Abstract
Background
The impact of corticosteroid therapy on outcomes of patients with coronavirus disease 2019 (COVID-19) is highly controversial. We aimed to compare the risk of death between COVID-19-related ARDS patients with corticosteroid treatment and those without.
Methods
In this single-center retrospective observational study, patients with ARDS caused by COVID-19 between January 20, 2020, and February 24, 2020, were enrolled. The primary outcome was 60-day in-hospital death. The exposure was prescribed systemic corticosteroids or not. Time-dependent Cox regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for 60-day in-hospital mortality.
Results
A total of 382 patients [60.7 ± 14.1 years old (mean ± SD), 61.3% males] were analyzed. The median of sequential organ failure assessment (SOFA) score was 2.0 (IQR 2.0–3.0). Of these cases, 94 (24.6%) patients had invasive mechanical ventilation. The number of patients received systemic corticosteroids was 226 (59.2%), and 156 (40.8%) received standard treatment. The maximum dose of corticosteroids was 80.0 (IQR 40.0–80.0) mg equivalent methylprednisolone per day, and duration of corticosteroid treatment was 7.0 (4.0–12.0) days in total. In Cox regression analysis using corticosteroid treatment as a time-varying variable, corticosteroid treatment was associated with a significant reduction in risk of in-hospital death within 60 days after adjusting for age, sex, SOFA score at hospital admission, propensity score of corticosteroid treatment, comorbidities, antiviral treatment, and respiratory supports (HR 0.42; 95% CI 0.21, 0.85; p = 0.0160). Corticosteroids were not associated with delayed viral RNA clearance in our cohort.
Conclusion
In this clinical practice setting, low-dose corticosteroid treatment was associated with reduced risk of in-hospital death within 60 days in COVID-19 patients who developed ARDS.
Keywords: Corticosteroids, Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus 2, Mortality, Propensity score, Methylprednisolone
Background
The World Health Organization (WHO) declared that the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) constitutes a pandemic [1]. Since the first confirmed case on January 22, 2020, the virus has been emerged in 216 countries. More than 14,765,000 laboratory-confirmed cases were reported, with an average mortality approaching 4.1% as of July 22, 2020 [2]. The spread of SARS-CoV-2 has led to serious socioeconomic consequences worldwide.
Currently, there is no specific treatment or vaccine for coronavirus disease 2019 (COVID-19). Up to 29% of the COVID-19 patients developed acute respiratory distress syndrome (ARDS) [3–5] as a consequence of cytokine storm. ARDS was the major cause of morbidity [6]. Adjunctive corticosteroids may be theoretically beneficial [7], and has been widely used by clinicians to suppression of hyperinflammation in COVID-19 patients, especially those with critical illness [3, 5, 8]. However, there was comprehensive controversy on its efficacy [9, 10], due to the results of observational studies that showed corticosteroid treatment was associated with increased mortality and nosocomial infections for influenza and delayed virus clearance for severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) [11].
In the early pandemic of COVID-19, lower mortality was reported among the critically ill subgroup of SARS patients treated with corticosteroids in a retrospective study [12]. Since then, evidence is growing that corticosteroid treatment is beneficial for some COVID-19 patients. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial from the UK reported reduced mortality in patients treated with oral or intravenous dexamethasone 6 mg/d for up to 10 days [13]. The efficacy was significant only in severe patients who receiving oxygen or invasive mechanical ventilation. A prospective meta-analysis of 7 RCTs of critically ill patients with COVID-19 also showed the association between systemic corticosteroids and lower all-cause mortality [14]. Other two randomized controlled trials (RCTs) did not show benefit on mortality from intravenous dexamethasone or methylprednisolone treatment, but corticosteroid treatment increased ventilator-free days and number of days alive in patients with moderate to severe ARDS [15, 16]. A meta-analysis of corticosteroids on the mortality of ARDS showed beneficial effects of corticosteroids on short-term mortality may be counteracted by the delayed onset of adverse effects [17], such as secondary infection due to immunosuppression and altered tissue repair. A follow-up period of 28 days in these randomized trials may underestimate the late adverse effects of corticosteroids on all-cause mortality. In addition, most of the aforementioned trials studied dexamethasone, and evidence for methylprednisolone was limited.
In this observational study, we thoroughly examined the associations of corticosteroid treatment with 60-day in-hospital mortality among a population of COVID-19 patients who have developed ARDS.
