Abstract
Objective
IPX203 is an investigational oral extended-release capsule formulation of carbidopa-levodopa (CD-LD). The aim of this study was to characterize the single-dose pharmacodynamics, pharmacokinetics, and safety of IPX203 in subjects with advanced Parkinson disease compared with immediate-release (IR) CD-LD and extended-release CD-LD (Rytary).
Methods
This was a randomized, open-label, rater-blinded, multicenter, single-dose crossover study. Blinded clinicians assessed subject's motor state and Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part III scores for up to 10 hours postdose. Duration of effect was determined using improvement thresholds in the MDS-UPDRS part III.
Results
Levodopa concentrations increased rapidly and similarly across all 3 treatments and were sustained for a longer duration after IPX203 dosing. All treatments exhibited a rapid onset of pharmacodynamic effect, whereas IPX203 had a significantly longer duration of effect based on MDS-UPDRS part III scores compared with IR CD-LD (P < 0.0001) and Rytary (P ≤ 0.0290). IPX203 had a 2.7-hour advantage over IR CD-LD (P < 0.0001) and a 0.9-hour advantage over Rytary in “off” time (P = 0.023) and in “good on” time (2.6 hours more than IR CD-LD, P < 0.0001; 0.9 hours more than Rytary, P = 0.0259) as measured by the Investigator Assessment of Subject's Motor State. Subjects were 77% more likely to require rescue following IR CD-LD treatment compared with IPX203 (hazard ratio, 0.23; P < 0.0001). More subjects reported treatment-emergent adverse effects during IR CD-LD (28.0%) and IPX203 (19.2%) than during Rytary (8.0%) treatment.
Conclusions
Compared with Rytary and IR CD-LD, IPX203 had a longer pharmacodynamic effect consistent with LD pharmacokinetics for the 3 treatments. The safety and tolerability of IPX203 were similar to those of IR CD-LD and Rytary.
Key Words: IPX203, levodopa, Parkinson disease, pharmacodynamics
Parkinson disease (PD) is characterized pathologically by progressive degeneration of dopaminergic nigrostriatal neurons and depletion of dopamine and manifests clinically in the core symptoms of rigidity, tremor, bradykinesia, postural instability, and gait disturbance. Levodopa (LD) in combination with an aromatic amino acid decarboxylase inhibitor such as carbidopa (CD) is considered a highly effective long-term symptomatic treatment of PD, and virtually all patients require this therapy during the course of their disease. Patients with advanced PD typically require frequent administration of immediate-release (IR) LD.1
As PD progresses and nigrostriatal dopaminergic transmission deteriorates, the brain becomes dependent on exogenous LD. Patients with advanced PD are typically treated with frequent administrations of oral IR CD-LD formulations,1 which result in fluctuating and variable plasma LD levels primarily due to its short half-life, variability in gastric emptying, and absorption limited to the small intestine.2 Most patients with advanced PD using short-acting dopaminergic therapies eventually develop motor complications, including slowness in turning “on,” end-of-dose wearing-off, unpredictable “on-off” motor fluctuations, and peak-dose dyskinesias.3,4 These motor complications are associated with episodes of poor mobility, slowness, stiffness, postural instability, and profound disability to perform activities of daily living.5 Increasingly, nonmotor symptoms are also being recognized as troubling and disabling to PD patients.4 Motor fluctuations are generally managed by optimization of dosing intervals, use of extended-release (ER) CD-LD formulations, and by the use of combination regimens that include CD-LD, longer-acting dopamine agonists, enzyme inhibitors (catechol-O-methyltransferase and monoamine oxidase), and amantadine.6 Eventually, for some patients, invasive therapies (direct continuous duodenal infusion and deep brain stimulation) are recommended when motor complications become refractory to standard treatments.7–9
IPX203 is a new investigational ER formulation of CD-LD that is being developed for the treatment of patients with PD. IPX203 was designed to rapidly deliver therapeutic LD plasma concentrations and to maintain them within the therapeutic range for a longer duration than current orally administered CD-LD products with minimal peak to trough fluctuations. IPX203 is designed to be dosed every 8 hours. The formulation has an improved LD bioavailability compared with ER CD-LD capsules (Rytary, Numient; Impax Laboratories, LLC, Hayward, CA), and has dose-proportional strengths, which should facilitate dosing.
