Abstract
A 44-year-old woman with no significant medical history presented with a 3-week history of high-grade fevers, fatigue and shortness of breath. Laboratory investigation was significant for lymphopenia and thrombocytopenia which progressively worsened during her hospital stay, along with new-onset anaemia, and elevated ferritin, transaminase and triglycerides. A computerized tomography (CT) scan of the abdomen revealed retroperitoneal lymphadenopathy. A bone marrow biopsy confirmed the diagnosis of haemophagocytic lymphohistiocytosis (HLH). Extensive infectious work-up revealed high IgG titres for Bartonella henselae and Coxiella burnetii. Interestingly, the left supraclavicular node was negative for both microbes by polymerase chain reaction (PCR), but the biopsy revealed anaplastic large T-cell lymphoma.
LEARNING POINTS
Haemophagocytic lymphohistiocytosis (HLH) is an important differential diagnosis to consider for fever of unknown origin in adults, especially in the setting of pancytopenia and hyperferritinaemia.
Q fever resulting from Coxiella burnetii can cause HLH and is also postulated to increase the risk of lymphoma.
Bartonella henselae infection can also trigger HLH, but the risk of lymphoma following infection by B. henselae is unknown.
Keywords: Haemophagocytic lymphohistiocytosis, Coxiella burnetii, Bartonella henselae, T-cell lymphoma
CASE PRESENTATION
A previously healthy 44-year-old woman with a remote history of exposure to farm animals was admitted for fever of unknown origin after she presented with a 3-week history of cough, night sweats, low-grade fevers, fatigue and sore throat. She had completed an outpatient course of antibiotics for suspected community-acquired pneumonia. Upon admission, she had a fever of 38.8°C. The physical examination was unremarkable apart from tender right cervical lymphadenopathy. The initial laboratory investigation revealed mild anaemia, but no leucocytosis, mild hyponatraemia and transaminitis. Blood cultures were drawn which remained negative and cerebrospinal fluid analysis following lumbar puncture showed no abnormalities. A chest x-ray and computed tomography (CT) scan showed a small right-sided pleural effusion, with associated right lower lobe consolidation and not the atelectasis which was found in a previous outpatient study. A CT scan of the abdomen was significant for para-aortic and mesenteric lymph node enlargement, which was interpreted as reactive in nature. Urine analysis was normal.
The patient was started on broad-spectrum antibiotics, which were discontinued later as the fever did not resolve. A battery of tests including an atypical viral panel and fungal cultures were performed to rule out infectious causes and were all negative except for high IgG titres for Bartonella henselae (BH) and Coxiella burnetii (CB). Despite treatment with doxycycline, the patient’s condition did not improve and rising levels of ferritin and triglycerides were noted. Progressively worsening bicytopenia was also seen with the total white blood cell count (WBC) and haemoglobin falling to 3.3 μ/l and 8.7 g/dl, respectively. A bone marrow biopsy was then performed which confirmed haemophagocytic lymphohistiocytosis (HLH) (Fig. 1). A left supraclavicular node was biopsied and submitted to polymerase chain reaction (PCR) to evaluate BH/CB as the possible trigger for HLH, but the results were negative for both microbes. However, histopathological examination revealed anaplastic large T-cell lymphoma (ALCL) (Fig. 2). The serological tests were considered false positive. The patient’s condition dramatically improved with dexamethasone and etoposide-based chemotherapy.
Figure 1.
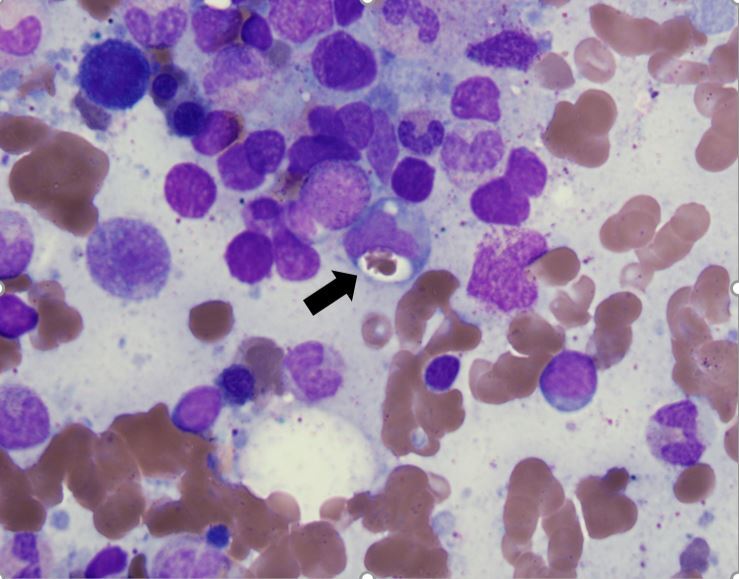