Materials and methods
Study design and patients
This single-center, retrospective, cohort study was conducted at the Jin Yin-tan Hospital, Wuhan, China. We identified all adult patients with confirmed COVID-19 according to WHO interim guidance [18] and patients were admitted between January 20, 2020, and February 24, 2020. Then, we identified those who developed ARDS according to the WHO definition for analysis [18]. Among these patients, 84 have been described previously by Wu et al. [8], and 91 participated in the open-label trial of Lopinavir–Ritonavir [19]. To avoid the influence of early mortality before cortical steroid presenting treatment efficacy, patients who died or discharged within 2 days on hospital admission were excluded. Other exclusion criteria were: (1) participating in any double-blind clinical trial, (2) under long-term corticosteroid therapy for at least 1 month as part of treatment for chronic underline diseases, or (3) could not provide valid medical history because of mental disease. The Jin Yin-tan Hospital Ethics Committee approved the study (No. KY-2020-44.01) and granted a waiver of informed consent from study participants.
In Jin Yin-tan hospital, systemic corticosteroids were considered if patients had progressive respiratory failure or laboratory findings indicated the presence of hyperinflammatory response. In patients receiving mechanical ventilation, preventive ventilation strategy of tidal volume 4–8 mL/kg of predicted body weight, inhale positive airway pressure < 30 cmH2O, and PEEP > 5 cmH2O was followed.
Data collection
Administration of corticosteroids was defined as systemic use (oral or intravenous) of corticosteroids, including methylprednisolone, dexamethasone, hydrocortisone, and prednisone. The primary outcome was 60-day in-hospital mortality. Patients were followed to death or discharge from hospital up to 60 days since hospital admission (last clinical outcome was observed on March 21, 2020). The secondary outcomes were time to SARS-CoV-2 viral clearance since symptom onset. Data on demographics, medical history, laboratory findings, chest radiology, medication use, and clinical outcomes were extracted retrospectively from electronic medical records using a standardized data collection form. All data were checked independently by two physicians (DH and XC). From January 11, 2020, to monitor the clearance of viral RNA, SARS-CoV-2 RNA was tested using polymerase chain reaction from throat-swab specimens for every other day after clinical remission of symptoms, including fever, cough, and dyspnea [8]. Viral clearance was defined as two consecutive negative results. The definitions of ARDS and other diseases were described in eMethods (Additional file 1).
Statistical analysis
Baseline characteristics were compared between patients with and without corticosteroid treatment. Data were reported as percentage for categorical variables and as mean ± standard deviation (SD) or median with interquartile range (IQR, 25–75%) for continuous variables. Categorical variables were compared by Fisher’s exact test or Pearson chi-square test, as appropriate, and continuous variables were compared by Mann–Whitney U test or Student’s t test.
Propensity score adjustment
To reduce the effect of steroids treatment bias and potential confounding factors, we performed propensity score analysis [20] to adjust the differences in baseline characteristics. For each patient, a propensity score indicating the likelihood of receiving systemic corticosteroid treatment was calculated by a logistic regression model. The model included 10 pre-selected baseline variables based on the clinical guidelines from National Health Commission of China, which recommended corticosteroids for patients with progressive respiratory failure and hyperinflammation response. Specifically, SpO2/FiO2 ratio and respiratory rate were included for indicating severe respiratory failure; temperature, heart rate, SOFA score, blood lymphocyte count, blood neutrophil count, and level of CRP at hospital admission were included for indicating systemic inflammatory response syndrome; age and sex were included as basic characteristics of each patient. The outcome variable was whether or not the patient received corticosteroid therapy in the current hospital stay. Goodness of fit was evaluated by the c-statistic and the Hosmer–Lemeshow test.
Cox proportional-hazard regression model
The effect of corticosteroid treatment on risk of 60-day in-hospital all-cause death was analyzed using a series of Cox proportional-hazard regression models. First, we constructed a univariable Cox regression model on hospital death by 60 days since hospital admission with corticosteroid treatment treated as a time-varying covariate. Then, we constructed a multivariable Cox model of 60-day hospital death with corticosteroid treatment as time-varying covariate and incorporated the individual propensity score into the model as a covariable to calculate the propensity adjusted hazard ratio (HR). In the final model, the effects of corticosteroids on 60-day in-hospital death were adjusted for propensity score of corticosteroid treatment, as well as the following pre-selected covariates: age, sex, sequential organ failure assessment (SOFA) score at hospital admission, comorbidities (diabetes, hypertension, coronary artery disease, chronic pulmonary disease, chronic renal or liver disease, solid malignant tumor, hematologic malignancy, and immunosuppressive status), antiviral treatment (Lopinavir–Ritonavir, oseltamivir, and ganciclovir), and respiratory supports (high-flow oscillation oxygen, noninvasive mechanical ventilation, and invasive mechanical ventilation) in hospital [9].
