Abstract
PURPOSE
Chimeric antigen receptor (CAR) T-cell therapy of B-cell malignancies has proved to be effective. We show how the same approach of CAR T cells specific for CD30 (CD30.CAR-Ts) can be used to treat Hodgkin lymphoma (HL).
METHODS
We conducted 2 parallel phase I/II studies (ClinicalTrials.gov identifiers: NCT02690545 and NCT02917083) at 2 independent centers involving patients with relapsed or refractory HL and administered CD30.CAR-Ts after lymphodepletion with either bendamustine alone, bendamustine and fludarabine, or cyclophosphamide and fludarabine. The primary end point was safety.
RESULTS
Forty-one patients received CD30.CAR-Ts. Treated patients had a median of 7 prior lines of therapy (range, 2-23), including brentuximab vedotin, checkpoint inhibitors, and autologous or allogeneic stem cell transplantation. The most common toxicities were grade 3 or higher hematologic adverse events. Cytokine release syndrome was observed in 10 patients, all of which were grade 1. No neurologic toxicity was observed. The overall response rate in the 32 patients with active disease who received fludarabine-based lymphodepletion was 72%, including 19 patients (59%) with complete response. With a median follow-up of 533 days, the 1-year progression-free survival and overall survival for all evaluable patients were 36% (95% CI, 21% to 51%) and 94% (95% CI, 79% to 99%), respectively. CAR-T cell expansion in vivo was cell dose dependent.
CONCLUSION
Heavily pretreated patients with relapsed or refractory HL who received fludarabine-based lymphodepletion followed by CD30.CAR-Ts had a high rate of durable responses with an excellent safety profile, highlighting the feasibility of extending CAR-T cell therapies beyond canonical B-cell malignancies.
INTRODUCTION
The majority of patients with classic Hodgkin lymphoma (HL) are cured with first-line therapy, but approximately 15% of patients have primary refractory disease or relapse after an initial response to treatment.1 The standard of care for patients whose first-line therapy fails is high-dose chemotherapy followed by autologous stem cell transplantation (aSCT), with about half of patients relapsing after transplantation.2 The prognosis for these individuals is dismal, with allogeneic stem cell transplantation (alloSCT) traditionally offering the best chance for sustained remission,3 but with high morbidity and mortality.4
CONTEXT
Key Objective
Are CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-Ts) effective against Hodgkin lymphoma (HL)? To our knowledge, only 2 small clinical trials investigating the activity of CD30.CAR-Ts for HL have been published until now: our first 7-patient report in which no lymphodepleting chemotherapy was given before CD30.CAR-Ts and another 16-patient report from China using various lymphodepletion regimens. Both studies showed limited efficacy, with only 2 complete responses. Our current, larger study demonstrates that autologous CD30.CAR-Ts infused after specific fludarabine-containing lymphodepletion regimens mediate complete and durable responses in patients with relapsed HL (59% complete responses).
Knowledge Generated
We show that CD30.CAR-Ts administered after lymphodepletion into patients with relapsed or refractory HL yield a high response rate and duration, with an excellent safety profile and minimal toxicity. Importantly, most of these patients had previously progressed on prior immunotherapies, including brentuximab vedotin and checkpoint inhibitors.
Relevance
CD30.CAR-Ts have clinical activity in relapsed or refractory HL with a limited adverse event profile. There is value in exploring the use of this therapy as an earlier line of treatment for patients with this disease.
Hodgkin/Reed-Sternberg (HRS) cells universally express CD30,5,6 which has proved to be an effective and safe target for novel therapies.7 The CD30-specific antibody drug conjugate brentuximab vedotin (BV) is active in HL,8 but offers sustained remissions in fewer than a quarter of patients with relapsed or refractory (r/r) disease.9 Patients with relapsed HL can also respond to checkpoint inhibitors (CPIs)10,11 and to the adoptive transfer of cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins,12,13 underlining the susceptibility of this tumor to T-cell–mediated immune control. Adoptive transfer of chimeric antigen receptor T cells (CAR-Ts), which combines antibody-mediated antigen specificity with the effector function and self-replication of T lymphocytes, offers the opportunity to infuse large numbers of T cells with defined antigen specificity and MHC-independent tumor targeting.14
In a previous phase I study aimed at assessing safety, we reported that CAR-Ts targeting CD30 (CD30.CAR-Ts) infused without lymphodepleting preconditioning were well tolerated but produced limited antitumor activity in patients with HL,15 with an overall response rate (ORR) of only 33%. We report the outcome of 41 patients with r/r HL treated at 2 independent centers with autologous CD30.CAR-Ts after lymphodepleting chemotherapy in 2 parallel phase I/II trials.
METHODS
Study Design and Patients
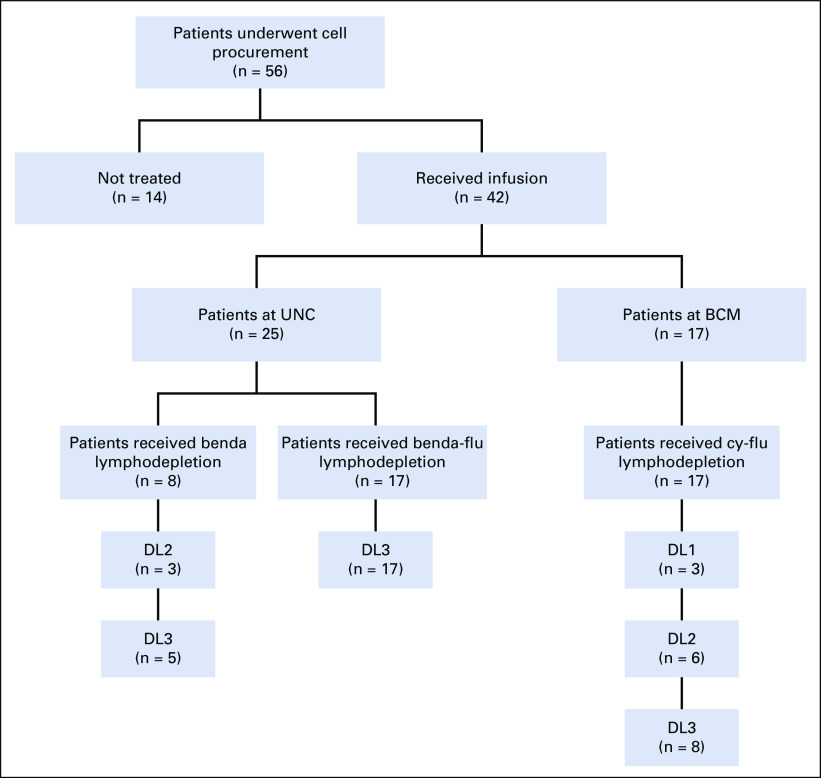
Patients were enrolled and treated at the University of North Carolina (UNC; Chapel Hill, NC) and Baylor College of Medicine (BCM; Houston, TX) in 2 institutional review board–approved independent protocols (ClinicalTrials.gov identifiers: NCT02690545 and NCT02917083, respectively) conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines for Good Clinical Practice. All patients provided written informed consent. Patients received autologous CD30.CAR-Ts manufactured at each Institution in Good Manufacturing Practice–compliant facilities (IND 14688 and IND 17272), using the same clinical grade gamma-retroviral vector and following the same Standard Operating Procedure for cell manufacturing (Appendix Table A1, online only). Patients with r/r CD30+ lymphomas who experienced disease progression after at least 2 lines of therapy were eligible for enrollment. Documented CD30 expression by immunohistochemistry was required, but there was no specific cutoff. Pediatric patients and patients with other CD30+ lymphomas were enrolled in both protocols, but we report only the outcome of 41 adult patients with HL enrolled at UNC (n = 25) and at BCM (n = 17). One patient was treated at UNC in the first cohort and 2 years later at BCM. Both infusions for this patient were included in the safety analyses, response and progression-free survival (PFS) calculations, but only the first infusion in overall survival (OS) calculations.
