Abstract
PURPOSE
Conventional wisdom has rendered patients with brain metastases ineligible for clinical trials for fear that poor survival could mask the benefit of otherwise promising treatments. Our group previously published the diagnosis-specific Graded Prognostic Assessment (GPA). Updates with larger contemporary cohorts using molecular markers and newly identified prognostic factors have been published. The purposes of this work are to present all the updated indices in a single report to guide treatment choice, stratify research, and define an eligibility quotient to expand eligibility.
METHODS
A multi-institutional database of 6,984 patients with newly diagnosed brain metastases underwent multivariable analyses of prognostic factors and treatments associated with survival for each primary site. Significant factors were used to define the updated GPA. GPAs of 4.0 and 0.0 correlate with the best and worst prognoses, respectively.
RESULTS
Significant prognostic factors varied by diagnosis and new prognostic factors were identified. Those factors were incorporated into the updated GPA with robust separation (P < .01) between subgroups. Survival has improved, but varies widely by GPA for patients with non–small-cell lung, breast, melanoma, GI, and renal cancer with brain metastases from 7-47 months, 3-36 months, 5-34 months, 3-17 months, and 4-35 months, respectively.
CONCLUSION
Median survival varies widely and our ability to estimate survival for patients with brain metastases has improved. The updated GPA (available free at brainmetgpa.com) provides an accurate tool with which to estimate survival, individualize treatment, and stratify clinical trials. Instead of excluding patients with brain metastases, enrollment should be encouraged and those trials should be stratified by the GPA to ensure those trials make appropriate comparisons. Furthermore, we recommend the expansion of eligibility to allow for the enrollment of patients with previously treated brain metastases who have a 50% or greater probability of an additional year of survival (eligibility quotient > 0.50).
INTRODUCTION
Exclusion of patients with brain metastases from clinical trials not only raises issues of equity and discrimination, but, given the high incidence of brain metastases, hinders accrual to and limits the generalizability of those trials. These patients are often excluded as a result of three main concerns. First, investigators fear that the historically poor outcomes associated with brain metastases could mask the benefit of the treatment under investigation.1-3 Second, because neurologic symptoms could occur, new investigational agents risk having these symptoms associated with the agent itself, a risk that many pharmaceutical companies avoid. Third, many pharmaceutical agents have poor blood-brain and brain-tumor penetrability, decreasing the likelihood of meaningful intracranial efficacy. An estimated 300,000 patients are diagnosed each year with brain metastases in the United States, and the incidence is growing because of advances that have resulted in patients living longer and hence at increased risk for brain metastases.4 For example, a recent review of patterns of failure in 1,549 patients with stage 1 to stage 3 non–small-cell lung cancer (NSCLC), 466 (30%) developed recurrence, most commonly brain metastases (37%).5
The brain metastases challenge is a complex problem because of the marked heterogeneity of this patient population: Brain metastases may arise from a wide variety of primary tumors, patients may have already received several different treatments for their primary cancer, and resistance to multiple lines of therapy is common. This heterogeneity has plagued the interpretation of clinical trials involving this patient population because of the difficulty in accurately stratifying those studies to ensure similar groups were being compared. The complexity is further compounded by the many possible combinations of available treatments for brain metastases (surgery, stereotactic radiosurgery [SRS], whole-brain radiation therapy [WBRT], chemotherapy, molecularly targeted therapeutics, and immunotherapies).
These concerns led to efforts to understand and quantitatively model prognosis. Our group has published a series of articles developing and refining a diagnosis-specific prognostic index, the Graded Prognostic Assessment (GPA), for patients with brain metastases. The GPA was first published in 20086 on the basis of 1,960 patients from five randomized Radiation Therapy Oncology Group (RTOG) trials (RTOG 7916, RTOG 8528, RTOG 8905, RTOG 9104, and RTOG 9508). Analysis showed that four prognostic factors—age, Karnofsky performance status (KPS), extracranial metastases, and number of brain metastases—were significant for survival. Those prognostic factors were weighted in proportion to their significance and scaled such that patients with the best or worst prognosis would have a GPA of 4.0 or 0.0, respectively. In 2010, we refined the GPA when we discovered that survival varies by primary diagnosis and diagnosis-specific prognostic factors based on a retrospective analysis of 3,940 patients.7 The Breast GPA was then further refined using tumor molecular subtype.8 In 2012, we published an executive summary report that was based on 3,940 patients with newly diagnosed brain metastases diagnosed between 1993 and 2010.9
Since the publication of the 2012 executive report, GPA indices have been updated for each diagnosis with larger contemporary cohorts using molecular markers and newly identified clinical prognostic factors.10-18 Of most importance, the treatment paradigms for most patients with brain metastases have evolved over the last decade. Our purpose is to report all the updated diagnosis-specific GPAs in a single report that can be used to individualize treatment, stratify clinical trials, and define the eligibility quotient (EQ) to expand clinical trial eligibility.
METHODS
Patient Population
A multi-institutional (18 institutions in three countries), institutional review board–approved, retrospective database of 6,984 patients with newly diagnosed brain metastases diagnosed between 2006 and 2017 was created using Research Electronic Data Capture software. Patients with recurrent brain metastases and/or leptomeningeal carcinomatosis were excluded.
Statistics
Survival was measured from the date of the first treatment of brain metastases to the date of death or last follow up. We used the Kaplan-Meier method to calculate all survival estimates. Treatment comparisons were made using a multiple Cox proportional hazards regression model that included categorical variables for initial treatment combination and GPA class. Analysis was performed using R software (version 3.4).
