Abstract
Aims
To estimate the proportion of patients with a recent myocardial infarction (MI) who would be eligible for additional lipid-lowering therapy according to the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines for the management of dyslipidaemias, and to simulate the effects of expanded lipid-lowering therapy on attainment of the low-density lipoprotein cholesterol (LDL-C) target as recommended by the guidelines.
Methods and results
Using the nationwide SWEDEHEART register, we included 25 466 patients who had attended a follow-up visit 6–10 weeks after an MI event, 2013–17. While most patients (86.6%) were receiving high-intensity statins, 82.9% of the patients would be eligible for expanded lipid-lowering therapy, as they had not attained the target of an LDL-C level of <1.4 mmol and a ≥50% LDL-C level reduction. When maximized use of high-intensity statins followed by add-on therapy with ezetimibe was simulated using a Monte Carlo model, the LDL-C target was reached in 19.9% using high-intensity statin monotherapy and in another 28.5% with high-intensity statins and ezetimibe, while 50.7% would still be eligible for proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. When use of alirocumab or evolocumab was simulated in those who were eligible for PCSK9 inhibitors, around 90% of all patients attained the LDL-C target.
Conclusion
Our study suggests that, even with maximized use of high-intensity statins and ezetimibe, around half of patients with MI would be eligible for treatment with PCSK9 inhibitors according to the 2019 ESC/EAS guidelines. Considering the current cost of PCSK9 inhibitors, the financial implications of the new guidelines may be substantial.
Keywords: ESC/EAS guidelines, Treatment goals, LDL cholesterol target, PCSK9 inhibitors, Ezetimibe, Statins
See page 3910 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa139)
Introduction
In August 2019, the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) released updated guidelines for the management of dyslipidaemias.1 For patients with a recent myocardial infarction (MI), the guidelines now recommend achieving a low-density lipoprotein cholesterol (LDL-C) reduction of ≥50% from baseline and an LDL-C level of <1.4 mmol/L (<55 mg/dL) (Class 1, Level A). To reach the LDL-C target, lifestyle modifications and treatment with high-intensity statins are recommended. If the target is not achieved after 4–6 weeks despite lifestyle modification and maximally tolerated statin therapy, add-on therapy with ezetimibe (Class 1, Level B) and thereafter a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (Class 1, level A), is recommended. The new guidelines present a lower LDL-C goal and recommend more aggressive LDL-C lowering therapy for patients with an MI, as compared with the previous 2016 ESC/EAS guidelines which recommended an LDL-C goal of <1.8 mmol/L and an LDL-C reduction of ≥50% if the baseline level was 1.8–3.5 mmol/L.2 The use of ezetimibe and thereafter a PCSK9 inhibitor were also given a lower recommendation level in the previous guidelines (Class IIa, Level B and Class IIb, Level C, respectively) for those who did not reach the LDL-C target with statin monotherapy.2 The implications of the new guidelines in terms of the proportion of patients who will be eligible for treatment with ezetimibe and PCSK9 inhibitors is unknown.
Using data from nationwide registers in Sweden, we sought to estimate the proportion of patients with a recent MI who would be eligible for additional LDL-C lowering therapy according to the new guidelines, and simulate the effects of expanded therapy with high-intensity statins, ezetimibe, and PCSK9 inhibitors on LDL-C target attainment.
Methods
Data sources and study population
All patients in Sweden admitted with an MI to a coronary care unit or other specialized inpatient facilities are continuously included in the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) register.3 During the study period, patients <75 years of age were invited to participate in the SWEDEHEART secondary prevention follow-up programme, including a first follow-up visit at 6–10 weeks after the MI. This follow-up visit largely corresponds to the 4–6 weeks of follow-up recommended in the 2019 ESC/EAS guidelines.1
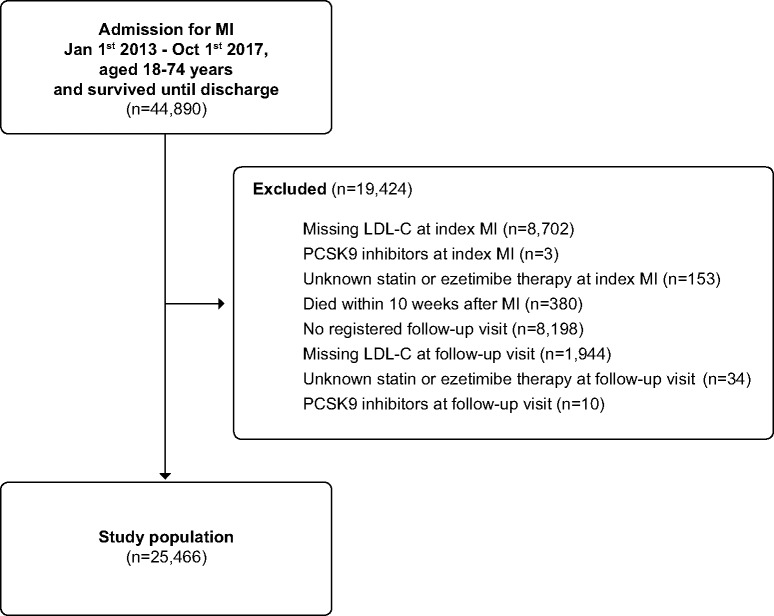
We included all patients in SWEDEHEART, aged 18–74 years, admitted with an MI between 1 January 2013 and 1 October 2017, and who survived until discharge. For patients with more than one admission during the study period, we randomly selected one admission. We excluded those who (i) had unknown statin or ezetimibe therapy at admission for the index MI, (ii) had missing LDL-C data at the index MI, (iii) died before 10 weeks after discharge, (iv) received PCSK9 inhibitors at the index MI or prior to the 6–10-week follow-up visit, (v) had no registration of a follow-up visit, (vi) had missing LDL-C data at the follow-up visit, or (vii) had unknown statin or ezetimibe therapy at the follow-up visit. The reason for excluding patients on PCSK9 inhibitors was that few patients (n = 13) were receiving these drugs during the study period.