Bridging chemotherapy was allowed before lymphodepletion. Patients who achieved a complete remission (CR) with bridging therapy were allowed to receive lymphodepletion and CAR-T infusion at UNC, but not at BCM. For patients enrolled at BCM, lymphodepletion consisted of cyclophosphamide 500 mg/m2/day and fludarabine 30 mg/m2/day for 3 days; at UNC, bendamustine 90 mg/m2/day for 2 days was used for the first cohort, and bendamustine 70 mg/m2/day and fludarabine 30 mg/m2/day for 3 days were used for the second cohort. Infusion of CD30.CAR-Ts occurred 2-5 days after finishing lymphodepletion. Patients enrolled at BCM received 1 of 3 dose levels (2 × 107 CAR-Ts/m2, 1 × 108 CAR-Ts/m2 or 2 × 108 CAR-Ts/m2), whereas at UNC, patients received 1 × 108 CAR-Ts/m2 or 2 × 108 CAR-Ts/m2. An expansion cohort of patients at both institutions received the highest dose level of 2 × 108 CAR-Ts/m2. A second infusion of CD30.CAR-Ts was allowed in patients who had stable disease (SD) or partial response (PR) after the first treatment.
End Points and Study Procedures
The primary objective of the studies was to establish a safe dose of CD30.CAR-Ts to infuse after lymphodepletion. Secondary end points included ORR, OS, and measurement of the expansion and persistence of CD30.CAR-Ts in the peripheral blood (PB) after infusion. Data were analyzed separately in patients who received nonfludarabine-based lymphodepletion and those who received regimens containing fludarabine. Cytokine release syndrome (CRS) was graded according to the criteria of Lee et al16 and American Society for Transplantation and Cellular Therapy consensus grading.17 All other toxicities, including neurologic, were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. All patients had baseline and post-treatment positron emission tomography/computed tomography scans, with response assessed at 6-8 weeks after CD30.CAR-T infusion using the Lugano criteria.18 Calculation of the response rate and PFS included only patients who had active disease at the time of lymphodepletion. PFS was defined as days from CD30.CAR-T infusion to relapse, progression, or death. Patients without events were censored at the last follow-up date or at the cutoff date of February 14, 2020, whichever was earlier. PFS was summarized using the Kaplan-Meier method in all patients with measurable disease at the time of infusion. CD30.CAR-T expansion and cytokine levels were measured as detailed in the Data Supplement. At BCM, dose escalation followed a modified continual reassessment method with cohorts of 3, allowing a maximum of 6 patients treated at each level. At UNC, a standard 3 + 3 design was used for the 2 dose levels.
RESULTS
Patients
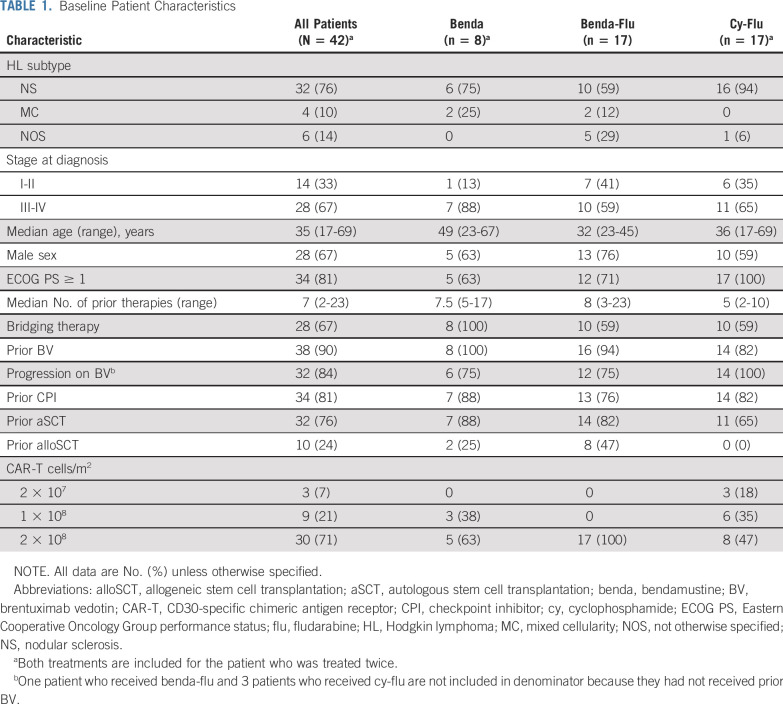
Between September 2016 and December 2019, 28 patients with HL were enrolled at UNC and 25 received CD30.CAR-Ts. Between June 2017 and November 2019, 28 patients were enrolled at BCM and 17 received CD30.CAR-Ts, including 1 patient treated at UNC 2 years earlier (Appendix Fig A1, online only). The median age for treated patients was 35 years (range, 17-69 years), and patients had a median of 7 prior lines of therapy (range, 2-23). Thirty-eight patients (90%) received prior BV, 32 (84%) of whom had experienced disease progression on BV. Thirty-four patients (81%) received prior CPIs, 32 (76%) received prior aSCT, and 10 (24%) received prior alloSCT. Twenty-eight patients (67%) received bridging therapy between cell collection and lymphodepletion (Table 1). The most common therapies used for bridging were bendamustine (32%), nivolumab (25%), BV (7%), and gemcitabine-based regimens (11%).
TABLE 1.
