Abstract
The global pandemic of COVID-19 pneumonia caused by the novel coronavirus (SARS-CoV-2) has strained healthcare resources across the world with emerging challenges of mass testing, resource allocation and management. While reverse transcriptase-polymerase chain reaction (RT-PCR) test is the most commonly utilized test and considered the current gold standard for diagnosis, the role of chest imaging has been highlighted by several studies demonstrating high sensitivity of computed tomography (CT). Many have suggested using CT chest as a first-line screening tool for the diagnosis of COVID-19. However, with advancement of laboratory testing and challenges in obtaining a CT scan without significant risk to healthcare providers, the role of imaging in diagnosis has been questioned. Several imaging societies have released consensus statements and guidelines on utilizing imaging resources and optimal reporting. In this review, we highlight the current evidence on various modalities in thoracic imaging for the diagnosis of COVID-19 and describe an algorithm on how to use these resources in an optimal fashion in accordance with the guidelines and statements released by major imaging societies.
Keywords: SARS-CoV-2, COVID-19, Thoracic imaging in COVID-19, Computed tomography, Chest radiograph
1. Introduction
We are amid an unprecedented healthcare pandemic caused by a novel strain of Coronavirus (SARS-CoV-2) with the disease it causes named COVID-19. Since the outbreak in Wuhan, China, it has turned into a global pandemic infecting more than 21 million people with over 760,000 deaths.1 To date, there has been no cure for the disease, though Remdesevir, an anti-viral drug, has most recently been approved by the United States Food and Drug Administration (US FDA) on compassionate grounds after clinical trials have shown it to reduce disease burden.2 , 3 A race to develop a vaccine has started in many countries as the most effective long-term preventive measure. While scientists worldwide are still grappling with excruciating details related to the pathogenesis of the virus, it is clear that the lungs remain the primary organ of injury. Understandably, there has been a significant interest in the medical community to study the role of thoracic imaging in COVID-19 pneumonia with respect to symptomatology and “confirmatory” laboratory tests for the virus. Major international imaging societies have come up with guidelines that take into consideration the current Center for Disease Control and Prevention (CDC) and World Health Organization (WHO) recommendations.
In this review, we summarize different imaging guidelines available to date and put forth an algorithm to guide the appropriate use of imaging resources based on existing knowledge of the disease. We also analyze the characteristic imaging features of the disease-related to temporal progression and emphasize that radiologists should be familiar with these findings.
2. Clinical features
The incubation period for the disease to manifest is estimated to be 5–14 days.4 Common symptoms of COVID-19 include cough (50–70%), fever (40–70%), sputum production (18–35%), fatigue (35–40%), shortness of breath (15–20%), and myalgia or arthralgia (10–15%).5 , 6 Characteristic early loss of sense of smell and taste has been seen in many patients, while gastrointestinal symptoms, including diarrhea, are also reported.7 , 8 Indeed, newer manifestations of the disease seem to be emerging with each passing day, with a hypercoagulable state, strokes in young patients, and Kawasaki-like autoinflammatory illnesses in children among the more notable.9 , 10 A substantial percentage of those infected can remain asymptomatic (40–50%) or manifest relatively mild symptoms (40%).11 COVID-19 often presents in a syndromic fashion with multiple organ involvement including renal failure, vascular thrombosis, ischemia, respiratory distress and gastrointestinal symptoms. The affinity of SARS-CoV-2 virus to angiotensin converting enzyme 2 (ACE-2) receptor has been proposed as a potential mechanism as a unifying mechanism for such diverse presentation.12
Moderate to severe symptoms are manifested in the remaining patients requiring hospitalization, supplemental oxygenation, and mechanical ventilation. The disease has proved to be fatal in a small percentage (<5%) of affected patients. Advanced age, smokers with structural lung disease, and the presence of comorbidities such as immunocompromised states, obesity, diabetes, and cardiovascular risk factors are among the most important parameters associated with worse prognosis.13 Viral shedding is variable, with infected personnel remaining infectious to others from 17 to 37 days after acquiring infection and dependent on the time of symptom onset and high fever.14 , 15
3. Laboratory testing
At this time, the reverse transcriptase-polymerase chain reaction (RT-PCR) test to detect viral RNA remains the most accurate diagnostic test. The test involves the collection of a nasopharyngeal or throat swab from suspected individuals and transportation of samples to a laboratory where real-time RT-PCR is employed on the collected sample. The most common SARS-CoV-2 genes targeted for testing are RdRp, E gene, and N gene. E gene is mainly used as a screening tool followed by a confirmatory test using RdRp gene.16