Derivation of GPA Indices
The common approach for deriving GPA indices was to use multiple Cox proportional hazards regression to identify an initial set of prognostic factors. These factors were then weighted, using half or full point increments, according to the magnitude of effect on survival—that is, hazard ratio. The final index was chosen by balancing criteria that included separation of prognostic classes, the percentage of patients in each class, and simplicity. Such metrics as the concordance index, R2, and log-rank test statistics were used to evaluate model performance. In general, marginally significant factors were retained only if they afforded nontrivial improvements to the final index. Factors initially considered included those that were already significant in previous GPA versions and additional factors that varied by primary cancer type that were hypothesized to be associated with survival—for example, molecular markers, sex, and body mass index. Details of the variables collected and specific methods used for each cohort may be found in the publication of each diagnosis-specific GPA.6-18
Role of Funding Source
The funding sources for this study had no role in the collection, analysis, or interpretation of the data; the writing of the report; or the decision to submit it for publication.
RESULTS
Patient Characteristics
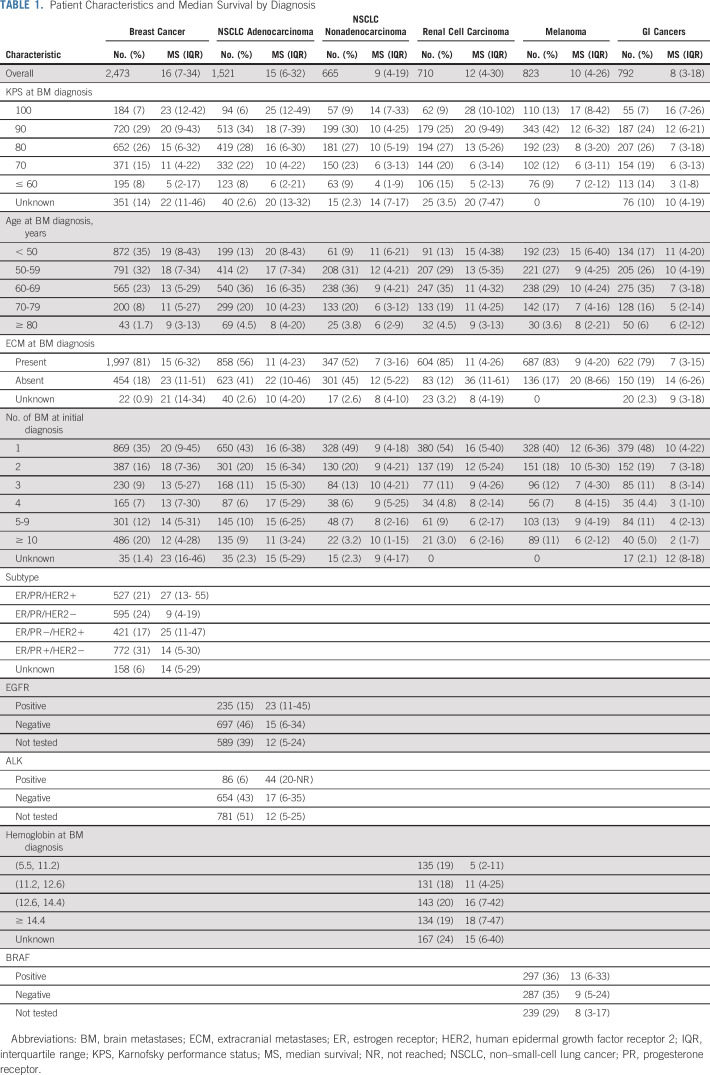
Table 1 lists patient characteristics for the overall data set. The proportions of patients who were asymptomatic (KPS = 100) for breast, lung adenocarcinoma, lung nonadenocarcinoma, renal, melanoma, and GI cancers were 7%, 6%, 9%, 9%, 13%, and 7%, respectively. Most patients had extracranial metastases. In lung adenocarcinoma, 15% and 6% of patients were EGFR and ALK positive, respectively. In melanoma, 36% were BRAF positive.
TABLE 1.