Baseline Patient Characteristics
Safety
There were no dose-limiting toxicities associated with CD30.CAR-T infusions in either study. For the safety assessment, the patient who was treated at UNC and later at BCM was considered twice (42 treatments total). CRS was observed in 10 patients (24%) and was more frequent with the cyclophosphamide-based conditioning regimen than with the bendamustine-based regimen (41% v 12%; Table 2). All CRS events were grade 1 and resolved spontaneously with no requirement for tocilizumab and/or steroid administration. The median time of onset of CRS was day 10 (range, 7-24 days) and median duration was 4 days (range, 1-6 days). Cytokines associated with the occurrence of CRS were elevated in the plasma of patients developing clinical signs of CRS (Appendix Fig A2, online only). Neurotoxicity was not observed. Twenty patients (48%) developed a nonpruritic, nontender, maculopapular skin rash, which was more commonly found in patients receiving cyclophosphamide (82%) versus bendamustine (24%; Fig 1). None of the rashes required specific treatment, and all resolved spontaneously within 7-10 days. The majority of grade 3 or higher toxicities (Table 2) reported during the first 6 weeks were hematologic and consistent with toxicities previously described in patients with lymphoma receiving lymphodepleting chemotherapy.19 One patient experienced grade 3 acute kidney injury and hypotension after starting chemotherapy and did not complete the scheduled lymphodepletion, but when symptoms resolved, was able to receive CD30.CAR-Ts. Grade 3-4 neutropenia that had not resolved by day 28 post–CAR-T infusion occurred in 4 patients (10%; Table 2); however, all resolved their neutropenia by day 90 without ongoing growth factor support. Ten patients (24%) had grade 3-4 thrombocytopenia that had not resolved by day 28. Four patients (10%) were platelet transfusion independent, but had grade 3-4 thrombocytopenia at month 3, with 1 patient having persistent grade 3, which improved to grade 2 at 1 year and grade 1 at 2 years post-therapy.
TABLE 2.
Grade 3 or Higher Adverse Events and Adverse Events of Special Interest
FIG 1.
Skin rash and biopsy. (A-B) Examples of the characteristic rash that develops in some patients given CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-T cells). (C-E) Biopsy revealed a spongiotic dermatitis with occasional eosinophils (epidermal edema with few intraepidermal blisters filled with neutrophils and eosinophils, with increased lymphocytes within the papillary dermis and occasional eosinophils within the deeper dermis surrounding skin adnexa). Immunohistochemistry demonstrated a mixed population of lymphocytes with a CD4:CD8 ratio of approximately 1.5:1. Apart from very rare scattered cells, CD30 stain was negative. Quantitative polymerase chain reaction for the CD30.CAR transgene was positive in DNA isolated from biopsy material.
Efficacy
The ORR for the 37 evaluable patients was 62% (Table 3; Appendix Fig A3, online only). Thirty-four patients underwent lymphodepletion containing fludarabine (17 together with bendamustine at UNC and 17 with cyclophosphamide at BCM). Of these 34 patients, 2 at UNC were in CR at the time of infusion, maintained CR, and were not included in the efficacy analysis. Of the remaining 32 patients evaluable for disease response, the ORR was 72%, with 19 patients (59%) achieving CR, 4 (13%) achieving PR, 3 (9%) showing SD, and 6 (19%) experiencing progressive disease (PD) at the time of the first response assessment. At BCM, the ORR for patients treated at the target dose level was similar to that of patients at lower dose levels (63% v 67%, respectively). Eight patients (5 active and 3 inactive disease) enrolled at UNC received lymphodepletion using bendamustine alone before CD30.CAR-T infusion. None of them showed objective clinical responses when treated with active disease (Table 3). All patients treated at UNC who were in CR from bridging therapy maintained their response at the first response assessment, with 1 patient still in CR 3 years after treatment. The 1-year OS for all 41 patients (counting the patient treated at UNC and subsequently at BCM only once) was 94% (95% CI, 79% to 99%), and no significant differences were observed between lymphodepletion regimens (Fig 2A; Appendix Fig A4A, online only).
TABLE 3.
Clinical Responses in Patients With Measurable Disease at the Time of Treatment
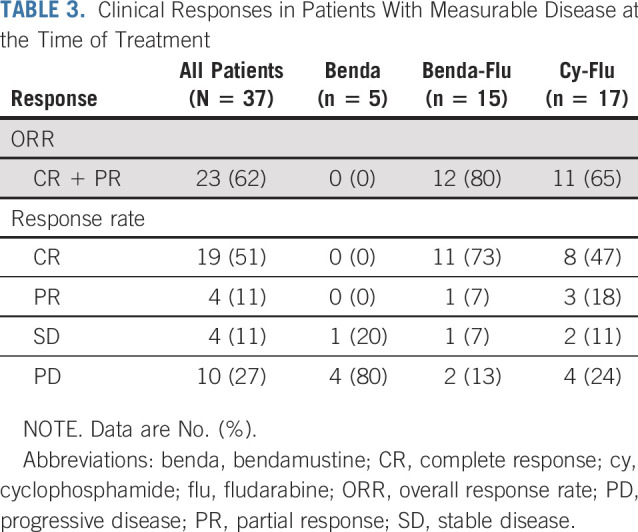
FIG 2.
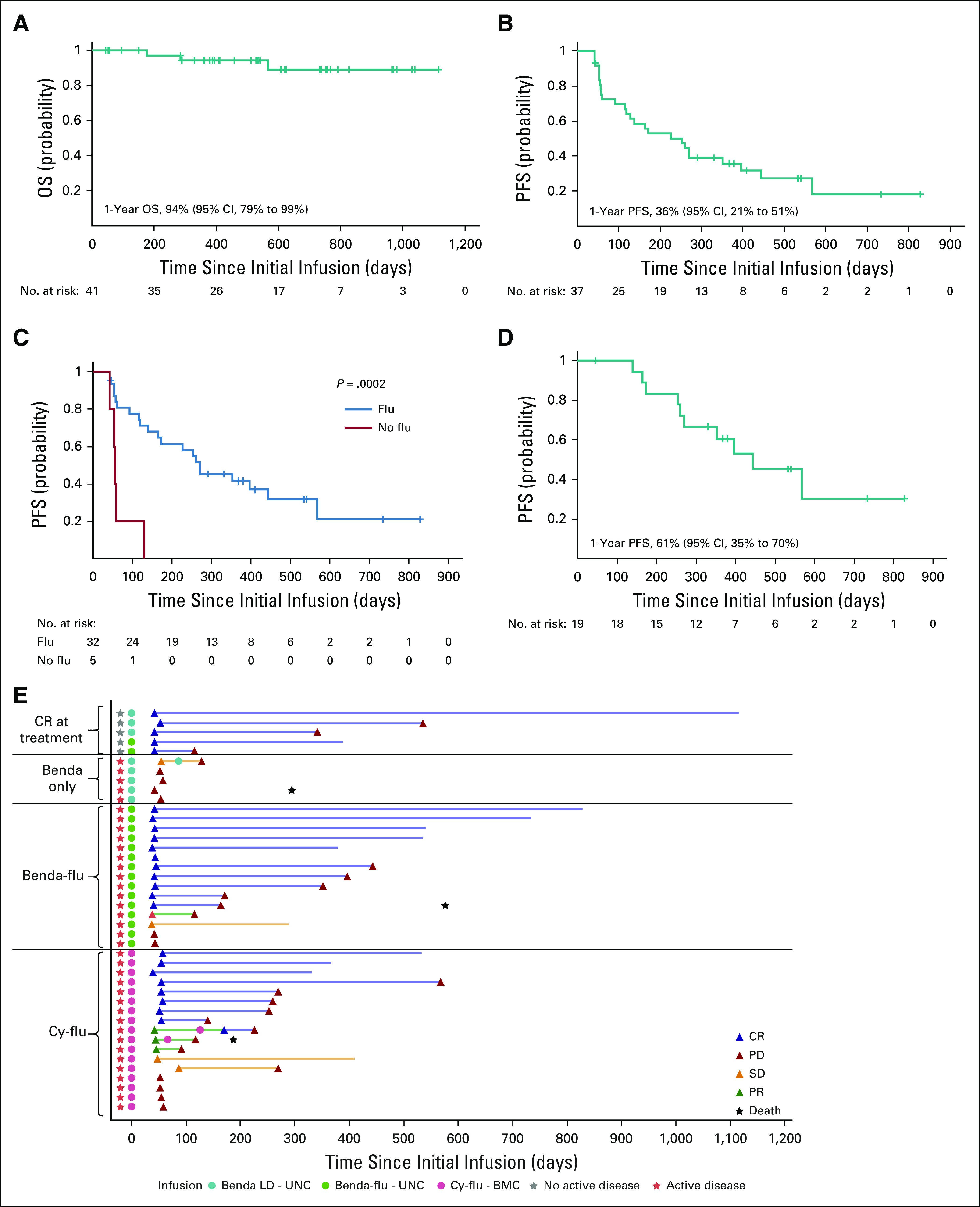
