Abstract
Objective
In the present study, we sought to better characterize the patients with coronavirus disease 2019 (COVID-19) most at risk of severe, outpatient thrombosis by defining the patients hospitalized with COVID-19 with arterial or venous thrombosis diagnosed at admission.
Methods
We conducted a single-center, retrospective analysis of COVID-19 patients. We found a shift in the proportions of thrombosis subtypes from 2019 to 2020, with declines in ST-segment myocardial infarction (from 22.0% to 10.1% of thrombotic events) and stroke (from 48.6% to 37.2%) and an increase in venous thromboembolism (from 29.4% to 52.7%). The patients with COVID-19–associated thrombosis were younger (age, 58 years vs 64 years; P = .043) and were less frequently women (31.3% vs 43.9%; P = .16). However, no differences were found in the body mass index or major comorbidities between those with and without COVID-19. COVID-19–associated thrombosis correlated with greater mortality (15.2% vs 4.3%; P = .016). The biometric profile of patients admitted with COVID-19–associated thrombosis compared with regular thrombosis showed significant changes in the complete blood count, liver function test results, D-dimer levels, C-reactive protein, ferritin, and coagulation panels.
Conclusions
Outpatients with COVID-19 who developed thrombosis requiring hospitalization had increased mortality compared with outpatients without COVID-19 who developed thrombosis requiring hospitalization. Given the significantly higher inflammatory marker levels, it is possible this is related to different mechanisms of thrombotic disease in these patients. The inflammation could be a therapeutic target to reduce the risk, or aid in the treatment, of thrombosis. We call for more studies elucidating the role that immunothrombosis might be playing in patients with COVID-19.
Keywords: Biomarkers, Coronavirus, COVID-19, Embolism, Ischemic stroke, Thrombosis
Article Highlights.
-
•
Type of Research: A single-center, retrospective, case-control study
-
•
Key Findings: A total of 129 patients were identified with thrombotic events (venous thromboembolism, stroke, or ST-segment myocardial infarction) in the first 48 hours of hospital admission in March and April of 2020 (44 per 1000 admissions) compared with 109 patients with thrombosis during the same period in 2019 (37 per 1000 admissions). A shift had occurred in the relative proportions of thrombosis subtypes, with declines in ST-segment myocardial infarction (from 22.0% to 10.1% of thrombotic events) and stroke (from 48.6% to 37.2%) and an increase in the proportion of patients with venous thromboembolism (from 29.4% to 52.7%). Patients with coronavirus disease 2019 (COVID-19)–associated thrombosis were younger (age, 58 years vs 64 years; P = .043) and were less frequently women (31.3% vs 43.9%; P = .16). However, no difference was found the body mass index or major comorbidities between those with and without COVID-19. COVID-19–associated thrombosis correlated with greater mortality (15.2% vs 4.3%; P = .016).
-
•
Take Home Message: In patients infected with COVID-19, the development of thrombosis as an outpatient portends for greater mortality than for patients without COVID-19 who develop outpatient thrombosis. The mechanism of thrombosis has not been completely understood. Given the variations in biometric profiles, immunothrombosis could be the major driver, which could mean that treatment and prevention strategies should vary.