There have been tremendous concerns related to the availability of adequate testing kits to meet the increasing and often overwhelming worldwide demands for testing, tracing, and isolating infected individuals. Experience in many countries, including the US, has shown that laboratory processing times may substantially increase when demand for these tests rise. This has augmented the importance of imaging tests as an alternate diagnostic tool to diagnose suspected COVID-19 patients, a strategy that has been successfully implemented in China when initial PCR test kits were exhausted early on in the course of the pandemic. Moreover, PCR testing has its limitation with a relatively low sensitivity of 60–70%.17 , 18 Initial test results can thus be falsely negative in a significant number of patients, and retesting these patients if they remain symptomatic results in further delays in PCR test turnaround times. Xiao et al., also demonstrated that up to 21% (15/70) of patients could have false-negative RT-PCR for SARS-CoV-2, which may be due to prolonged nucleic acid conversion rather than a recurrence of infection.19 Interestingly, a Bayesian analysis of Chinese studies found a sensitivity of 77% and specificity of 98% for the Chinese CDC approved RT-PCR for SARS-CoV-2.20
Moreover, not all RT-PCR tests are equivalent. China approved 11 different tests between January 26 and March 12, 2020. A version of the test that has been widely used is an RT-PCR adaptation of the CDC assay and validated by FDA-EUA protocol.21 As the RT-PCR tests require identifying a primer in different viral genes, the results can be influenced by genetic variations, mutations and evolution of the virus.22 There is a need for reference standards in asymptomatic patients so that all RT-PCR tests can be standardized and improve overall sensitivity for screening purposes.23Most of us who have suffered from a common cold and have antibodies in our blood to many unrelated strains of Coronaviruses, which cause the common cold. This has proved to be a challenge as far as antibody-based detection tests for the specific strain of coronavirus implicated in COVID-19. More recently, several newer tests have been developed to detect the presence of IgG or IgM antibodies against the virus in human serum. While these tests have the advantage of being relatively “rapid” with faster turnaround time, they are limited by relatively low negative predictive values and high false negatives, necessitating some to advocate a cautionary approach before relying on their accuracy.24 Antibody testing is a great tool to assess previous exposure or infection but cannot reliably diagnose active COVID-19 infection, disease activity or transmissibility.
4. Thoracic imaging in COVID-19
4.1. Computed tomography (CT) of the chest
The role of chest CT in its application to suspected COVID-19 cases has continued to evolve since the pandemic began. This has primarily been explored in parallel with our increasing understanding of laboratory testing, given the rapid spread of infection over a short period. Earlier studies from China demonstrated high sensitivity for Computed Tomography (CT) of the chest for COVID-19, suggesting its use as a potential screening test at the time [Table 1 ].
Table 1.
Key imaging findings of COVID-19 pneumonia on computed tomography (CT) of the chest.
| Key imaging findings of COVID-19 pneumonia on computed tomography (CT) of the chest | |
|---|---|
| Diverse pattern of lung disease on CT with some key imaging features | |
| Distribution | Bilateral, multilobar, subpleural, peripheral and basilar predominant |
| Pattern | Rounded morphology, ground-glass opacities (GGO) and multilobar consolidations |
| Uncommon findings | Mediastinal lymphadenopathy, pleural effusions, cavitations and pulmonary nodules |
| Initial findings | Typical pattern Normal in up to 25% patients |
| Progression | Lobar consolidations, pleural effusions, subpleural blebs and bullae may develop in severe illness |
| Organization | Early fibrosis and traction bronchiectasis may develop in severe ARDS in two to four weeks |
Fang et al., in their cohort of 51 patients (29 men, 22 women), demonstrated that difference in detection rate for initial CT chest was 98% (50/51) compared to 71% for RT-PCR test (36/51).17 While 72% (36/50) of admitted patients had typical findings of peripheral, subpleural ground-glass opacities (GGO), often in the lower lobes, 28% (14/50) of patients had atypical CT manifestations. One patient had a normal CT chest. Pulmonary vascular prominence, particularly in the areas of ground-glass opacities has been identified as a key feature and can be found in 45% to 90% of cases.25 , 26
In another study by Tao et al., with a larger cohort of 1014 patients, the sensitivity of CT chest was higher compared to RT-PCR.18 Of 1014 patients, 59% (601/1014) had positive RT-PCR results, and 88% (888/1014) had positive chest CT scans. The sensitivity of chest CT in suggesting COVID-19 was 97% (95%CI, 95–98%, 580/601 patients) based on positive RT-PCR results. In patients with negative RT-PCR results, 75% (308/413) had positive chest CT findings; of 308, 48% were considered as highly likely cases, with 33% as probable cases. By analysis of serial RT-PCR assays and CT scans, the mean interval time between the initial negative to positive RT-PCR results was 5.1 ± 1.5 days; the initial positive to subsequent negative RT-PCR result was 6.9 ± 2.3 days. 60% to 93% of cases had initial positive CT consistent with COVID-19 prior (or parallel) to the initial positive RT-PCR results. 42% (24/57) cases showed improvement in follow-up chest CT scans before the RT-PCR results turned negative.
While these initial studies suggested frequent use of chest CT as a screening tool, other studies advocated a more conservative approach. Adam et al., studied 121 patients to assess CT chest findings within two days of symptom onset and found that 56% (20/36) of the patients had normal CT chest.27 Barring one patient, almost all of these patients had positive RT-PCR suggesting the presence of infection even when the chest CT was normal. This study highlighted the variability in the negative predictive value of the CT.