Clinical outcome. (A) Overall survival (OS) for the 41 patients receiving lymphodepletion with bendamustine alone (benda LD), bendamustine and fludarabine (benda-flu LD), or cyclophosphamide and fludarabine (cy-flu LD). One patient was treated with benda LD before CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-T cells) only at University of North Carolina and then 2 years later received cy-flu LD before CD30.CAR-T cells at Baylor College of Medicine. For the OS analysis, this patient was counted only according to the first treatment. (B) Progression-free survival (PFS) for all 37 patients with measurable disease at the time of treatment. (C) PFS for the 37 patients with measurable disease at the time of treatment and receiving lymphodepletion with bendamustine alone (no flu, red line) or fludarabine containing lymphodepleting regimens (flu, blue line). For the PFS comparison, the patient who received benda before CD30.CAR-T cells only and then cy-flu before CD30.CAR-T cells 2 years later was counted in each treatment group. (D) PFS for the 19 patients with measurable disease at the time of treatment and achieving complete response (CR). The median PFS for these patients was 444 days (95% CI, 260 to infinity). (E) Swimmer plot for all 42 patients (including the one treated at the both institutions). Gray stars indicate patients treated in CR.
Three patients died of PD. The 1-year PFS for patients with measurable disease at the time of treatment was 36% (95% CI, 21% to 51%; Fig 2B) and significantly longer in patients receiving a fludarabine-based conditioning versus bendamustine alone (P = .0002; Fig 2C). The 1-year PFS for patients with measurable disease was 41% (95% CI, 24% to 58%) for all patients who received fludarabine-based lymphodepletion and 61% (95% CI, 35% to 79%) for those who achieved CR as initial response (Appendix Fig A4B). The median PFS for the 19 patients with active disease at the time of lymphodepletion/infusion who achieved CR was 444 days (95% CI, 26 to infinity; Fig 2D). Ten patients with active disease at the time of treatment had not experienced disease progression after therapy at the time of data analysis, including 5 who continue to be in CR more than a year (15, 16, 16, 22, and 25 months) after initial response assessment (Fig 2E). The patient treated at UNC and later at BCM experienced PD after bendamustine and 1 × 108 CAR-Ts/m2, but achieved CR with cyclophosphamide and fludarabine followed by 2 × 108 CAR-Ts/m2 (both treatment instances are included in PFS analyses). Three patients received a second infusion without lymphodepletion, with 2 having PD at subsequent assessment and 1 having CR but progression several months later, suggesting limited benefit of a second infusion of CD30.CAR-Ts without lymphodepletion.
CD30.CAR-T Cell Expansion and Persistence
In patients receiving fludarabine-based lymphodepletion, CD30.CAR-Ts in the PB peaked within the first 2-3 weeks post infusion. CD30.CAR-T persistence, measured as area under the curve, was higher in patients receiving 2 × 108 CAR-Ts/m2 than in patients receiving 2 × 107 CAR-Ts/m2 or 1 × 108 CAR-Ts/m2 (P < .001) regardless of type of lymphodepletion (Fig 3A; Appendix Fig A5A, online only). Polymerase chain reaction results correlated with flow cytometry (Fig 3B; Appendix Fig A5B). A positive correlation was observed between the number of infused CD30.CAR-Ts and peak expansion (P = .008), which, however, did not correlate with clinical response. Serum CCL17, a predictive marker of early response assessment in HL,20 was elevated before CAR-T infusion and had a significant decrease (P = .009) after treatment in responding patients (Fig 3C). A significant increase of the homeostatic cytokines interleukin (IL)-7 and IL-15 was detected over a period of 4 weeks post lymphodepletion with bendamustine and fludarabine (Fig 3D). Fludarabine was essential in promoting the homeostatic cytokine milieu because patients receiving fludarabine-based regimens showed higher levels of IL-7 (P = .013) and IL-15 (P = .003; Appendix Fig A5C), which also corresponded with higher CD30.CAR-T persistence (P = .016; Appendix Fig A5), versus bendamustine alone. Biopsies obtained at the time of relapse demonstrated continued expression of CD30 by tumor cells.
FIG 3.

Detection of CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-T cells) and thymus and activation-regulated chemokine (TARC, also known as CCL17) in the peripheral blood. (A) Detection of CD30.CAR-T cell molecular signals by quantitative polymerase chain reaction in patients treated with fludarabine (flu)-based lymphodepletion regimens. Data points represent postinfusion intervals after the administration of CD30.CAR-T cells at different dose levels and type of lymphodepletion. Lines denote mean ± SEM. (B) Percent of CD3+ CAR+ cells (after gating on CD45+ cells) detected in the peripheral blood using flow cytometry for treated patients at the indicated time points. Each dot denotes a single patient, and the line represents the mean value. (C) Detection of TARC in the serum by specific enzyme-linked immunosorbent assay for responding patients (achieving either complete remission [CR] or partial response [PR]) versus nonresponder patients (showing stable disease [SD] or progressive disease [PD]) pre-CAR-T cell infusion versus 6 weeks post–CAR-T cell infusion. P values shown are 2-tailed paired t-test. (D) Detection of interleukin (IL)-2, IL-7, and IL-15 in the plasma after lymphodepletion with bendamustine (benda) and flu in 4 representative patients. cy, cyclophosphamide; ns, nonsignificant; UNC, University of North Carolina.