Thrombosis is a frequent complication during hospitalization for the novel coronavirus disease 2019 (COVID-19) and occurs in ≤31% of cases.1 Even in patients without overt thrombosis, COVID-19 is frequently characterized by abnormal coagulation parameters, including elevated D-dimer, which are associated with an adverse prognosis.2, 3, 4, 5 Prophylactic anticoagulation for patients with COVID-19 might be beneficial. In an observational study, patients with severe COVID-19 and elevated D-dimer levels or evidence of coagulopathy who had received thromboprophylaxis during hospitalization had decreased in-hospital mortality,6 supporting associations between the occurrence of thrombosis and poor outcomes. Autopsy studies also noted fatal thromboses in patients with COVID-19.7 Thus, the current society recommendations support chemical thromboprophylaxis to mitigate the thrombotic risk in hospitalized patients with COVID-19.6 , 8 , 9
Catastrophic thrombotic events, including pulmonary embolism (PE), stroke, and myocardial infarction, have been also reported in patients with COVID-19 at hospital presentation, suggesting the prehospital initiation of thrombus.7 , 10, 11, 12 The excess out-of-hospital deaths observed during the COVID-19 pandemic could also be an indication of fatal thromboses in outpatients with COVID-19.13, 14, 15
Despite the clinical implications of thrombosis, little is known regarding the clinical presentations and outcomes of patients with COVID-19 experiencing outpatient thrombosis.16 Data from the pre–COVID-19 era have suggested that venous thromboembolism (VTE) occurring in outpatients has a more favorable prognosis than inpatient VTE. The outcomes of patients with outpatient thromboses associated with COVID-19 have not yet been evaluated.17 Therefore, we aimed to characterize patients hospitalized with COVID-19 with an arterial or a venous thrombosis diagnosed within 48 hours of admission and to compare these patients with those with a similar thrombotic presentation who did not have COVID-19.
Methods
We conducted a retrospective, observational study of patients admitted to the New York University Langone Health Manhattan Campus with and without COVID-19 from March 1, 2020 to April 30, 2020 with thrombosis. Thrombosis was defined as one or more imaging-confirmed acute VTE, consisting of deep vein thrombosis (DVT) or acute PE, ischemic stroke, or ST-segment myocardial infarction (STEMI). Only radiographically proven events were included to ensure accurate diagnoses. Eligible imaging studies to identify thrombotic events included computed tomography angiography for PE, lower extremity duplex ultrasonography for DVT, computed tomography or magnetic resonance imaging for stroke, and invasive coronary angiography for myocardial infarction. Other types of thrombosis were not included, secondary to an inability to reliably capture all events. Given the possibility of imaging delays because of the isolation precautions at the initial presentation, we included only those events that had been confirmed by imaging findings within 48 hours of hospital admission. Patients who had been administered empiric anticoagulation with radiographic confirmation within 48 hours were included; however, patients without radiographic confirmation were not included. To compare the patients with and without COVID-19, we analyzed the data from patients who had had one or more nasopharyngeal swabs taken for severe acute respiratory syndrome-associated coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) testing during a hospital admission in 2020. Patients with positive test results were included in the COVID-19 cohort. Patients with a negative test results were assumed to not have COVID-19. Patients without SARS-CoV-2 test results during hospitalization in 2020 were excluded from subsequent analyses. Consecutive patients with VTE, ischemic stroke, and STEMI who had been hospitalized before the COVID-19 global pandemic, from March 1, 2019 to April 30, 2019, were also included and were considered not infected with SARS-CoV-2.
A medical record review was performed to abstract the demographics, comorbidities, clinical presentation, relevant laboratory findings, treatment, and outcomes. The endpoints of the present study included in-hospital mortality, hospital length of stay, and the need for critical care services. The New York University School of Medicine institutional review board approved the present study and waived the requirement for written informed consent, given the retrospective study design.
Statistical analysis
Continuous variables are presented as the median and interquartile range (IQR) and were compared using the Mann-Whitney U test. Categorical data are shown as numbers and percentages and were compared using χ 2 tests or Fisher's exact tests. Logistic regression was performed to estimate the associations between a COVID-19 diagnosis and in-hospital mortality after adjustment for demographics and thrombosis type. Other clinical covariates with a univariate P value of ≤.1 were added to the demographics and thrombosis type in a separate model. All statistical analyses were performed using SPSS, version 25 (IBM Corp, Armonk, NY).
