Abstract
Objectives:
To develop a clinically actionable predictive model to quantitate the risk of estimated glomerular filtration rate decline to ≤ 45 ml/min/1.73m2 following radical nephrectomy in order to better inform decisions between radical and partial nephrectomy.
Patients and Methods:
Our prospectively maintained kidney cancer registry was reviewed for patients with pre-operative estimated glomerular filtration rate > 60 ml/min/1.73m2 who underwent radical nephrectomy for a localized renal mass. New baseline renal function was indexed. We used multivariable logistic regression to develop a predictive nomogram and evaluated it utilizing receiver operating characteristic analysis. Decision curve analysis assessed the net clinical benefit.
Results:
668 patients met inclusion criteria. 183 patients (27%) experienced estimated glomerular filtration rate decline to ≤ 45 ml/min/1.73m2. On multivariable analysis, increasing age (p=0.001), female gender (p<0.001), and increasing pre-operative creatinine (p<0.001) were associated with functional decline. We constructed a predictive nomogram that included these variables in addition to comorbidities with a known association with kidney disease but found that a simplified model excluding comorbidities was equally robust (cross-validated area under receiver operating curve was 0.78). Decision curve analysis demonstrated the net-clinical benefit at probabilities >~11%.
Conclusions:
The decision to perform radical vs. partial nephrectomy is multifaceted. We provide a simple quantitative tool to help identify patients at risk of a post-operative eGFR of ≤ 45 ml/min/1.73m2 who may be stronger candidates for nephron preservation.
Keywords: renal cell carcinoma, radical nephrectomy, chronic kidney disease, AUA guidelines, estimated glomerular filtration rate, nomogram
1. Introduction
The American Urological Association (AUA) Guideline for Management of Localized Renal Masses suggests that an anticipated estimated glomerular filtration rate (eGFR) below 45 ml/min/1.73m2 upon renal unit removal should help guide decision-making between radical nephrectomy (RN) or partial nephrectomy (PN) in patients for whom risk trade-offs between RN and PN are uncertain.[1] Delayed risks associated with renal functional decline following RN weighed against immediate higher perioperative risks of PN underscore the complexity of decision-making surrounding the optimal surgical management of localized renal masses,[2, 3] particularly those larger than 4 cm.[4] The AUA Guideline states that RN is “preferred” in the setting of high tumor complexity, a lack of pre-existing renal dysfunction, and – notably – where “new baseline eGFR will likely be >45 ml/min/1.73m2.”[1] Long-term preservation of renal function and potential chronic kidney disease (CKD) associated adverse events[5, 6] must be appropriately balanced against immediate perioperative risks associated with complex PN, especially in the frail co-morbid patient.[7] Yet, a clinically-useful predictive tool for anticipated eGFR decline following RN in patients with a normal contralateral kidney is not currently available. As such, we sought to determine clinical factors predictive for eGFR decline ≤45 ml/min/1.73m2 among patients with normal preoperative renal function (eGFR >60 ml/min/1.73m2) undergoing RN and to develop a predictive nomogram that provides actionable information to contextualize nuanced surgical decision-making.
2. Patients & Methods
2.1. Study population
We reviewed our prospectively maintained Institutional Review Board-approved Fox Chase Cancer Center kidney cancer registry. Patients undergoing RN between 1990 and 2015 for suspected renal cell carcinoma at a tertiary referral center were indexed (n=1065). Procedures were performed by experienced urologic oncologists and included minimally-invasive and open techniques according to surgeon discretion. Patients with incomplete eGFR or co-morbidity data were excluded (n=109).
2.2. Variables and outcome definition
Demographic and comorbidity data was indexed and included patient age, gender, race, presence of diabetes (ICD 9 code 250 [Diabetes Mellitus]), peripheral vascular disease (ICD 9 code 443.9 [Peripheral Vascular Disease], and hypertension (ICD 9 code 401.9 [unspecified hypertension]). A history of cardiac disease was indexed using the following codes: cardiac disease (ICD 9 code 427.31 [atrial fibrillation], 427.9 [Cardiac dysrhythmia], 429.3 [cardiomegaly], 414 [coronary atherosclerosis of native or graft vessel], 426 [atrioventicular block], 412 [old myocardial infarction]).
Serum creatinine (sCr) was extracted from the database and eGFR was calculated using the Modification of Diet in Renal Disease formula.[8] eGFR was calculated preoperatively and postoperatively. New baseline eGFR was defined as the last reported eGFR within a year of surgery.
