjects with low baseline IOP (KRONUS)32 and sustained reductions in IOP during use through 1 year in subjects with OAG or OHT (JUPITER).11
The APOLLO and LUNAR studies were nearly identically designed phase 3 efficacy and safety studies of LBN 0.024% in patients with OAG or OHT, each consisting of a 3-month active-(timolol) comparator double-masked efficacy phase followed by a 9-month (APOLLO) or 3-month (LUNAR) open-label safety extension phase with LBN 0.024% only. Individual study results for the 3-month double-masked efficacy phase of APOLLO and LUNAR have been published33,34 and demonstrated noninferiority of LBN 0.024% once daily (qd) in the evening to timolol 0.5% twice daily (bid). The APOLLO study also showed significantly greater IOP lowering at all 9 timepoints assessed over 3 months of treatment with LBN 0.024% qd versus timolol 0.5% bid, while the LUNAR study showed significantly greater IOP lowering with LBN 0.024% qd versus timolol 0.5% bid at 8 of 9 timepoints assessed. Here, we report a pooled analysis of the IOP-lowering effect of LBN 0.024% in the active-comparator double-masked efficacy through the open-label safety extension phases of these 2 studies, as well as safety findings over the entirety of the 2 studies.
PATIENTS AND METHODS
Study Design
This is a pooled analysis of data from two phase 3, randomized, multicenter, double-masked, parallel-group, noninferiority clinical trials with an open-label safety extension phase: the APOLLO study (NCT01749904) and the LUNAR study (NCT01749930). These studies were conducted at 91 unique sites (APOLLO, 45 sites; LUNAR, 46 sites) in the United States and the EU. Both studies consisted of a 3-month, double-masked efficacy phase followed by an open-label safety extension phase for 3 months in the LUNAR study and 9 months in the APOLLO study (Fig. 1). Institutional Review Board/Ethics Committee approval was obtained at each participating site. Details of the study methods and independent findings for the double-masked efficacy phase of these studies have been previously reported.33,34
FIGURE 1.
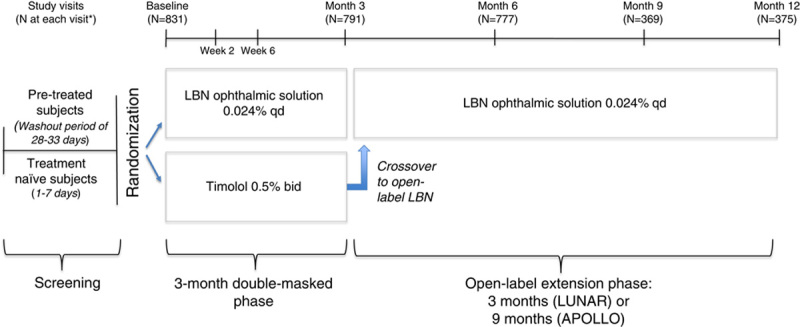
APOLLO and LUNAR study designs. bid indicates twice daily; LBN, latanoprostene bunod; qd, once daily. *Intent-to-treat population. Figure 1 can be viewed in color online at www.glaucomajournal.com.
Subjects
Each study enrolled subjects 18 years of age and above with OAG or OHT in 1 or both eyes. Eligible subjects had a mean IOP ≥24 mm Hg in 1 eye, and ≤36 mm Hg in both eyes at all 3 measurement timepoints (8 am, 12 pm, 4 pm) at baseline. The baseline visit occurred following a washout period in subjects receiving topical hypotensive treatment at enrollment (maximum of 28 +5 d). Subjects were also required to have a best-corrected visual acuity (BCVA) of +0.7 logMAR units or better in at least 1 eye. Additional inclusion/exclusion details were described previously.33,34
Treatment
For the 3-month double-masked efficacy phase, subjects were randomized 2:1 to LBN 0.024% instilled qd in the evening (~8 pm) and vehicle qd in the morning (~8 am) or timolol 0.5% instilled bid. The investigator, study personnel, and subjects were masked to treatment. Following assessment at month 3, all subjects continued with open-label LBN 0.024% qd for the safety extension phase, which was 9 months (APOLLO) or 3 months (LUNAR) in duration. Subjects previously on treatment with timolol 0.5% bid were crossed over to treatment with open-label LBN 0.024% qd in the evening in the safety extension phase.
Assessments
Subjects completed 3 study visits during the double-masked efficacy phase (week 2, week 6, month 3) and every 3 months thereafter through month 12 (APOLLO) or month 6 (LUNAR) during the open-label safety extension phase. IOP was measured in both eyes at 8 am, 12 pm, and 4 pm at each study visit using a Goldmann applanation tonometer. Whenever possible, the same operator measured IOP at each visit using the same tonometer. Safety assessments were conducted on an ongoing basis throughout the study and included vital sign measurements, BCVA, conjunctival hyperemia assessment, slit-lamp examination, gonioscopy, ophthalmoscopy, and treatment-emergent adverse events (hereafter, “AEs”). In addition, conjunctival hyperemia was graded at each visit on a scale from 1 (none) to 4 (severe) using photographic standards. Additional assessment details were described previously.33,34
Endpoints
The primary efficacy endpoint was the IOP in the study eye measured at 8 am, 12 pm, and 4 pm at week 2, week 6, and month 3 of double-masked treatment. Secondary efficacy endpoints included: the proportion of subjects with IOP ≤18 mm Hg at all 9 timepoints in the first 3 months; the proportion of subjects with IOP reduction ≥25% at all 9 timepoints in the first 3 months; the change in mean diurnal IOP (defined as the average of IOP measurements at 8 am, 12 pm, and 4 pm) from prerandomization baseline to months 3, 6, 9, and 12; and, for subjects randomized to timolol 0.5% and crossed over to LBN, the change in mean diurnal IOP from month 3 to months 6, 9, and 12 of open-label treatment. Mean percent reductions from baseline in diurnal IOP, and the proportion of subjects with IOPs ≤18, ≤17, ≤16, ≤15, and ≤14 mm Hg at the month 3 visit were post hoc endpoints.