Low-density lipoprotein cholesterol levels were measured using standard methods at each hospital. Information on previously diagnosed diseases was retrieved from the SWEDEHEART register and the National Patient Register, which includes the diagnoses of all hospital admissions in Sweden since 1987. Data regarding medications at admission and discharge, as well as use of lipid-lowering therapies at the follow-up visit were collected in the SWEDEHEART register. Data regarding filled prescriptions and the type of statin treatment were retrieved from the Prescribed Drugs Register which includes all dispensed drugs in Sweden since 2005, and date of death was obtained from the Swedish population registry, which includes the vital status of all Swedish residents. The regional ethics committee in Stockholm approved the study (2018/1957/32).
Exposure to lipid-lowering therapies and baseline low-density lipoprotein cholesterol levels
Patients who had a registered use of statins in SWEDEHEART and had filled a statin prescription within the previous 180 days were considered as exposed to statins at the index MI and at the 6–10-week follow-up, respectively. If more than one prescription was filled within the previous 180 days, the statin of the latest filled prescription was used. Similarly, patients who had a registered use of ezetimibe in SWEDEHEART and who had filled a prescription for the drug within the previous 180 days were considered as exposed to ezetimibe.
In order to assess the target of a ≥50% reduction in LDL-C level, a baseline LDL-C level is needed. The 2019 ESC/EAS guidelines define the baseline as the pre-treatment LDL-C level for patients not receiving lipid-lowering therapy and the extrapolated pre-treatment value for those with ongoing lipid-lowering therapy.1 Thus, for patients not receiving lipid-lowering therapy (80.2% of the study population), we used the LDL-C level as measured at the index MI. For those with ongoing lipid-lowering therapy (19.8%), we extrapolated the baseline LDL-C level using the LDL-C level at the index MI and the LDL-C reduction of the lipid-lowering therapy that the patient was receiving. This LDL-C reduction was sampled from β probability density functions derived from clinical trials, specific for each drug and dose, as presented and validated by Cannon et al.4 (Supplementary material online, Table S1).
Monte Carlo simulation model for intensification of lipid-lowering therapies
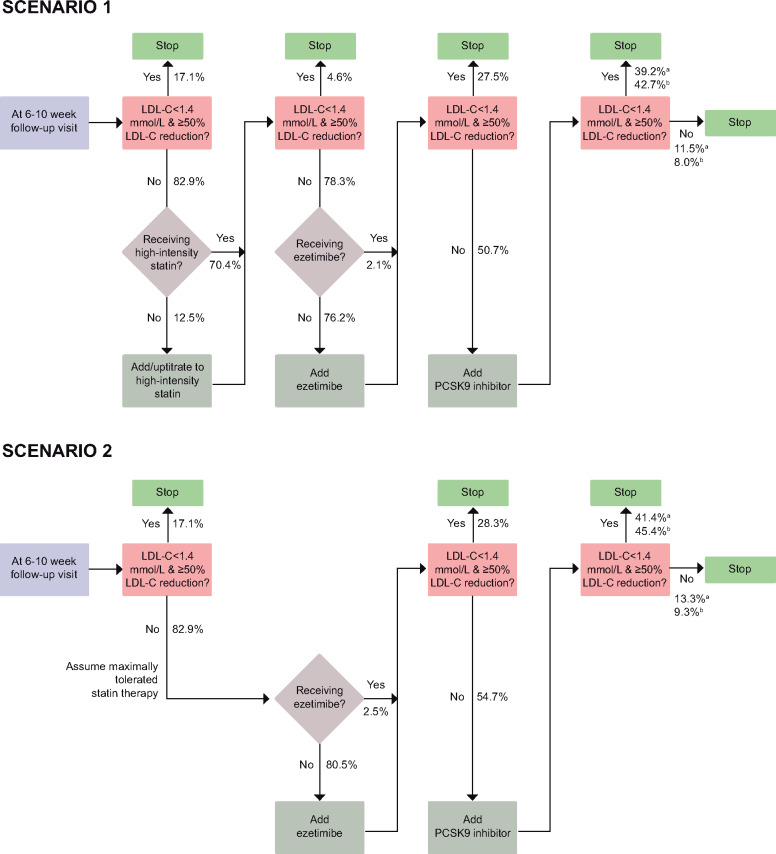
We used a Monte Carlo simulation model, which was based on methods and data previously presented and validated by Cannon et al.,4 to simulate two treatment intensification scenarios to achieve the 2019 ESC/EAS LDL-C target. Monte Carlo simulation is a method for estimating outcomes in scenarios using repeated random sampling. The logic of each treatment intensification scenario in our study is shown in Figure 1. In the first scenario, we simulated a maximized uptake of high-intensity statin therapy followed by maximized use of ezetimibe and then add-on therapy with a PCSK9 inhibitor [alirocumab (75 mg biweekly)5 or evolocumab (140 mg biweekly or 420 mg monthly)6] (Scenario 1). Patients who initiated a high-intensity statin in the simulation were set to receive atorvastatin 80 mg.