DISCUSSION
The outcome for patients with r/r HL whose salvage therapy has failed is poor.21 In this independent 2-center study, we demonstrated that autologous CD30.CAR-Ts infused after fludarabine-based lymphodepletion is well tolerated and have significant clinical activity in heavily pretreated patients with r/r HL, with an ORR of 72%, CR rate of 59%, and PFS of 41% at the 1-year follow-up. Excellent responses were seen despite the substantial number of prior therapies that patients received, which included the most recent immunotherapy-based approaches, BV, and/or CPIs.
Recent trials have assessed newer therapies in patients with r/r HL. Younes et al8 administered BV to patients who experienced progression after aSCT or at least 2 prior regimens, reporting an ORR of 75%, CR rate of 34%, and median duration of response of 20.5 months for patients achieving CR8. The Checkmate 205 study evaluated treatment with nivolumab in patients with HL who experienced disease progression after aSCT,22 reporting an ORR of 69%, CR rate of 16%, and PFS of 22.2 months in patients achieving CR. Our ORR of 72%, CR rate of 59%, and median PFS of 14.8 months for patients achieving CR after infusion of CD30.CAR-Ts compares favorably with those receiving BV and CPI therapy, with our population having been more heavily pretreated. Of note, 14 of the 29 patients in whom BV failed achieved CR post–CAR-Ts. The 1-year OS of 94% highlights that even patients who had disease progression after CAR-T therapy may have a prolonged life expectancy. Although it is possible that there is a selection bias in patients who are able to participate in cell therapy clinical trials having more indolent disease, OS in this trial was similar to patients who experienced disease progression after CPI therapy22,23 and likely reflects instead the natural history of HL. Patients received different treatments after relapse. Additional studies are required to assess whether the effect of CAR-T therapy on tumor biology and the immune response will affect the tumor’s susceptibility to subsequent therapies.
Treatment with CD19- or B-cell maturation antigen (BCMA)-redirected CAR-Ts preceded by lymphodepletion achieved robust clinical responses in patients with acute lymphoblastic leukemia (ALL),24 diffuse large B-cell lymphoma,19,25 and multiple myeloma.26 We found that targeting CD30 in HL with CAR-Ts can be similarly effective. Although CD30.CAR-Ts showed modest activity in HL when infused without lymphodepletion,15 robust clinical responses were achieved when these cells were infused in hosts lymphodepleted with fludarabine-containing regimens. In contrast, no objective clinical responses were observed when lymphodepletion contained only bendamustine.27 Although fludarabine and cyclophosphamide do not have significant anti-HL activity, bendamustine is a potential therapy for r/r HL, with an ORR of 53%. However, this benefit is generally short lived, with a median duration of response of 5 months.28 Even though most patients in our study had chemotherapy-refractory disease, with almost half previously treated with bendamustine, responses to CD30.CAR-Ts were more durable than responses to bendamustine. Moreover, there was a significant benefit with the addition of fludarabine to bendamustine. Therefore, it is unlikely that the antitumor activity of bendamustine has a meaningful contribution to the responses presented here.
We established a direct correlation between the number of infused CAR-Ts and their persistence. Our findings are consistent with the data reported in patients with multiple myeloma treated with BCMA-redirected CAR-Ts,26 but contrast with those with CD19-specific CAR-Ts in patients with ALL, in whom clinical efficacy seems independent of the number of infused CAR-Ts.24 Interestingly, the correlation between CAR-T numbers and persistence did not extend to clinical outcomes in our study. These differences demonstrate the difficulty of correlating outcomes across CAR-T studies that use different targets and diverse single-chain variable fragments, and treat patients with different tumor types. Previous clinical studies also suggested a correlation between the development of CRS and the efficacy of CAR-T therapy. This was not evident in the current study, with a modest incidence and intensity of CRS. CRS is mediated at least in part by induction of a proinflammatory milieu by myeloid cells. Patients with HL are generally immunosuppressed,29,30 which may play a role in mitigating CRS without impairing effector T-cell responses, thus calling for future in-depth evaluations of the dysregulated microenvironment of HL pre– and post–CAR-T therapy. In addition, HL is unique in that there is only a small proportion of malignant CD30+ cells in the tumor.31
Other toxicities included transient skin rash. Skin keratinocytes have modest expression of CD30, which can be found in some inflammatory conditions,32 and CD30.CAR-Ts may transiently target these or other cells in skin. Irrespective of the mechanism, the rash was largely asymptomatic and transient, and not associated with long-term toxicity. More work is needed to characterize the relationship between cutaneous toxicity and CD30.CAR-Ts. Moreover, a small proportion of patients had prolonged cytopenias, particularly thrombocytopenia. Although in most cases, these can be attributed to lymphodepletion, some patients had more prolonged cytopenias, including 4 with grade 3-4 thrombocytopenia for greater than 3 months, which could not be explained by the acute effects of lymphodepletion alone. Although CD30 is expressed on activated hematopoietic stem and progenitor cells, these are generally protected from CAR-T attack because of low levels of antigen expression and intrinsic protection mechanisms.33 Our study supports these findings because prolonged cytopenias were rare, self-limiting, and without significant complications. We propose that prolonged cytopenias are more likely related to limited hematopoietic reserve due to extensive prior therapy, which needs to be evaluated in larger clinical studies. No other significant on-target toxicities were observed in patients infused with CD30.CAR-Ts, even at the highest dose, including no neurologic adverse effects.
Disease relapse after achieving CR post–CAR-T therapies can occur because of antigen escape and/or insufficient persistence of the CAR-Ts at the tumor site. Although our protocol did not mandate tumor biopsies at relapse, CD30 expression was retained in relapsing tumors, suggesting that recurrence is attributable to insufficient persistence of CAR-Ts within the highly immunosuppressive tumor microenvironment of HL. The expression of programmed death-1 on CD30.CAR-Ts15 indicates that these cells remain susceptible to the programmed death-ligand 1 inhibition exerted by HRS cells and surrounding infiltrating macrophages at the tumor site.10 CPIs have efficacy in treating patients with HL. Future studies could investigate whether the combination of CD30.CAR-Ts and CPIs improves the likelihood of patients remaining in CR post-therapy.
In summary, in a 2-center study of a heavily pretreated population of patients with HL, administration of 2 × 108 CD30.CAR-Ts/m2 after lymphodepletion (with an alkylating agent and fludarabine) produced remarkable antitumor activity without significant toxicity. This approach provides a new therapeutic option that could be administered in earlier stages of r/r disease.