Patient Characteristics and Median Survival by Diagnosis
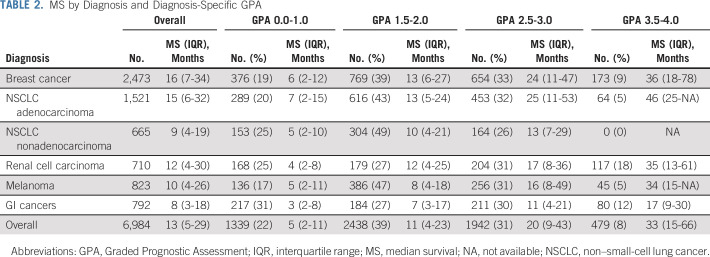
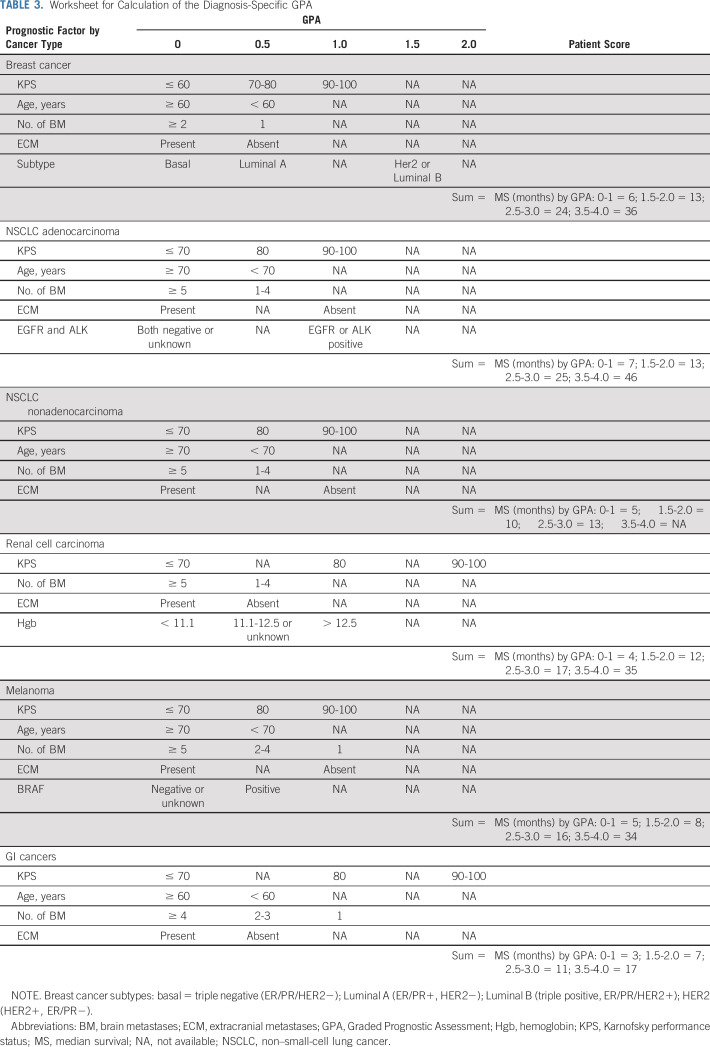
Table 2 lists median survival by diagnosis and GPA. Compared with our prior summary report,9 overall median survival for patients with brain metastases from all diagnoses has improved in more recent analyses, from 7-12 months—15 months for lung adenocarcinoma—for NSCLC; from 14-16 months for breast cancer; from 7-10 months for melanoma; from 5-8 months for GI cancers; and from 10-12 months for renal cell carcinoma. Table 2 also shows that the range of median survival by GPA is wide, 7-46 months for NSCLC, 3-36 months for breast cancer, 5-34 months for melanoma, 3-17 months for GI cancers, and from 4-35 months for renal cell carcinoma. Figure 1 shows the Kapan-Meier curves for survival by diagnosis and the updated GPA, demonstrating excellent separation between groups (P < .01) and the median survival for each GPA group (0-1 month, 1.5-2.0 months, 2.5-3.0 months, and 3.5-4.0 months). Table 3 provides a user-friendly worksheet with which to calculate the GPA for an individual patient. A free online application is also available at brainmetgpa.com.
TABLE 2.
MS by Diagnosis and Diagnosis-Specific GPA
FIG 1.
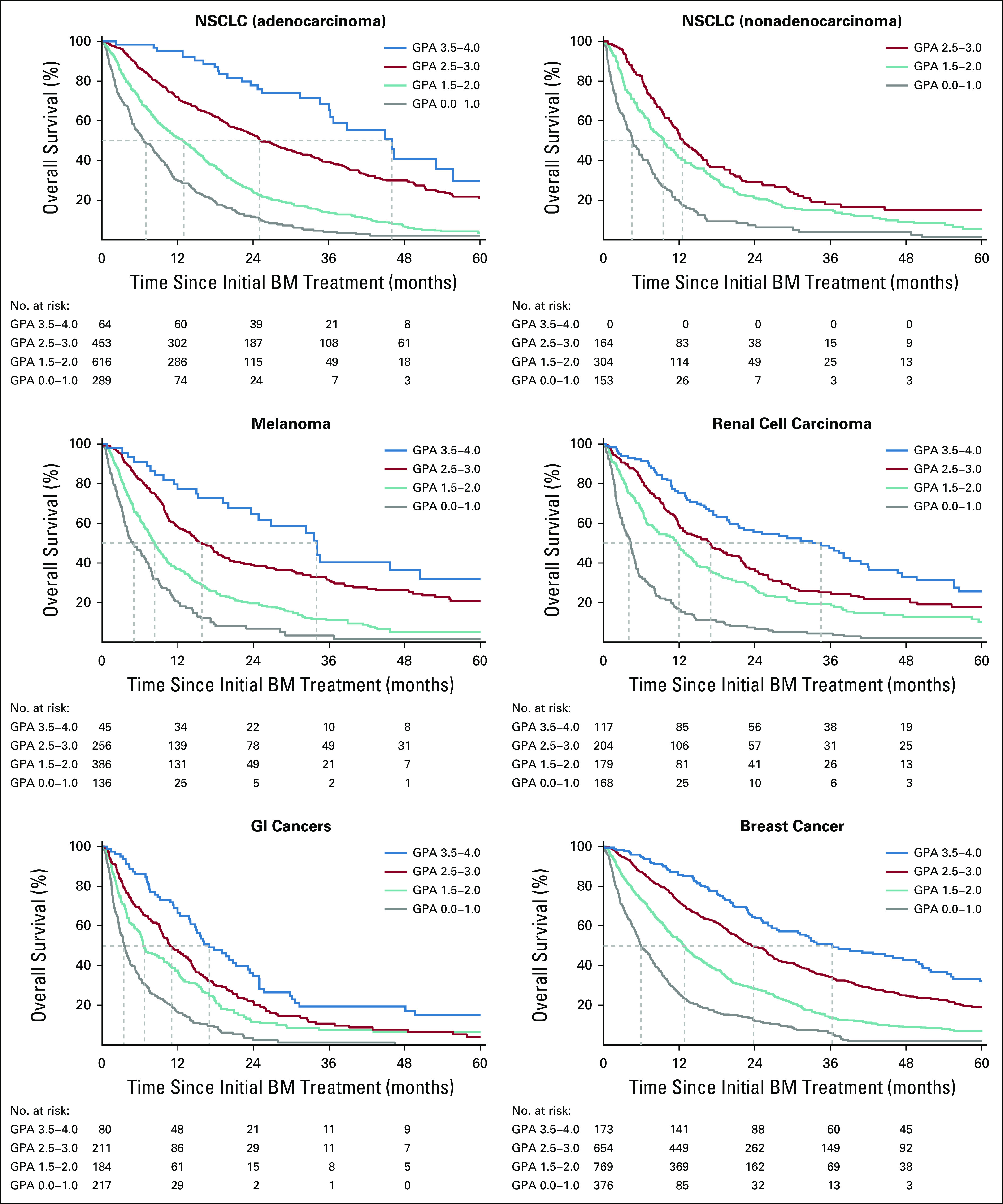
Kaplan-Meier curves for survival by diagnosis and the updated diagnosis-specific Graded Prognostic Assessment (GPA). BM, brain metastases; NSCLC, non–small-cell lung cancer;
TABLE 3.