The objectives for this investigation were to characterize the single-dose pharmacodynamics and pharmacokinetics (PK) of IPX203 in patients with advanced PD and to compare the profile to IR CD-LD and Rytary.
MATERIALS AND METHODS
This randomized, open-label, rater-blinded, multicenter, 3-treatment, 3-period, single-dose crossover study was designed to assess the pharmacodynamics/efficacy, PK, and safety of a single dose of IPX203 compared with IR CD-LD and Rytary. Approximately 26 qualified subjects with advanced PD and motor fluctuations who were on a stable dose of IR CD-LD were randomized to 1 of 3 dosing sequences to receive IR CD-LD, Rytary, and IPX203.
Study Participants
Eligible subjects had a diagnosis of idiopathic PD by UK Parkinson Disease Brain Bank criteria10 with onset after age 40 years, a Hoehn and Yahr5 stage 2 to 4, a Montreal Cognitive Assessment score of at least 24,11 and a total score of at least 20 units on part III of the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) in the “off” state12 and that was at least 25% or 10 units greater than in the “on” state. Subjects were required to be on a stable dose of LD for at least 4 weeks and required a morning IR LD dose of 100 to 250 mg, a total IR LD daily dose of at least 400 mg but not exceeding a maximum daily LD dose of 1600 mg during waking hours, and an IR CD-LD dosing frequency of at least 4 doses per day excluding nighttime dosing. By history, subjects were responsive to LD, typically experiencing an “on” response with the first morning dose of IR CD-LD. In addition, subjects experienced daily “wearing off” episodes with periods of bradykinesia and rigidity and an “off” state upon awakening on most mornings; the first morning dose of IR CD-LD usually lasted less than 4 hours, typically “wearing off” prior to the next dose, or the subject took a second dose of PD medication prior to 4 hours to avoid an “off” state. Based on a 3-day PD diary,13 subjects experienced an average of at least 2 hours of “off” time per day with at least 1 hour “off” on each day. Exclusion criteria included prior functional neurosurgical treatment for PD or history of a recent seizure or epilepsy, psychosis, peptic ulcer disease or gastrointestinal hemorrhage or any medical condition or prior surgery that would interfere with LD absorption. Subjects were also excluded if they had received any morning dose of controlled-release or ER LD, additional CD or benserazide, any doses of catechol-O-methyltransferase inhibitors or nonselective monoamine oxidase inhibitors, apomorphine, or dopaminergic-blocking agents within the previous 4 weeks.
Study Design
Subjects were randomly assigned to 1 of 3 sequences to receive IPX203, IR CD-LD, and Rytary. During each treatment, subjects presented to the clinic after fasting and in the “off” state with their last dose of CD-LD and other PD medication taken no later than 10 pm the previous evening. Subjects received a single dose of their study medication in the clinic, with washout periods of approximately 1 week between treatments. Subjects continued taking IR CD-LD between study visits. Pharmacodynamic, PK, and safety assessments were conducted for 10 hours after each treatment. If a subject experienced an “off” state for more than 3 consecutive hours, the subject could receive rescue medication with his/her usual CD-LD treatment and no further pharmacodynamic or PK assessments were conducted for that treatment visit. Safety evaluations were conducted throughout the study duration.
Selection of Doses
The dose of IR CD-LD was the same as the subject's prestudy (stable) morning baseline dose. The dose of IPX203 and Rytary was chosen based on previous PK findings in healthy subjects and was intended to attain peak LD plasma concentrations (Cmax) that were approximately ±20% of the Cmax obtained after administration of the subjects' IR CD-LD dose. A 100-mg dose of IR CD-LD converted to 360 mg of IPX203 and to 340 mg of Rytary; doses greater than 100 mg of IR CD-LD were converted to IPX203 or Rytary on a proportional basis.