Patients with preoperative eGFR ≥60 ml/min/1.73m2 were our cohort of interest (n=668). The outcome of the study was a new baseline post-operative eGFR < 45 ml/min/1.73m2. eGFR values utilized were the last laboratory evaluations within the one-year post-operative period. For the ease of presentation, we omit the “ml/min/1.73m2” units for much of the remainder of this manuscript.
2.3. Statistical analysis
We used Fisher’s Exact tests to assess the relationship of comorbidities (cardiac disease/coronary artery disease [CAD], hypertension [HTN], peripheral vascular disease [PVD], and diabetes mellitus [DM] coded as binary yes/no variables) with patients categorical preoperative eGFR status (preoperative eGFR ≥ 60, 45–60, and ≤45). We used multiple logistic regression analysis to determine clinical factors predicting postoperative the new baseline eGFR ≤45 among those with preoperative eGFR ≥ 60. We used a logistic regression, rather than a time-to-event model, since the outcome was a binary measure at the last follow-up time within one year of surgery. We did not evaluate the time when eGFR fell below 45, as many individuals had short term eGFR loss which rebounded by the end of the year post-surgery (183, 27.4%, had such GFR decline on the last assessment within a year, but an additional 123 [18.4%] had initial GFR decline which increased to > 45 by the end of the first year) (Supplemental figure 1).
We assessed logistic model fit both by the full sample area under the receiver operator curve (ROC) and by 20-fold cross-validation of the area under (AUC) the ROC curve. We used Decision Curve Analysis (DCA) to evaluate the net benefit that our models would provide when investigating interventions to reduce eGFR decline outcomes in this population; specifically, the net benefit of preserving eGFR by not removing the whole kidney. We entered continuous variables into the models via restricted cubic spline terms with three knots at the empirical cut-points. The use of splines is standard in nomogram development.[9]
As a sensitivity analysis to confirm the strength of our model, we also examined the model using a logistic regression estimated by generalized estimating equations to account for the repeated measures nature of the model. In the GEE estimated model, we entered time via restricted cubic splines and interacted the predictors with each time spline term.
Nominal P-values of 0.05 were used as the criteria for statistical significance. Statistical analysis, including the decision curve analysis, was conducted using STATA (Statacorp, College Station, Texas) software.
3. Results
3.1. Pre-operative patient characteristics
Of 1065 patients undergoing RN, 956 had adequate pre-operative eGFR and comorbidity data for analysis. Of these, 668 patients had a pre-operative eGFR ≥60 (median 81; IQR 72–95). The median age was 61 yrs (IQR 52–69 yrs) with the cohort being 64% (n=430) male and 9% (n=57) black. There was no difference between median pre-operative eGFR in men compared to women (83 vs 81, p=0.3). The median tumor diameter at presentation was 6 cm (IQR 4–9.5 cm). The incidence of patient reported CAD, HTN, PVD, and DM was 17% (n=112), 56% (n=373), 1% (n=9), and 16% (n=105), respectively (Table 1).
TABLE 1:
Univariate and Multivariable Model predicting decline in eGFR ≤45 among patients with preoperative eGFR ≥60
| Clinical Variable (n = 668) | Univariate Model |
Final Multivariable Model |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio (HR) | 95% HR Confidence Limits | p-value | Hazard Ratio (HR) | 95% HR Confidence Limits | p-value | |
| Age | Linear Term | Spline Coefficient 11.07 | ||||
| Median 61 yrs (IQR 52–69 yrs) | Only 1.05 | 1.03–1.06 | <0.001 | 1.03–1.12 | 0.001* | |