Worksheet for Calculation of the Diagnosis-Specific GPA
Effect of Treatment
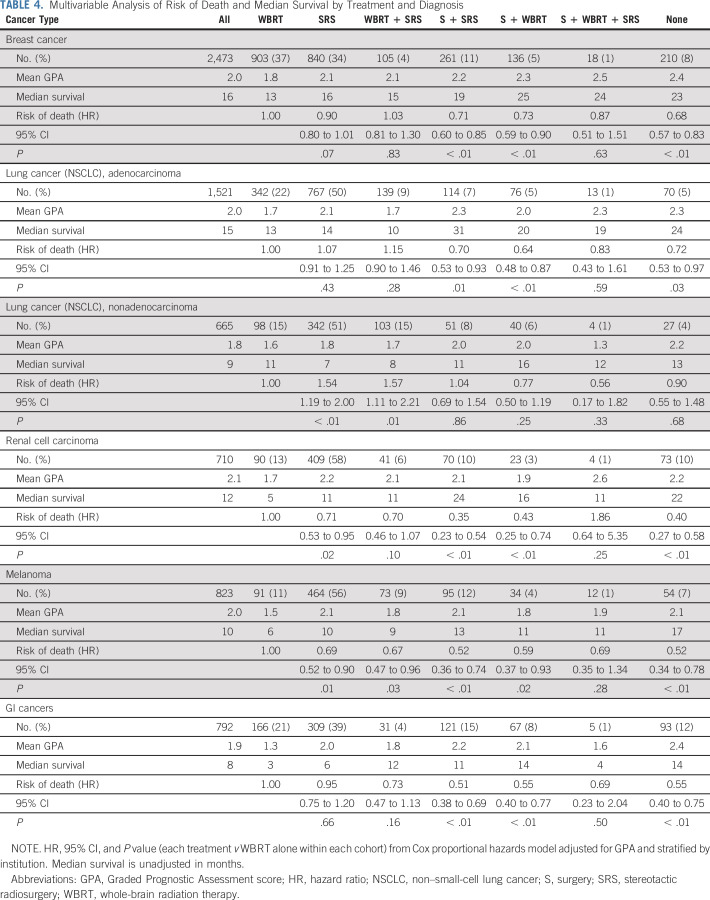
The purpose of prognostic indices is to predict outcomes before treatment, thereby helping the clinician select treatment by balancing risks versus benefit with knowledge of anticipated survival, which is in contrast to predictive tools that predict outcomes after treatment. Therefore, the data presented in Table 4, a multivariable analysis of the risk of death and median survival by treatment and diagnosis, are not intended to show that one treatment is better than another. These data are retrospective with inherent selection bias and therefore cannot be used to compare treatments. Nonetheless, Table 4 does show patterns of care and can be compared with those patterns in the prior era. For instance, the percentage of patients receiving WBRT as part of initial therapy in the prior era compared with our current data decreased from 75%-37% in NSCLC adenocarcinoma and from 67%-47% in breast cancer.7,9 This is consistent with randomized trials demonstrating that SRS alone is equally effective in terms of survival and associated with fewer neurocognitive deficits, albeit with a much greater likelihood of intracranial relapse than SRS plus WBRT19,20
TABLE 4.
Multivariable Analysis of Risk of Death and Median Survival by Treatment and Diagnosis
Summary of Changes to the Indices
The larger contemporary database allowed us to identify new and more robust prognostic factors, including mutations, which have been incorporated into the updated GPA indices. All of the previously identified factors were confirmed.
NSCLC.
Changes in the updated Lung GPA (n = 2,186)10,11 compared with the original Lung GPA (NSCLC, n = 1,833)7,9 include, for lung adenocarcinoma, the addition of two new factors (EGFR and ALK gene alterations). There were no changes in the Lung GPA for nonadenocarcinoma.
Breast cancer.
Changes in the updated Breast GPA12 compared with the original Breast GPA8,9 include the addition of two new factors, extracranial metastases and the number of brain metastases, which were found to be significant in the new breast data (n = 2,473).
Melanoma.
Changes in the updated Melanoma-mol GPA (n = 823)13,14 compared with the original Melanoma GPA (n = 481)7,9 include the addition of three new factors (BRAF status, age, and extracranial metastases).