Sensitivity analysis
Several sensitivity analyses were performed to assess the robustness of our findings. To test whether the findings were influenced by the time point of baseline variables used in propensity score analysis, Cox proportional-hazard regression models were repeated in sensitivity analyses: (1) comparing patients receiving corticosteroids within 2 days after hospital admission versus no corticosteroids; (2) comparing patients receiving corticosteroids versus no corticosteroids using ARDS onset date as baseline, where values of SpO2/FiO2 ratio, respiratory rate, temperature, heart rate, respiratory rate, SOFA score, blood lymphocyte count, blood neutrophil count, and level of CRP at ARDS onset were used; (3) comparing patients receiving corticosteroids within 2 days after ARDS diagnosis versus no corticosteroids with ARDS onset as baseline. To test whether the findings might be influenced by ARDS definition, we conducted survival analysis using the same model among patients diagnosed with ARDS by Berlin definition [21]. The difference between Berlin Definition and WHO definition was that the latter included patients with SpO2/FiO2 ≤ 315 when PaO2 is not available, and did not restrict to ventilated patients.
Viral shedding and inflammation
Differences in the time to SARS-CoV-2 RNA clearance were analyzed using Cox proportional-hazard regression adjusted for the same covariables with corticosteroid therapy as a time-varying covariate. Patients died without viral shedding or discharged alive before they had two consecutive negative SARS-CoV-2 RNA tests were censored.
The associations between c-reactive protein with corticosteroids treatment after ARDS onset were analyzed using the interaction between corticosteroids and days after ARDS onset based on a linear regression model.
Results were analyzed with SAS (version 9.4, SAS Institute, Cary, NC). Unadjusted and adjusted hazard ratios and their 95% confidence intervals (Cis) were reported. Two-sided P values less than 0.05 were considered statistically significant.
Results
A total of 1147 patients with COVID-19 were screened for the study. Forty patients were excluded for participating in any double-blind clinical trial (n = 15), death or discharge from the hospital within 2 days after hospital admission (n = 21), underwent long-term corticosteroid therapy for chronic kidney disease or rheumatic disease (n = 3), or no valid medical history provided because of mental disease (n = 1). From 1107 patients remained, 382 patients were identified as ARDS (Additional file 1: Fig. S1).
Baseline characteristics of the ARDS patients at hospital admission by receiving systemic corticosteroid treatment are shown in Table 1. In the entire cohort, the mean age was 60.7 ± 14.1 years, and 234 (61.3%) patients were male. 147 (38.5%) were treated with NIMV, 94 (24.6%) with IMV, and 11 (2.9%) with ECMO. All but one of the patients reached end point of decease (53.1%) or discharge (46.6%) during the follow-up period of 60 days. The median duration of follow-up was 12.0 (IQR 7.0–18.0) days. A total of 226 (59.2%) ARDS patients had a prescription of systemic corticosteroids. Corticosteroids were more likely prescribed to the younger (p = 0.0077) and males (p = 0.0135). Corticosteroids group had lower lymphocyte count and higher levels of CRP and lactate dehydrogenase at hospital admission than non-corticosteroids group, indicating a propensity in prescribing corticosteroids to patients with more severe immune dysfunction and inflammatory response (Table 1). The 60-day hospital death in patients who ever used corticosteroids was higher than the patients who did not use corticosteroids [135 (59.7%) vs. 68 (43.6%), p = 0.0019]. However, the median survival duration was longer in corticosteroid group [19.0 (IQR 15.0–21.0) vs. 15.0 (IQR 12.0–23.0), p = 0.0239].
Table 1.