ACKNOWLEDGMENT
We thank the patients who participated in these trials and their families. We also thank Todd Maguire, John West, Desirae Shelley, and the staff of the Lineberger Advanced Cellular Therapeutics Facility (University of North Carolina [UNC], Chapel Hill, NC); Huimin Zhang, Zhuyong Mei, Olga Dakhova and the staff of the Center for Cell and Gene Therapy facility (Baylor College of Medicine [BCM], Houston, TX); Spencer Laing, E. Samantha Sharf, Deborah Covington, and Maurice Alexander of the Cellular Therapeutic Program (UNC, Chapel Hill, NC), Mrinalini Bilgi and Vicky Torrano of the Center for Cell and Gene Therapy (BCM, Houston, TX) for help with clinical trial management; George Hucks (UNC, Chapel Hill, NC) and George Carrum and Rammurti Kamble (BCM, Houston, TX) for referring patients. We thank Dr Alessandro Rambaldi (Department of Oncology and Hematology, Azienda Socio Sanitaria Territoriale Papa Giovanni XXIII, Bergamo, Italy) for the critical revision of the manuscript. We thank Bambi J. Grilley, Kaitlin Morrison for their contribution to regulatory support. We also thank Kenneth Cornetta and the National Gene Vector Biorepository at the Indiana University School of Medicine for postinfusion testing of the product for replication-competent retrovirus, the data and safety monitoring board, and the hematology-oncology and critical care faculty for providing clinical support; and the nurses, residents, and fellows at the 2 institutions.
Appendix
Methods
Study design and patients.
To generate CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-Ts), autologous peripheral blood (PB) mononuclear cells were stimulated with immobilized CD3 and CD28 agonistic antibodies, and an average of 2 ×107 activated T cells were transduced with the gamma-retroviral vector encoding the CD30.CAR, including the CD28 costimulatory endodomain, and expanded using recombinant cytokines interleukin (IL)-7 and IL-1515 (Appendix Table A1, online only).
Study oversight.
The studies were approved by the local institutional review boards at the University of North Carolina (UNC) and Baylor College of Medicine (BCM).
End points and study procedures.
Cytokines, such as interleukin (IL)-7, IL-15, IL-6, were measured in the plasma by Luminex assay (R&D Systems, Minneapolis, MN), whereas serum CCL17 (thymus and activation-regulated chemokine or TARC) was measured by a specific enzyme-linked immunosorbent assay. Log values were analyzed and a t test used for those comparisons because stem and leaf plot of log values looked normal, and the sample variances were not different. The persistence of CD30.CAR-Ts in vivo was determined by quantitative polymerase chain reaction (PCR) and flow cytometry from peripheral blood samples collected before and at different time points after infusion, as previously described.15 PCR data were log transformed and the area under the curve calculated up to 8 weeks post–CAR-T infusion for each cohort.
Results
Patients.
For the 3 patients enrolled at UNC who did not receive treatment, 2 elected not to proceed with the clinical trial, and 1 who was heavily pretreated with prior autologous stem cell transplantation, allogeneic stem cell transplantation (alloSCT), and multiple donor lymphocyte infusions failed CAR-T cell manufacturing (Appendix Fig A1). Of the 11 patients who did not receive treatment at BCM, 5 achieved remission or had too little disease to be treated since procurement because of bridging therapy; 4 were unable to receive treatment because of abnormal pulmonary function tests, patient preference, lack of compliance, or opting for alloSCT; and 2 died of rapidly progressive disease before receiving lymphodepletion (Appendix Fig A1).
Safety.
Grade 3 or higher toxicities included lymphopenia (100%), leukopenia (57%), neutropenia (48%), thrombocytopenia (26%), anemia (12%), hypoalbuminemia (7%), hyponatremia (5%), hyperkalemia, dyspnea, pharyngitis, lung infection, and headache (all 2%; Table 2).
CD30.CAR-T cell expansion and persistence.
No correlation was observed between the CD8+ cell content of the cellular product and the peak value of PCR. Five of 8 patients with available data at/around relapse had CAR-Ts detectable in PB, albeit at low levels (range, 10-493 copies/μg of DNA).
Fig A1.
Flowchart for CD30-specific chimeric antigen receptor (CAR) T cell (CD30.CAR-T cell) trials including cell procurement and treatment. Dose level (DL) 1, 2 × 107 CAR-T cells/m2; DL2, 1 × 108 CAR-T cells/m2; DL3, 2 × 108 CAR-T cells/m2. One patient received bendamustine (benda) lymphodepletion before CD30.CAR-T cells at University of North Carolina (UNC), and 2 years later, received cyclophosphamide-fludarabine (cy-flu) lymphodepletion before CD30.CAR-T cells at Baylor College of Medicine (BCM).
FIG A2.
(A) Detection of biologic markers of cytokine release syndrome (CRS). Fold difference in the plasma levels of (A) interleukin (IL)-6 and (B) IL-1Ra pre–CD30-specific chimeric antigen receptor (CAR) T cell (CD30.CAR-T cell) infusion and 2 weeks post–CD30.CAR-T cell infusion or at the time of grade 1 CRS. Each dot denotes a single patient, and the line represents the mean value. (C) Peak levels of plasma C-reactive protein (CRP) in patients developing grade 1 CRS versus patients who did not develop CRS. Significance determined using 2-tailed unpaired t test.
FIG A3.
Antitumor effects of CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-T cells). Two patients with relapsed Hodgkin lymphoma: (A) one with several bone lesions in the pelvis and elsewhere, and (B) the other with numerous hypermetabolic lymph nodes, including cervical, right axillary, mediastinal, portacaval, and retroperitoneal before treatment. Six weeks after CD30.CAR-T cell infusion, positron emission tomography–computed tomography scan showed complete responses to therapy (Deauville 2).
FIG A4.

(A) Overall survival (OS) and (B) progression-free survival (PFS) of 37 patients receiving lymphodepletion (LD) with bendamustine alone (benda LD; blue line), benda and fludarabine (benda-flu; red line), or cyclophosphamide and flu (cy-flu; black line). For this PFS comparison, the patient who received benda LD before CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-T cells) only and then cy-flu LD before CD30.CAR-T cells 2 years later was counted in each treatment group. BCM, Baylor College of Medicine; UNC, University of North Carolina.
FIG A5.