Renal cell carcinoma.
Changes in the updated Renal GPA (n = 710)15,16 compared with the original Renal GPA (n = 286)7,9 include the addition of two new factors (extracranial metastases and hemoglobin).
GI carcinomas.
Changes in the updated GI GPA (n = 792)17,18 compared with the original GI GPA (n = 209)7,9 include the addition of three factors (age, number of brain metastases, and extracranial metastases).
DISCUSSION
Guidelines
Three current evidence-based guidelines assert the primary role of local therapies—resection, SRS, and WBRT with or without hippocampal avoidance—in the management of patients with brain metastases.21-23 These treatments are supported by multiple randomized clinical trials that have changed the standard of care.19,20,24-27
Regarding systemic therapy in patients with breast cancer with brain metastases, an ASCO Clinical Practice Guideline Update recommended that patients with human epidermal growth factor receptor 2 (HER2)–positive breast cancer and brain metastases receive appropriate local therapies—resection, SRS, or WBRT—and HER2-targeted therapies, making it clear that local therapies remain the mainstay of initial management for brain metastases.23
Each of these guidelines emphasizes the importance of understanding prognosis to optimally individualize treatment. This report refines our understanding of prognosis and summarizes newly discovered prognostic factors, confirming that prognosis varies by both histopathologic and molecular diagnosis. The updated diagnosis-specific GPA offers a more accurate method with which to estimate survival. Such information will guide clinical decision making, patient choice, and end-of-life care, and will be useful in the stratification of future clinical trials to ensure that those trials are comparing comparable patients.
Improvement in survival.
Comparison of survival between our prior summary report9 and this report shows improvement for all included diagnoses. The cause is undoubtedly multifactorial. In patients with lung adenocarcinoma with EGFR or ALK gene alterations and brain metastases, survival in the best prognostic group (GPA, 3.5-4.0) is now a remarkable 47 months. This is consistent with randomized data showing the efficacy of third-generation tyrosine kinase inhibitors (osimertinib) in these patients.28
In patients with breast cancer with brain metastases, not only has overall survival improved, but for patients in the best prognostic group (Breast GPA, 3.5-4.0), median survival is now 36 months. Furthermore, in the HER2 subtype, our data show that median survival has improved from 18 months to 25 months.8,9 For HER2-positive patients with CNS progression after initial local therapy, a number of HER2-directed regimens offer the potential for durable responses in some patients.29-31 Whereas our data set has limitations, in light of the frequent exclusions of patients with brain metastases in prospective clinical trials and the inclusion only of de novo stage IV patients in the population-based SEER registry,32 we believe that our observations represent perhaps the most persuasive evidence to date of the impact of advances in local and systemic therapy on the outcomes of patients with HER2-positive breast cancer and brain metastases in a real-world scenario.
In patients with melanoma with brain metastases, randomized phase II data show that dual immune checkpoint inhibition—ipilimumab and nivolumab—has some efficacy in the small percentage of patients without symptoms.33,34 Our data show that only 13% of patients with melanoma brain metastases do not have symptoms (KPS = 100; Table 1), again affirming the primary role of local therapies—SRS with or without craniotomy—for the vast majority of patients.
The search for drug therapies for these patients has been thwarted by poor blood-brain and brain-tumor penetrability. Another complicating factor is receptor status or gene alteration discordance between the primary tumor and brain metastasis. In breast cancer, for instance, the frequency of receptor gain for estrogen receptor, progesterone receptor, and HER2 was 18%, 14%, and 13%, respectively. The frequency of receptor loss for estrogen receptor, progesterone receptor, and HER2 was 26%, 49%, and 7%, respectively.35 If actionable receptors are lost, survival would decline. If actionable receptors are gained, targeted therapies may be of benefit. These discordance data lead to the practical clinical recommendation that whenever a patient undergoes resection of a brain metastasis, relevant receptors or gene alteration studies should be performed. This represents another layer of heterogeneity, and because the GPA incorporates molecular factors, the prognostic impact of receptor discordance is also reflected in these indices.
Has the GPA been used in clinical trials?
Multiple reports have used the GPA. Among these are four secondary analyses of prospective randomized clinical trials that have defined the current standard of care ([RTOG 9508,36 JROSG 99-1,37 EORTC 22952-26001,38 and NCCTG-N057439) and two trials that incorporated the GPA into the initial analysis (RTOG 032040 and RTOG 111941). Two trials in development stratify by the GPA: NRG-BN-2026 (SRS plus ipilimumab/nivolumab in melanoma brain metastases) and Canadian Cancer Trials Group (SRS for five to 15 brain metastases).42
How can adoption of the GPA enhance enrollment of patients with brain metastases in clinical trials?
These data have several specific implications for clinical management and future research. Patients with brain metastases have been routinely considered to be ineligible for clinical trials because of the obsolete conventional wisdom that their outcomes are uniformly grim and inclusion would mask the benefit of otherwise promising treatments. This obstructs progress and discriminates against patients with brain metastases. Including patients with brain metastases in clinical trials would be consistent with the recommendations of the ASCO-Friends of Cancer Research eligibility working group1 and the Response Assessment in Neuro-Oncology Brain Metastasis Working Group,43 and in line with US Food and Drug Administration guidance.44
The GPA can be used to enhance the enrollment of patients with brain metastases in clinical trials, including not only trials investigating treatments for brain metastases, but also a much broader spectrum of trials investigating treatments unrelated to brain metastases. We recommend the expansion of eligibility to include any otherwise eligible patient with brain metastases if their median survival, as defined by the GPA, is 1 year or greater. If the primary focus of a proposed trial is unrelated to brain metastases, the trial would ideally be—but need not be—stratified by the GPA as long as the eligibility is expanded as suggested and a subgroup analysis is performed by GPA class. Failure to stratify by the GPA, however, could render the trial uninterpretable, resulting in an enormous waste of time and resources. Improved survival for subsets of patients with brain metastases should be sufficient for enrolling them in clinical trials of systemic agents.