| Spline Coefficient 20.99 | 0.95–1.03 | 0.6* | ||||
| Tumor size | Linear Term | Spline Coefficient 11.02 | ||||
| Median 6 cm (IQR 4–9.5 cm) | Only 0.97 | 0.93–1.02 | 0.2 | 0.89–1.18 | 0.8* | |
| Spline Coefficient 20.95 | 0.77–1.16 | 0.6* | ||||
| Race | Tab | |||||
| Non-Black (n=611; 91%) | Ref | Ref | ||||
| Black (n=57; 9%) | 1.62 | 0.92–2.85 | 0.097 | 0.95 | 0.48–1.89 | 0.9 |
| Gender | ||||||
| Male (n=430; 64%) | Ref | Ref | ||||
| Female (n=238; 36%) | 1.06 | 0.74–1.51 | 0.7 | 5.58 | 3.20–9.75 | <0.001 |
| Cardiac Disease | ||||||
| No (n=556; 83%) | Ref | Ref | ||||
| Yes (n=112; 17%) | 1.68 | 1.10–2.59 | 0.017 | 1.05 | 0.63–1.75 | 0.9 |
| Hypertension | ||||||
| No (n=295; 44%) | Ref | Ref | ||||
| Yes (n=373; 56%) | 1.54 | 1.08–2.18 | 0.016 | 0.93 | 0.62–1.41 | 0.7 |
| Peripheral Vascular Disease | ||||||
| No (n=659; 99%) | Ref | Ref | ||||
| Yes (n=9; 1%) | 5.45 | 1.35–22.0 | 0.017 | 4.89 | 1.06–22.59 | 0.042 |
| Diabetes | ||||||
| No (n=563; 84%) | Ref | Ref | ||||
| Yes (n=105; 16%) | 1.40 | 0.90–2.19 | 0.14 | 1.24 | 0.72–2.13 | 0.4 |
| Pre-operative Creatinine | Spline Coefficient 11902 | |||||
| Median 0.9 mg/dL (IQR 0.8–1.05) | Linear term 38.7 | 14.1–106.4 | <0.001 | 127–28,596 | <0.001 * | |
| Spline Coefficient 20.19 | 0.0009–37.5 | 0.6* | ||||
p-values for joint tests that both spline coefficients are equal to zero: Age p<0.001, Size p=0.8, Pre-operative Creatinine p<0.001
IQR=Interquartile Range
Patients with preoperative eGFR ≥60 had lower baseline rates of CAD (15.8% vs 27.8% vs 32.9%, p<0.001), HTN (54.3% vs 60.9% vs 76.8%, p<0.001), PVD (1.3% vs 3% vs 6.1%, p = 0.01), and DM (15.2% vs 21.1% vs 26.8%, p = 0.02) compared with patients with preoperative eGFR 45–60 and eGFR ≤45, respectively.
3.2. Uni- and multivariate analysis predicting eGFR decline to ≤45 ml/min/1.732
183 patients (27%) with preoperative eGFR ≥60 experienced an eGFR decline to ≤45. Median time to last recorded eGFR was 86 days (IQR 5–239 days). There was no difference in median decline in eGFR between men and women (−32 vs −32, p=0.4). On univariate analysis, age (p<0.001), presence of CAD (p=0.02), presence of HTN (p=0.016), presence of PVD (p=0.02), and increasing preoperative sCr (p<0.001) were associated with eGFR decline to ≤45. In the multivariable model, increasing age (Odds Ratio [OR] 1.07, 95% CI 1.03–1.12, p=0.001 for linear first spline term, p<0.001 for joint test of coefficients), female gender (OR 5.58, 95% CI 3.20–9.75, p<0.001), presence of PVD (OR 4.89, 95% CI 1.06–22.59, p=0.04), and increasing pre-operative sCr (OR 1902, 95% CI 127–28,596, p<0.001 for linear first spline term, p<0.001 for joint test of coefficients) were associated with eGFR decline to ≤45.
3.3. Nomogram construction, decision curve analysis, and sensitivity analysis
Based on these variables, we constructed a predictive nomogram integrating pre-operative characteristics. The first model, inclusive of all variables such as comorbidities (CAD, HTN, DM, & PVD), is presented in Supplemental Figure 2 and has a ROC-AUC of 0.793. The 20-fold cross validation of the ROC-AUC of the full model was 0.775 (Supplemental Figure 3). Supplemental Figure 4 shows the results of model calibration. The second model, simplified for ease of clinical application to only include age, sex, and pre-operative sCr (Figure 1) had a ROC-AUC of 0.787 and a slightly better 20-fold cross validation AUC of 0.777 (Figure 2) and similarly robust calibration when compared to the full model (Figure 3). The decision analysis curves (Figure 4) suggest that the reduced model afford nearly identical performance to the full model in assessing strategies that can prevent eGFR decline at probabilities >11%.