Characteristics of patients with acute respiratory distress syndrome associated with coronavirus disease 2019
| Characteristics | All | Corticosteroids | No corticosteroids | p value |
|---|---|---|---|---|
| N | 382 (100.0) | 226 (59.2) | 156 (40.8) | – |
| Age (year) | 60.7 ± 14.1 | 59.1 ± 14.0 | 63.0 ± 14.0 | 0.0077 |
| Male sex | 234 (61.3) | 150 (66.4) | 84 (53.8) | 0.0135 |
| Smoking history | 35 (9.2) | 24 (10.6) | 11 (7.1) | 0.2347 |
| Days from onset at hospital admission | 11.0 (8.0–15.0) | 10.0 (7.0–14.0) | 12.0 (9.0–16.5) | 0.0029 |
| Medical history | ||||
| Chronic pulmonary disease | 20 (5.2) | 12 (5.3) | 8 (5.1) | 0.9376 |
| Hypertension | 136 (35.6) | 79 (35.0) | 57 (36.5) | 0.7508 |
| Diabetes | 67 (17.5) | 36 (15.9) | 31 (19.9) | 0.3193 |
| Chronic liver disease | 15 (3.9) | 11 (4.9) | 4 (2.6) | 0.2546 |
| Chronic renal disease | 6 (1.6) | 2 (0.9) | 4 (2.6) | 0.1945 |
| Cardiovascular disease | 28 (7.3) | 12 (5.3) | 16 (10.3) | 0.0682 |
| Malignant tumor | 12 (3.1) | 7 (3.1) | 5 (3.2) | 0.9527 |
| Hematological malignant tumor | 2 (0.6) | 1 (0.5) | 1 (0.7) | 0.8339 |
| Immunosuppressive conditions | 14 (3.7) | 9 (4.0) | 5 (3.2) | 0.6911 |
| SOFA score at hospital admission | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 0.1383 |
| Corticosteroid therapy before hospital admission | 40 (10.5) | 28 (12.4) | 12 (7.7) | 0.1405 |
| Vital signs at hospital admission | ||||
| Temperature (°C) | 36.8 ± 0.7 | 36.9 ± 0.8 | 36.7 ± 0.5 | 0.0069 |
| Heart rate (min−1) | 90.9 ± 15.3 | 92.9 ± 16.3 | 88.0 ± 13.1 | 0.0018 |
| Respiratory rate (min−1) | 24.0 ± 6.3 | 24.5 ± 7.1 | 23.3 ± 5.0 | 0.0609 |
| Laboratory findings | ||||
| Blood leukocyte count (× 109/L) | 8.1 (5.2–11.3) | 8.4 (5.1–11.7) | 7.5 (5.4–10.0) | 0.2593 |
| Lymphocyte count (× 109/L) | 0.7 (0.5–1.0) | 0.6 (0.5–0.8) | 0.8 (0.6–1.1) | < 0.0001 |
| Neutrophil count (× 109/L) | 6.9 (4.0–10.2) | 7.4 (4.2–10.7) | 5.7 (4.0–8.7) | 0.0508 |
| SpO2/FiO2 | 229.3 (175.5–352.4) | 218.6 (170.0–366.7) | 241.5 (184.0–332.8) | 0.2133 |
| CRP (mg/L) | 89.0 (38.0–159.9) | 96.7 (45.9–160.0) | 68.7 (28.6–138.5) | 0.0026 |
| D-dimer (mg/L) | 1.5 (0.7–8.0) | 1.5 (0.6–9.5) | 1.5 (0.7–7.1) | 0.9913 |
| Lactate dehydrogenase (U/L) | 409.0 (304.0–545.0) | 429.0 (320.0–569.0) | 386.5 (277.0–509.5) | 0.0124 |
| Bilateral involvement | 358 (93.7) | 214 (94.7) | 144 (92.3) | 0.3455 |
| Severity of ARDSa | ||||
| Mild | 120 (31.4) | 60 (26.5) | 60 (38.5) | 0.0297 |
| Moderate | 157 (41.1) | 99 (43.8) | 58 (37.2) | |
| Severe | 105 (27.5) | 67 (29.6) | 38 (24.4) | |
| Antivirus drugs | ||||
| Lopinavir | 91 (24.0) | 72 (31.9) | 19 (12.4) | < 0.0001 |
| Ganciclovir | 32 (8.4) | 19 (8.4) | 13 (8.5) | 0.9754 |
| Interferon | 103 (27.2) | 65 (28.8) | 38 (24.8) | 0.3994 |
| Oseltamivir | 64 (16.9) | 52 (23.0) | 12 (7.8) | 0.0001 |
| Antibiotics | 371 (97.1) | 224 (99.1) | 147 (94.2) | 0.0126 |
| Respiratory support during hospital stay | ||||
| High-frequency oscillation ventilation | 146 (38.8) | 100 (45.2) | 46 (29.7) | 0.0023 |
| NIMV | 147 (38.5) | 104 (46.0) | 43 (27.6) | 0.0003 |
| IMV | 94 (24.6) | 59 (26.1) | 35 (22.4) | 0.4130 |
| ECMO | 11 (2.9) | 8 (3.5) | 3 (1.9) | 0.3530 |
| Hyperglycemia | 32 (8.4) | 20 (8.8) | 12 (7.7) | 0.6882 |
| In-hospital 60-day mortality | 203 (53.1) | 135 (59.7) | 68 (43.6) | 0.0019 |
| In-hospital days for all patients | 12.0 (7.0–18.0) | 14.0 (9.0–21.0) | 10.0 (6.0–13.0) | < 0.0001 |
| In-hospital days for survivors | 13.0 (10.0–19.0) | 16.0 (11.0–24.0) | 11.0 (8.5–15.0) | < 0.0001 |
| Median survival time (days) | 18.0 (15.0–20.0) | 19.0 (15.0–21.0) | 15.0 (12.0–23.0) | 0.0239 |
| Duration of viral shedding from symptom onset (days) | 18.0 (14.0–23.0) | 19.0 (14.0–23.0) | 18.0 (14.0–24.0) | 0.7217 |