Definition of the EQ.
Clinical trialists should ask how exactly the GPA can be used to allow enrollment of patients with previously treated brain metastases in clinical trials. The GPA is based on patients with newly diagnosed brain metastases. Nonetheless, the GPA scale can also be used for patients with previously treated brain metastases. Kaplan-Meier GPA curves can be used to estimate remaining survival time, which is conditional on surviving some amount of time after the initial treatment of brain metastases. For example, consider a GPA of 2.0 in a patient with breast cancer who is eligible for a clinical trial 6 months after the treatment of brain metastases. Should she be eligible for that trial? Yes, because her expected survival of 6 months after the treatment of brain metastases would be approximately 75% (Fig 1). The survival percentage 1 year later—18 months after the treatment of brain metastases—is approximately 40%. Using the conditional probability equation, EQ = (survival percentage at time t + 12 months)/survival percentage at time t, where EQ = eligibility quotient and t = survival percentage at the time (months) after the treatment of brain metastases, we can calculate EQ = 40/75 = 0.54. We recommend that any patient with an expected survival of 50% or greater for at least 1 additional year (EQ ≥ 0.50) be eligible for clinical trials. This is a conservative cutoff, given that the end points of most phase I and II trials are tumor response or progression-free survival, which are usually determined before 1 year.
Guiding supportive care.
The GPA also identifies patients with the worst prognosis. Patients with a GPA of 0.0-1.0 have a poor prognosis and conservative management and/or hospice should be considered. Randomized data suggest that supportive care is not inferior to WBRT in patients with a poor prognosis.45 Patients with brain metastases from GI cancers had the highest percentage (31%) of extremely poor prognosis (GPA = 0.0-1.0).
Limitations
Limitations of this study include the retrospective design and inherent selection biases; because of selection bias, these data cannot be used to conclude the superiority of one treatment over another. In addition, patients who were not treated for their brain metastases may not be included in this database and hence these data may slightly overestimate survival for the overall population with brain metastases.
In conclusion, survival for patients with brain metastases has improved, but varies widely by histopathologic and molecular diagnosis and by diagnosis-specific prognostic factors. New prognostic factors, including molecular markers, have been identified and incorporated into the updated GPA indices. For each diagnosis, robust separation between GPA subgroups/score was discerned, confirming marked heterogeneity. The updated GPA, which was based on large contemporary cohorts, provides an accurate method with which to estimate survival and stratify clinical trials for patients with brain metastases. By including these patients in clinical trials, we expand eligibility, democratize research, enhance accrual, and speed progress. Furthermore, results of those trials will be more generalizable and thus better reflect the real world. The GPA is also useful for clinicians as they individualize treatment or guide supportive care on the basis of prognosis, regardless of whether the patient is in a clinical trial.
Lastly, instead of excluding patients with brain metastases from clinical trials, enrollment should be encouraged, and those trials should be stratified by the GPA to ensure that those trials make appropriate comparisons, and eligibility should be expanded by application of the EQ.
ACKNOWLEDGMENT
The authors thank Susan Lowry, database programmer/analyst and REDCap administrator (Clinical and Translational Science Institute, University of Minnesota), for database support and management.
DISCLAIMER
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Minnesota. The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication was solely the responsibility of the authors and does not necessarily represent the official views of the funders/sponsors.
SUPPORT
Supported by National Institutes of Health Grants No. UL1TR002494 from the National Center for Advancing Translational Sciences and P30CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and the National Center for Advancing Translational Sciences. No author received support for the work in this manuscript other than R.S. for statistical analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Paul W. Sperduto, Jing Li, Steve Braunstein, Arjun Sahgal, Ryan Shanley, David Roberge, Yi An, Minesh P. Mehta
Administrative support: Arjun Sahgal
Provision of study material or patients: Paul W. Sperduto, Jing Li, John P. Kirkpatrick, Paul D. Brown, Arjun Sahgal, David Roberge, Laurie E. Gaspar, Michael Chuong, Hidefumi Aoyama, Minesh P. Mehta
Collection and assembly of data: Paul W. Sperduto, Shane Mesko, Jing Li, Daniel Cagney, Eric Nesbit, Tim J. Kruser, Jason Chan, Steve Braunstein, Jessica Lee, John P. Kirkpatrick, Will Breen, Diana Shi, Helen A. Shih, Hany Soliman, Ryan Shanley, Emil Lou, Ashlyn Everett, Drexell H. Boggs, Laura Masucci, David Roberge, Jill Remick, Kristin Plichta, John M. Buatti, Supriya Jain, Laurie E. Gaspar, Cheng-Chia Wu, Tony J.C. Wang, John Bryant, Michael Chuong, Yi An, Veronica Chiang, Toshimichi Nakano, Hidefumi Aoyama
Data analysis and interpretation: Paul W. Sperduto, Shane Mesko, Jing Li, Ayal Aizer, Nancy U. Lin, Tim J. Kruser, Steve Braunstein, Paul D. Brown, Arjun Sahgal, Ryan Shanley, William A. Sperduto, Emil Lou, David Roberge, Supriya Jain, Laurie E. Gaspar, Cheng-Chia Wu, Tony J.C. Wang, Michael Chuong, Minesh P. Mehta