Safety endpoints were: the incidence of ocular and systemic AEs as well as ocular AEs of special interest (changes in iris pigmentation, eyelid pigmentation, and eyelash growth); vital signs; BCVA; conjunctival hyperemia assessment; and slit-lamp examination, gonioscopy, and ophthalmoscopy.
Statistical Analysis
Primary efficacy analyses were performed using analysis of covariance (ANCOVA), with missing data imputed using the last observation carried forward (LOCF). The ANCOVA model included fixed effect terms for study, baseline IOP, and treatment, and baseline IOP as a covariate. The 2 study treatments were compared for each timepoint by visit, and the least squares (LS) mean, difference in LS mean (LBN ophthalmic solution 0.024% minus timolol 0.5%), and the 2-sided 95% confidence intervals (CIs) for the mean difference were obtained. Noninferiority was determined if the upper limit of the CIs for the difference did not exceed 1.5 mm Hg at all 9 timepoints and did not exceed 1.0 mm Hg for ≥5 of the 9 timepoints. If noninferiority was determined, superiority at each timepoint was demonstrated if the upper limit of the 95% CI did not exceed 0 mm Hg at all 9 timepoints. The primary efficacy analysis was performed in the intent-to-treat (ITT) population, which consisted of all randomized subjects who instilled ≥1 dose of study drug and had ≥1 postbaseline IOP assessment, and was repeated in the per-protocol population (PP), which comprised all subjects in the ITT population who remained in the study through month 3.
Secondary endpoints of proportions of subjects with IOP ≤18 mm Hg or with IOP reductions ≥25% at all of the 9 timepoints during the efficacy phase were based on the ITT population with LOCF. The 2-sided 95% CI around the difference in proportions (LBN ophthalmic solution 0.024% minus timolol 0.5%) and the P-value from Cochran-Mantel-Haenszel test adjusted by study factor were determined, with multiplicity of the P-values adjusted by using Hochberg’s method at 0.05 2-sided level.
Analysis of IOP-lowering efficacy in the safety extension phase was based on the ITT population using observed data. For each treatment group, the reduction from baseline in mean diurnal IOP were evaluated using an analysis of variance model with a fixed study factor for data at months 3 and 6 (and for completeness, weeks 2 and 6 of the efficacy phase), and using a 2-sided t test for data at months 9 and 12. In addition, for subjects randomized to timolol in the efficacy phase who were crossed over to LBN 0.024% in the safety extension phase, the change from month 3 diurnal IOP was evaluated using a 2-sided paired t test.
Least squares mean percent reductions from baseline in mean diurnal IOP at 3 months, a post hoc endpoint, were determined for the ITT population and compared using ANCOVA with treatment as a fixed effect and baseline as covariate. The proportion of subjects with IOPs ≤18, ≤17, ≤16, ≤15, and ≤14 mm Hg at the month 3 visit, also a post hoc endpoint, was based on subjects attaining the target IOP at ≥1 of the 3 assessment timepoints (8 am, 12 pm, 4 pm) at that visit. Differences between treatment groups were analyzed in the ITT population with LOCF using a Cochran-Mantel-Haenszel test adjusted by study factor.
All efficacy analyses used a 2-sided α=0.05 test.
Safety analyses were conducted in the safety population, which consisted of all subjects who received ≥1 dose of study drug. Safety data were reported according to received, rather than assigned, treatment. All safety data were summarized using descriptive statistics or categorically (conjunctival hyperemia). AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) terminology, and were summarized by severity (mild, moderate, severe) and relationship to study drug (unrelated, unlikely, possibly, probably, definitely). AEs with unknown severity were counted as severe and with unknown relationship to study drug were counted as probably related to study drug.
All statistical analyses were performed using SAS software (SAS Institute Inc.) version 9.2 or higher.
RESULTS
Subjects
Of the 1435 subjects screened across the 2 studies, 840 were randomized (LBN 0.024%, n=569; timolol 0.5% crossover to LBN 0.024%, n=271). A total of 832 subjects received at least 1 instillation of drug and comprised the pooled safety population (LBN 0.024%, n=561; timolol 0.5% crossover to LBN 0.024%, n=271) and 831 subjects were included in the pooled ITT population (LBN 0.024%, n=562; timolol 0.5% crossover to LBN 0.024%, n=269). Of subjects in the ITT population, 774 [LBN 0.024%, n=523 (93.1%); timolol 0.5% crossover to LBN 0.024%, n=251 (93.3%)] completed the efficacy phase and 738 [LBN 0.024%, n=503 (89.5%); timolol 0.5% crossover to LBN 0.024%, n=235 (87.4%)] completed both the efficacy and safety extension phase. The most frequent reasons for discontinuation were AE [LBN 0.024%, n= 12 (2.1%); timolol 0.5% crossover to LBN 0.024%, n=12 (4.5%)]; withdrawal of consent [LBN 0.024%, n= 12 (2.1%); timolol 0.5% crossover to LBN 0.024%, n=6 (2.2%)]; and failure to follow the required study procedures [LBN 0.024%, n=10 (1.8%); timolol 0.5% crossover to LBN 0.024%, n=4 (1.5%)]. Other reasons for discontinuation occurred in ≤1.3% of subjects in total.