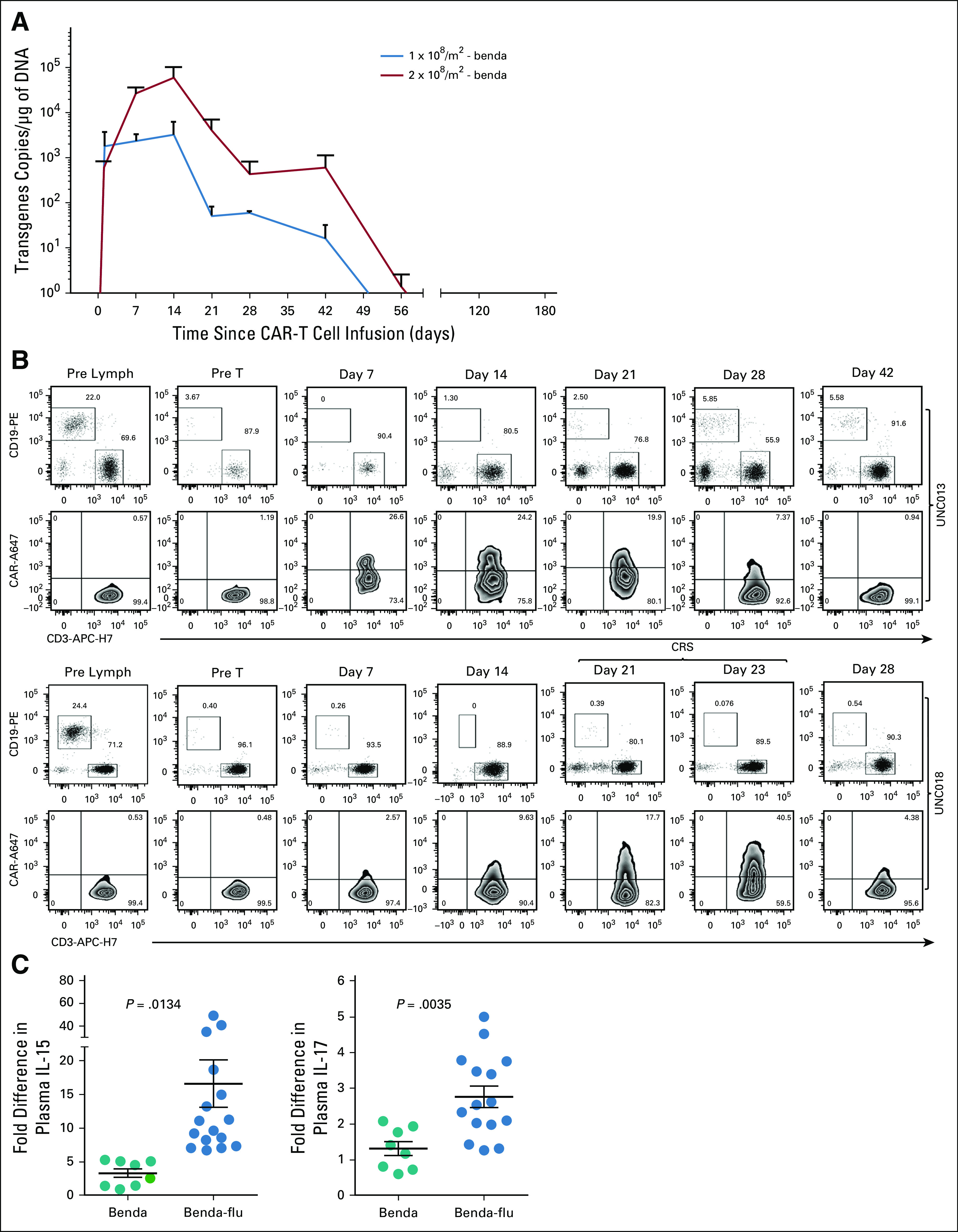
Detection of CD30-specific chimeric antigen receptor (CAR) T cells (CD30.CAR-T cells) in the peripheral blood. (A) Detection of CD30.CAR-T cell molecular signals by quantitative polymerase chain reaction in patients receiving bendamustine (benda) alone as a lymphodepletion regimen. Data points represent postinfusion intervals after the infusion of CD30.CAR-T cells at different dose levels. Lines denote mean ± SEM for the various dose levels and lymphodepletion regimens. (B) Flow plots of CD30.CAR-T cell detection in the peripheral blood of 2 representative patients using flow cytometry for patients infused with 2 × 108 CAR-T cells/m2 post–benda-fludarabine (flu) at the indicated time point. Upper plots for each donor were gated on lymphocytes and on CD45bright cells. Lower plots were gated on CD3+ cells. (C) Fold increase in plasma levels of interleukin (IL)-15 and IL-7 pre- and postlymphodepletion with benda versus lymphodepletion with flu and before CD30.CAR-T cell infusions. Each dot denotes a patient, and the line represents the mean value. P values shown are 2-tailed unpaired t test. CRS, cytokine release syndrome; UNC, University of North Carolina.
TABLE A1.
CD30.CAR-T Cell Product Characteristics for Each Patient Enrolled
SUPPORT
Supported at University of North Carolina (UNC) by Grant No. RO1 HL114564 and University Cancer Research Fund at the Lineberger Comprehensive Cancer Center, and at Baylor College of Medicine by National Cancer Institute Grants No. P50 CA126752 and P30 CA125123, and a Specialized Center of Research grant from the Leukemia & Lymphoma Society. N.S.G. was supported by UNC Oncology Grant No. K12 (K12 CA120780).
CLINICAL TRIAL INFORMATION
NCT02690545 (ATLAS) and NCT02917083 (RELY-30).