Figure 1.
Logic of scenarios for lipid-lowering treatment intensification and percentage of patients flowing through the treatment intensification logic in the simulation. High-intensity statin was defined as atorvastatin ≥40 mg, rosuvastatin ≥20 mg, or simvastatin 80 mg; patients who initiated high-intensity statin therapy in the simulation were set to receive atorvastatin 80 mg. aAlirocumab 75 mg biweekly. bEvolocumab 140 mg biweekly/420 mg monthly.
Second, as high-intensity statins were recommended for patients with MI also in previous guidelines,2,7 we performed another simulation in which we assumed that patients were already receiving the maximally tolerated intensity of statin therapy at the follow-up visit. In this scenario, patients eligible for additional lipid-lowering therapy first received ezetimibe, followed by a PCSK9 inhibitor (Scenario 2).
At each step in the treatment intensification pathway, patients received the treatment if they had not attained the LDL-C target in the previous step and were not already receiving the treatment. The achieved LDL-C level following an add-on treatment was modelled probabilistically from the distribution of LDL-C level reduction with a given lipid-lowering therapy. The treatment effect of each drug and dose combination used in the simulation was sampled from β probability density functions (Supplementary material online, Table S1) derived from clinical trials.4 As such, each patient followed a unique path in the simulation model depending on their LDL-C levels at the index MI and at the follow-up visit, and probabilistic sampling of LDL-C level reduction.
We performed additional analyses for the two scenarios, as described in the Supplementary material online. First, we simulated use of alirocumab 150 mg in patients who had not reached the LDL-C target despite receiving alirocumab 75 mg. Second, we modelled statin intolerance in 9.2% of the patients based on the proportion of patients not using statins at 12–14 months after the event. Third, we accounted for a potential depression of LDL-C levels during the admission for MI. Finally, we estimated the effect of statins and ezetimibe on LDL-C levels in the study population (Supplementary material online, Table S2) and used these estimates in the simulations of treatment intensification.
Statistical analyses
We assessed the lipid-lowering therapy, LDL-C levels and characteristics of the study population by eligibility for additional lipid-lowering therapy at the 6–10-week follow-up visit. Next, we performed the simulations of treatment intensification. At each step in the simulation, we assessed the proportion of the patients who had attained the LDL-C target, who had reached an LDL-C level of <1.4 mmol/L but not achieved a ≥50% LDL-C level reduction and who had an LDL-C level of ≥1.4 mmol/L, as well as the proportion of patients by lipid-lowering therapy. While statin therapy and ezetimibe are well-established treatments and available at a relatively low cost, PCSK9 inhibitors have a higher cost and some physicians may not consider these drugs for patients who are close to their LDL-C target. Therefore, we also assessed the proportion who had an LDL-C level of ≥1.8 mmol/L at the step in the simulations when eligibility for PCSK9 inhibitors was considered. We performed the simulations 1000 times and report 95% confidence interval based on the resulting distributions of the proportions of patients attaining the LDL-C target and receiving add-on lipid-lowering therapies at each step of treatment intensification. We present the point estimates as the value constituting the median in these distributions. Analyses were performed in Stata version 15.0 (StataCorp LP, College Station, TX, USA).
Results
Study population
We identified 44 890 patients, aged 18–74 years, who had been hospitalized with MI between 1 January 2013 and 1 October 2017 and who survived until discharge. In total, 19 424 patients (43.3%) were excluded, the majority due to missing LDL-C data at the index MI (n = 8702), or no registered follow-up visit (n = 8198). The final study population included 25 466 patients (Figure 2). The baseline characteristics of the patient population at the index MI, those who were excluded, and the study population are shown in Supplementary material online, Table S3. Compared to the patients who were included in the analyses, those who were excluded tended to be older, more likely to have a non-ST-elevation MI, diabetes, previous MI, previous percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG) and to receive high-intensity statins at admission, and less likely to receive PCI and ticagrelor. Lipid levels at the index MI were similar among those included vs. excluded. At the index MI, the mean [standard deviation (SD)] age of the study population was 62.4 (8.6) years, 24.6% were women, 41.5% presented with an ST-elevation MI, and 14.7% had a previous MI.
Figure 2.
A flowchart for study population.
Lipid-lowering therapy and low-density lipoprotein cholesterol levels
At admission for the index MI, most patients (80.2%) had no ongoing lipid-lowering therapy; 13.7% were receiving monotherapy with low- or moderate-intensity statins and 5.0% were receiving monotherapy with high-intensity statins. Few patients (1.2%) were receiving ezetimibe as monotherapy or in combination with statins. At the 6–10-week follow-up visit, only 3.9% of the patients had no lipid-lowering therapy; most patients (84.3%) were receiving monotherapy with high-intensity statins, followed by monotherapy with low- or moderate-intensity statins (8.8%), combination therapy of ezetimibe and high-intensity statins (2.3%) or low- or moderate-intensity statins (0.4%), and ezetimibe monotherapy (0.5%). The mean (SD) LDL-C level was 3.1 (1.1) mmol/L at the index MI and 1.9 (0.7) mmol/L at the 6–10-week follow-up visit.