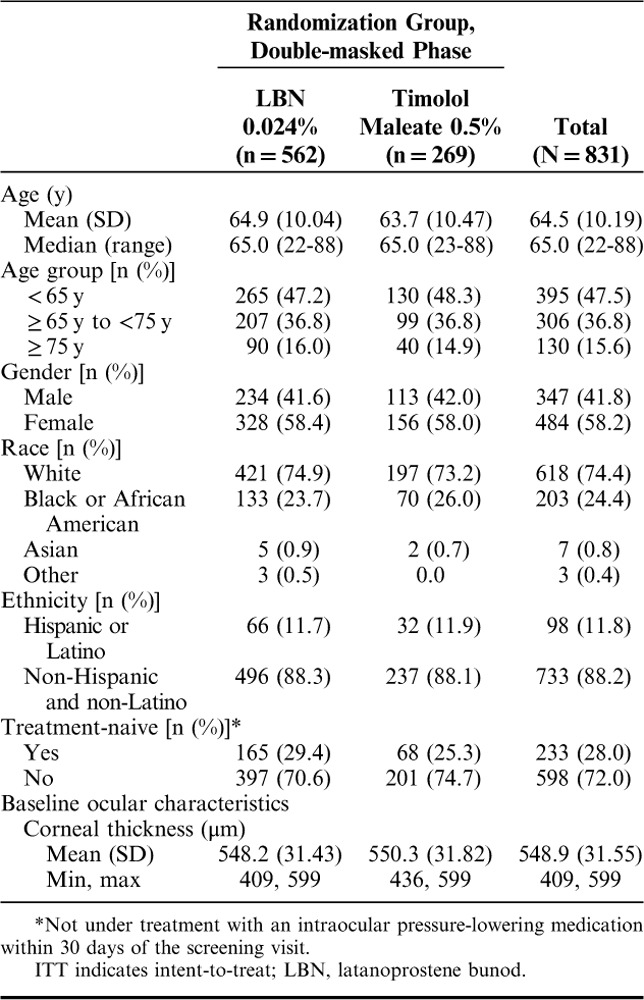
Demographics in the pooled ITT population were similar across treatment groups (Table 1). Subjects had a mean (SD) age of 64.5 (10.2) years and were predominantly White (74.4%) and female (58.2%). Most patients (72.0%) were taking a topical IOP-lowering medication at screening or had used IOP-lowering medication within 30 days of the screening visit and, therefore, participated in a washout. As previously reported,33,34 the most common prior ophthalmic medications were PGAs followed by beta-blockers and carbonic anhydrase inhibitors. The mean (SD) diurnal IOP at baseline was 26.7 (2.43) mm Hg in subjects randomized to LBN 0.024% qd and 26.5 (2.35) mm Hg in subjects randomized to timolol 0.5% bid.
TABLE 1.
Subject Demographics and Baseline Characteristics (ITT Population)
Efficacy
Primary Efficacy Endpoint
During the double-masked efficacy phase, the LS mean of the mean IOP in the study eye was significantly lower in the LBN 0.024% qd group (range, 17.8 to 18.9 mm Hg) than in the timolol 0.5% bid group (range, 19.0 to 19.7 mm Hg) at all timepoints measured (8 am, 12 pm, and 4 pm) at week 2, week 6, and month 3 (P<0.001 at all timepoints; Fig. 2). The upper limits of the 95% CIs for between-treatment comparisons of LS means of mean IOP were <0 mm Hg at all timepoints and visits, demonstrating not only noninferiority but also superiority of LBN 0.024% compared with timolol 0.5% for IOP lowering. Results in the PP population were consistent with those for the ITT population.
FIGURE 2.
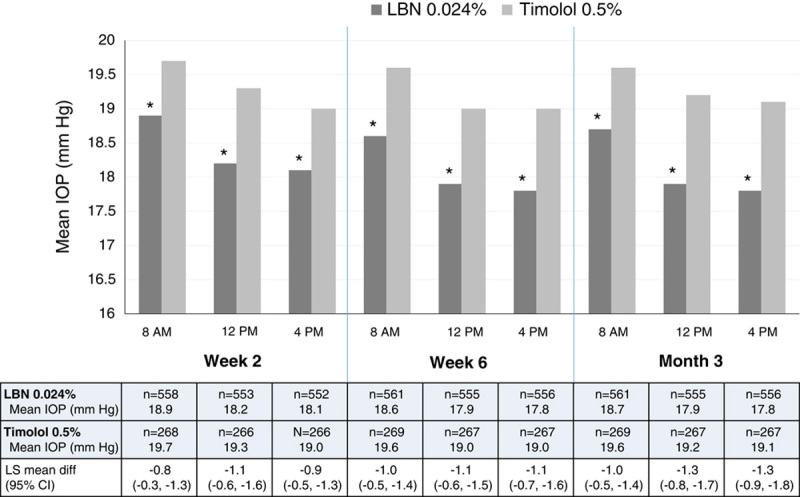
LS mean IOP in the study eye by visit, timepoint, and treatment group (ITT population; LOCF). CI indicates confidence interval; IOP, intraocular pressure; ITT, intent-to-treat; LBN, latanoprostene bunod; LOCF, last observation carried forward; LS, least squares. *P<0.001. Figure 2 can be viewed in color online at www.glaucomajournal.com.
Secondary Efficacy Endpoints
A significantly greater percentage of subjects treated with LBN 0.024% versus timolol 0.5% had their IOP reduced to ≤18 mm Hg at all of the 9 evaluation timepoints during the first 3 months of treatment (20.2% vs. 11.2%, P=0.001). In addition, the percentage of subjects with IOP reduction ≥25% from baseline at all 9 timepoints during the first 3 months of treatment was significantly greater in the LBN 0.024% group compared with the timolol 0.5% group (32.9% vs. 19.0%, P<0.001).