Ethical Conduct
The study was reviewed and approved by the institutional review boards of the participating institutions. It was conducted in compliance with the Declaration of Helsinki, the clinical protocol, and Good Clinical Practice guidelines promulgated by the International Conference of Harmonization. All subjects provided written informed consent. The study was registered with a clinical trials registry (ClinicalTrials.gov identifier NCT02271503).
Pharmacodynamic and PK Assessments
The Investigator Assessment of Subject's Motor State is similar to the patient Hauser Diary13 but modified for this 10-hour series of in-clinic assessments by qualified site staff who were blinded to treatment assignment. Assessments were performed predose and half-hourly up to 10 hours postdose, and motor states were assigned as asleep, “off,” “on” without dyskinesia, “on” with nontroublesome dyskinesia, or “on” with troublesome dyskinesia. The state of “good on” was defined as the sum of “on” without dyskinesia and “on” with nontroublesome dyskinesia.14 The MDS-UPDRS part III (motor examination) was assessed predose and hourly up to 10 hours postdose by a qualified clinician blinded to treatment assignment. In addition, plasma samples for measurement of LD concentrations were obtained for up to 10 hours.
Statistical Analysis
Statistical analyses comparing pharmacodynamic responses by treatment were conducted in subjects who received all 3 treatments. For analyses purposes, all time points after rescue were imputed “off” for the Investigator Assessment of Subject's Motor State and baseline observation carried forward for MDS-UPDRS part III. The primary pharmacodynamics end point was the mean total “off” time, with the primary comparison being IPX203 and IR CD-LD. The total duration of each motor state based on the Investigator Assessment of Subject's Motor State was compared using a mixed-model analysis of variance with treatment, period, and sequence as fixed effects and subject-within-sequence as a random effect. In addition, treatment differences in the proportion of subjects with “good on” state by various time durations were compared. Postdose treatment differences in the MDS-UPDRS part III total scores were analyzed using a mixed-model analysis of covariance with treatment, period, and sequence as fixed effects, baseline as covariate, and subject-within-sequence as a random effect. Duration-of-effect results, that is, duration of improvements in MDS-UPDRS part III score of at least 4, 7, or 13 points from treatment predose average, were summarized. Time to rescue across the 3 treatment groups was analyzed using a Cox regression model adjusting for treatment, period, and sequence. Statistical analyses were conducted using SAS version 9.4 (Cary, NC).
Safety Assessments
Adverse events (AEs) were monitored throughout the study. Adverse events were assessed in terms of severity (mild, moderate, severe) and relationship to study drug. Additional safety assessments included physical examinations, vital signs, electrocardiograms, and clinical laboratory tests.
RESULTS
Patient Disposition and Baseline Characteristics
A total of 46 subjects were screened, and 26 subjects were randomized at 10 study sites in the United States; 25 subjects (96.2%) completed all 3 treatments. Baseline demographic and clinical characteristics of the cohort of subjects who completed all treatments included a mean age of 66.2 years, mean age at PD onset at 57.4 years, and an average duration of PD of 9 years, and all subjects were Hoehn and Yahr stage 2 or 3. Participants were primarily white (92.0%) and equally split by sex (52.0% male). The mean IR LD total daily dose was 745 mg, and on average subjects received 4.8 doses of IR CD-LD per day. Subjects had a mean “off” time of 6.1 hours per day based on the 3-day diary prior to the first treatment visit.
Levodopa Dose and PK
The mean LD doses administered to subjects who completed all 3 treatments were 168, 587, and 538 mg for IR CD-LD, IPX203, and Rytary, respectively. Levodopa plasma concentrations increased rapidly after dosing, and peak LD concentrations were generally comparable (within approximately ±20%) across treatments (2492, 3161, and 2839 ng/mL for IR CD-LD, IPX203, and Rytary, respectively). Levodopa plasma concentrations were sustained above 50% of peak LD concentrations for 1.9, 4.7, and 3.9 hours for IR CD-LD, IPX203, and Rytary, respectively. The bioavailability of LD from IPX203 and Rytary relative to IR CD-LD based on the ratio of geometric mean AUC values was 85.5% and 71.8%, respectively.