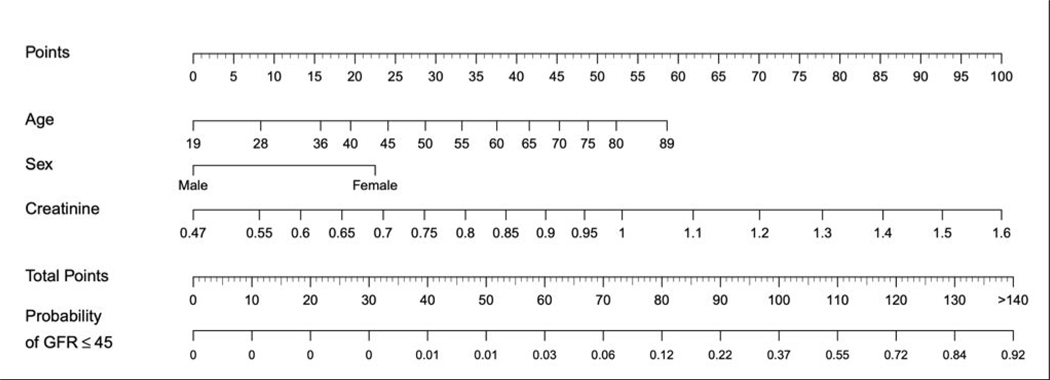
Figure 1:
Nomogram (reduced model) for the prediction of baseline eGFR reduction to ≤45 within one year of surgery, based on the limited multivariable model. Point values are calculated for each predictive variable and then applied to the probability scale at the bottom. For example, a 65 year-old male with a preoperative creatinine of 0.8 mg/dL would have a probability of eGFR decline to ≤45 of ~10% (76 points) as opposed to a 70 year old female with a preoperative creatinine of 1.2 mg/dL (~90%; 137 points).
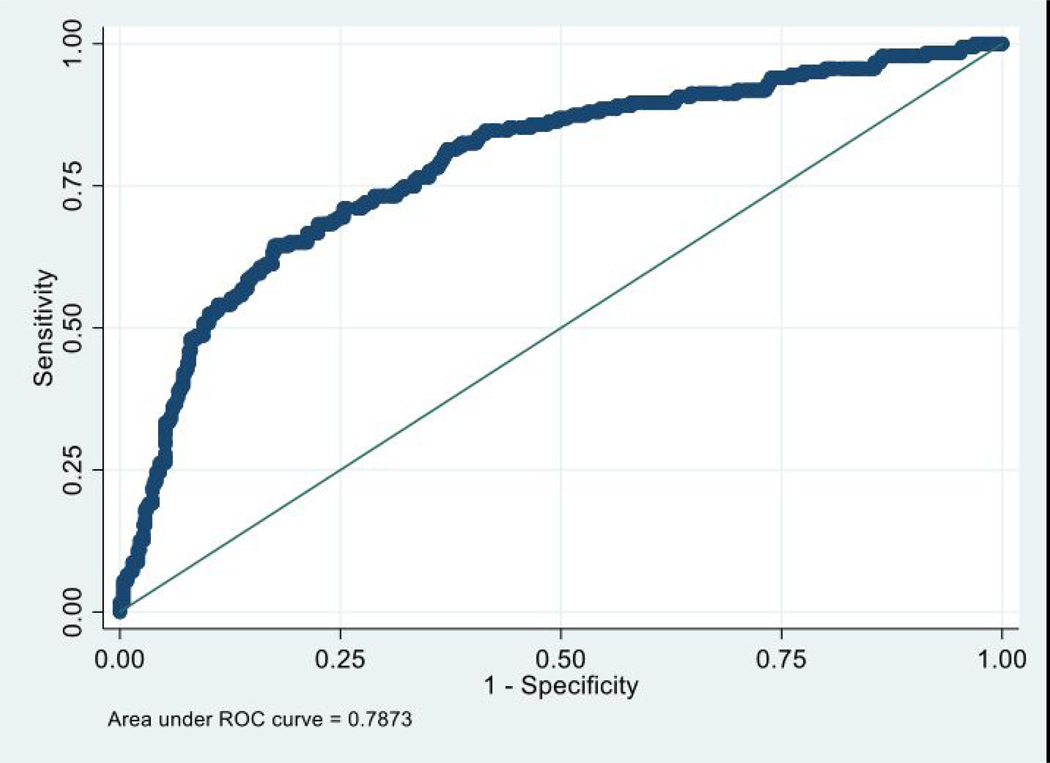
Figure 2:
ROC curve for nomogram (reduced model) demonstrated an area under the curve (AUC) of 0.79. The 20-fold cross validated ROC-AUC was 0.78 (95% CI: 0.74–0.82).