Figure 3 presents mean diurnal IOP at baseline and at each study visit of the double-masked efficacy phase through the open-label safety extension phase. There were statistically significant reductions from baseline in mean diurnal IOP for both treatment groups at all study visits during both phases of the study (P<0.001 vs. baseline for all visits, both treatments). Subjects assigned to LBN 0.024% during the double-masked efficacy phase maintained consistently lowered IOP during the open-label safety extension phase with a mean (SD) diurnal IOP of 18.1 (2.9), 18.2 (3.3), and 17.9 (3.0) mm Hg at months 6, 9, and 12, respectively, of the open-label extension phase, compared with 18.1 (2.9) at month 3 of the efficacy phase. Corresponding reductions from baseline were 8.6 (3.0), 8.5 (3.5), and 8.8 (3.2) mm Hg at months 6, 9, and 12 (open-label extension phase), respectively, and 8.6 (3.0) mm Hg at month 3 (efficacy phase). Subjects randomized to timolol 0.5% in the double-masked efficacy phase demonstrated an additional decrease in mean diurnal IOP when crossed over to LBN 0.024% in the open-label safety extension phase that was maintained through the open-label extension phase. Mean (SD) diurnal IOP in these subjects was 18.0 (3.5), 17.6 (2.7), and 17.6 (2.6) at months 6, 9, and 12, respectively (open-label extension phase), compared with 19.2 (2.9) at month 3 of the efficacy phase. Corresponding reductions from baseline were 8.5 (3.3), 8.7 (2.8), and 8.7 (3.0) at months 6, 9, and 12, respectively (open-label extension phase), compared with 7.3 (2.9) mm Hg at month 3. The additional reduction in IOP at these visits, ranging in 1.1 to 1.2 mm Hg IOP lowering from the last on-timolol treatment at month 3, was statistically significant (P≤0.009 for all vs. month 3).
FIGURE 3.
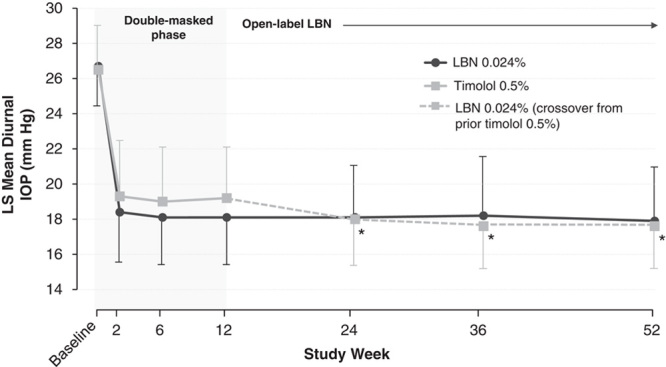
Mean (SD) diurnal IOP for subjects randomized to LBN 0.024% and subjects randomized to timolol 0.5% in the double-masked efficacy phase and crossed over to LBN 0.024% in the open-label safety extension phase (ITT population; data as observed). IOP indicates intraocular pressure; ITT, intent-to-treat; LBN, latanoprostene bunod; LS, least squares. *P≤0.009 versus month 3 for subjects randomized to timolol 0.5% in the efficacy phase.
Further analysis of IOP lowering at 3 months (the last visit of the double-masked efficacy phase) showed LS mean percent reductions from baseline in mean diurnal IOP for subjects randomized to LBN 0.024% and timolol 0.5%, respectively, were 32.0% and 27.6% at month 3 (P<0.001 for the difference). During the safety extension phase, percent reductions from baseline were similar to the percent reduction reported for treatment with LBN at month 3.
The proportion of subjects attaining IOP targets ranging from ≤18 to ≤14 mm Hg at the month 3 visit, a post hoc endpoint, were significantly greater with LBN 0.024% treatment compared with timolol 0.5% treatment. Specifically, respective proportions were 71.9% versus 60.2%, 60.1% versus 41.6%, 46.4% versus 33.5%, 31.0% versus 19.0%, and 19.0% versus 9.3% for subjects attaining targets of ≤18, ≤17, ≤16, ≤15, and ≤14 mm Hg, respectively, at the month 3 visit (P<0.001 for all).
Safety
Duration of Exposure
The mean (SD) duration of exposure for subjects treated with LBN 0.024% was 90.3 (16.0) days during the double-masked efficacy phase and 231.9 (110.1) days for the entire study duration. During the efficacy phase of the study, the mean duration of exposure for subjects treated with timolol 0.5% was 90.4 (16.7) days.
AEs
Ocular AEs that occurred in the study eye of ≥2% of subjects in any treatment group for the pooled safety population are summarized in Table 2. For subjects who received timolol during the efficacy phase and crossed over to LBN in the safety extension phase, AEs reported after the first dose of LBN 0.024% were counted in association with LBN. The most common ocular AE in the study eye for subjects during treatment with LBN 0.024% was conjunctival hyperemia (5.9% of subjects; timolol 0.5%, 1.1% of subjects). The majority of ocular AEs occurring in the study eye were considered at least possibly related to study drug in the LBN (80.2%) and timolol (86.5%) groups. Most ocular AEs occurring in the study eye were mild to moderate in severity in the LBN (97.0%) and timolol 0.5% (97.3%) groups. During treatment with LBN 0.024%, a total of 6 ocular AEs in the study eye were considered severe: blepharospasm, conjunctival hyperemia, allergic conjunctivitis, retinal vein occlusion, intraocular pressure increased, and eyelid tumor, occurring in 1 subject (0.1%) each. During treatment with timolol 0.5%, 1 severe ocular AE in the study eye, instillation site pain, was reported for 1 subject (0.4%).
TABLE 2.