Results
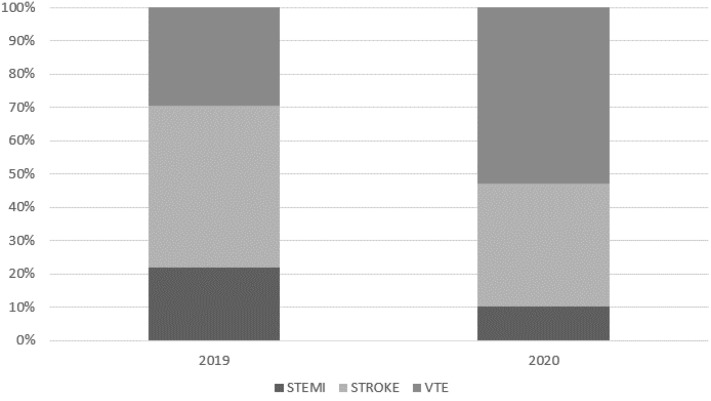
A total of 129 patients were identified with thrombotic events (VTE, stroke, or STEMI) in the first 48 hours of hospital admission in March and April 2020 of a total 2945 hospital admissions through the emergency department (44 per 1000 admissions). In contrast, 109 patients with thrombosis had been identified during the same period in 2019 of a total 2930 hospital admissions through the emergency department (37 per 1000 admissions). From 2019 to 2020, we observed a shift in the relative proportions of thrombosis subtypes, with declines in STEMI (from 22.0% to 10.1% of thrombotic events) and stroke (from 48.6% to 37.2%) and an increase in VTE (from 29.4% to 52.7%; Fig ). Of the 129 patients hospitalized in 2020 with thrombosis, RT-PCR testing for SARS-CoV-2 was performed for 103 (80%), of whom 48 (46.6%) tested positive for COVID-19. The remaining 55 patients had had negative COVID-19 RT-PCR test results. All 109 patients with thrombosis in 2019 were included as comparators without COVID-19.
Fig.
Relative percentages of ST-segment myocardial infarction (STEMI), stroke, and venous thromboembolism (VTE) at hospital presentation in March and April in 2019 and 2020.
The demographics of the patients with thrombosis with and without COVID-19 are presented in Table I . The patients with COVID-19–associated thrombosis were younger (age, 58 years vs 64 years; P = .043) and less frequently women (31.3% vs 43.9%; P = .16). The body mass index of the thrombosis patients was not different between those with and without COVID-19. The comorbidities are also presented in Table I. The patients with thrombosis were frequently hypertensive and one fifth had diabetes mellitus. The proportions of patients with preexisting cardiovascular risk factors and disease were not different between the patients with and without COVID-19. The patients with thrombosis in the setting of COVID-19 were less likely to have any history of malignancy (4.2% vs 22.0%; P = .009). Prehospital medication use is shown in Table I. Patients with COVID-19–associated thrombosis were less likely to be receiving antiplatelet therapy at the event than were patients with thrombosis without COVID-19 (2.1% vs 15.3%; P = .03).
Table I.
Demographics and clinical characteristics of patients with thrombus diagnosed within 48 hours of hospital presentation stratified by COVID-19 diagnosis
| Variable | COVID-19 |
P value | |
|---|---|---|---|
| No (n = 164) | Yes (n = 48) | ||
| Demographics | |||
| Age, years | 64 (53-74) | 58 (50-66) | .043 |
| Female sex | 72 (43.9) | 15 (31.3) | .161 |
| BMI, kg/m2 | 27.8 (23.4-31.1) | 27.0 (23.4-31.5) | .83 |
| Comorbidities | |||
| Hypertension | 82 (50.0) | 23 (47.9) | .93 |
| Diabetes mellitus | 33 (19.5) | 11 (22.9) | .76 |
| Heart failure | 14 (8.6) | 1 (2.1) | .20 |
| Asthma | 8 (4.9) | 2 (4.2) | 1.00 |
| COPD | 10 (6.1) | 0 (0) | .12 |
| CKD | 17 (10.4) | 1 (2.1) | .08 |
| ESRD | 2 (1.2) | 0 (0) | 1.00 |
| Malignancy | 36 (22.0) | 2 (4.2) | .009 |