Hematoxylin and eosin stain of bone marrow biopsy with hemophagocytosis. A red blood cell indenting a macrophage nucleus can be seen (black arrow)
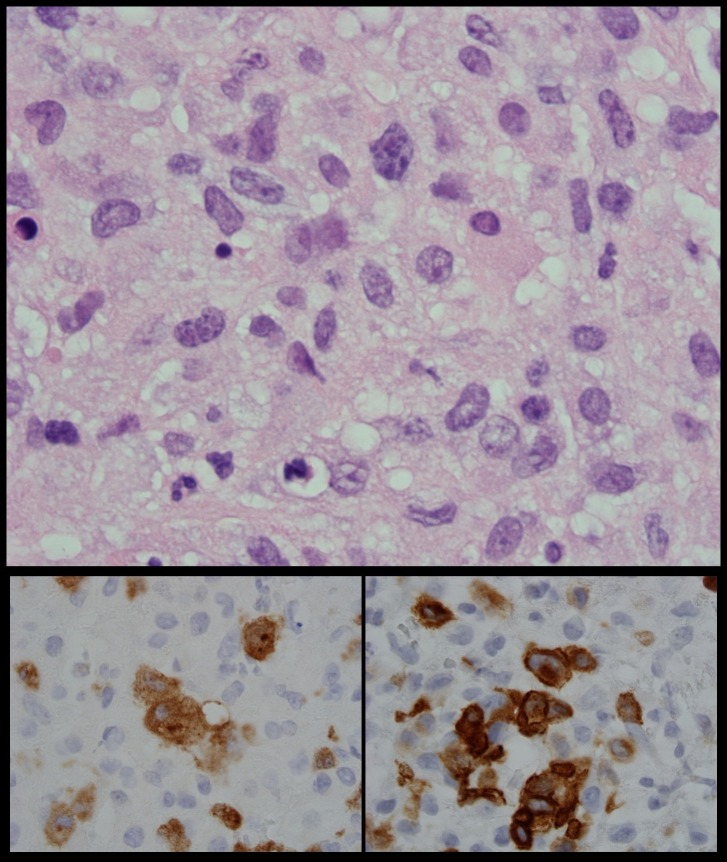
Figure 2.
Hematoxylin and eosin staining of the supraclavicular node showing atypical lymphocytes of variable sizes with prominent nuclei with hyperchromatic chromatin (top panel). Further immunohistochemical staining revealed the atypical lymphocytes cells to stain positive for ALK (bottom left panel) and CD 30 (bottom right panel) consistent with anaplastic T cell lymphoma.
DISCUSSION
HLH involves uncontrolled activation of macrophages, which engulf erythrocytes, leukocytes and platelets in the bone marrow. HLH can be familial or secondary to malignancy, infections or autoimmune disorders [1, 2]. At least five out of the following are required for diagnosis: fever, splenomegaly, hypofibrinogenaemia, cytopenias, hypertriglyceridaemia, hyperferritinaemia, haemophagocytosis, low NK-cell activity and an elevated soluble CD25 level [1, 2]. Q fever resulting from CB infection is associated with a higher risk for both lymphomas and HLH. An observational study from 2016 reported seven cases of B-cell NHL after CB infection [3]. In fact, Q fever patients were found to have a 25-fold increased risk of developing B-cell NHL compared with the general population [3]. Pathogenesis was attributed to overproduction of interleukin 10 (IL-10) by infected macrophages leading to overexpression of Bcl-2 protein, a B-cell growth factor [3]. However, cases of CB associated with ALCL (a T-cell lymphoma) have not been documented in the literature thus far. Furthermore, case reports also have highlighted that an exaggerated immune response in the setting of both Q fever and BH infection is a trigger for HLH [4–6].
In our patient, whether the serological tests for CB and BH were false positives or the result of a true past infection remains uncertain, although the negative lymph node PCR supports the former. Similarly to CB, the question of whether BH has a role in lymphomagenesis remains unanswered. Our case raises important questions of association and causality between these zoonotic organisms and tumorigenesis, which needs further investigation.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
REFERENCES
- 1.Ibrahim U, Saqib A, Rehan M, Atallah JP. ALK-negative anaplastic large cell lymphoma presenting as disseminated intravascular coagulation and hemophagocytic lymphohistiocytosis: a potentially fatal presentation. Case Rep Hematol. 2018;2018 doi: 10.1155/2018/3465351. 3465351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris P, Dixit R, Norton R. Coxiella burnetii causing haemophagocytic syndrome: a rare complication of an unusual pathogen. Infection. 2011;39(6):579–582. doi: 10.1007/s15010-011-0142-4. [DOI] [PubMed] [Google Scholar]
- 3.Melenotte C, Million M, Audoly G, Gorse A, Dutronc H, Roland G, et al. B-cell non-Hodgkin lymphoma linked to Coxiella burnetii. Blood. 2016;127(1):113–121. doi: 10.1182/blood-2015-04-639617. [DOI] [PubMed] [Google Scholar]
- 4.Chen TC, Chang K, Lu PL, Liu YC, Chen YH, Hsieh HC, et al. Acute Q fever with hemophagocytic syndrome: case report and literature review. Scand J Infect Dis. 2006;38(11–12):1119–1122. doi: 10.1080/00365540600684405. [DOI] [PubMed] [Google Scholar]
- 5.Khateeb J, Stern A, Yaseen H, Levi Y, Khamaisi M. Chronic Q fever infection mimicking hematological malignancy. Infect Dis Clin Pract. 2018;26(6):e77–e79. [Google Scholar]
- 6.Poudel A, Lew J, Slayton W, Dharnidharka VR. Bartonella henselae infection inducing hemophagocytic lymphohistiocytosis in a kidney transplant recipient. Pediatr Transplant. 2014;18(3):E83–E87. doi: 10.1111/petr.12235. [DOI] [PubMed] [Google Scholar]