Ocular AEs Occurring in ≥2% of Study Eyes in Either Treatment Group (Safety Population)
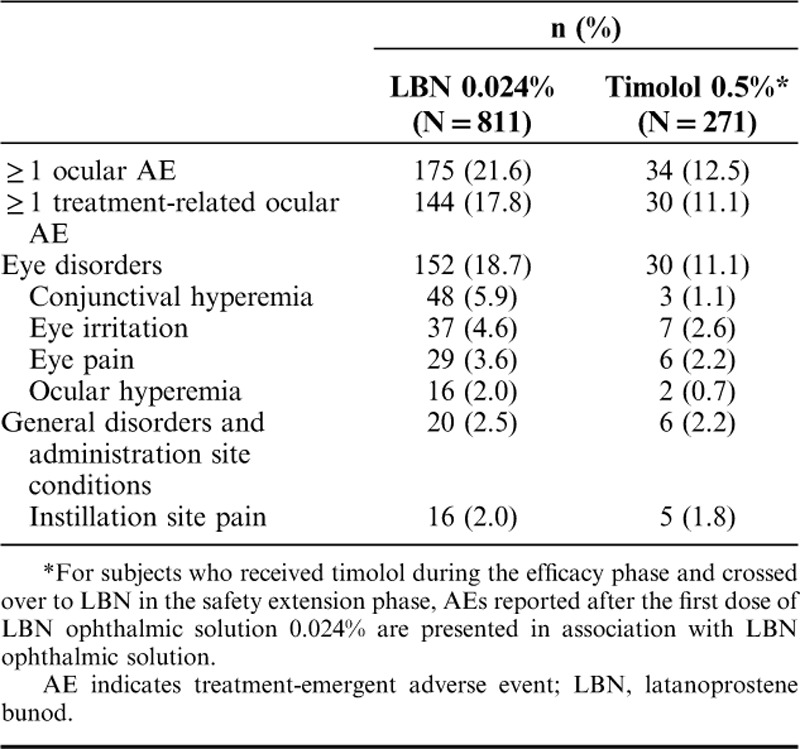
As observed with the study eye, the most common AE in the treated fellow eye during LBN 0.024% treatment was conjunctival hyperemia (6.5% of subjects; timolol 0.5% 1.5% of subjects), followed by eye irritation (4.2% LBN 0.024%, 2.6% timolol 0.5%), and eye pain (3.9% LBN 0.024%, 1.9% timolol 0.5%). Also, most ocular AEs in the treated fellow eye were considered at least possibly related to study drug and mild to moderate in severity. A total of 5 ocular AEs in the treated fellow eye of patients receiving LBN 0.024% were considered severe: conjunctival hyperemia, conjunctivitis allergic, scleritis, foreign body in eye, and IOP increased, occurring in 1 subject (0.1%) each. During treatment with timolol 0.5%, 2 ocular AEs occurring in the treated fellow were considered severe: instillation site pain and IOP increased, occurring in 1 subject (0.4%) each.
During treatment with LBN 0.024%, growth of eyelashes was reported for both eyes of 1 subject (both probably related), and iris hyperpigmentation was reported for both eyes of another subject (both definitely related). No ocular AEs of special interest occurred in the timolol 0.5% group.
No nonocular AEs occurred in ≥2% of subjects in either treatment group. The most common nonocular AE was headache, which occurred in 5 subjects (1 unrelated, 3 possibly related, 1 probably related) during treatment with LBN 0.024% and 5 subjects (2 unrelated, 3 possibly related) receiving timolol 0.5%. With the exception of 1 event of dysgeusia in a subject treated with LBN 0.024%, there were no other nonocular AEs considered definitely related to study treatment.
No serious AEs (SAEs) occurred in the study eye of subjects in either treatment group, while 1 subject (0.1%) in the LBN 0.024% group experienced an ocular SAE of device dislocation (dislocation of intraocular lens) in the treated fellow eye which was not considered treatment related. At least 1 nonocular SAE occurred in 16 (2.0%) subjects in the LBN 0.024% group and 2 (0.7%) subjects in the timolol 0.5% group; none were considered related to study drug. Two deaths occurred during the studies, both in subjects treated with LBN 0.024% (one following study exit). Both deaths (cardiac arrest in one subject, sepsis in another) were considered unrelated to study treatment.
There were few study discontinuations secondary to AEs. During LBN 0.024% treatment, ocular AEs in the study eye that led to discontinuation in 11 (1.4%) subjects included: increased IOP (n=3); ocular hyperemia (n=2); and 1 event each of conjunctival irritation, conjunctival edema, allergic conjunctivitis, eye irritation, eye pain, foreign body sensation, punctate keratitis, instillation site hypersensitivity, vision blurred, and rash. Study eye ocular AEs leading to discontinuation in 4 (1.5%) subjects during treatment with timolol 0.5% included 1 event each of allergic conjunctivitis, eye allergy, eye irritation, and eyelid edema.
Five (0.6%) subjects in the LBN 0.024% group and 1 (0.4%) in the timolol 0.5% group had at least 1 nonocular AE leading to discontinuation. Nonocular AEs leading to discontinuation in the LBN 0.024% group included chest discomfort, fatigue, malignant lung neoplasm, dizziness, headache, insomnia, and dyspnea; one subject in the timolol 0.5% discontinued the study due to nonocular AEs of dizziness, headache, and somnolence.
Vital sign measurements and BCVA were comparable between treatment groups during the efficacy phase and did not vary across study visits. No safety concerns emerged during the safety extension phase based on vital signs, BCVA, ocular signs, or gonioscopy results.