Data are n (%), mean (SD), or median (IQR). For continuous variables, t test or Mann–Whitney U test was used to calculate the p value unless otherwise noted. For categorical variables, chi-square test was used to calculate the p value unless otherwise noted
ARDS acute respiratory distress syndrome, SOFA sequential organ failure assessment, CRP c-reactive protein, MV mechanical ventilation, NIMV noninvasive mechanical ventilation, IMV invasive mechanical ventilation, ECMO extracorporeal membrane oxygenation, SpO2 pulse oxygen saturation, FIO2 fraction of inspired oxygen. ARDS was defined according to World Health Organization interim guidance
aPaO2/FiO2 was estimated from SpO2/FiO2 if PaO2/FiO2 was not available [37]
Among patients prescribed corticosteroids, methylprednisolone was the most frequently administered corticosteroids (213/226, 94.2%) (Table 2). Corticosteroid treatment lasted for 7.0 (IQR 4.0–12.0) days in total. The maximum dose in methylprednisolone equivalent was 80.0 (IQR 40.0–80.0) mg per day and duration of maximum dose was 3.0 (IQR 2.0–5.0) days. Corticosteroids were initiated 13.0 (IQR 10.0–16.0) days after symptom onset. 82.3% (186/226) of the patients received corticosteroids started the therapy within 2 days after ARDS diagnosis. Survivors had shorter duration from symptom onset to corticosteroids [11.0 (9.0–14.0) vs. 14.00 (IQR 11.0–18.0), p = 0.0031] and had earlier initiation of corticosteroids with regard to the date of ARDS onset [0.0 (IQR − 1.0 to 1.0) vs. 1.00 (IQR 0.0–2.0), p = 0.0102] when compared with non-survivors. Clinical characteristics of survivors and non-survivors received corticosteroid are summarized in eTable 1 (Additional file 1).
Table 2.
Administration of corticosteroids, stratified by outcome
| All (n = 226) | Non-survivors (n = 135) | Survivors (n = 91) | p value | |
|---|---|---|---|---|
| Corticosteroid prescribed | ||||
| Methylprednisolone | 213 (94.2) | 132 (62.0) | 83 (38.0) | 0.0004 |
| Prednisolone | 41 (18.1) | 11 (73.2) | 30 (26.8) | 0.0007 |
| Dexamethasone | 5 (2.2) | 4 (80.0) | 1 (20.0) | 0.4470 |
| Maximum dose (methylprednisolone equivalent, mg) | 80.0 (40.0–80.0) | 80.0 (40.0–80.0) | 80.0 (40.0–80.0) | 0.0821 |
| Days of corticosteroid treatment | 7.0 (4.0–12.0) | 6.0 (3.0–11.0) | 9.0 (5.0–12.0) | 0.0069 |
| Days of maximum dose | 3.0 (2.0–5.0) | 3.0 (1.0–5.0) | 4.0 (2.0–5.0) | 0.0287 |
| Days from symptom onset to corticosteroid treatment | 13.0 (10.0–16.0) | 14.0 (11.0–18.0) | 11.0 (9.0–14.0) | 0.0031 |
| Days from hospital admission to corticosteroid treatment | 1.0 (0.0–3.0) | 1.0 (0.0–4.0) | 1.0 (0.0–2.0) | 0.1892 |
| Days from ARDS to corticosteroid treatment | 0.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.0 (− 1.0 to 1.0) | 0.0102 |
| Days from ventilation to corticosteroid treatment | − 1.0 (− 3.0 to 0.0) | − 1.0 (− 3.0 to 0.0) | − 2.0 (− 4.0 to 1.0) | 0.7576 |
Data are n (%) or medium (IQR). For continuous variables, t test or Mann–Whitney U test was used to calculate the p value unless otherwise noted. For categorical variables, chi-square test was used to calculate the p value unless otherwise noted
In the logistic regression model generating propensity score, the pre-selected variables most closely correlated with prescription of systemic corticosteroids included age, blood lymphocyte count, heart rate and CRP (eTable 2, Additional file 1). The multivariable regression model of propensity for corticosteroid treatment had area under the receiver operating characteristic curve (ROC) of 0.71.
In survival analysis, univariable time-dependent Cox regression model showed the prescription of corticosteroids was associated with a lower risk of death (HR 0.48; 95% CI 0.25, 0.93; p = 0.0285) (Table 3). In full model adjusted for age, sex, SOFA score, propensity score, comorbidities, antiviral drugs, and respiratory supports, the association remained (HR 0.42; 95% CI 0.21, 0.85; p = 0.0160) (Fig. 1).