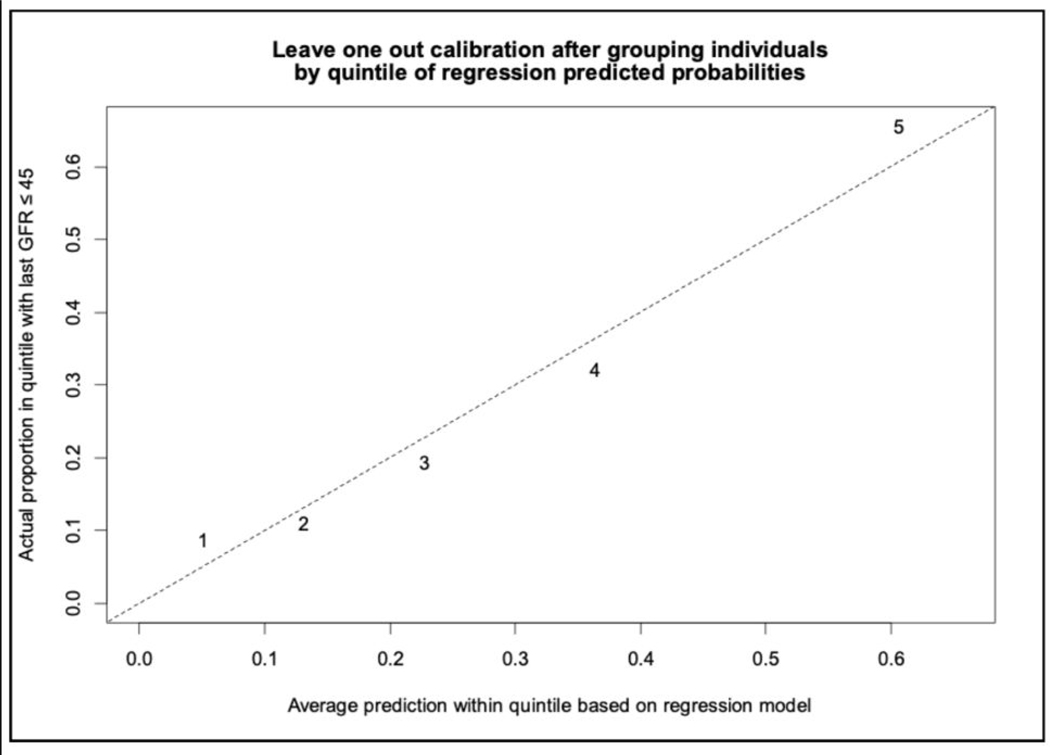
Figure 3:
The calibration curve, performed by leave one out calibration method after grouping individuals by quintile of regression predicted probabilities, demonstrates good calibration of the reduced model.
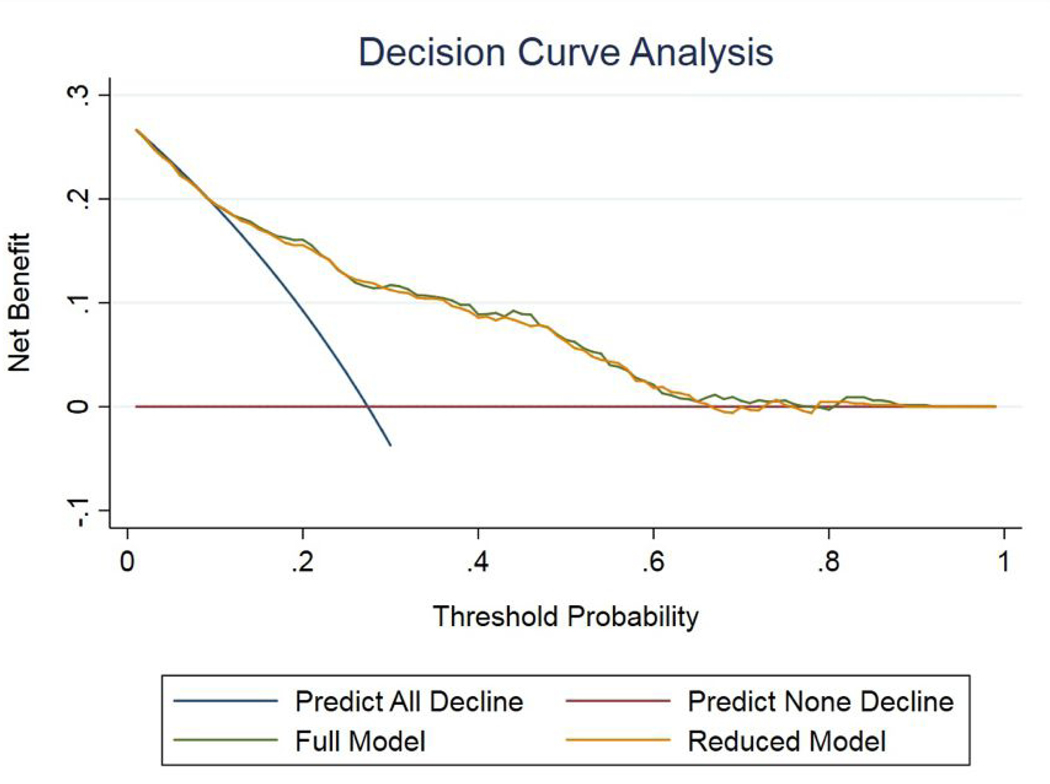
Figure 4:
Decision Curve Analysis demonstrating the net benefit to renal functional preservation associated with the use of the nomogram-derived probability. The model weighs the net benefit (y-axis) of the choice to forgo radical nephrectomy at various threshold probabilities (x-axis) can be observed, based both the full and reduced model, for the prediction of eGFR reduction to ≤ 45. The curves are shown nearly overlapping, highlighting the equal clinical utility of the reduced model relative to the model that includes the patient comorbidity profile.
It is possible that some renal functional data was measured before renal eGFR fully equilibrated, thus over or under-estimating renal function following RN. We thus analyzed a subset of patients in whom renal functional data could be verified to be > 60 days postoperatively. The AUC of the nomogram remained high (0.76) suggesting this is not a major shortcoming of our dataset. In our sensitivity analysis using a repeated measures regression estimated by generalized estimating equations, sex, age, and pre-operative sCr values retained their salience as predictors of decline (data not shown).
4. Discussion
The decision to perform RN versus PN in patients with large or anatomically complex renal mass is nuanced and complex, balancing theoretical benefits of long-term renal parenchymal preservation against perioperative and potential oncological considerations.[7] Enthusiasm for nephron-sparing (NSS) is fueled by long-term data supporting better renal function and a potential overall survival (OS) benefit in patients undergoing PN.[2, 4, 10–13] This body of literature has lead providers to adopt PN as the standard approach for masses where NSS is technically feasible.[1, 14] However, the only prospective randomized trial (EORTC 30904) comparing RN versus PN suggests a superior OS for patients undergoing RN in an intention-to-treat analysis (10-yr OS: 81.1% vs 75.7%; HR 1.51; p=0.02).[15] Although the data from EORTC 30904 are imperfect, these and other data suggest that many patients are unharmed by RN.[16–19] Yet, PN, especially for complex renal masses, is associated with higher perioperative risks.[20, 21] To this end the urologic community has worked to calibrate risk trade-offs surrounding the PN vs RN decision.[7]