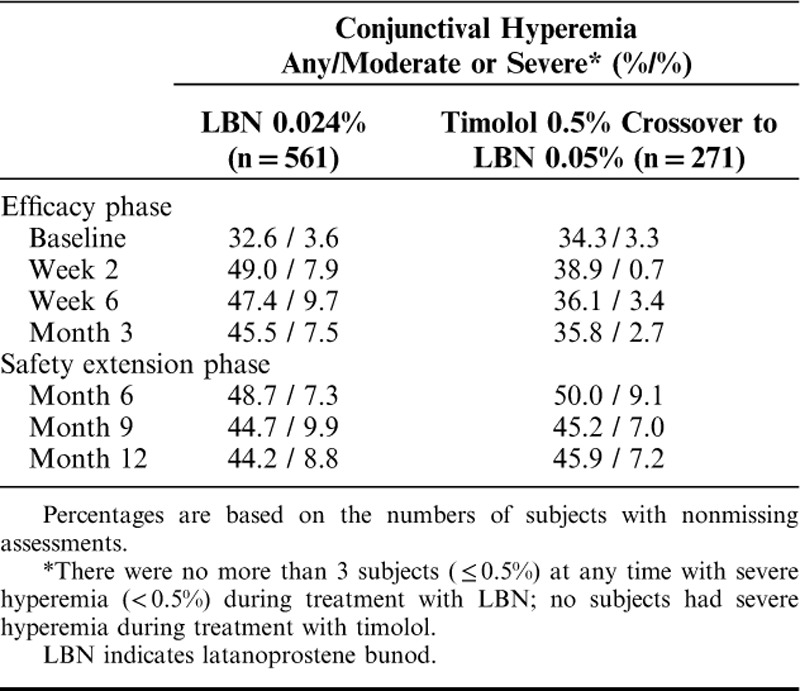
Conjunctival Hyperemia
At baseline, 33% to 34% of study eyes were assessed by investigators as having conjunctival hyperemia, mostly mild-to-moderate. During the efficacy phase, there was an increase from baseline in the proportion of subjects in the LBN 0.024% group with any hyperemia and moderate or severe hyperemia based on investigator assessments which persisted through the safety extension phase (Table 3). In contrast, the proportions of subjects with any conjunctival hyperemia and with moderate or severe conjunctival hyperemia in the study eye increased only slightly from baseline during the efficacy phase in the timolol group 0.5% but then increased further following crossover to LBN 0.5%. Few subjects, ranging from none to a maximum of 3 (≤0.5%) had severe conjunctival hyperemia at any visit during treatment with LBN 0.024%, while no subjects had conjunctival hyperemia considered severe during treatment with timolol 0.5%.
TABLE 3.
Percent of Subjects With Conjunctival Hyperemia per Investigator Assessment in the Study Eye (Safety Population)
DISCUSSION
Pooling data from nearly identical phase 3 trials offers a robust assessment of the safety and IOP-lowering potential of LBN, a novel NO-donating prostaglandin F2α analog. These data provide both a controlled comparison against an active comparator, timolol, as well as long-term safety data for LBN 0.024% for up to 12 months of use. In the pooled analysis, LBN 0.024% instilled qd in the evening was superior to timolol 0.5% instilled bid in reducing IOP over 3 months of treatment in subjects with OAG or OHT. In addition, a greater proportion of subjects attained a mean IOP ≤18 mm Hg consistently across all 9 timepoints or IOP reductions ≥25% from baseline across all 9 timepoints with LBN 0.024% compared with timolol 0.5%. At month 3, the last evaluation timepoint during double-masked treatment, the mean percent reduction from baseline with LBN was 32.0%. The mean percent reduction with timolol at this same timepoint was 27.6% and consistent with results reported for prior studies with this beta-blocker.35,36 In agreement with the JUPITER study,11 pooled results from the open-label safety extension phases demonstrated that IOP reduction with LBN 0.024% was maintained through 12 months, with no apparent loss of IOP-lowering effect over time. Subjects initially randomized to timolol 0.5% in the double-masked phase and crossed over to LBN 0.024% in the open-label phase demonstrated an additional 1.2 mm Hg decrease in mean diurnal IOP after switching to LBN that was significant and likewise sustained throughout the safety extension phase.
Intraocular pressure-lowering is a primary management strategy in patients with OAG or patients with OHT at risk for developing OAG, and is the only modifiable risk factor. While pharmacotherapy is not the only strategy for lowering IOP, PGAs or beta-blockers are commonly used as initial therapy.7 The magnitude of desired IOP reduction, or target IOP, needs to be individualized for each patient, taking into account baseline IOP, age, and clinical status, and presence of glaucoma-induced structural damage. In this pooled analysis of more than 500 subjects treated with LBN 0.024% in the efficacy through the safety extension phases, IOP reductions of up to 9 mm Hg were observed from a mean baseline IOP of ∼27 mm Hg. At the 3-month visit, the mean percentage reduction from baseline in LBN-treated subjects was 32% and the majority (72%) of subjects reached a target IOP ≤18 mm Hg.
Although sustained IOP lowering has been demonstrated with latanoprost over follow-up periods as long as 5 years,37–39 many patients will require additional therapies over the long term to maintain target IOP.1,8,9 LBN offers a novel dual mechanism of action, namely, the well-known activity of latanoprost along with the pharmacologic effect from the NO-donating moiety. The pharmacological benefits of NO are consistent with the documented role of NO in regulating IOP homeostasis.24,40 Studies indicate that NO activates the soluble-guanylyl cyclase/cyclic guanosine monophosphate signaling pathway, triggering numerous downstream processes that relax and enhance the permeability of cells in the TM and Schlemm’s canal.23,41 The triggered activity includes, among many other mechanisms, Rho-kinase and overall Rho pathway inhibition.24,42 Activation of the soluble-guanylyl cyclase/cyclic guanosine monophosphate signaling pathway by the NO moiety is thought to be responsible for the additive IOP-lowering effect of LBN compared with latanoprost observed in various animal models of glaucoma and/or OHT,10 and in a well-controlled dose-ranging study in patients with OAG and OHT.4
Safety is an important consideration for long-term IOP-lowering drug therapy. Topical PGAs have an excellent safety profile with regard to systemic side effects. Ocular side effects can include conjunctival hyperemia, increases in iris pigmentation, elongation and darkening of the eyelashes, periocular skin pigmentation, and ocular surface effects or irritation.43,44 This pooled analysis showed that LBN 0.024% demonstrated a high degree of safety through 12 months of treatment and exhibited a safety profile typical of topical PGA therapy, with mainly mild-to-moderate ocular AEs and no systemic SAEs considered treatment-related. No new ocular AEs were identified and the overall frequency of ocular AEs was comparable to other PGAs.43,44 The most common ocular AEs in the study in the LBN and timolol treatment groups were conjunctival hyperemia, eye irritation, and eye pain. The lack of clinically significant systemic side effects and of significant changes in laboratory parameters also suggests that the NO-donating moiety of LBN 0.024% ophthalmic solution does not alter the safety profile of this novel PGA.