| HIV | 1 (1.1) | 0 (0) | 1.00 |
| Previous stroke | 15 (9.1) | 1 (2.1) | .13 |
| Previous MI | 7 (6.4) | 1 (2.1) | .44 |
| Previous VTE | 11 (9.9) | 3 (6.3) | .55 |
| Cigarette smoking | 43 (27.3) | 8 (17.0) | .22 |
| Electronic cigarette use (“vaping”) | 4 (2.8) | 0 (0) | .66 |
| Heavy alcohol use | 22 (14.6) | 1 (2.4) | .031 |
| Medications before admission | |||
| Anticoagulation therapy | 14 (8.6) | 2 (4.2) | .54 |
| Direct oral anticoagulant | 11 (78.6) | 0 (0) | |
| Warfarin | 3 (21.4) | 2 (100) | |
| Low-molecular-weight heparin | 0 (0) | 0 (0) | |
| Antiplatelet therapy | 25 (15.3) | 1 (2.1) | .030 |
| Aspirin | 20 (80) | 1 (100) | |
| P2Y12 inhibitor | 5 (20) | 0 (0) | |
| NSAIDs | 5 (3.1) | 0 (0) | .59 |
| Steroids | 8 (4.9) | 0 (0) | .20 |
| Other immunosuppression medication | 10 (6.2) | 0 (0) | .12 |
| Clinical presentation | <.001 | ||
| STEMI | 30 (18.3) | 0 (0) | |
| Stroke | 74 (45.1) | 8 (16.7) | |
| VTE | 60 (36.6) | 40 (83.3) | |
BMI, Body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; MI, myocardial infarction; NSAIDs, nonsteroidal anti-inflammatory drugs; STEMI, ST-segment myocardial infarction; VTE, venous thromboembolism.
Data presented as number (%) or median (interquartile range).
The laboratory findings for the patients with COVID-19–associated thrombosis compared with those for patients with non–COVID-19 thrombosis are shown in Table II . COVID-19 patients had significantly higher markers of inflammation (eg, white blood cell count, C-reactive protein, ferritin), and liver function test results (eg, bilirubin, aspartate aminotransferase, alanine transaminase) and lower levels of albumin. The platelet counts were also significantly higher in patients with thrombosis and COVID-19. The coagulation panels also varied between those with COVID-19 and those without COVID-19 (ie, international normalized ratio, partial thromboplastin time). The D-dimer levels were significantly higher in the patients with thrombosis in the setting of COVID-19 than those patients with thrombosis but without COVID-19.
Table II.
Laboratory features of patients with thrombus diagnosed within 48 hours of hospital presentation stratified by COVID-19 diagnosisa
| Laboratory finding | COVID-19 negative (n = 164) |
COVID-19 positive (n = 48) |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| Patients, No. | Median (IQR) | Abnormal, % | Patients, No. | Median (IQR) | Abnormal, % | Overallb | Abnormal vs normal | |
| WBC count, 103/μL | 161 | 8.2 (6.3-10.3) | 36.0 | 48 | 10.4 (8.0-12.6) | 66.7 | <.001 | <.001 |
| Absolute lymphocyte count, 103/μL | 160 | 1.5 (1-2.2) | 38.1 | 48 | 1.1 (0.7-1.7) | 54.2 | .011 | .070 |
| Hemoglobin, g/dL | 161 | 13.5 (11.8-14.4) | 55.9 | 48 | 13.45 (12.33-14.7) | 54.2 | .304 | .96 |
| Platelet count, 103/μL | 161 | 222 (171.5-279.5) | 14.3 | 48 | 276.5 (206-348.3) | 8.3 | .003 | .40 |
| Sodium, mmol/L | 162 | 139 (137-141) | 4.9 | 48 | 139 (137-142.8) | 8.3 | .113 | .48 |