One consistent deliverable of PN across both prospective and retrospective series is the preservation of renal function.[3, 4, 16] Yet, despite being a surrogate marker of OS,[5] retention of maximal renal function following renal surgery does not appear to benefit all patients. For instance, Demirjian et al illustrated that a lower risk of progressive decline in eGFR for patients with surgically induced (CKD-S) as opposed to medically induced (CKD-M) chronic kidney disease.[22] An analysis by the same group suggested that the overall survival is better for CKD-S than for medical CKD-M, particularly if the postoperative GFR is ≥45 ml/min/1.73 m2.[6] Even with normal preoperative renal function (eGFR ≥ 60), about a third of patients may experience eGFR decline to <60 (CKD-S) and 10% may experience a 50% reduction in renal function or require dialysis after renal surgery [6]. Accordingly, AUA guidelines have suggested that PN should be favored where feasible when post-operative eGFR is expected to decline to ≤45 as this constitutes CKD stage 3b.[1]
Despite this recommendation, there are no validated clinical tools to help risk stratify patients with normal renal function for an eGFR decline to below 45. Sorbellini et al developed a nomogram to predict renal insufficiency following RN or PN[23] but utilized a stringent definition of renal insufficiency (two sCr values of >2 mg/dL) which likely overlooks many patients with eGFR <45. More recently, a large retrospective analysis from the Mayo Clinic identified patient age, pre-operative eGFR, pre-operative proteinuria, tumor size, and time from surgery, as predictive of renal functional decline or renal failure following RN.[24] We, in turn, have developed a simple, clinically intuitive nomogram to predict which patients with normal preoperative renal function are likely to have an eGFR decline to ≤45 if they were to undergo RN. Despite building predictive models that incorporated granular clinical variables that included comorbidities (supplemental material), we found that a simplified model that only integrated age, sex, and preoperative creatinine, provided equally robust prediction of eGFR decline to ≤45. An interesting finding of our study was that female gender was associated with eGFR decline. Considering there were no differences between pre-operative eGFR or median post-operative eGFR decline between men and women, this finding cannot be readily explained; however, the association is strong and warrants further exploration. Furthermore, this finding is consistent with data reported in the transplant literature demonstrating a >3-fold increase in the risk of stage III CKD in healthy female renal donor patients when compared to male counterparts.[19] Nevertheless, it is important to note that ultimate progression to end stage renal disease in such donor nephrectomy patients appears to occur more frequently in men.[17]