Interestingly, chest CT may show variable sensitivity depending on the time when the scan is performed during illness. To look at the temporal evolution of COVID-19 pneumonia, Wang et al., found the sensitivity for chest CT to be 84% (95% confidence interval: 73%–92%) when conducted in 0–5 days of symptom onset. The sensitivity increased to 99% (95% confidence interval: 93%- 100%) if the chest CT was obtained on day 6–11.28 Yu et al. observed that more severe disease had more lung segment involvement, more extensive opacities, and frequent findings of interlobular septal thickening, air bronchograms, and even pleural effusions.29 Another study by Pan et al. showed similar findings with greater severity peaking at day 10 of illness.30
Inui et al., studied the chest CT findings on cases from “Diamond Cruise Ship” and found that imaging abnormalities were more common in symptomatic (22/28 = 79%) compared to asymptomatic (41/76 = 54%) laboratory-confirmed COVID-19 patients. However, ground-glass opacities were common in asymptomatic patients (83%) compared to consolidative opacities in symptomatic patients (41%).31 Meng et al., from Wuhan, studied chest CT evolution in asymptomatic laboratory-confirmed COVID-19 patients for over 54 days and found similar findings of GGO and interlobular septal thickening. They found that 27% (16/58) of patients eventually became symptomatic and had abnormal inflammatory markers and lymphopenia.32
Ultimately, key imaging features of COVID-19 on chest CT are now understood to include bilateral (multilobar), peripheral, and basilar distribution, of opacities with rounded morphology. Pertinent imaging negatives to note are the absence of lymphadenopathy, effusions, cavitation, or nodules, which potentially suggest alternative diagnoses (Table 1).27 However, the imaging presentation is often variable, and the commonly associated imaging features are inherently non-specific. Imaging features alone cannot differentiate COVID-19 from other viral pneumonias. Additionally, diagnoses such as cryptogenic organizing pneumonia, eosinophilic pneumonia, and pulmonary infarcts can also present with these imaging features. Given this lack of specificity in imaging features when present and the variable sensitivity of chest CT related to phase of the disease the patient is scanned in, CT is not considered an effective screening tool by the major radiology societies. Instead, clinical symptomology and PCR laboratory findings will decide whether a patient is likely positive for COVID-19 and requires isolation33 (Table 2 ).
Table 2.
RSNA expert consensus statement on structured reporting for chest CT in COVID-19.
| RSNA expert consensus statement on structured reporting for chest CT in COVID-1933 | ||
|---|---|---|
| Classification | Rationale | Suggested reporting language |
| Typical | Imaging features with high specificity and commonly reported for COVID-19 pneumonia | “Commonly reported imaging features of COVID-19 pneumonia are present. Other processes such as influenza pneumonia and organizing pneumonia, as can be seen with drug toxicity and connective tissue disease can cause a similar imaging pattern.” |
| Indeterminate | Non-specific imaging features reported in COVID-19 pneumonia | “Imaging features can be seen in COVID-19 pneumonia, though are non-specific and can occur with a variety of infectious and non-infectious processes.” |
| Atypical | Uncommon or imaging features not reported in COVID-19 pneumonia | “Imaging features are atypical or uncommonly reported for COVID-19 pneumonia. Alternative diagnosis should be considered.” |
| Negative | No features of pneumonia |
“No CT findings to indicate pneumonia” (Note: CT may be negative in initial stage of COVID-19) |
Chest CT, therefore, is primarily recommended to be used judiciously when required as a problem-solving tool only in specific clinical situations that will change management decisions, such as worsening respiratory status or if there are concerns for additional diagnoses. The risk profile of CT scanning in suspected COVID-19 patients is also uniquely higher than the vast majority of common indications for chest CT scanning, given concerns regarding infection spread in transit to the CT scanner and within the CT scanner. This risk also requires an increased usage of clinical resources for infection control precautions to minimize infection spread. Additionally, institutions that have been impacted most severely by the pandemic may have a constraint on imaging resources. In this setting, in particular, unneeded chest CTs in COVID-19 suspected patients may limit the ability of other patients to receive a chest CT in a timely fashion for whom the study is indicated, whether in the setting of suspected COVID-19 or not. Additionally, the decision to perform a contrasted study to evaluate for concomitant pulmonary embolism (PE) versus a non-contrasted study should be at the clinician's discretion based on clinical presentation. Covid-19 has been identified as a prothrombotic state with incidence of venothromboembolism (VTE).34 In PUIs presenting with chest pain, dyspnea, leg swelling, tachycardia and elevated D-dimer, CT angiography of the chest should be considered to evaluate for PE as early initiation of anticoagulation can be lifesaving.