Table 3.
Estimated effects of corticosteroid treatment on 60-day mortality in patients with ARDS associated with COVID-19
| Nos. | Hazard ratio | 95% CI | p value | |
|---|---|---|---|---|
| All ARDS patients | ||||
| Full multivariate modela | 355 | 0.421 | 0.21, 0.85 | 0.0160 |
| Sensitivity analysis | ||||
| ARDS patients defined by Berlin definition | 168 | 0.43 | 0.21, 0.88 | 0.0208 |
| Initiated ≤ 2 days after hospital admission versus no corticosteroids (reference) | 262 | 0.37 | 0.18, 0.76 | 0.0072 |
| Full multivariate model, ARDS onset as baselineb | 335 | 0.48 | 0.24, 0.97 | 0.0399 |
| Initiated ≤ 2 days after ARDS onset versus no corticosteroids, ARDS onset as baselineb (reference) | 279 | 0.45 | 0.22, 0.92 | 0.0275 |
All of the models assessed the effects of corticosteroids as a time-varying covariate
ARDS acute respiratory distress syndrome, FIO2 fraction of inspired oxygen, SOFA sequential organ failure assessment, SpO2 pulse oxygen saturation
aAdjusted for age, sex, SOFA score at hospital admission, propensity score of corticosteroid treatment, comorbidities (diabetes, hypertension, chronic pulmonary disease, chronic renal or liver disease, solid malignant tumor, hematologic malignancy, and immunosuppressive status), antiviral treatment (Lopinavir–Ritonavir, oseltamivir, and ganciclovir), and respiratory supports (high-flow oscillation oxygen, noninvasive mechanical ventilation, and invasive mechanical ventilation). Propensity score was calculated by a non-parsimonious logistic regression model that included: age, sex, SOFA score, temperature, respiratory rate, SpO2/FiO2 ratio, blood lymphocyte count, blood neutrophil count, and level of c-reactive protein at hospital admission
bUsing values of SpO2/FiO2 ratio, respiratory rate, temperature, heart rate, respiratory rate, SOFA score, blood lymphocyte count, blood neutrophil count, and level of CRP at ARDS onset
Fig. 1.
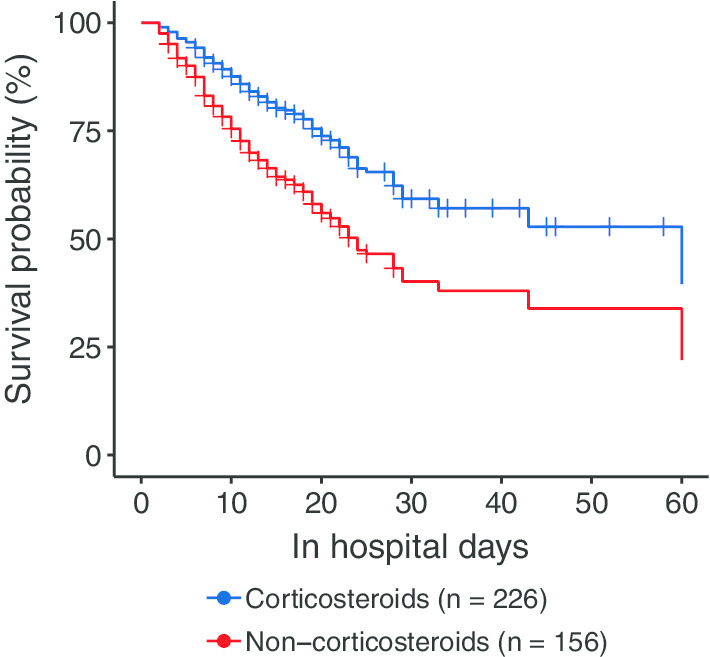
Estimated survival probability of multivariable Cox regression model with time-dependent corticosteroid treatment. Cox regression model with corticosteroid treatment was time-varying variable, adjusting for age, sex, SOFA score at hospital admission, propensity score of corticosteroid treatment, comorbidities (diabetes, hypertension, chronic pulmonary disease, chronic renal or liver disease, solid malignant tumor, hematologic malignancy, and immunosuppressive status), antiviral treatment (Lopinavir–Ritonavir, oseltamivir, and ganciclovir), and respiratory supports (high-flow oscillation oxygen, noninvasive mechanical ventilation, and invasive mechanical ventilation). Propensity score was calculated by a non-parsimonious logistic regression model that included: age, sex, SOFA score, temperature, respiratory rate, SpO2/FiO2 ratio, blood lymphocyte count, blood neutrophil count, and level of c-reactive protein at hospital admission. ARDS, acute respiratory distress syndrome; SOFA, sequential organ failure assessment
In sensitivity analysis, narrowing to patients meet the Berlin definition of ARDS did not alter the association between corticosteroids and lower risk of death (HR 0.43; 95% CI 0.21, 0.88; p = 0.0208). The HR of corticosteroids on risk of death was constant when using hospital admission as baseline and excluding patients received corticosteroids 2 days after hospital admission (HR 0.37; 95% CI 0.18, 0.76; p = 0.0072). When using ARDS onset date as baseline, the associations between corticosteroids and the risk of death were also significant (Table 3).