We evaluated whether comorbid conditions with a known clinical relationship to renal function (HTN, CAD, DM, and PVD) were predictive of eGFR decline. On multivariate analysis, only presence of PVD demonstrated a statistically significant relationship with functional outcomes, however, only ~1.5% patients carried this diagnosis limiting the predictive power of the model. Further, when co-morbidity data was added to the nomogram model, the 20-fold cross-validation of the ROC-AUC actually slightly decreased when compared to the reduced model (including only age, sex, and pre-operative creatinine), suggesting that these conditions have little predictive value in patients without clinically-significant CKD. Our findings dovetail with a recent report by Isharwal et al demonstrating that comorbidity status fails to predict renal functional decline following renal surgery.[25] The literature in this space is contradictory,[23, 26, 27] but the greatest predictor of eGFR decline to remains radical nephrectomy.[28, 29] Woldu et al recently compared renal functional outcomes after NSS and RN among patients with CKD I and II. [30] CKD II patients were 2.3-fold more likely to develop eGFR ≤45 when RN rather than NSS was performed. Similar to the proportion of patients in our series (27%), the investigators report that 21% of RN patients developed eGFR ≤45. Our predictive model only included individuals with normal preoperative renal function, thus likely selecting for patients whose kidney are especially resistant to decline in face of an unfavorable comorbidity profile and screening out those with disease profiles significant enough to induce CKD. Indeed, our findings underscore that in a cohort of patients with eGFR >60 risk factors classically associated with CKD do not factor into the risks of post-operative renal functional decline. In the final nomogram (Figure 1), we paired down the predictive model to its essential elements in order to facilitate its use at the point of care.
Our study has several important limitations. Although our renal cancer database is prospectively maintained, the retrospective nature of the data analysis subjects our findings to selection biases and confounding. Because we intentionally excluded patients with baseline eGFR <60, for whom decision between radical and partial nephrectomy is more heavily weighted towards nephron preservation, our model should not be applied to patients with pre-operative CKD stage ≥ 3. Another potential criticism is that we do not integrate pre-operative nuclear medicine renal scan split renal function into our model. Yet, recent data suggest that preoperative renal scans can significantly over-estimate split renal function of kidneys with large tumors putting the clinical utility of such test results in this setting into question [31].
5. Conclusions
The decision to perform RN vs. PN remains a clinical challenge. We provide a simple point-of-care tool to help clinicians objectify critical clinical decision making to address criteria outlined in the AUA guidelines. As with any predictive model built on a single dataset, this nomogram awaits external validation.