At the follow-up visit, 82.9% of the patients would be eligible for intensified lipid-lowering therapy according to the 2019 ESC/EAS guidelines, as they had not reached an LDL-C level of <1.4 mmol/L (76.6%) or had reached an LDL-C level of <1.4 mmol/L but had not achieved a ≥50% LDL-C level reduction (6.3%). Patient characteristics by eligibility for additional lipid-lowering therapy are shown in Table 1. Compared to the patients who were not eligible for additional therapy, those who were eligible tended to have higher LDL-C levels at the index MI, were more likely to have had a previous MI, as well as previously having undergone PCI and CABG, and were less likely to have diabetes and to have received ticagrelor at discharge. Of the patients who were not eligible for additional lipid-lowering therapy, 94.3% received high-intensity statins and 4.3% received low- or moderate-intensity statins. The corresponding numbers for those who were eligible were 85.0% and 10.1%.
Table 1.
Population characteristics by eligibility for increased lipid-lowering therapy according to the 2019 ESC/EAS guidelines for the management of dyslipidaemias at 6–10 weeks after the myocardial infarction
| Eligible | Not eligible | |
|---|---|---|
| [n = 21 122 (82.9%)] | [n = 4344 (17.1%)] | |
| At/during admission for MI | ||
| Age (years), mean (SD) | 62.5 (8.5) | 61.9 (8.9) |
| Age group (years) | ||
| 18–44 | 677 (3) | 188 (4) |
| 45–54 | 3220 (15) | 722 (17) |
| 55–64 | 6944 (33) | 1412 (33) |
| 65–74 | 10 281 (49) | 2022 (47) |
| Women | 5301 (25) | 953 (22) |
| Body mass index (kg/m2) | ||
| <25 | 5699 (28) | 1105 (26) |
| 25 to <30 | 9454 (46) | 1927 (45) |
| ≥30 | 5419 (26) | 1205 (28) |
| Smoking status | ||
| Never smoker | 7203 (35) | 1595 (37) |
| Former smoker | 7459 (36) | 1525 (36) |
| Current smoker | 6055 (29) | 1148 (27) |
| Lipid levels (mmol/L), mean (SD) | ||
| Total cholesterol | 5.1 (1.3) | 4.8 (1.0) |
| LDL cholesterol | 3.2 (1.2) | 2.9 (0.8) |
| HDL cholesterol | 1.2 (0.4) | 1.21 (0.4) |
| Triglycerides | 1.6 (0.9) | 1.7 (1.0) |
| Type of MI | ||
| STEMI | 8827 (42) | 1741 (40) |
| NSTEMI | 12 295 (58) | 2603 (60) |
| Received PCI | 18 118 (86) | 3793 (87) |
| Lipid-lowering therapya | ||
| High-intensity statins | 1195 (6) | 189 (4) |
| Low/moderate-intensity statins | 3071 (15) | 467 (11) |
| Ezetimibe | 223 (1) | 56 (1) |
| Comorbidities | ||
| Diabetes | 4000 (19) | 1081 (25) |
| Previous MI | 3303 (16) | 450 (10) |
| Previous PCI | 2871 (14) | 383 (9) |
| Previous CABG | 802 (4) | 98 (2) |
| Congestive heart failure | 568 (3) | 73 (2) |
| Stroke | 963 (5) | 159 (4) |
| COPD | 1027 (5) | 149 (3) |
| Renal insufficiencyb | 2063 (10) | 419 (10) |
| Peripheral artery disease | 578 (3) | 88 (2) |
| Cancer | 296 (1) | 69 (2) |
| Medications at discharge | ||
| Ticagrelor | 16 348 (77) | 3659 (84) |
| Clopidogrel | 2996 (14) | 362 (8) |
| Other P2Y12 inhibitor | 240 (1) | 36 (1) |
| Aspirin | 20 353 (96) | 4224 (97) |
| ACE inhibitors | 13 936 (66) | 2859 (66) |
| Angiotensin II receptor blockers | 4149 (20) | 898 (21) |
| Beta-blockers | 19 013 (90) | 3911 (90) |
| Calcium channel blockers | 3073 (15) | 606 (14) |
| Diuretics | 2722 (13) | 495 (11) |
| Oral anticoagulation | 1568 (7) | 248 (6) |
| At 6–10-week follow-up visit | ||
| LDL cholesterol (mmol/L), mean (SD) | 2.0 (0.7) | 1.1 (0.2) |
| Lipid-lowering therapya | ||
| High-intensity statins | 17 945 (85) | 4098 (94) |
| Low/moderate-intensity statins | 2134 (10) | 188 (4) |
| Ezetimibe | 628 (3) | 168 (4) |
Numbers are shown as n (%) if not otherwise indicated. Missing values were body mass index (n = 657), smoking status (n = 481), total cholesterol (n = 165), triglycerides (n = 1989), HDL cholesterol (n = 217), renal insufficiency (n = 346), and medications at discharge (ranging from n = 5 to n = 13).
ACE, angiotensin-converting enzyme; CABG, coronary artery bypass surgery; COPD, chronic obstructive lung disease; MI, myocardial infarction; NSTEMI, non-ST-elevation MI; PCI, percutaneous coronary intervention; STEMI, ST-elevation MI.