Ocular AEs of special interest (changes in iris pigmentation, eyelid pigmentation, and eyelash growth) were uncommon in the phase 3 study populations. Although these AEs may be more apparent with long-term use of PGAs, the observed low incidence during exposure to LBN 0.024% in this pooled analysis suggests that there is no increased risk of these AEs with LBN 0.024% ophthalmic solution relative to other PGAs.43,44 However, the findings in these studies contrast with those of the JUPITER study of LBN 0.024% qd in Japanese patients with OAG or OHT, in which 10.0% of study eyes and 8.8% of treated fellow eyes had a clear increase in iris pigmentation from baseline to week 52.11 Differences in ethnic populations may have contributed to the greater iris pigmentation observed in the JUPITER study. In addition, iris pigmentation in the JUPITER study was assessed based on analysis of photographs taken at baseline and week 52, whereas the current study captured changes in iris pigmentation that were recorded as ocular AEs. Further study is warranted to better understand potential differences of these AEs among study populations.
In both APOLLO and LUNAR studies, conjunctival hyperemia was assessed by investigators at each visit using a photographic reference scale. Consistent with individual study findings, approximately one third of subjects in the pooled data set had conjunctival hyperemia at baseline, mostly mild to moderate, before treatment initiation. An increase in hyperemia relative to baseline was noted by week 2 among subjects assigned to LBN 0.024% without much further increase beyond that point. Among subjects initially randomized to timolol, hyperemia did not increase from baseline during 3 months of timolol treatment, but did increase initially following crossover to LBN 0.024%. These findings are not surprising given that conjunctival hyperemia is a common side effect of PGA therapy.43,44 Very few (≤0.5%) subjects had conjunctival hyperemia rated as severe during treatment with LBN 0.024%.
The current study measured IOP only during daytime hours (8 am, 12 pm, and 4 pm) and therefore did not assess potential differences between LBN 0.024% and timolol 0.5% in controlling nocturnal IOP levels. Latanoprost has been shown to reduce nocturnal IOP relative to both baseline and to timolol in patients with OHT or early glaucomatous changes, whereas timolol failed to reduce nocturnal IOP.45 Similarly, in a phase 2, randomized crossover study in 25 subjects, 40 to 90 years of age, with OAG or OHT, LBN 0.024%, but not timolol 0.5%, decreased nocturnal IOP relative to baseline.31 Further, LBN 0.024% significantly decreased nocturnal IOP compared with timolol.
CONCLUSIONS
These pooled findings demonstrate that the NO-donating prostaglandin F2α analog LBN provided greater IOP-lowering compared with timolol over 3 months in patients with OAG and OHT. Reduction in IOP with LBN was sustained through 1 year. This novel monotherapy also has a safety profile comparable with that of PGAs, with mild-to-moderate ocular AEs, and without serious systemic side effects.
ACKNOWLEDGMENTS
The authors thank Adrienne Drinkwater, PhD of Churchill Communications for writing and editorial support.
Footnotes
Data presented in part at the 26th annual meeting of the American Glaucoma Society, March 3-6, 2016, Ft. Lauderdale, FL; the annual meeting of the Association for Research in Vision and Ophthalmology, May 1-5, 2016, Seattle, WA; and the 120th meeting of the American Academy of Ophthalmology, October 15-18, 2016, Chicago, IL.
Supported by Bausch & Lomb Incorporated. The phase 3 studies described here and the integrated analysis of pooled data were funded by Bausch & Lomb Incorporated.