Supplementary Material
REFERENCES
- 1.Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. The Journal of urology. 2017;198(3):520–9. [DOI] [PubMed] [Google Scholar]
- 2.Kim SP, Murad MH, Thompson RH, et al. Comparative Effectiveness for Survival and Renal Function of Partial and Radical Nephrectomy for Localized Renal Tumors: A Systematic Review and Meta-Analysis. The Journal of urology. 2012. [DOI] [PubMed] [Google Scholar]
- 3.Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65(2):372–7. [DOI] [PubMed] [Google Scholar]
- 4.Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, Autorino R. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol. 2017;71(4):606–17. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 6.Lane BR, Demirjian S, Derweesh IH, et al. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol. 2015;68(6):996–1003. [DOI] [PubMed] [Google Scholar]
- 7.Kim SP, Campbell SC, Gill I, et al. Collaborative Review of Risk Benefit Trade-offs Between Partial and Radical Nephrectomy in the Management of Anatomically Complex Renal Masses. Eur Urol. 2017;72(1):64–75. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical chemistry. 2007;53(4):766–72. [DOI] [PubMed] [Google Scholar]
- 9.Nieboer D, Vergouwe Y, Roobol MJ, et al. Nonlinear modeling was applied thoughtfully for risk prediction: the Prostate Biopsy Collaborative Group. Journal of Clinical Epidemiology. 2015;68(4):426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75(12):1236–42. [DOI] [PubMed] [Google Scholar]
- 11.Scosyrev E, Wu K, Levey HR, et al. Overall Survival after Partial Versus Radical Nephrectomy for a Small Renal Mass: Systematic Review of Observational Studies. Urology Practice. 2014;1(1):27–34. [DOI] [PubMed] [Google Scholar]
- 12.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. The Journal of urology. 2000;163(2):442–5. [PubMed] [Google Scholar]
- 13.Sun M, Trinh QD, Bianchi M, et al. A non-cancer-related survival benefit is associated with partial nephrectomy. Eur Urol. 2012;61(4):725–31. [DOI] [PubMed] [Google Scholar]
- 14.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–24. [DOI] [PubMed] [Google Scholar]
- 15.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–52. [DOI] [PubMed] [Google Scholar]
- 16.Gershman B, Thompson RH, Boorjian SA, et al. Radical Versus Partial Nephrectomy for cT1 Renal Cell Carcinoma. Eur Urol. 2018;74(6):825–32. [DOI] [PubMed] [Google Scholar]
- 17.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. Jama. 2014;311(6):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smaldone MC, Egleston B, Uzzo RG, Kutikov A. Does partial nephrectomy result in a durable overall survival benefit in the Medicare population? The Journal of urology. 2012;188(6):2089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. The New England journal of medicine. 2009;360(5):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2007;51(6):1606–15. [DOI] [PubMed] [Google Scholar]
- 21.Simhan J, Smaldone MC, Tsai KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011;60(4):724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demirjian S, Lane BR, Derweesh IH, Takagi T, Fergany A, Campbell SC. Chronic kidney disease due to surgical removal of nephrons: relative rates of progression and survival. The Journal of urology. 2014;192(4):1057–62. [DOI] [PubMed] [Google Scholar]
- 23.Sorbellini M, Kattan MW, Snyder ME, Hakimi AA, Sarasohn DM, Russo P. Prognostic nomogram for renal insufficiency after radical or partial nephrectomy. The Journal of urology. 2006;176(2):472–6; discussion 6. [DOI] [PubMed] [Google Scholar]
- 24.Bhindi B, Lohse CM, Schulte PJ, et al. Predicting Renal Function Outcomes After Partial and Radical Nephrectomy. Eur Urol. 2018. [DOI] [PubMed] [Google Scholar]
- 25.Isharwal S, Ye W, Wang A, et al. Impact of Comorbidities on Functional Recovery from Partial Nephrectomy. The Journal of urology. 2018;199(6):1433–9. [DOI] [PubMed] [Google Scholar]
- 26.Ngo TC, Hurley MP, Thong AE, Jeon SH, Leppert JT, Chung BI. Estimating the risk of chronic kidney disease after nephrectomy. The Canadian journal of urology. 2013;20(6):7035–41. [PubMed] [Google Scholar]
- 27.Yokoyama M, Fujii Y, Takeshita H, et al. Renal function after radical nephrectomy: development and validation of predictive models in Japanese patients. International journal of urology : official journal of the Japanese Urological Association. 2014;21(3):238–42. [DOI] [PubMed] [Google Scholar]
- 28.Reinstatler L, Klaassen Z, Barrett B, Terris MK, Moses KA. Body mass index and comorbidity are associated with postoperative renal function after nephrectomy. International braz j urol : official journal of the Brazilian Society of Urology. 2015;41(4):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. The Lancet Oncology. 2006;7(9):735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woldu SL, Weinberg AC, Korets R, et al. Who really benefits from nephron-sparing surgery? Urology.2014;84(4):860–7. [DOI] [PubMed] [Google Scholar]
- 31.Badri A, McIntosh A, Hsu L, et al. PD23–06: Prediction of Post-Operative eGFR in Patients Undergoing Radical Nephrectomy: Harder Than It Looks (On Scans). Journal of Urology. 2019;201(Supplement 4):e395-e. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.