Several imaging findings have been suggested as typical for COVID-19, while other findings are considered atypical as they are seen uncommonly (Table 1).27 Although these findings may hold high sensitivity for COVID-19 during a pandemic, one must be cognizant of other diseases that can cause similar findings. Most viral pneumonias, cryptogenic organizing pneumonia, and drug-induced lung injury can also present similarly. Thus, it becomes imperative to endorse a detailed history and physical examination before settling on the diagnosis of COVID-19 purely based on chest imaging. Major imaging societies such as the Radiological Society of North America (RSNA) released an expert consensus on how to document these findings. This statement has been endorsed by the American College of Radiology (ACR) and Society of Thoracic Radiology33 (Table 2).
4.2. Our experience with progression of COVID-19 disease and CT imaging
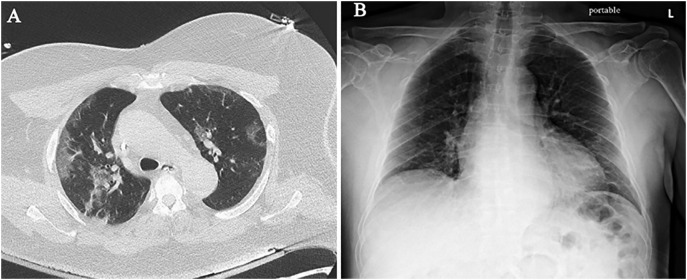
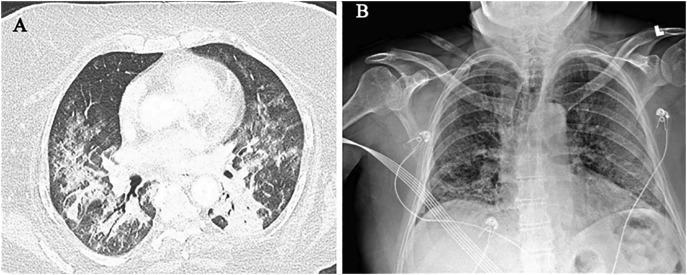
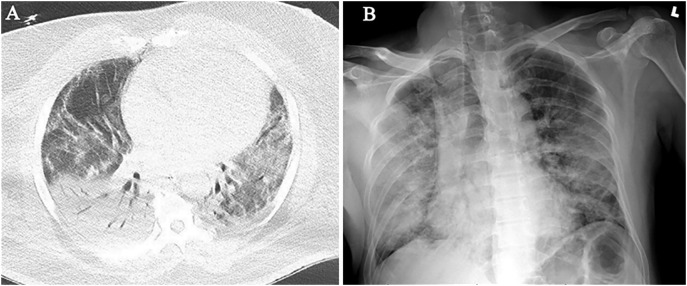
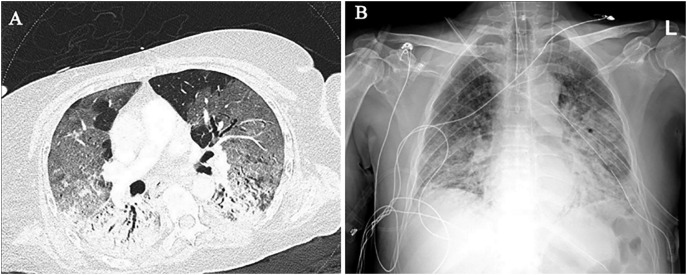
Based on our experience in New York and Cleveland, initial findings in laboratory-confirmed COVID-19 patients include typical patterns of peripheral, patchy ground-glass opacities in most cases (Fig. 1A–B). Some patients experienced rapid progression to acute respiratory distress syndrome (ARDS) with worsening consolidation more pronounced in the lower lobes (Fig. 2A–B). Secondary bacterial pneumonia has been observed in autopsy findings and can manifest as lobar consolidation35 (Fig. 3A–B). Since the pathophysiology of lung injury is severe diffuse alveolar damage (DAD) that starts with the exudative phase and evolves into a fibroproliferative phase, a longer in-hospital follow-up on these patients has demonstrated persistent ground-glass opacities with organized focal consolidations (Fig. 4A–B). In severe cases, early-onset extensive fibrosis and bronchiectasis have been observed (Fig. 5 ). In patients with severe ARDS who develop secondary bacterial and fungal infections, necrotizing cavitary lesion (Fig. 6 ), and large bullous disease with pneumothoraces (Fig. 7A–B) have been observed. These patterns highlight the importance of CT imaging during the clinical course, especially if a secondary infection or COVID-19 related complication is suspected. However, there is always a logistic constraint in obtaining CT imaging, which includes intrahospital transit, decontamination of the CT room, and risk of exposure to healthcare providers. This may become further complicated in the future when there are sporadic cases of unconfirmed COVID-19, which can lead to increased exposure to radiology technician and CT imaging.
Fig. 1.
Initial imaging findings in COVID-19. (A) Chest CT shows bilateral, peripheral, patchy ground-glass opacities in both lungs, right worse than left. (B) Portable CXR is near normal with very subtle peripheral opacities in the mid lung zones and left base.
Fig. 2.