See accompanying article on page 3816
AUTHOR CONTRIBUTIONS
Conception and design: Carlos A. Ramos, Natalie S. Grover, Anastasia Ivanova, Thomas C. Shea, Cliona M. Rooney, Steven I. Park, Bambi J. Grilley, Kaitlin Morrison, Malcolm K. Brenner, Jonathan S. Serody, Gianpietro Dotti, Helen E. Heslop, Barbara Savoldo
Provision of study materials or patients: Carlos A. Ramos, Natalie S. Grover, Thomas C. Shea, Adrian P. Gee, Birju Mehta, Faith B. Buchanan, Gianpietro Dotti, Barbara Savoldo
Collection and assembly of data: Carlos A. Ramos, Natalie S. Grover, Premal D. Lulla, Anastasia Ivanova, Thomas C. Shea, Christopher Dittus, Steven I. Park, Adrian P. Gee, Paul W. Eldridge, Kathryn L. McKay, Birju Mehta, Catherine J. Cheng, Faith B. Buchanan, Malcolm K. Brenner, Jonathan S. Serody, Gianpietro Dotti, Barbara Savoldo
Data analysis and interpretation: Carlos A. Ramos, Natalie S. Grover, Anne W. Beaven, Meng-Fen Wu, Anastasia Ivanova, Thomas C. Shea, Tao Wang, Cliona M. Rooney, Steven I. Park, Jonathan S. Serody, Gianpietro Dotti, Helen E. Heslop, Barbara Savoldo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carlos A. Ramos
Consulting or Advisory Role: Novartis
Research Funding: Tessa Therapeutics
Natalie S. Grover
Stock and Other Ownership Interests: Sangamo Therapeutics
Consulting or Advisory Role: Seattle Genetics, Tessa Therapeutics
Research Funding: Genentech
Uncompensated Relationships: Tessa Therapeutics
Anne W. Beaven
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Research Funding: Roche (Inst), Spectrum Pharmaceuticals (Inst), Seattle Genetics (Inst), MorphoSys (Inst), Genentech (Inst)
Premal D. Lulla
Stock and Other Ownership Interests: Johnson & Johnson (I)
Research Funding: Tessa Therapeutics
Thomas C. Shea
Consulting or Advisory Role: Amgen, Jazz
Research Funding: Novartis (Inst), Pharmacyclics (Inst), Jazz (Inst)
Travel, Accommodations, Expenses: Amgen, Jazz
Cliona M. Rooney
Employment: Tessa Therapeutics
Leadership: Tessa Therapeutics (I)
Stock and Other Ownership Interests: Marker (I), Marker, Bluebird Bio (I)
Consulting or Advisory Role: Tessa Therapeutics
Research Funding: Tessa Therapeutics
Patents, Royalties, Other Intellectual Property: Royalties from Takeda, Allogene, and Bellicum
Travel, Accommodations, Expenses: Tessa Therapeutics, Tessa Therapeutics (I)
Christopher Dittus
Research Funding: Seattle Genetics, AstraZeneca, Genentech
Patents, Royalties, Other Intellectual Property: Royalty payment from Springer Publishing for a medical textbook publication
Steven I. Park
Consulting or Advisory Role: Bristol Myers Squibb, Rafael Pharmaceuticals, G1 Therapeutics, Teva
Speakers' Bureau: Gilead Sciences, Seattle Genetics
Research Funding: Seattle Genetics, Bristol Myers Squibb
Bambi J. Grilley
Employment: South Texas Nuclear Pharmacy (I), Q B Regulatory Consulting
Leadership: Allovir
Stock and Other Ownership Interests: Allovir
Travel, Accommodations, Expenses: TESSA
Kaitlin Morrison
Consulting or Advisory Role: Vesselon
Malcolm K. Brenner
Stock and Other Ownership Interests: Bluebird Bio, Tessa Therapeutics, Maker Therapeutics, Allovir
Honoraria: Merck (I)
Consulting or Advisory Role: Tessa Therapeutics, Memgen, Torque, Umun, NantKwest, Poseida Therapeutics, Cell Medica (I), Formula Pharmaceuticals, Walking Fish Therapeutics, Tscan, Allogene, Maker Therapeutics, Turnstone Biologics
Research Funding: Cell Medica (I)
Travel, Accommodations, Expenses: Merck (I), Tessa Therapeutics, Bluebird Bio, Torque
Jonathan S. Serody
Stock and Other Ownership Interests: Incyte, Bellicum, Genentech
Consulting or Advisory Role: Pique
Research Funding: Merck, Incyte, Glaxo Smith Kline
Patents, Royalties, Other Intellectual Property: I have pending patents for the use of IL-C2 for the treatment/prevention of acute graft versus host disease of the lower GI tract. I also have a patent pending for the use of stimulator of interferon genes (STING) agonists with CAR T cells to treat solid tumors (Inst)
Gianpietro Dotti
Consulting or Advisory Role: Molmed, Bellicum Pharmaceutical, Tessa Therapeutics
Research Funding: Bellicum Pharmaceuticals, Cell Medica
Patents, Royalties, Other Intellectual Property: Patents in the field of T/NKT cell therapies and royalties derived from licensed patents
Helen E. Heslop
Stock and Other Ownership Interests: Marker Therapeutics, Allovir
Consulting or Advisory Role: Gilead Sciences, Novartis, Kiadis Pharma, Tessa Therapeutics, Marker Therapeutics, PACT Pharma
Research Funding: Cell Medica (Inst), Tessa Therapeutics (Inst)
Barbara Savoldo
Consulting or Advisory Role: Tessa Therapeutics
Research Funding: Cell Medica (I), Bluebird Bio (I), Bellicum pharmaceutical (I)
Patents, Royalties, Other Intellectual Property: Patent filed for some CAR molecules (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Glimelius I, Ekberg S, Jerkeman M, et al. Long-term survival in young and middle-aged Hodgkin lymphoma patients in Sweden 1992-2009-trends in cure proportions by clinical characteristics. Am J Hematol. 2015;90:1128–1134. doi: 10.1002/ajh.24184. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 3.Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: A retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115:3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 4.Devetten MP, Hari PN, Carreras J, et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15:109–117. doi: 10.1016/j.bbmt.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dürkop H, Latza U, Hummel M, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 6.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 7.Grover NS, Savoldo B. Challenges of driving CD30-directed CAR-T cells to the clinic. BMC Cancer. 2019;19:203. doi: 10.1186/s12885-019-5415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34:3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollard CM, Tripic T, Cruz CR, et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J Clin Oncol. 2018;36:1128–1139. doi: 10.1200/JCO.2017.74.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu Rev Med. 2016;67:165–183. doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos CA, Ballard B, Zhang H, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127:3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DW. doi: 10.1182/blood-2014-05-552729. Gardner R, Porter DL, et al: Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [Erratum: Blood 126:1048, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidetti A, Mazzocchi A, Miceli R, et al. Early reduction of serum TARC levels may predict for success of ABVD as frontline treatment in patients with Hodgkin Lymphoma. Leuk Res. 2017;62:91–97. doi: 10.1016/j.leukres.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Mehta-Shah N, Bartlett NL. Management of relapsed/refractory classical Hodgkin lymphoma in transplant-ineligible patients. Blood. 2018;131:1698–1703. doi: 10.1182/blood-2017-09-772681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134:1144–1153. doi: 10.1182/blood.2019000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. doi: 10.1056/NEJMoa1407222. Maude SL, Frey N, Shaw PA, et al: Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [Erratum in N Eng J Med 374:998, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turtle CJ, Hanafi L-A, Berger C, et al. Addition of fludarabine to cyclophosphamide lymphodepletion improves in vivo expansion of CD19 chimeric antigen receptor-modified T cells and clinical outcome in adults with B cell acute lymphoblastic leukemia. Blood. 2015;126:3773. [Google Scholar]
- 28.Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31:456–460. doi: 10.1200/JCO.2012.45.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 30.Baráth S, Aleksza M, Keresztes K, et al. Immunoregulatory T cells in the peripheral blood of patients with Hodgkin’s lymphoma. Acta Haematol. 2006;116:181–185. doi: 10.1159/000094678. [DOI] [PubMed] [Google Scholar]
- 31.Küppers R, Engert A, Hansmann M-L. Hodgkin lymphoma. J Clin Invest. 2012;122:3439–3447. doi: 10.1172/JCI61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caproni M, Bianchi B, D’Elios MM, et al. In vivo relevance of CD30 in atopic dermatitis. Allergy. 1997;52:1063–1070. doi: 10.1111/j.1398-9995.1997.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 33. Hombach AA, Görgens A, Chmielewski M, et al: Superior therapeutic index in lymphoma therapy: CD30(+) CD34(+) hematopoietic stem cells resist a chimeric antigen receptor T-cell attack. Mol Ther 24:1423-1434, 2016. [DOI] [PMC free article] [PubMed]