As monotherapy or as part of combination therapy with statins and ezetimibe.
Defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2.
Simulation of treatment intensification
Figure 3 shows the proportion of the population by lipid-lowering therapy and LDL-C target achievement at the 6–10-week follow-up as well as at each step in the two simulated scenarios for treatment intensification. The 95% CIs are shown in Supplementary material online, Tables S4 and S5.
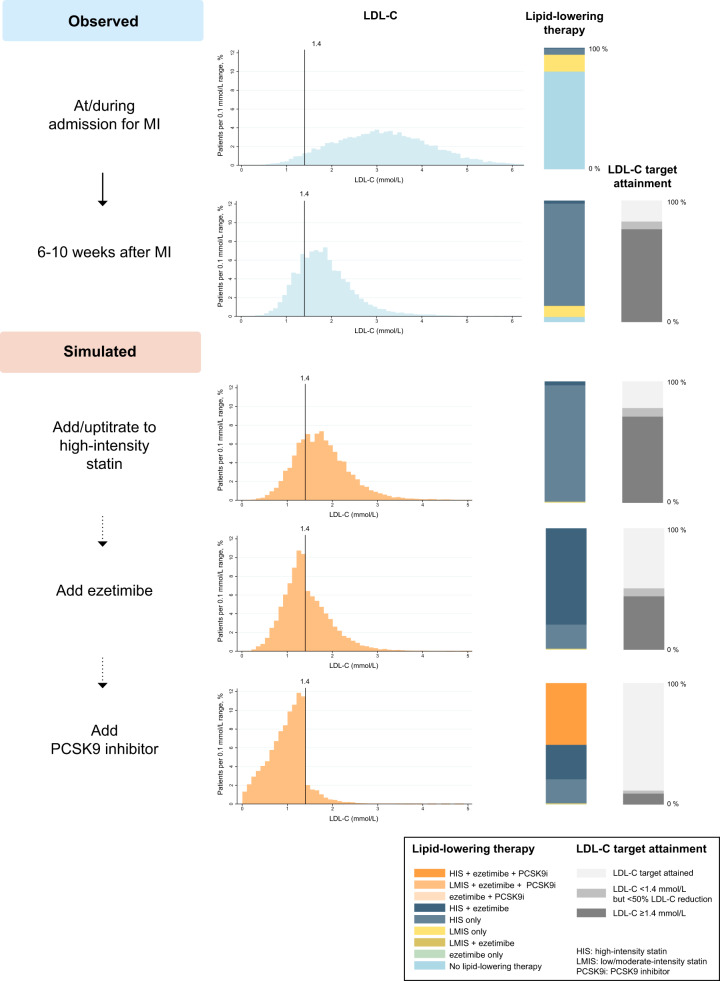
Figure 3.
Observed and simulated proportions of the population by lipid-lowering therapy and low-density lipoprotein cholesterol target achievement. HIS, high-intensity statins; LMIS, low/moderate-intensity statins. aBiweekly and bmonthly.
In Scenario 1, when all patients who had not reached the LDL-C target and who were not receiving high-intensity statins were treated with atorvastatin 80 mg, the proportion not attaining the LDL-C target decreased only slightly, from 82.9% to 78.3%. In the next step, as use of ezetimibe was maximized, 50.7% of the patients would be eligible for PCSK9 inhibitors as they had not reached the LDL-C target, with 21.1% of the patients having an LDL-C level of ≥1.8 mmol/L. When using PCSK9 inhibitors, the proportion not attaining the LDL-C target decreased to 11.5% for alirocumab and 8.0% for evolocumab. At this stage, 19.9% received monotherapy with high-intensity statins, 28.5% received high-intensity statins with ezetimibe and 50.7% received PCSK9 inhibitors.
In Scenario 2, which assumed that all patients were already receiving their maximally tolerated statin therapy, maximizing the use of ezetimibe resulted in 54.7% of the patients being eligible for PCSK9 inhibitors as they had not attained the LDL-C target, with 24.1% of the patients having an LDL-C level of ≥1.8 mmol/L. When using PCSK9 inhibitors, the proportion not reaching the target decreased to 13.3% for alirocumab and 9.3% for evolocumab. At this stage, 15.5% received monotherapy with high-intensity statins, 26.2% received high-intensity statins with ezetimibe, and 54.7% received PCSK9 inhibitors.