Rapid progression to ARDS in COVID-19. (A) Chest CT shows worsening consolidation which is more pronounced in the bilateral lower lobes. (B) Portable CXR obtained a few days prior to CT shows early development of consolidative changes in the bilateral posterior lung bases.
Fig. 3.
Secondary bacterial pneumonia in patient of COVID-19. (A) Chest CT shows dense right lower lobe consolidation with air-bronchograms due to secondary bacterial pneumonia. Mild consolidation is seen in the left lower lobe. (B) Portable CXR obtained a day later, shows progression of dense consolidation in the right lower lobe as well as right upper lobe and left lung.
Fig. 4.
Persistent opacities with imaging signs of early organization. (A) Chest CT shows persistent ground-glass opacities with lobular areas of sparing in the non-dependent areas and organized consolidations in the dorsal/dependent portions of both lungs. (B) Portable CXR obtained a few days after CT, shows progression of dense consolidations in both lungs with mid and lower zone predominance.
Fig. 5.
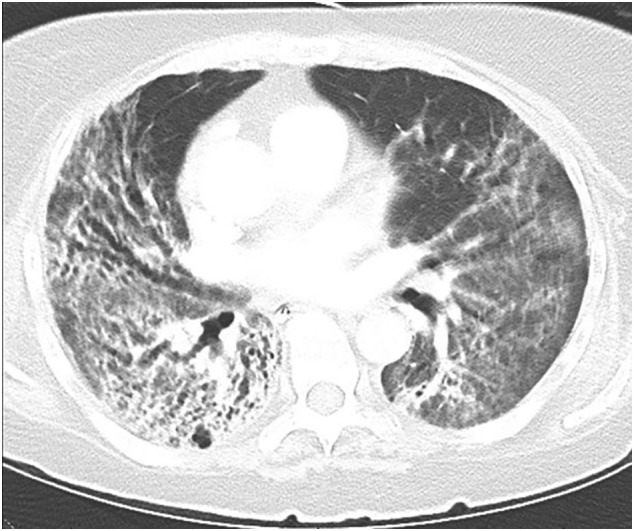
Early-onset extensive fibrosis and bronchiectasis in COVID-19. Chest CT shows peribronchial fibrotic consolidations and ground-glass opacities with development of traction bronchiectasis mainly in the right lower and middle lobes.
Fig. 6.
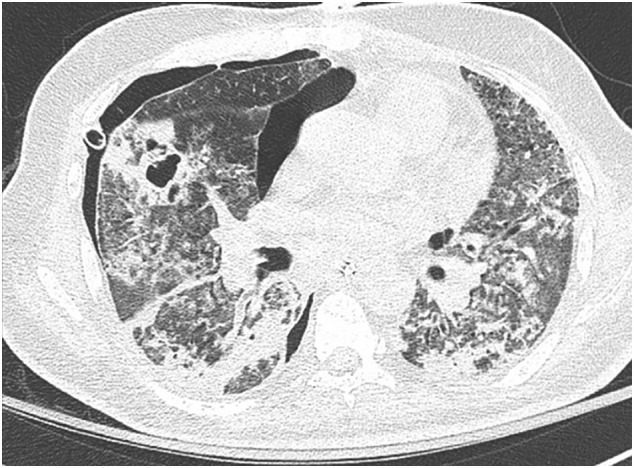
Necrotizing pneumonia with COVID-19. CT chest shows a cavitary lesion in the middle lobe suggestive of necrotizing pneumonia with lung abscess due to secondary bacterial pneumonia. There are diffuse ground-glass opacities and lower lobe consolidations due to COVID-19. Note, small right pneumothorax.
Fig. 7.
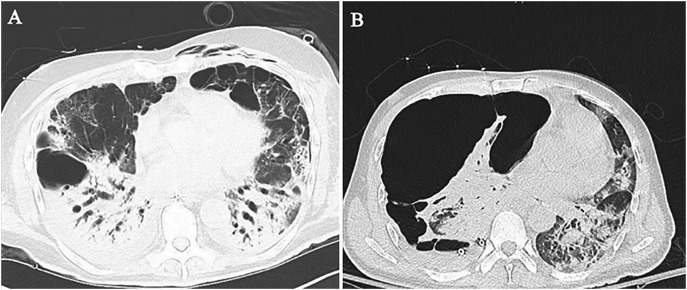
Large bullous disease with pneumothorax in COVID-19. (A) Chest CT shows bullae in the anterior basilar segment right lower lobe and superior lingula, with dense consolidations in both lower lobes. (B) Follow-up chest CT shows development of a large multi-loculated, tension right pneumothorax due to ruptured bulla, resulting in contralateral mediastinal shift.