Investigator Assessment of Subject Motor State
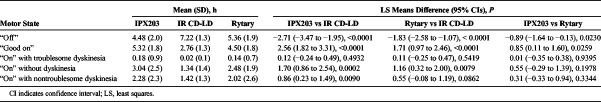
The mean “off” time was 4.5 hours following IPX203 treatment, 7.2 hours following IR CD-LD, and 5.4 hours following Rytary, demonstrating a 2.7-hour advantage for IPX203 over IR CD-LD (P < 0.0001) and a 0.9-hour advantage over Rytary (P = 0.023) (Table 1). The reduction in “off” time with IPX203 was accompanied by a corresponding increase in “good on” time—2.6 hours more than for IR CD-LD (P ≤ 0.0001) and 0.9 hour more than for Rytary (P ≤ 0.0259). Following treatment with IPX203, a significantly larger proportion of subjects achieved at least 4, 5, 6, and 7 hours of “good on” time compared with IR CD-LD and Rytary (Fig. 1).
TABLE 1.
Investigator Assessment of Subject's Motor State Following Single Doses of IPX203, IR CD-LD, and Rytary in Subjects With Advanced Parkinson Disease
FIGURE 1.
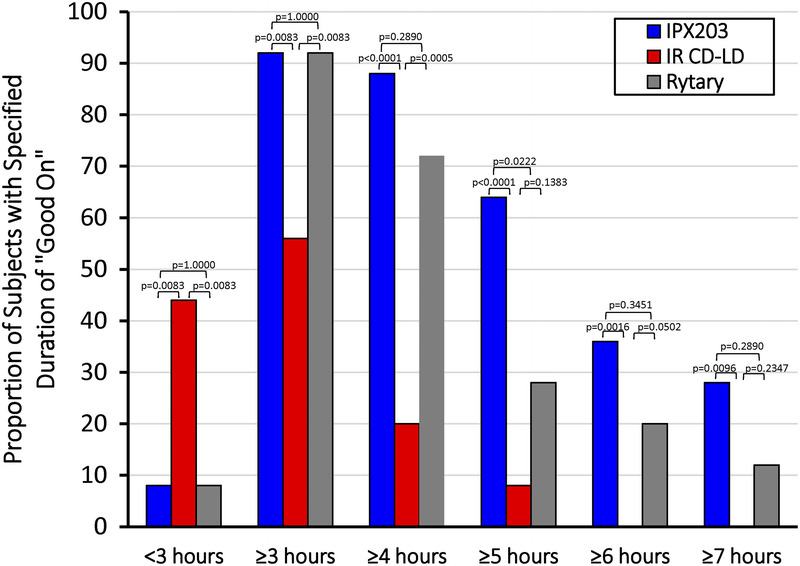
Proportion of subjects with “good on” of various time durations following single doses of IPX203, IR CD-LD, and Rytary. The calculated state of “good on” is the sum of “on” without dyskinesia and “on” with nontroublesome dyskinesia.
Movement Disorders Society Unified Parkinson's Disease Rating Scale Part III
After IPX203 treatment, subjects experienced significantly greater improvement (decrease) from average predose MDS-UPDRS part III scores over 10 hours compared with IR CD-LD (−12.70 vs −6.62 units; P < 0.0001) and also compared with Rytary (−12.70 vs −9.33 units; P = 0.0333). Improvements in MDS-UPDRS part III scores were similar for all 3 treatments during the first 2 hours (Fig. 2), consistent with the PK findings and suggestive of a similar onset of effect. When examined hour-by-hour, the decrease in MDS-UPDRS part III scores following IPX203 was significantly improved compared with IR CD-LD from 3 to 10 hours (all P ≤ 0.029; Fig. 2), and Rytary showed a significant difference compared with IR CD-LD from 3 to 5 hours (all P ≤ 0.0042). IPX203 showed significant differences compared with Rytary from 5 to 10 hours (all P ≤ 0.0352) except at 7 hours where the improvement did not reach statistical significance (P = 0.0601). Based on MDS-UPDRS part III results, IPX203 had a significantly longer duration of effect compared with IR CD-LD (P < 0.0001) and compared with Rytary (P ≤ 0.0290), measured by the duration of 4-, 7-, and 13-point improvements from average predose value (Fig. 3).