| Potassium, mmol/L | 162 | 4 (3.7-4.4) | 14.2 | 48 | 4.2 (3.8-4.5) | 25.0 | .288 | .12 |
| Bicarbonate, mmol/L | 161 | 23 (21-26) | 30.4 | 48 | 21 (19-23.8) | 52.1 | .001 | .010 |
| Creatinine, mg/dL | 162 | 0.9 (0.8-1.2) | 17.3 | 48 | 1 (0.9-1.4) | 27.1 | .100 | .20 |
| Direct bilirubin, mg/dL | 127 | 0.6 (0.4-0.8) | 12.6 | 46 | 0.8 (0.5-1.2) | 28.3 | .004 | .027 |
| AST, U/L | 127 | 23 (18-32) | 13.4 | 46 | 37 (24-56.3) | 43.5 | <.001 | <.001 |
| ALT, U/L | 127 | 22 (15-31) | 10.2 | 46 | 31 (19.3-62.3) | 34.8 | .002 | <.001 |
| Albumin, g/dL | 127 | 3.9 (3.6-4.3) | 17.3 | 45 | 3.5 (3.1-3.9) | 48.9 | <.001 | <.001 |
| Ferritin, ng/mL | 20 | 282 (76-716.8) | 70.0 | 43 | 882 (358-2046) | 90.7 | .002 | .061 |
| ESR, mm/h | 18 | 47 (25-72.5) | 88.9 | 7 | 79 (53-90) | 100 | .108 | .99 |
| Maximal D-dimer,c ng/mL | 50 | 1044 (346.5-2931) | 82.0 | 44 | 4084.5 (2176.3-10,000) | 100 | <.001 | .003 |
| Maximal CRP, mg/L | 29 | 67 (19.4-132.4) | 82.8 | 43 | 148 (78.4-238) | 100 | .001 | .008 |
| INR | 136 | 1.1 (1-1.2) | 29.4 | 40 | 1.3 (1.2-1.5) | 80.0 | <.001 | <.001 |
| PTT, seconds | 131 | 29.8 (27.4-32.3) | 77.1 | 38 | 28 (25.9-30.1) | 63.2 | .025 | .13 |
ALT, Alanine transaminase; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; INR, international normalized ratio; PTT, partial thromboplastin time; WBC, white blood cell.
Thresholds for abnormal values: WBC count, >9.1 103/μL; absolute lymphocyte count, <1300 103/μL; hemoglobin, <13.7 g/dL; platelet count, <150 103/μL; sodium, <132 mmol/L or >4.5 mmol/L; potassium, <3.5 mmol/L or >5.0 mmol/L; bicarbonate, <22 mmol/L or >30 mmol/L; creatinine, >1.3 mg/dL; bilirubin, 0.3-1.2 mg/dL; AST, >40 U/L; ALT, >50 U/L; albumin, <3.5 g/dL or >5.2 g/dL; ferritin, 22-248 ng/mL; ESR, >15 mm/h; D-dimer, >230 ng/mL; CRP, >5 mg/L; INR >1.1; PTT >27 seconds.
Mann-Whitney-Wilcoxon test.
D-dimer assay highest reported value was 10,000.00 ng/mL.
The symptoms of patients presenting with thrombosis in the setting of COVID-19 were significantly different from those of the patients who had presented with thrombosis without COVID-19. A greater proportion of COVID-19 patients had had fever (50% vs 8.9%; P < .001), cough (54.2% vs 12.8%; P < .001), chest pain (47.9% vs 22.6%; P = .001), and nausea, vomiting, and/or diarrhea (18.8% vs 3.0%; P < .001). Among the 48 patients with COVID-19, 39 (81.3%) had had one or more of these symptoms. The median time to presentation after the onset of respiratory or gastrointestinal COVID-19 symptoms was 14 days (IQR, 10-14.75 days; range, 2-21 days) for the 28 patients with COVID-19 for whom these data were recorded.
In-hospital mortality or discharge to hospice care was significantly more common for the patients with COVID-19–associated thrombosis than for patients with thrombosis in the absence of COVID-19 (15.2% vs 4.3%; P = .016). A positive COVID-19 status was significantly associated with in-hospital all-cause mortality after adjustment for age, sex, and subtype of thrombosis (adjusted odds ratio, 35.7; 95% confidence interval, 2.0-621.8).