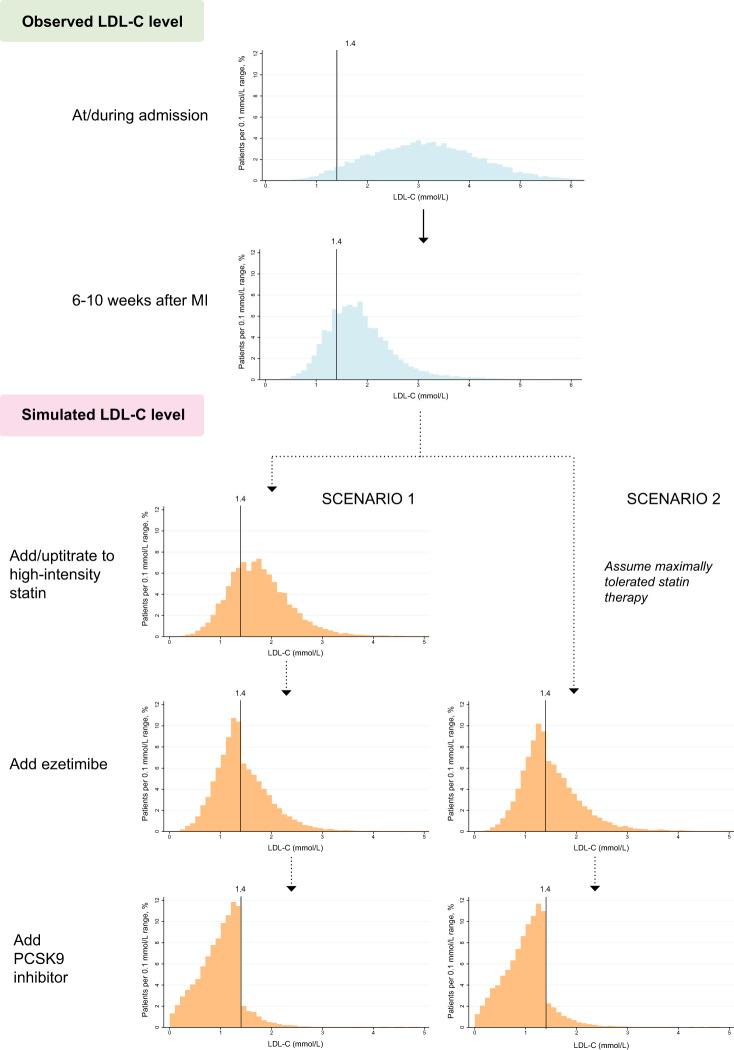
In the additional analyses in which the use of alirocumab 150 mg was simulated in patients not reaching the LDL-C target despite receiving alirocumab 75 mg, 97.1% and 96.5% of the patients reached the LDL-C target in the first and second scenario, respectively (Supplementary material online, Table S6). When statin intolerance was modelled in 9.2% of the patients, 54.9% and 56.7% of the patients would be eligible for PCSK9 inhibitors in the first and second scenario, respectively (Supplementary material online, Table S7). The corresponding numbers were 46.8% and 50.8% for simulations modelling a depression in LDL-C levels during the admission for MI (Supplementary material online, Table S8) and 65.2% and 66.4% when add-on therapy with statins and ezetimibe was simulated using effect estimates obtained from the study population (Supplementary material online, Table S9). The distribution of LDL-C levels at the index MI, the 6–10-week follow-up and at each step in the simulated scenarios are shown in Figure 4 and Supplementary material online, Table S10.
Figure 4.
Observed and simulated distribution of low-density lipoprotein cholesterol levels. The distribution of low-density lipoprotein cholesterol levels for proprotein convertase subtilisin/kexin type 9 inhibitors is shown for alirocumab 75 mg. The low-density lipoprotein cholesterol level distribution for evolocumab is shown in Supplementary material online, Figure S1.
Discussion
The recently released 2019 ESC/EAS guidelines for the management of dyslipidaemias present a lowered LDL-C goal and strengthened recommendations for use of ezetimibe and PCSK9 inhibitors in patients with a recent MI. In our analyses using nationwide register data from 25 466 patients, we found that while most of the patients were receiving high-intensity statins, four out of five were eligible for escalated lipid-lowering therapy according to the new guidelines at 6–10 weeks after an MI. In a simulation in which the use of high-intensity statins and ezetimibe was maximized, the target of a ≥50% reduction in LDL-C and an LDL-C level of <1.4 mmol/L could be reached in about 20% of the patients using high-intensity statin monotherapy and in another 30% of the patients by adding ezetimibe, while half of the patients would still be eligible for treatment with PCSK9 inhibitors as they had not reached the LDL-C target. When we simulated use of alirocumab or evolocumab among those who were eligible for PCSK9 inhibitors, 9 out of 10 patients attained the LDL-C target.