FIGURE 2.
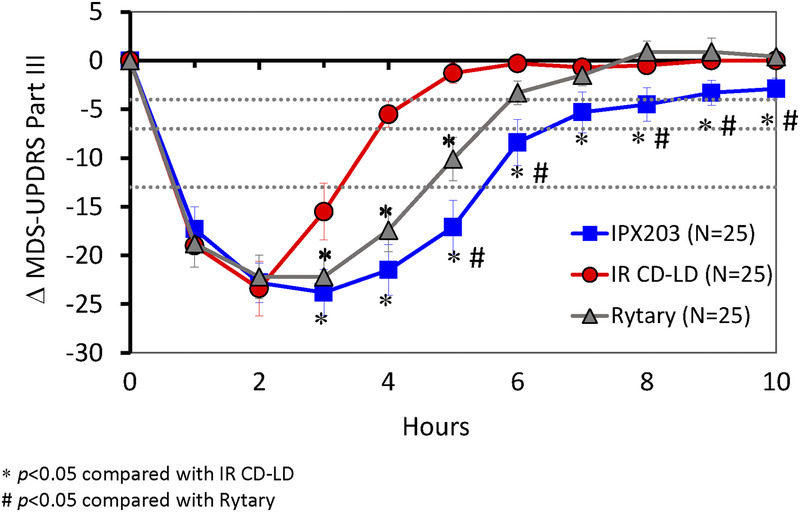
Change in MDS-UPDRS part III following single doses of IPX203, IR CD-LD, and Rytary. Horizontal dashed lines represent 4-, 7-, and 13-point improvements from average predose value.
FIGURE 3.
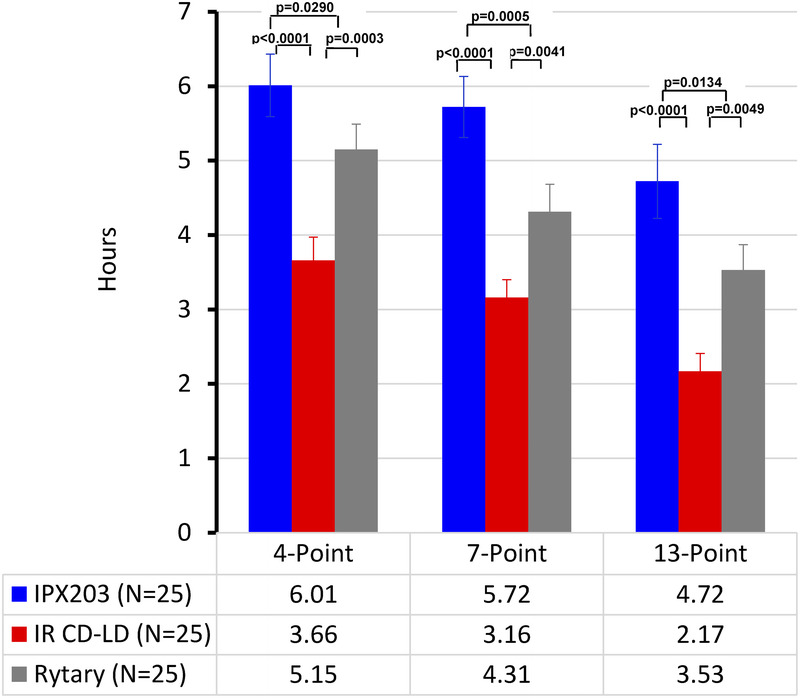
Duration of effect based on 4-, 7-, or 13-point improvements in MDS-UPDRS part III Score.
Use of Rescue Medication
Subjects were 77% more likely to require rescue during IR CD-LD treatment compared with IPX203 (hazard ratio, 0.23; P < 0.0001).