VTE patients
Of the 100 patients with VTE analyzed, 40 had had COVID-19 and 60 had not had COVID-19 (28 had tested negative for SARS-CoV-2 in 2020 and 32 had had VTE in 2019). Of the 40 patients with COVID-19, 27 had had radiographically confirmed PE (3 saddle, 11 central, 10 segmental, and 3 subsegmental) and 19 had had radiographically confirmed above-the-knee DVT (13 unilateral and 6 bilateral; 15 with multiple vessels in one leg and 4 with a single vessel in one leg; and 17 occlusive and 2 nonocclusive). Significant differences were present in the laboratory findings among the VTE patients with and without COVID-19. D-dimer was measured in 63 patients and was significantly higher in those with COVID-19–associated VTE (median, 3406.5 ng/mL [IQR, 2176.3-10,626.3 ng/mL] vs median, 1956 [IQR, 737-3283 ng/mL]; P = .017). For the 48 patients with C-reactive protein measured, a trend was found toward higher values in the setting of COVID-19 (median, 152 mg/L [IQR, 83.0-242.5 mg/L] vs 91.8 [IQR, 20.3-214.8 mg/L]; P = .086). The severity markers of VTE differed between those with and without COVID-19. The PE severity index scores were higher for the patients with COVID-19 (median, 99.5 [IQR, 74.8-119.3] vs median, 81.0 [IQR, 57.0-104.0]; P = .024). Troponin was measured in 71 VTE patients, and the peak troponin level was higher in the COVID-19 patients with VTE than in those with VTE without COVID-19 (median, 0.035 ng/mL [IQR, 0.01-0.28 ng/mL] vs median, 0.009 ng/mL [IQR, 0.009-0.14 ng/mL]; P = .033). Brain natriuretic peptide was measured in 56 patients with VTE and did not differ by COVID-19 status (median, 19.5 pg/mL [IQR, 10-79 pg/mL] vs median, 53.0 pg/mL [IQR, 14-169 pg/mL]; P = .184). In-hospital mortality was significantly greater for the patients with COVID-19 (12.5% vs 0%; P = .009).
STEMI patients
Fewer patients presented with STEMI during the COVID-19 pandemic compared with the same period in 2019 (24 with STEMI in 2019 vs 13 with STEMI in 2020). Of the 13 patients with STEMI during the COVID-19 pandemic in 2020, 6 had undergone testing for COVID-19, none of whom tested positive for the virus. All patients with ST-segment elevations had had obstructive, thrombotic coronary artery disease with the culprit vessel identified. The culprit vessel was the left anterior descending artery in 20.8% of cases in 2019 and 61.5% of cases in 2020. Fewer patients in 2020 had had multivessel coronary artery disease than in 2019 (23.1% in 2020 vs 58.3% in 2019; P = .087). TIMI (thrombolysis in myocardial infarction) 0 flow was observed in 78.4% of patients before coronary intervention (76.9% in 2020 and 79.2% in 2019; P = .98). Primary percutaneous coronary intervention was performed in all cases. Aspiration thrombectomy was performed in 66.7% of the cases in 2019 and 76.9% in 2020 (P = .78). Glycoprotein IIb/IIIa inhibitors were used for 7.7% of those with STEMI in 2020 and 4.2% in 2019 (P = .99). No patient received thrombolytic therapy. Coronary stents were placed in 97.3% of the patients. After coronary intervention, TIMI 3 flow had been achieved in 91.7% in 2019 and 76.9% in 2020 (P = .27). A trend was found toward a lower left ventricular ejection fraction by echocardiography in 2020 vs 2019 (median left ventricular ejection fraction, 52.5% in 2019 vs 40% in 2020; P = .067). The peak troponin level was significantly greater in patients presenting with STEMI in 2020 than in those presenting in 2019 (median, 105 ng/mL [IQR, 50.9-185.3 ng/mL] vs median, 34.4 ng/mL [IQR, 13.6-76.0 ng/mL]; P = .009). A trend was found toward longer delays from symptom onset to presentation in 2020 vs 2019 (median, 5 hours in 2020 vs median, 1.5 hours in 2019; P = .694).