Although adherence to clinical guidelines varies depending on patient and physician preferences, our findings have important implications for the treatment of lipids in patients with MI. Statins and ezetimibe are available at a relatively low cost. For example, the annual cost of treatment per patient in Sweden is 24 Euro with atorvastatin 80 mg and 34 Euro with ezetimibe.8 In contrast, the annual cost of PCSK9 inhibitors (alirocumab 75 mg/150 mg, evolucumab 140 mg) is ∼4555 Euro.8 If half of the patients with MI would be eligible for PCSK9 inhibitors, the financial burden on health systems throughout Europe, and other countries using the ESC/EAS guidelines, may be substantial unless the cost of treatment is reduced. We estimated that the use of alirocumab 75 mg or 150 mg in patients who had not reached the LDL-C target despite maximized use of high-intensity statins and ezetimibe, would result in an average LDL-C reduction of 1.1 mmol/L. An analysis of the ODYSSEY trials showed that each mmol/L reduction in LDL-C with alirocumab or ezetimibe was associated with a 24% relative risk reduction for major adverse cardiovascular events, although this number should be interpreted with caution due to small number of events and differences in follow-up time across studies.9 Assuming an incidence of major adverse cardiovascular events of 2066 per 100 000 patient-years, as observed in previous analyses of SWEDEHEART data (patients aged <75 years who survived until 6–10 weeks post-MI),10 the cost of one prevented event with alirocumab would be around 846 000 Euro in Sweden. Recent analyses in US populations using a higher LDL-C level threshold for treatment with PCSK9 inhibitors (1.8 mmol) than that recommended in the 2019 ESC/EAS guidelines (but also a higher cost for PCSK9 inhibitors) have indicated that the cost of PCSK9 inhibitors must be dramatically reduced in order to reach generally accepted cost-effectiveness thresholds.11,12
This is the first study to assess the eligibility for additional or intensified lipid-lowering therapy based on the LDL-C target of the 2019 ESC/EAS guidelines. Notably, little is known regarding attainment of LDL-C goals and lipid-lowering therapies among patients with a recent MI as previous studies, which have assessed attainment of an LDL-C level of <1.8 mmol/L, have been based on selected samples with limited generalizability,13,14 used data on LDL-C levels collected a long period after the event,15 or included patients with a broad range of cardiovascular diseases or cardiovascular risk factors.4,16 Largely in line with our findings, an analysis of a convenience sample of 1071 patients with acute coronary syndrome from 18 countries showed that only 37% had reached an LDL-C level of <1.8 mmol/L at 4 months after the event, although over 90% received statin therapy13; these findings were similar in analyses limited to the 439 patients from European countries.14 Moreover, in analyses including 7824 patients from 27 countries who were interviewed 0.5–2 years after an elective CABG, elective PCI, or an acute coronary syndrome, only 29.0% of the patients had an LDL-C level of <1.8 mmol/L, even though 34.1% received low- or moderate-intensity statins and 49.9% received high-intensity statins.15
Strengths of our study include the use of nationwide registers and the assessment of LDL-C levels and lipid-lowering therapies of patients at the time of the MI and at 6–10 weeks after discharge; this roughly corresponds to the guideline recommendation to examine LDL-C levels and initiate or modify treatment during the admission and at 4–6 weeks after the event.1 Moreover, we used a simulation model with validated estimates of the LDL-C level reduction of lipid-lowering therapies.4 Importantly, this model accounted for the large interindividual variation in the percentage of LDL-C level reduction, as observed in clinical trials of statins,17,18 ezetimibe,19 and PCSK9 inhibitors.20 Compared to simulations applying an average effect of a given treatment to all patients,16 this approach provides a better estimate of the proportion of patients who become eligible for specific therapies, and their expected outcomes in terms of attainment of the LDL-C target.4
Our study has limitations. Of the 44 890 patients meeting the inclusion criteria 19 424 (43.3%) were excluded, the majority due to missing data on LDL-C levels or no registered follow-up visit. The excluded patients tended to be slightly older, less likely to have ST-elevation MI and to receive PCI and more likely to have had a previous MI, although their lipid levels were similar to those of the study population. Patients who do not attend follow-up visits may have poorer adherence to drugs and lifestyle recommendations and we may thus have underestimated the eligibility for additional lipid-lowering therapy. Moreover, estimates of the effect of LDL-C lowering therapies were taken from clinical trials. As adherence tends to be lower and issues related to tolerability and adverse events might be more common in routine clinical practice,21 we may have overestimated the effect of LDL-C reduction in our simulation models. The proportion of patients eligible for PCSK9 inhibitors increased only slightly when the recorded statin use (or non-use) at the 6–10-week follow-up was considered as the patient’s maximally tolerated statin therapy and when modelling statin intolerance. However, when simulating use of statins and ezetimibe using LDL-C reductions estimated in the study population, this proportion increased such that two out of three patients were eligible for PCSK9 inhibitors. We also assumed that decisions to increase lipid-lowering therapy in response to LDL-C levels were immediate and strictly according to the simulated treatment logics, although these assumptions would not be fully met in routine clinical practice. As such, our study should be considered as a reference point for the implications of the 2019 ESC/EAS guidelines based on well-defined assumptions.
Conclusions
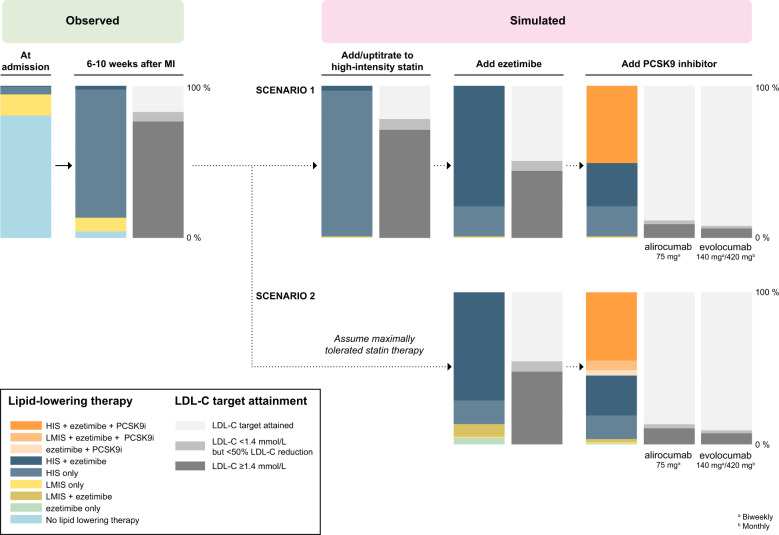
In this analysis of nationwide registers in Sweden, most patients with a recent MI were receiving high-intensity statins. Still, four out of five would be eligible for escalated lipid-lowering therapy according to the 2019 ESC/EAS guidelines at 6–10 weeks after the event. Even with maximized use of high-intensity statins and ezetimibe, our study suggests that half of the patients would be eligible for additional treatment with a PCSK9 inhibitor (Take home figure). Given the current cost of PCSK9 inhibitors, the financial implications of the new guidelines may be substantial, and our findings highlight an urgent need for cost-effectiveness analyses of the 2019 ESC/EAS guideline recommendations.