4.3. Role of chest radiography in the diagnosis of COVID-19
Chest radiography (CXR) plays a role in the imaging management of pneumonia in immunocompetent patients, despite known low sensitivity. A recent multi-site study of 636 symptomatic patients from the greater New York area with confirmed Covid-19 demonstrated a normal chest radiograph in 58.3% (371/636) of patients and a normal or only mildly abnormal chest radiograph in 89% (566/636) of patients.36 , 37 In patients with COVID, the indication for CXR are no different than in other pneumonias. CXR is considered appropriate as an initial imaging diagnostic test in patients with lower respiratory tract infection, including those with suspicion for COVID infection. CXR should not be indicated to rule out COVID-19 infection due to its low sensitivity. Wong et al., observed the sensitivity of CXR to be 69% (44/64) compared to 91% (58/64) for RT-PCR in their cohort of 64 COVID positive patients.38 Peripheral, lower lobe predominant consolidations and ground-glass opacities were the most common findings. Ippolito et al., demonstrated that out of 68 patients who had a CT chest after a CXR with a mean lag time of 2 days; the CXR was able to detect abnormal findings in 89.7% (61/68) of the patients. They also had 10% (7/68) of patients who had negative CXR but were found to have GGO on CT scan.39 A normal CXR does not rule out the possibility of pneumonia in general and, expressly, does not exclude the diagnosis of pneumonia in patients with suspected COVID. However, chest radiography is valuable to image the evolution of COVID-19 pneumonia, which can be followed using serial CXR. The advantages of CXR include portability and easy accessibility. Particularly in institutions that are constrained in CT imaging resources, it can also preclude the need for additional CTs. The associated increased risk of infection in transporting a patient to the scanner, or infection spread within the scanner, and subsequent mandatory decontamination measures, are also mitigated.40 , 41
4.4. Lung ultrasonography
The current guidelines in use by RSNA, Fleischner, and ACR /STR for lung imaging in COVID-19 do not prescribe any role for lung ultrasonography. The authors would like to emphasize that point-of-care lung ultrasonography is an investigational tool in imaging COVID-19 patients at this time. Small case series have suggested pleural line irregularities, multiple B-lines and subpleural consolidations and absence of pleural effusions as common findings in COVID-19.42 , 43 Zhang et al. found B-lines in 100% (n = 28) of their patients while thickened pleural lines in 60.7% and subpleural consolidations in 67% of their patients.44 While data is limited to small case series, there is no larger studies to assess the accuracy of lung ultrasonography in COVID-19 and the evidence for its routine use for diagnosis of COVID-19 is scant. There is a role for ultrasound in evaluating complications related to pneumonia such as evaluating for parapneumonic effusions and/or empyema, though these are not specific for COVID-19.
5. Current guidelines issued by major imaging societies
In the light of the COVID-19 pandemic and emerging evidence on serologic and imaging diagnosis of COVID-19 pneumonia, all major imaging societies have released expert consensus statement and guidelines. The Society of Thoracic Radiology (STR) and the American Society of Emergency Radiology (ASER) released a position statement on March 11, 2020, on the utility of CT chest as a screening tool for COVID-19. They do not recommend routine CT chest for screening patients under investigation (PUI) for COVID-19 and suggest using it to evaluate complications such as abscess or empyema.45
The American College of Radiology (ACR) in concordance with the CDC released guidelines echoing similar sentiments in avoiding CT chest as a first-line screening test to diagnose COVID-19 pneumonia.46 They recommend the limited role of CT chest in symptomatic and hospitalized patients who fulfill clinical indications for a CT scan.47 Alternatively, they suggest using portable CXR as it is quick, efficient, and easy to decontaminate. The ACR also cautions against using CT chest to guide decisions on RT-PCR testing for COVID-19 or quarantining patients, as normal imaging does not exclude infection, and abnormal CT imaging is not specific for COVID-19 diagnosis. They do, however, suggest a possible role of CT chest for diagnosis and management of COVID-19 in critically ill patients or if RT-PCR is not available.46
The Radiological Society of North America (RSNA) in endorsement with STR, recommended not using CT chest to screen for COVID-19 and suggested four categories for standardized CT chest reporting language for COVID-19.33 In anticipation of mixed and atypical imaging findings due to either complications or co-infection, the interpretation towards COVID-19 may become challenging. Table 2 highlights the RSNA suggested reporting language for COVID-19 imaging.33 The RSNA recommends using “viral pneumonia” as an alternative term for incidentally discovered imaging findings that are compatible with COVID-19. They also recommend radiologists to follow ACR Practice Parameter and Communication for Diagnostic Imaging Findings for reporting.48
The Fleischner Society has also released a multinational statement on using chest imaging during the COVID-19 pandemic based on the severity of illness, resource availability, and pre-test probability for COVID-19.41 They recommend against the use of any imaging in mild COVID-19 case while reserving imaging for patients with severe or progressive respiratory failure. In a resource-constrained situation, imaging may be utilized for triaging patients with moderate to severe symptoms and high pre-test probability of COVID-19 pneumonia.41 Imaging may also be utilized for assessment of disease progression or complications in a resource-constrained environment.