Stroke patients
A total of 101 strokes were identified, 53 from 2019 and 48 from 2020. Of the 48 patients with stroke during the COVID-19 pandemic, 29 had undergone testing for COVID-19, of whom 8 had tested positive for COVID-19. The difference in the peak troponin level in the patients with vs without COVID-19 was not significantly different statistically (median, 0.12 ng/mL [IQR, 0.009-0.58 ng/mL] vs median, 0.01 ng/mL [IQR, 0.009-0.04 ng/mL]; P = .347; n = 69; 8 with COVID-19 and 61 without COVID-19) or brain natriuretic peptide (median, 98 pg/mL [IQR, 3.25-251.3 pg/mL] vs median, 147 pg/mL [IQR, 56-556.3 pg/mL]; P = .45). The maximal D-dimer level was significantly greater among the patients who had presented with out-of-hospital thrombosis in the setting of COVID-19 compared with that for those without COVID-19 (median, >10,000 ng/mL [IQR, 1883.5-10,000 ng/mL] vs median, 417.5 ng/mL [IQR, 140.8-2286.8 ng/mL]; P = .001). The National Institutes of Health Stroke Scale scores were significantly higher for the stroke patients with COVID-19 (median, 24 [IQR, 2.5-27.0] vs median, 2.0 [IQR, 0-6]; P = .031) compared with those without COVID-19 (n = 71). The COVID-19 patients who presented with stroke were as likely to have a premorbid modified Rankin scale score of ≥1 as were patients without COIVD-19 (12.5% with COVID-19 vs 32.4% without COVID-19; P = .424). Of the 80 patients with strokes included in the final analysis, the in-hospital mortality for the patients with COVID-19 was numerically greater than that for those without COVID-19 (33.3% vs 6.8%; P = .084).
Discussion
In the present study of patients with thrombosis before and after the COVID-19 pandemic, we observed that the number of STEMI activations had decreased,18 , 19 severe strokes in younger patients had increased,20 , 21 and the incidence of DVT and PE had increased,22 , 23 as previously described in other cohorts. However, unlike previous studies, our data have demonstrated that these occurred among patients with thrombosis in the outpatient setting. Although patients with thrombosis and COVID-19 were younger than those without COVID-19, we observed few other differences in demographics and clinical characteristics between the two groups. These data suggest that patients at risk before the COVID-19 pandemic remain at increased risk with COVID-19, with the notable exception of those with malignancy. We found a large reduction in the proportion of patients with thrombosis requiring hospitalization with malignancy and COVID-19 compared with those without COVID-19–associated thrombi. COVID-19-associate thrombosis patients were identified within 21 days of COVID-19 symptom onset, with most identified within 14 days. This finding might inform the optimal duration of thromboprophylaxis for those who might derive benefit.
Changes in thrombosis epidemiology during the COVID-19 era were also complicated by the stay-at-home quarantine orders in the New York City area. This city mandate increased the time that residents were indoors and limited their mobility. Home quarantine might increase the risk of thrombosis; however, this has not been proved and the degree to which quarantine might increase risk is not known.
We found increased mortality in COVID-19 patients who develop outpatient thrombosis compared with patients with thrombosis without COIVD-19. This might be related to the different mechanisms of thrombotic disease in these patients or other non–thrombotic in-hospital complications of SARS-CoV-2 infection. We observed that the biomarkers of inflammation were significantly elevated in COVID-19 patients compared with patients without COVID-19 who had experienced thrombotic events. Given the degree of increase in inflammatory markers around the thrombotic events, it is possible that the inflammation itself is a large risk factor for thrombus generation; thus, inflammation could be a possible target to reduce the risk of thrombus generation. This was previously noted in studies examining pneumonia as a risk factor for DVT and PE.24
Respiratory infections, and pneumonia specifically, have been shown to increase the incidence of VTE.24 Samama25 reported that mobility changes, cancer, and infectious disease were identified as risk factors for an increased incidence of thrombosis. The degree to which COVID-19 confers thrombotic risk relative to other infections is not known but is an area of ongoing interest.
Although thromboprophylaxis might yield clinical benefit for patients with COVID-19, the optimal antithrombotic therapy is unknown, and bleeding complications with the use of anticoagulation therapy for patients with COVID-19 have been reported.26 , 27 To determine which patients should receive anticoagulation therapy, the risks and benefits must be well-established. Understanding the clinical characteristics of outpatients who develop thrombosis in the setting of COVID-19 will be imperative to this effort, and our report is one of the first steps in generating these data.