Viral shedding was observed in 49.2% (188/382) of the whole population, including 69.3% (124/179) of survivors and 31.5% (64/203) of the non-survivors. In Cox regression model, we found no difference in time to viral shedding between corticosteroids-treated group and the corresponding group (HR 1.43, 95% CI 0.43, 4.80; p = 0.5593).
Blood CRP level decreased among corticosteroids group on the first 4 days after ARDS onset (Fig. 2), while an increase was found in non-corticosteroids group. CRP levels were significantly lower in corticosteroids-treated group after 2 days of ARDS onset (p for interaction = 0.0434).
Fig. 2.

Changes in c-reactive protein in patients with ARDS associated with coronavirus disease 2019. ARDS, acute respiratory distress syndrome. Values are means ± SD
Discussion
In this observational study, prescription of low-to-moderate dose systemic corticosteroids was associated with lower risk of 60-day in-hospital death among COVID-19 patients who developed ARDS. The efficiency of corticosteroids was further supported by the reduction of CRP, as the marker for suppressed systemic inflammation responses. No associations between corticosteroid treatment with viral shedding were found in our study.
Our study demonstrates the association between corticosteroid treatment with long-term (60 days) risk of death in severe COVID-19 patients. It is biologically plausible that suppression of inflammatory response by corticosteroids may be beneficial for patients with ARDS, which was caused by dysregulated systemic inflammation [3–5] and proved the main cause of death. The RECOVERY trial and a metanalysis of ongoing RCTs showed reduced 28-day mortality and longer ventilation-free days in patients with corticosteroid treatment [13]. We used 60-day in-hospital death as primary endpoint. To our knowledge, it was longer than previously reported RCTs. The results showed significant association between corticosteroids and risk of death, which further provide evidence on the long-term benefit of corticosteroids. It was compatible with studies that indicated corticosteroid treatment was not associated with increases in secondary infections in COVID-19-related ARDS patients [15, 16].
Type, dosage, and duration of corticosteroids therapy were fundamental variables of corticosteroid treatment regimens. Different from the most of the published RCTs using dexamethasone, most of the patients in our study were treated with methylprednisolone. Methylprednisolone is a rapid onset glucocorticoid with shorter half-life and less mineralocorticoid effects than dexamethasone, which indicate shorter effects on systemic immunity and preventing corticosteroids-related fluid retention. Meduri et al. [22] firstly promoted the early use of corticosteroids in ARDS. They found that methylprednisolone reduced the duration of mechanical ventilation, ICU stay, and ICU mortality in early severe ARDS patients. In our study, a similar maximum dose (equivalent to methylprednisolone of 1–2 mg/kg) of corticosteroids was used, which was also close to the dose in RECOVERY trial and recommended by Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) for ARDS patients [23, 24]. Our results were in line with the RCTs of COVID-19 [13, 14] and previous studies of other ARDS patients, which showed low-dose corticosteroid treatment (equivalent to methylprednisolone of 1–2 mg/kg) accelerates the resolution of ARDS [23, 25, 26], indicating low-dose methylprednisolone as an alternative to dexamethasone in COVID-19-related ARDS. Higher dose may increase risks of immune-suppression and corticosteroid-induced complications [27–29]. In this cohort, tapering strategy was performed as has been suggested by the guidelines for the ARDS-related corticosteroid insufficiency (CIRCI) to reduce deterioration from the development of a reconstituted inflammatory response and febrile response. Of note, a randomized trial included mild COVID-19 patients using a similar dose and shorter course of methylprednisolone (0.5 mg/kg twice daily for 5 days) than in the regimens of our study found no benefit on mortality. More research is needed to determine the best duration of corticosteroid therapy. A recent analysis of four trials showed prolonged corticosteroids therapy reduced mortality [25]. However, chronic side effects of corticosteroids including secondary infection and osteoporosis may occur in prolonged course of treatment.