Safety and Tolerability
All subjects who received at least 1 treatment were included in the safety evaluation. No serious AEs were reported, and none of the AEs led to discontinuation from the study. More subjects reported treatment-emergent AEs (TEAEs) following IR CD-LD (28.0%) and IPX203 (19.2%) than following Rytary (8.0%). The majority of TEAEs were categorized as mild, and none were classified as severe. Treatment-emergent AEs reported by 2 or more subjects included dizziness (1 subject each during IPX203 and Rytary treatment and 2 subjects during IR CD-LD treatment), nausea (2 subjects during IR CD-LD treatment), and hypertension (2 subjects each during IPX203 and IR CD-LD treatment and 1 subject during Rytary). There were no clinically meaningful changes in laboratory values or in vital signs (systolic or diastolic blood pressure or heart rate). Within 6 hours after dosing, median changes from predose in standing orthostatic systolic and diastolic blood pressure and heart rate were negligible and similar between the 3 treatments. Overall, IPX203 was generally well tolerated.
DISCUSSION
IPX203 is being developed as an oral ER CD-LD formulation to provide rapid initial absorption of LD and then to sustain LD plasma concentrations for an extended period compared with currently available CD-LD products. The current study compared the single-dose PK and rater-blinded pharmacodynamics profiles of IPX203, IR CD-LD, and Rytary in patients with advanced PD experiencing motor fluctuations.
The PK data demonstrated that the initial increase in LD plasma concentrations from IPX203 was comparable to that seen with IR CD-LD and Rytary, and pharmacodynamic improvements were likewise similar between treatments during the first 2 hours postdose. Following the initial increase, LD concentrations were sustained for longer with IPX203 than with IR CD-LD or Rytary. Overall, IPX203 sustained LD concentrations above 50% of the peak for approximately 0.8 hour longer than Rytary and 2.8 hours longer than IR CD-LD. In addition, IPX203 demonstrated improved LD bioavailability compared with Rytary.
There was good concordance between LD PK and pharmacodynamic responses as assessed by the Investigator Assessment of Subject's Motor State and changes in MDS-UPDRS part III scores. A rapid onset of effect following treatment with IPX203 was demonstrated by the clinician-rated MDS-UPDRS part III scores. The Investigator Assessment of Subject's Motor State demonstrated significant improvements in “off” time and in “good on” time following IPX203, both of which approximated 2.7 hours and 0.9 hour compared with IR CD-LD and Rytary, respectively. The IPX203-related improvements in the Investigator Assessment of Subject's Motor State “on” and “off” times were independently corroborated by the unequivocal improvements in the duration of effect as measured with prespecified improvement thresholds in MDS-UPDRS part III scores. The PK and pharmacodynamic advantages of IPX203 may have also manifested in fewer subjects requiring rescue treatment and a longer time to rescue for IPX203 compared with IR CD-LD and Rytary.
In this study, patients were administered their individualized usual morning dose of IR CD-LD in order to ensure that study participants achieved an adequate “on” state and to allow an equitable comparison. The doses of Rytary and of IPX203 were based on their morning dose of IR CD-LD using a dose-conversion algorithm intended to result in LD concentrations that were comparable to the peak IR CD-LD dose. Because the different subjects received different doses of CD-LD, it is difficult to compare the LD concentrations across patients to assess variability without accounting for differences in dose. Historically, some studies have administered fixed doses of CD-LD to patients. Although this study design allows comparison of the PK, a limitation to this approach is that some subjects may be underdosed or overdosed. We have taken the approach of using an individualized dose in this study because in our view this allows the most rigorous assessment of the pharmacodynamics, which ultimately is the focus of patient therapy. The mean LD dose during IR CD-LD treatment in this study was 168 mg, which is similar to the LD dose (165 mg) for IR CD-LD noted in a previous comparative pharmacodynamics study.15 In this study comparing the PK of Rytary with IR CD-LD, peak concentrations of LD were 3000 and 2360 ng/mL, for mean LD doses of 664 and 165 mg for Rytary and IR CD-LD.15 This corresponds to 2431 and 2403 ng/mL for mean LD doses 538 and 165 mg for Rytary and IR CD-LD noted in the present study. The estimated LD concentrations using data from this published study are very comparable to the LD concentrations noted for Rytary and IR CD-LD in the current study.
Overall, there were no differences in the safety and tolerability between the 3 treatments. The most common TEAEs were dizziness, nausea, and hypertension.