6. Diagnostic algorithm based on RT-PCR testing and imaging
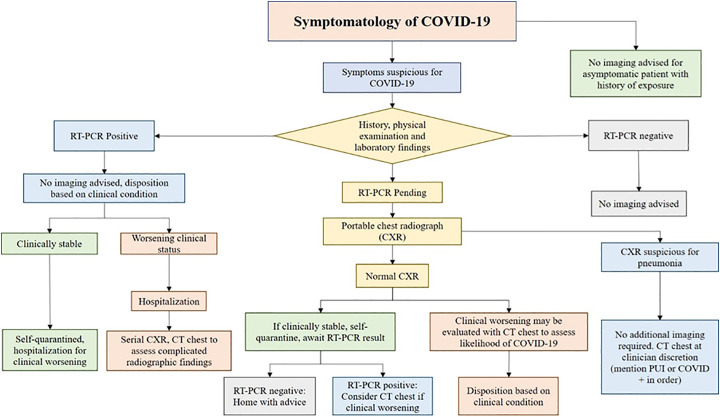
We propose a diagnostic algorithm utilizing RT-PCR and thoracic imaging in accordance with the expert consensus and statements from major imaging societies (Fig. 8 ). For most patients, imaging has little practical influence on their disposition. RT-PCR testing has remained the reference standard and initial screening test of choice. We use clinical symptoms to guide care in most cases, given that 20–25% of CT scans will be normal in the early stages of the disease.
Fig. 8.
Proposed algorithm for imaging in patients with suspected COVID-19 pneumonia.
The current standard is to test for the respiratory syncytial virus (RSV), Influenza A, and B as part of the nasopharyngeal swab for COVID-19 to evaluate for alternate etiology. If RT-PCR is positive for COVID-19, clinical symptoms are used to triage patients to quarantine at home or admit to the hospital. No imaging is advised at this stage as it will not change management in most individuals.
If RT-PCR is negative for COVID-19, no specific imaging recommendation is made. RT-PCR is generally repeated 5–6 days after negative results if the patient remains symptomatic (the approach may not always be practical with limited access to testing).
In symptomatic patients, if RT-PCR status is unknown or pending, a portable CXR can be considered as an initial test of choice. If typical features are present on CXR, a CT chest is not recommended. The decision to scan the patient can be made based on the presence of suspected radiographic abnormalities such as lung abscess formation, empyema, pneumothorax, etc. In symptomatic patients with normal chest radiographs who have a high pre-test probability of COVID-19, high-risk individuals such as elderly, smokers, history of chronic lung disease, or those with high suspicion for clinical worsening, a CT chest may be considered for the diagnosis of COVID-19.
6.1. Key points related to Imaging of suspected COVID-19 patients
-
•
CXR is the initial imaging modality of choice in every suspected case regardless of laboratory status.
-
•
If CT is indicated for reasons as previously stated, a non-contrast CT chest should be the standard test.
-
•
Referring services should enter discriminators such as “COVID-19 positive” or “COVID-PUI” when ordering the CT chest.
6.2. Reporting: radiologist responsibilities
-
•
Radiologists should familiarize themselves with the most common imaging findings of COVID-19 pneumonia.
-
•
Radiologists must remember that the imaging findings are non-specific and may be secondary to other viral or infectious pathogens or other non-infectious disease processes. To that end, with the relevant imaging findings, an appropriate format for reporting must be used such as, “the imaging findings are consistent with an infectious process, possibly viral” rather than making a specific reference to COVID-19 or another specific pathogen.
-
•
As with the imaging findings of any lung illness, knowing the clinical context is essential to providing a probable or correct diagnosis. Diagnostic concern about a specific organism should be communicated directly with the patient's clinician, allowing him/her to put that in the proper context.
7. Conclusion
As the diagnostic techniques for COVID-19 continue to evolve, laboratory confirmation of COVID-19 remains the initial screening test of choice with a limited role of CT chest in diagnosis or screening. CXR appears to be a reasonable imaging modality of choice in patients with suspected and pending RT-PCR for COVID-19 (PUI). Imaging may be considered to triage patients in the resource-constrained environment as recommended by the Fleischner Society expert statement.41 The ACR, CDC, RSNA, and STR at this point do not see the advantage of screening CT as it is non-specific and will not change management and quarantine status, which is dictated by the patient's history and symptoms. Initial CT chest can be negative in up to 25% of patients with COVID-19; however, sensitivity increases with disease progression with abnormal findings in 95% of cases after 5–6 days of infection. CT chest should only be performed if there is a clinical indication for it in accordance with ACR appropriateness criteria for acute respiratory illness in immunocompetent patients.47
In hospitalized patients, CXR remains the imaging modality of choice as a baseline imaging and monitoring disease progression and complications. CT chest may be considered for evaluation of complications as superimposed bacterial pneumonia, abscess, or empyema. Point of care lung ultrasonography for the diagnosis of COVID-19 remains an investigational tool and is currently not recommended as a diagnostic test by major professional imaging societies.
Financial and conflict of interest disclosures
SG, HD, MBS, VKM, SR and ACM, have no financial disclosure or conflict of interest to declare.