Take home figure.
Observed and simulated levels of low-density lipoprotein cholesterol, lipid-lowering therapy, and low-density lipoprotein cholesterol target attainment according to the 2019 ESC/EAS dyslipidaemia guidelines.
Funding
A.A. was supported by a research scholarship from The Heart Foundation for Danderyd Hospital (Stiftelsen Hjärtat). P.U. was supported by grants from the Swedish Heart-Lung Foundation and the Swedish Society for Medical Research.
Conflict of interest: A.A. reports institutional grants from MSD, outside the submitted work. T.J. reports grants from Novartis and MSD; personal fees from Astra-Zeneca, MSD, Novartis, Sanofi, Bayer, and Aspen, outside the submitted work. E.H. reports grants, personal fees, and other from Amgen and Sanofi; personal fees from Bayer, outside the submitted work.
Supplementary Material
References
- 1. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 2. Catapano AL, Graham I, Backer GD, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen M-R, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 3. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J 2015;169:906–915.e13. [DOI] [PubMed] [Google Scholar]
- 6. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 7. Reiner Z, Catapano AL, Backer G, De Graham I, Taskinen M-R, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 8.The Dental and Pharmaceutical Benefits Agency, Sweden. https://tlv.se/in-english.html (26 November 2019).
- 9. Ray KK, Ginsberg HN, Davidson MH, Pordy R, Bessac L, Minini P, Eckel RH, Cannon CP. Reductions in atherogenic lipids and major cardiovascular events. Circulation 2016;134:1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohm J, Hjemdahl P, Skoglund PH, Discacciati A, Sundström J, Hambraeus K, Jernberg T, Svensson P. Lipid levels achieved after a first myocardial infarction and the prediction of recurrent atherosclerotic cardiovascular disease. Int J Cardiol 2019;296:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Kazi DS, Penko J, Coxson PG, Guzman D, Wei PC, Bibbins-Domingo K. Cost-effectiveness of alirocumab a just-in-time analysis based on the ODYSSEY outcomes trial. Ann Intern Med 2019;170:221–229. [DOI] [PubMed] [Google Scholar]
- 12. Kazi DS, Penko J, Coxson PG, Moran AE, Ollendorf DA, Tice JA, Bibbins-Domingo K. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial. JAMA 2017;318:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gitt AK, Lautsch D, Ferrières J, Ferrari GM, De Vyas A, Baxter CA, Bash LD, Ashton V, Horack M, Almahmeed W, Chiang F-T, Poh KK, Brudi P, Ambegaonkar B. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: results from the Dyslipidemia International Study II. Atherosclerosis 2017;266:158–166. [DOI] [PubMed] [Google Scholar]
- 14. Ferrieres J, Ferrari GD, Hermans MP, Elisaf M, Toth PP, Horack M, Brudi P, Lautsch D, Bash LD, Baxter CA, Ashton V, Ambegaonkar B, Gitt AK. Predictors of LDL-cholesterol target value attainment differ in acute and chronic coronary heart disease patients: results from DYSIS II Europe. Eur J Prev Cardiol 2018;25:1966–1976. [DOI] [PubMed] [Google Scholar]
- 15. Backer GD, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Ž, Rydén L, Tokgözoğlu L, Wood D, Bacquer DD. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019;285:135–146. [DOI] [PubMed] [Google Scholar]
- 16. Virani SS, Akeroyd JM, Smith SC, Al-Mallah M, Maddox TM, Morris PB, Petersen LA, Ballantyne CM, Grundy SM, Stone NJ. Very high-risk ASCVD and eligibility for nonstatin therapies based on the 2018 AHA/ACC cholesterol guidelines. J Am Coll Cardiol 2019;74:712–714. [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM, Mora S, Rose L. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J 2016;37:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, Larosa JC, Waters DD, Demicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Ridker PM, Grundy SM, Kastelein J. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Descamps O, Tomassini JE, Lin J, Polis AB, Shah A, Brudi P, Hanson ME, Tershakovec AM. Variability of the LDL-C lowering response to ezetimibe and ezetimibe + statin therapy in hypercholesterolemic patients. Atherosclerosis 2015;240:482–489. [DOI] [PubMed] [Google Scholar]
- 20. Qamar A, Giugliano RP, Keech AC, Kuder JF, Murphy SA, Kurtz CE, Wasserman SM, Sever PS, Pedersen TR, Sabatine MS. Interindividual variation in low-density lipoprotein cholesterol level reduction with evolocumab: an analysis of FOURIER trial data. JAMA Cardiol 2019;4:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vonbank A, Drexel H, Agewall S, Lewis BS, Dopheide JF, Kjeldsen K, Ceconi C, Savarese G, Rosano G, Wassmann S, Niessner A, Schmidt TA, Saely CH, Baumgartner I, Tamargo J. Reasons for disparity in statin adherence rates between clinical trials and real-world observations: a review. Eur Heart J Cardiovasc Pharmacother 2018;4:230–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.