This exploratory crossover pharmacodynamic study in patients with PD had some methodological limitations. This was a single-dose study in a relatively small number of patients in which the identity of IR CD-LD, Rytary, and IPX203 was not blinded to the subjects. The doses of Rytary and of IPX203 were based on a dose-conversion algorithm intended to result in LD concentrations that were comparable to the peak IR CD-LD dose; however, the doses of these treatment were not optimized with a titration. Hence, the results from this single-dose study may not reflect the outcomes from multiple dosing where patients are able to adjust their treatment regimen to optimize efficacy and tolerability. This study used the Investigator Assessment of Motor State pharmacodynamic measure rather than a typical 3-day Hauser PD diary used in a longer-term efficacy study.
Despite these limitations, the results from this single-dose study are very informative and present comparative pharmacodynamic data in the patient population of interest, supporting further investigation in a multiple-dose study.
In summary, IPX203 was well tolerated in this single-dose study in patients with advanced PD with motor fluctuations. IPX203 provided a rapid onset of effect, comparable to IR CD-LD and Rytary, and improved bioavailability compared with Rytary. IPX203 treatment resulted in a longer duration of effect compared with IR CD-LD and Rytary.
ACKNOWLEDGMENTS
The authors acknowledge study investigators Jason Aldred, MD, Victor Biton, MD; John Campbell, MD; Aaron Ellenbogen, DO, MPH; Steven Gunzler, MD; Robert Hauser, MD, MBA, FAAN; Stuart Isaacson, MD; John Morgan, MD, PhD; Omid Omidvar, MD; Holly Shill, MD; Mark Stacy, MD; and Daniel Truong, MD, for their contributions to the conduct of this study; Phillip Dinh for statistical analysis and contributing to the drafting of the manuscript; and Karen Getz of Impax Laboratories for medical writing support in drafting the manuscript.
Footnotes
Conflicts of Interest and Source of Funding: N.B.M., S.K., and S.G. are employees of Impax Laboratories, LLC, and hold company stocks. A.M. and R.R. were employees of Impax Laboratories, LLC, during study execution and initial manuscript development and submission. The study was funded by Impax Laboratories, LLC, from the design of the studies through writing of the final publication.
REFERENCES
- 1.Verhagen Metman L, Stover N, Chen C, et al. Gastroretentive carbidopa/levodopa, DM-1992, for the treatment of advanced Parkinson's disease. Mov Disord 2015;30:1222–1228. [DOI] [PubMed] [Google Scholar]
- 2.Nyholm D, Askmark H, Gomes-Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol 2003;26:156–163. [DOI] [PubMed] [Google Scholar]
- 3.Schapira AH, Emre M, Jenner P, et al. Levodopa in the treatment of Parkinson's disease. Eur J Neurol 2009;16:982–989. [DOI] [PubMed] [Google Scholar]
- 4.Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord 2015;30:80–89. [DOI] [PubMed] [Google Scholar]
- 5.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 6.LeWitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med 2008;359:2468–2476. [DOI] [PubMed] [Google Scholar]
- 7.Kurlan R, Nutt JG, Woodward WR, et al. Duodenal and gastric delivery of levodopa in Parkinsonism. Ann Neurol 1998;23:589–595. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson D, Nyholm D, Aquilonius SM. Duodenal levodopa infusion in Parkinson's disease: long-term experience. Acta Neurol Scand 2001;104:343–348. [DOI] [PubMed] [Google Scholar]
- 9.Nyholm D, Aquilonius SM. Levodopa infusion therapy in Parkinson disease: state of the art in 2004. Clin Neuropharmacol 2004;27:245–256. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 12.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 13.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 2000;23:75–81. [DOI] [PubMed] [Google Scholar]
- 14.Hauser RA, Deckers F, Lehert P. Parkinson's disease home diary: further validation and implications for clinical trials. Mov Disord 2004;19:1409–1413. [DOI] [PubMed] [Google Scholar]
- 15.Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson's disease. Mov Disord 2011;26:2246–2252. [DOI] [PubMed] [Google Scholar]