References
- 1.WHO . World Health Organization; 2020. World Health Organization coronavirus disease (COVID-19) situation Report-158. [Google Scholar]
- 2.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — preliminary report. New England Journal of Medicine. 2020;383:1813–1826. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 3.Grein J., Ohmagari N., Shin D. Compassionate use of Remdesivir for patients with severe Covid-19. New England Journal of Medicine. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauer S.A., Grantz K.H., Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. [DOI] [PMC free article] [PubMed]
- 7.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. American Journal of Gastroenterology. 2020;115 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the young. New England Journal of Medicine. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet. [DOI] [PMC free article] [PubMed]
- 11.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England Journal of Medicine. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni W., Yang X., Yang D. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi L, Yang Y, Jiang D, et al. Factors associated with duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. [DOI] [PMC free article] [PubMed]
- 15.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Y., Zhang H., Xie J. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao A.T., Tong Y.X., Zhang S. 2020. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padhye N.S. Reconstructed diagnostic sensitivity and specificity of the RT-PCR test for COVID-19. medRxiv. 2020 [2020.2004.2024.20078949] [Google Scholar]
- 21.ACCELERATED EMERGENCY USE AUTHORIZATION(EUA) SUMMARYCOVID-19 by RT-PCRTEST (FULGENT THERAPEUTICS, LLC) for In vitro diagnostic UseRx OnlyFor use under emergency use Authorization (EUA) only. U.S Food and Drug Administration (FDA); 2020. [Google Scholar]
- 22.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications. New England Journal of Medicine. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 24.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S., Wang Y., Zhu T. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Xu Z., Wang J. CT characteristics of patients infected with 2019 novel coronavirus: association with clinical type. Clin Radiol. 2020;75:408–414. doi: 10.1016/j.crad.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernheim A., Mei X. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Dong C., Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020:200843. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M., Xu D., Lan L. Thin-section chest CT imaging of coronavirus disease 2019 pneumonia: comparison between patients with mild and severe disease. Radiology: Cardiothoracic Imaging. 2020;2:e200126. doi: 10.1148/ryct.2020200126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inui S., Fujikawa A., Jitsu M. Chest CT findings in cases from the cruise ship “diamond princess” with coronavirus disease 2019 (COVID-19) Radiology: Cardiothoracic Imaging. 2020;2:e200110. doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng H., Xiong R., He R. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020;81:e33–e39. doi: 10.1016/j.jinf.2020.04.004. [S0163-4453(0120)30211-30215] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson S., Kay F.U., Abbara S. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiology: Cardiothoracic Imaging. 2020;2:e200152. doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational research : the journal of laboratory and clinical medicine. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [S1931-5244(1920)30070-30070] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstock M.B.E.A., Russell J.W., Leib A. Chest x-ray findings in 636 ambulatory patients with COVID-19 presenting to an urgent care center: a normal chest x-ray is no guarantee. Journal of Urgent Care Medicine. 2020;14:13–18. [Google Scholar]
- 37.Self W.H., Courtney D.M., McNaughton C.D. High discordance of chest x-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med. 2013;31:401–405. doi: 10.1016/j.ajem.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong H.Y.F., Lam H.Y.S., Fong A.H.-T. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2020:201160. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ippolito D., Maino C., Pecorelli A. Chest X-ray features of SARS-CoV-2 in the emergency department: a multicenter experience from northern Italian hospitals. Respir Med. 2020;160:106036. doi: 10.1016/j.rmed.2020.106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mossa-Basha M., Meltzer C.C., Kim D.C. Radiology department preparedness for COVID-19: radiology scientific expert panel. Radiology. 2020:200988. doi: 10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin G.D., Ryerson C.J., Haramati L.B. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Radiology. 2020:201365. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buda N., Segura-Grau E., Cylwik J. Lung ultrasound in the diagnosis of COVID-19 infection - a case series and review of the literature. Adv Med Sci. 2020;65:378–385. doi: 10.1016/j.advms.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng Q.-Y., Wang X.-T., Zhang L.-N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46:849–850. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Xue H., Wang M. Lung ultrasound findings in patients with coronavirus disease (COVID-19) Am J Roentgenol. 2020:1–5. doi: 10.2214/AJR.20.23513. [DOI] [PubMed] [Google Scholar]
- 45.The American Society of Emergency Radiology (ASER) (2020) STR / ASER COVID-19 position statement. https://thoracicrad.org/wp-content/uploads/2020/03/STR-ASER-Position-Statement-1.pdf 2020.
- 46.ACR Recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 47.Jokerst C., Chung J.H., Ackman J.B. ACR appropriateness criteria: acute respiratory illness in Immunocompetent patients. J Am Coll Radiol. 2018;15:S240–S251. doi: 10.1016/j.jacr.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 48.ACR . The American College of Radiology (ACR); 2014. Practice parameterfor communication of diagnostic imaging findings.https://www.acr.org/-/media/ACR/Files/Practice-Parameters/CommunicationDiag.pdf [Google Scholar]