Our study had several important limitations. It was a retrospective, observational analysis of a single, large academic hospital center in the epicenter of the early COVID-19 pandemic in the United States. Data were acquired during the surge of cases in New York City, before the understanding of the complex pathophysiology of the disease. The concern for infectious spread might have led to fewer radiology studies performed for at-risk patients. Fewer patients presented for in-person medical evaluations, and other studies have suggested that rates of outpatient cardiac arrest increased significantly during this period.15 These issues could have confounded the clinical presentation, especially if patients had allowed symptoms to persist for longer and outpatient providers were unable to establish the diagnosis via telemedicine. Although we were not able to quantify the reluctance, our data showed evidence of this, with a trend found toward an increase in the interval from symptom onset to presentation for myocardial infarction. Even with these limitations, our findings highlight the risk of outpatient thrombosis in patients with COVID-19. We have also demonstrated that the thrombosis risk appears to be greatest within 3 weeks of COVID-19 symptom onset, highlighting the need to identify which patients might derive the greatest benefit from outpatient thromboprophylaxis.
Author contributions
Conception and design: SB
Analysis and interpretation: SB, NS, KI, JT, SY
Data collection: SB, NS, NA, MB, JB, RG, KI, NT, JT, SY, EY, TM
Writing the article: SB, NS
Critical revision of the article: SB, NS, NA, MB, JB, RG, KI, NT, JT, SY, EY, TM
Final approval of the article: SB, NS, NA, MB, JB, RG, KI, NT, JT, SY, EY, TM
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: SB
Footnotes
New York University provided divisional support for faculty time and effort.
Author conflict of interest: J.S.B. conducts research with Astra Zeneca, Janssen, and Amgen. S.B.B., N.R.S., N.E.A., M.B., R.G., K.I., N.T., J.T., S.Y., E.Y., and T.S.M. have no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., China Medical Treatment Expert Group for COVID-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox S., Akmatbekov A., Harbert J., Li G., Brown J., Vander Heide R. Pulmonary and cardiac pathology in COVID-19: the first autopsy series from New Orleans. MedRxiv. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz I., Sanger-Katz M. The New York Times; New York, NY: 2020. N.Y.C. Deaths Reach 6 Times the Normal Level, Far More Than Coronavirus Count Suggests. [Google Scholar]
- 14.Gillum J.S., Song L., Koa J. ProPublica; New York, NY: 2020. There’s Been a Spike in People Dying at Home in Several Cities. That Suggests Coronavirus Deaths Are Higher Than Reported. [Google Scholar]
- 15.Lai P.H., Lancet E.A., Weiden M.D., Webber M.P., Zeig-Owens R., Hall C.B. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emert R., Shah P., Zampella J.G. COVID-19 and hypercoagulability in the outpatient setting. Thromb Res. 2020;192:122–123. doi: 10.1016/j.thromres.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maestre A., Sánchez R., Rosa V., Aujesky D., Lorenzo A., Barillari G. Clinical characteristics and outcome of inpatients versus outpatients with venous thromboembolism: findings from the RIETE Registry. Eur J Intern Med. 2010;21:377–382. doi: 10.1016/j.ejim.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.De Filippo O., D’Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A. Reduced rate of hospital admissions for ACS during COVID-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon M.D., McNulty E.J., Rana J.S., Leong T.K., Lee C., Sung S.-H. The COVID-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383:691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 20.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of COVID-19 in the Young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaghi S., Ishida K., Torres J., MacGrory B., Raz E., Humbert K. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 23.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro D.D., Lijfering W.M., Van Hylckama Vlieg A., Rosendaal F.R., Cannegieter S.C. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost. 2012;10:1179–1182. doi: 10.1111/j.1538-7836.2012.04732.x. [DOI] [PubMed] [Google Scholar]
- 25.Samama M.M. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the SIRIUS study. Arch Intern Med. 2000;160:3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 26.Dogra S., Jain R., Cao M., Bilaloglu S., Zagzag D., Hochman S. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conti C.B., Henchi S., Coppeta G.P., Testa S., Grassia R. Bleeding in COVID-19 severe pneumonia: the other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147–149. doi: 10.1016/j.ejim.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]