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical hypotensive medication delays or prevents the onset of primary open angle glaucoma. Arch Ophthalmol. 2002;120:701–703. [DOI] [PubMed] [Google Scholar]
- 2.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1679. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study Group. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126:1030–1036. [DOI] [PubMed] [Google Scholar]
- 6.Heijl A. Glaucoma treatment: by the highest level of evidence. Lancet. 2015;385:1264–1266. [DOI] [PubMed] [Google Scholar]
- 7.Prum BE Jr, Rosenberg LF, Gedde SJ. Primary Open-Angle Glaucoma Preferred Practice Pattern® Guidelines. Ophthalmology. 2016;123:P41–P411. [DOI] [PubMed] [Google Scholar]
- 8.Schmier JK, Hulme-Lowe CK, Covert DW. Adjunctive therapy patterns in glaucoma patients using prostaglandin analogs. Clin Ophthalmol. 2014;8:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman AL, Lum FC, Velentgas P, et al. Practice patterns and treatment changes for open-angle glaucoma: the RiGOR study. J Comp Eff Res. 2016;5:79–85. [DOI] [PubMed] [Google Scholar]
- 10.Krauss AH, Impagnatiello F, Toris CB, et al. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2α agonist, in preclinical models. Exp Eye Res. 2011;93:250–255. [DOI] [PubMed] [Google Scholar]
- 11.Kawase K, Vittitow JL, Weinreb RN, et al. Long-term safety and efficacy of latanoprostene bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: the JUPITER study. Adv Ther. 2016;33:1612–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman PL. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin Pharmacother. 2017;18:433–444. [DOI] [PubMed] [Google Scholar]
- 13.Lütjen-Drecoll E, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2a. Exp Eye Res. 1988;47:761–769. [DOI] [PubMed] [Google Scholar]
- 14.Gabelt BT, Kaufman PL.Levin LA, Nilsson SFE, Ver Hoeve J, Wu SM, Alm A, Kaufman PL. Production and flow of aqueous humor. Adler’s Physiology of the Eye, 11th ed St Louis: Mosby Elsevier; 1989:274–307. [Google Scholar]
- 15.Nilsson SF, Samuelsson M, Bill A, et al. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2 alpha-1-isopropylester in the cynomolgus monkey. Exp Eye Res. 1989;48:707–716. [DOI] [PubMed] [Google Scholar]
- 16.Lindsey JD, Kashiwagi K, Kashiwagi F, et al. Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest Ophthalmol Vis Sci. 1997;38:2214–2223. [PubMed] [Google Scholar]
- 17.Richter M, Krauss AH, Woodward DF, et al. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003;44:4419–4426. [DOI] [PubMed] [Google Scholar]
- 18.Weinreb RN, Kashiwagi K, Kashiwagi F, et al. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophtahlmol Vis Sci. 1997;38:2772–2780. [PubMed] [Google Scholar]
- 19.Weinreb RN, Toris CB, Gabelt BT, et al. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(suppl 1):S53–S64. [DOI] [PubMed] [Google Scholar]
- 20.Sagara T, Gaton DD, Lindsey JD, et al. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol. 1999;117:794–801. [DOI] [PubMed] [Google Scholar]
- 21.Schachtschabel U, Lindsey JD, Weinreb RN. The mechanism of action of prostaglandins in uveoscleral outflow. Curr Opin Ophthalmol. 2000;11:112–125. [DOI] [PubMed] [Google Scholar]
- 22.Gaton DD, Sagara T, Lindsey JD, et al. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F(2 alpha)-isopropyl ester treatment. Arch Ophthalmol. 2001;119:1165–1170. [DOI] [PubMed] [Google Scholar]
- 23.Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994;35:2515–2520. [PubMed] [Google Scholar]
- 24.Cavet ME, Vittitow JL, Impagnatiello F, et al. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest Ophthalmol Vis Sci. 2014;55:5005–5015. [DOI] [PubMed] [Google Scholar]
- 25.Toris CB, Camras CB, Yablonski ME. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 1993;100:1297–1304. [DOI] [PubMed] [Google Scholar]
- 26.Stamer WD, Lei Y, Boussommier-Calleja A, et al. eNOS, a pressure-dependent regulator of intraocular pressure. Ophthalmol Vis Sci. 2011;52:9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995;36:1765–1773. [PubMed] [Google Scholar]
- 28.Saeki T, Tsuruga H, Aihara M, et al. Dose-response profile of PF-03187207 (PF-207) and peak IOP lowering response following single topical administration to FP receptor knockout mice vs. wild type mice. Invest Ophthalmol Vis Sci. 2009;50:4064 E-Abstract. [Google Scholar]
- 29.Weinreb RN, Ong T, Scassellati Sforzolini B, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eveleth D, Starita C, Tressler C. A 4-week, dose-ranging study comparing the efficacy, safety and tolerability of latanoprost 75, 100 and 125 µg/ml (Xalatan) in the treatment of primary open-angle glaucoma and ocular hypertension. BMC Ophthalmol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JH, Slight JR, Vittitow JL, et al. Efficacy of Latanoprostene Bunod 0.024% compared with timolol 0.5% in lowering intraocular pressure over 24 hours. Am J Ophthalmol. 2016;169:249–257. [DOI] [PubMed] [Google Scholar]
- 32.Araie M, Sforzolini BS, Vittitow J, et al. Evaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24 h in healthy Japanese subjects. Adv Ther. 2015;32:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinreb RN, Scassellati Sforzolini B, Vittitow J, et al. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016;123:965–973. [DOI] [PubMed] [Google Scholar]
- 34.Medeiros FA, Martin KR, Peace J, et al. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol. 2016;168:250–259. [DOI] [PubMed] [Google Scholar]
- 35.Hedman K, Alm A. A pooled-data analysis of three randomized, double-masked, six-month clinical studies comparing the intraocular pressure reducing effect to latanoprost and timolol. Eur J Ophthalmol. 2000;10:95–104. [DOI] [PubMed] [Google Scholar]
- 36.Zhang WY, Po AL, Dua HS, et al. Meta-analysis of randomized controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2001;85:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedman K, Watson PG, Alm A. The effect of latanoprost on intraocular pressure during 2 years of treatment. Surv Ophthalmol. 2002;47(suppl 1):S65–S76. [DOI] [PubMed] [Google Scholar]
- 38.Kashiwagi K, Tsumura T, Tsukahara S. Long-term effects of latanoprost monotherapy on intraocular pressure in Japanese glaucoma patients. J Glaucoma. 2008;17:662–666. [DOI] [PubMed] [Google Scholar]
- 39.Kara C, Şen EM, Elgin KU, et al. Does the intraocular pressure-lowering effect of prostaglandin analogues continue over the long term? Int Ophthalmol. 2017;37:619–626. [DOI] [PubMed] [Google Scholar]
- 40.Buys ES, Potter LR, Pasquale LR, et al. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front Mol Neurosci. 2014;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneemann A, Dijkstra BG, van den Berg TJ, et al. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:936–941. [DOI] [PubMed] [Google Scholar]
- 42.Thoonen R, Sips PY, Bloch KD, et al. Pathophysiology of hypertension in the absence of nitric oxide/cyclic GMP signaling. Curr Hypertens Rep. 2013;15:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(suppl 1):S93–S105. [DOI] [PubMed] [Google Scholar]
- 44.Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol. 2004;138:389–395. [DOI] [PubMed] [Google Scholar]