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paul W. Sperduto
Employment: Minneapolis Radiation Oncology PA
Leadership: Minneapolis Radiation Oncology PA
Stock and Other Ownership Interests: Minneapolis Radiation Oncology PA
Shane Mesko
Stock and Other Ownership Interests: Dexcom (I)
Consulting or Advisory Role: Oscar Health
Jing Li
Research Funding: Bristol Myers Squibb (Inst)
Daniel Cagney
Research Funding: NH Theraguix
Ayal Aizer
Consulting or Advisory Role: Novartis
Research Funding: Varian Medical Systems
Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, California Institute for Regenerative Medicine, Denali Therapeutics
Research Funding: Genentech, Pfizer, Seattle Genetics, Merck
Patents, Royalties, Other Intellectual Property: Royalties for chapter in UpToDate regarding management of breast cancer brain metastases; royalties, Jones & Bartlett
Tim J. Kruser
Leadership: Elsevier
Honoraria: OncLive
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
John P. Kirkpatrick
Employment: Duke University
Stock and Other Ownership Interests: ClearSight RT
Research Funding: Varian Medical Systems (Inst), BioMimetix (Inst)
Other Relationship: American Society for Radiation Oncology
Paul D. Brown
Honoraria: UpToDate
Helen A. Shih
Honoraria: UpToDate, Prime Oncology
Expert Testimony: Cleveland Clinic
Hany Soliman
Honoraria: Elekta
Arjun Sahgal
Leadership: Elekta AB
Honoraria: BrainLAB, Varian Medical Systems, AbbVie, Elekta AB
Consulting or Advisory Role: AbbVie, Merck, Roche, BrainLAB, Varian Medical Systems, Elekta AB, VieCure
Research Funding: Elekta AB (Inst), Varian Medical Systems (Inst)
Patents, Royalties, Other Intellectual Property: Clustering software for Gamma Knife treatment planning (Inst)
Travel, Accommodations, Expenses: Elekta AB, Varian Medical Systems, BrainLAB
Other Relationship: Elekta AB, Accuray, Varian Medical Systems, Elekta MR Linac Research Consortium, International Stereotactic Radiosurgery Society, BrainLAB, Medtronic Kyphon, Elekta Spine, Oligometastases and Linac Based SRS Consortia
Emil Lou
Honoraria: Novocure, GlaxoSmithKline, Boston Scientific
Consulting or Advisory Role: Novocure, Boston Scientific
Research Funding: Novocure
Travel, Accommodations, Expenses: GlaxoSmithKline
Uncompensated Relationships: Minnetronix, Nomocan Pharmaceuticals
Drexell H. Boggs
Honoraria: Varian Medical Systems
Research Funding: Varian Medical Systems, Novocure
Travel, Accommodations, Expenses: Varian Medical Systems
Laura Masucci
Research Funding: Bristol Myers Squibb (Inst)
David Roberge
Stock and Other Ownership Interests: Croton Healthcare, Arctic Fox AI, Miso Chip
Honoraria: Pfizer, EMD Serono, BrainLAB, Siemens Healthineers
Research Funding: Varian Medical Systems (Inst), Siemens Healthineers (Inst), Elekta AB (Inst), Bristol Myers Squibb (Inst)
John M. Buatti
Patents, Royalties, Other Intellectual Property: UpToDate
Laurie E. Gaspar
Employment: BannerMDA Colorado
Speakers' Bureau: Cancer Expert Now
Other Relationship: National Cancer Institute Thoracic Malignancy Steering Committee
Open Payments Link: https://openpaymentsdata.cms.gov/physician/275039/summary
Tony J.C. Wang
Stock and Other Ownership Interests: Doximity
Honoraria: AstraZeneca, Elekta AB, Novocure, Cancer Panels, University of Iowa, Rutgers University
Consulting or Advisory Role: AbbVie, Merck, AstraZeneca, Doximity, Elekta AB, Novocure
Research Funding: AbbVie, RTOG Foundation
Patents, Royalties, Other Intellectual Property: Wolters Kluwer
Travel, Accommodations, Expenses: Novocure, AbbVie, Elekta AB, Cancer Panels
Michael Chuong
Employment: Fertility and IVF Center of Miami (I)
Honoraria: Accuray, ViewRay, Sirtex
Consulting or Advisory Role: ViewRay
Speakers' Bureau: Accuray, ViewRay, Sirtex
Research Funding: AstraZeneca, MedImmune, ViewRay
Travel, Accommodations, Expenses: Accuray, ViewRay
Veronica Chiang
Consulting or Advisory Role: Monteris Medical, MRI Interventions
Speakers' Bureau: Monteris Medical
Minesh P. Mehta
Leadership: Oncoceutics
Stock and Other Ownership Interests: Oncoceutics
Consulting or Advisory Role: Tocagen, Blue Earth Diagnostics, Karyopharm, Mevion Medical Systems
Patents, Royalties, Other Intellectual Property: WARF patent 14/934,27, topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy
Uncompensated Relationships: Xcision Medical Systems, ViewRay
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research brain metastases working group. J Clin Oncol. 2017;35:3760–3773. doi: 10.1200/JCO.2017.74.0761. [DOI] [PubMed] [Google Scholar]
- 2.Harvey RD, Rubinstein WS, Ison G, et al. Impact of broadening clinical trial eligibility criteria for advanced non-small cell lung cancer patients: Real-world analysis. J Clin Oncol. 2019;37(suppl; abstr LBA108) doi: 10.1158/1078-0432.CCR-20-3857. [DOI] [PubMed] [Google Scholar]
- 3.Patel RR, Verma V, Miller AB, et al. Exclusion of patients with brain metastases from cancer clinical trials. Neuro-oncol. 2020;22:577–579. doi: 10.1093/neuonc/noz246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 5.Karacz CM, Yan J, Zhu H, et al. Timing, sites, and correlates of lung cancer recurrence. Clin Lung Cancer. 2020;21:127–135.e3. doi: 10.1016/j.cllc.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 7.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the Graded Prognostic Assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperduto PW, Kased N, Roberge D, et al. Summary report on the Graded Prognostic Assessment (GPA): An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperduto PW, Yang TJ, Beal K, et al. The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys. 2016;96:406–413. doi: 10.1016/j.ijrobp.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the Graded Prognostic Assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperduto PW, Mesko S, Li J, et al. Beyond an updated Graded Prognostic Assessment (Breast GPA): A prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107:334–343. doi: 10.1016/j.ijrobp.2020.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperduto PW, Jiang W, Brown PD, et al. The prognostic value of BRAF, C-KIT, and NRAS mutations in melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;98:1069–1077. doi: 10.1016/j.ijrobp.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: An update of the Graded Prognostic Assessment for melanoma using molecular markers (Melanoma-molGPA) Int J Radiat Oncol Biol Phys. 2017;99:812–816. doi: 10.1016/j.ijrobp.2017.06.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperduto PW, Deegan BJ, Li J, et al. Effect of targeted therapies on prognostic factors, patterns of care, and survival in patients with renal cell carcinoma and brain metastases. Int J Radiat Oncol Biol Phys. 2018;101:845–853. doi: 10.1016/j.ijrobp.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperduto PW, Deegan BJ, Li J, et al. Estimating survival for renal cell carcinoma patients with brain metastases: An update of the Renal Graded Prognostic Assessment tool. Neuro-oncol. 2018;20:1652–1660. doi: 10.1093/neuonc/noy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperduto PW, Fang P, Li J, et al. Survival and prognostic factors in patients with gastrointestinal cancers and brain metastases: Have we made progress? Transl Res. 2019;208:63–72. doi: 10.1016/j.trsl.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperduto PW, Fang P, Li J, et al. Estimating survival in patients with gastrointestinal cancers and brain metastases: An update of the Graded Prognostic Assessment for gastrointestinal cancers (GI-GPA) Clin Transl Radiat Oncol. 2019;18:39–45. doi: 10.1016/j.ctro.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 20.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson JJ, Kalkanis SN, Ryken TC. Congress of Neurological Surgeons systematic review and evidence-based guidelines for the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84:550–552. doi: 10.1093/neuros/nyy540. [DOI] [PubMed] [Google Scholar]
- 23.Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor-2-positive breast cancer and brain metastases: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36:2804–2807. doi: 10.1200/JCO.2018.79.2713. [DOI] [PubMed] [Google Scholar]
- 24.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 25.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 26.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 27.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small cell lung cancer: Data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36:2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 29.Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37:1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 31.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [Erratum: N Engl J Med 382:586, 2020] [DOI] [PubMed] [Google Scholar]
- 32.Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: A population-based study. JAMA Oncol. 2017;3:1069–1077. doi: 10.1001/jamaoncol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 34.Tawbi HAH, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperduto PW, Mesko S, Li J, et al. Estrogen, progesterone and HER2 receptor discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival Neuro-oncol22:1359-1367, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA) Int J Radiat Oncol Biol Phys. 2014;90:526–531. doi: 10.1016/j.ijrobp.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with and without whole-brain radiotherapy for brain metastases: Secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015;1:457–464. doi: 10.1001/jamaoncol.2015.1145. [DOI] [PubMed] [Google Scholar]
- 38.Churilla TM, Handorf E, Collette S, et al. Whole brain radiotherapy after stereotactic radiosurgery or surgical resection among patients with one to three brain metastases and favorable prognoses: A secondary analysis of EORTC 22952-26001. Ann Oncol. 2017;28:2588–2594. doi: 10.1093/annonc/mdx332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Churilla TN, Ballman KV, Brown PD, et al. Stereotactic radiosurgery with or without whole brain radiotherapy for limited brain metastases: A secondary analysis of the North Central Cancer Treatment Group N0574 (Alliance) randomized controlled trial. Int J Radiat Oncol Biol Phys. 2017;99:1173–1178. doi: 10.1016/j.ijrobp.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim IA, Moughan J, Sperduto PW, et al. NRG ONCOLOGY/RTOG 1119: Phase II randomized study of whole brain radiotherapy/stereotactic radiosurgery with concurrent lapatinib in patients with brain metastases from HER2-positive breast cancer—A collaborative study of NRG and KROG ( NCT01622868). 2020 American Society for Radiation Oncology Annual Meeting; October 23-29, 2020; Miami, FL (abstr). [Google Scholar]
- 42.Roberge D, Brown PD, Whitton A, et al. The future is now: Prospective study of radiosurgery for more than 4 brain metastases to start in 2018! Front Oncol. 2018;8:380. doi: 10.3389/fonc.2018.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: A guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19:e20–e32. doi: 10.1016/S1470-2045(17)30693-9. [DOI] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration Cancer clinical trial eligibility criteria: Brain metastases—Guidance for industry. https://www.fda.gov/media/121317/download
- 45.Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]