Delayed virus clearance was reported in corticosteroid-treated patients with both SARS, MERS, and influenza [30–32], which was a major concern for the immune suppressive effects of corticosteroids, albeit its uncertain clinical relevance. We found no difference in viral shedding duration from symptom onset between corticosteroid and non-corticosteroid groups, which may explain the heterogeneity in efficacy of corticosteroids between COVID-19 and other virus infections. Of note, positive SARS-CoV-2 test results have been reported after two consecutive negative results [33]. Viral tests of throat swabs were not monitored after two consecutive negative tests in our cohort. More evidence is needed for assessing the effects of corticosteroids on clearance of SARS-CoV-2 RNA.
We used rigorous statistical method to control for survival and indication bias. Survivors-treated bias exists in observational studies that assess exposure after the start of follow-up, where only patients survived long enough had an opportunity to receive the intervention. Therefore, the patients died early are more likely to be misclassified to the no-treatment group, leading to overestimation of the effectiveness of medicine [34]. This study was specifically designed to address survivors-treated bias of corticosteroid treatment, by using a time-dependent variable for corticosteroids initiation to define corticosteroids group and non-corticosteroids group [35]. In addition, there was a propensity of clinicians to give corticosteroids to patients who were critically ill in non-randomized clinical condition. The imbalance in baseline characteristics may introduce confounders in comparison of mortality between corticosteroids and non-corticosteroids group. In this cohort, lymphopenia and elevation of CRP and lactate dehydrogenase levels were more severe in patients who received corticosteroids therapy. Propensity score is a validated method to account for baseline confounding and control selection bias in this case [36]. In this study, we performed a rigorous propensity adjustment analysis accounting for the baseline variables related to propensity of corticosteroid treatment. These added to the strength of our results that found associations between corticosteroid treatment and risk of death.
Our study had some limitations. First, unlike randomized controlled trials, the selection bias and potential confounding effects might exist. We used propensity analysis rather than standard multivariable analysis to rigorously adjust for selection bias, and time-dependent model to avoid survivors-treated bias. Nonetheless, only measured factors were controlled for due to the nature of observational study design. Second, secondary infections were not monitored in this study, because microbiological culture results needed for definite diagnosis of secondary infection were possibly affected by antibiotic treatment the patients received simultaneously. To include the delayed effects of secondary infections on mortality, a longer follow-up period of 60 days was used. Third, this study was single center and patients were sicker and transferred from other hospital, so might lacking of generality. Forth, some of the patients were treated with Lopinavir–Ritonavir; it might be a confounder because the efficacy of Lopinavir–Ritonavir in COVID-19 was unclear. Fifth, our cohort was collected in the early outbreak of COVID-19; thus, the mortality was relatively higher than other studies, which limited generalization of our results.
Conclusion
Our findings suggest administration of low dose of corticosteroids might reduce the risk of death in COVID-19 patients who developed ARDS.
Supplementary information
Additional file 1: eMethods, eTable 1 and 2, and eFigure 1 were included.
Acknowledgements
We thank Yixiu Tao M.D. (Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China), Jingjing Zhou (Department of Clinical Medicine, Wannan Medical College, Anhui, China), and Guohua Shi (Department of Respiratory Medicine, traditional Chinese medicine hospital of Qingpu District, Shanghai, China) for data collection.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CI
Confidence intervals
- COVID-19
Coronavirus disease 2019
- CRP
c-reactive protein
- ECMO
Extracorporeal membrane oxygenation
- FIO2
Fraction of inspired oxygen
- HR
Hazard ratios
- IMV
Invasive mechanical ventilation
- IQR
Interquartile range
- MERS
Middle East respiratory syndrome
- MV
Mechanical ventilation
- NIMV
Noninvasive mechanical ventilation
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SD
Standard deviation
- SOFA
Sequential organ failure assessment
- SpO2
Pulse oxygen saturation
Authors’ contributions
CW, CD, YC, and JZ contributed substantially to the study design, data collection, and patient management. DH contributed substantially to the study design, data collection, statistical analysis and interpretation, and the writing of the manuscript. XC contributed to statistical analysis and the writing of the manuscript. JX, CC, XH, YZ, JS, LW, and YC contributed to the acquisition, analysis, or interpretation of data. YF, WX, DC, MZ, JH, JJ, CB, and XZ contributed to critical revision of the manuscript for important intellectual content; JX, YS, and FG contributed to administrative, technical, or material support. All authors read and approved the final manuscript.
Funding
This study was supported by The National Natural Science Foundation of China (82041003, 81630001, 81770075, 81800008), Science and Technology Commission of Shanghai Municipality (20411950402, 20XD1401200), Shanghai Municipal Key Clinical Specialty (shslczdzk02201), Shanghai Key Discipline of Medicine (ZK2019B06), Shanghai Sailing Program (18YF1404300), Academic Leader of Shanghai Qingpu District Healthcare Commission (WD2019-36), and Sub-specialist Project of Qingpu Branch of Zhongshan Hospital, Fudan University (YZK 2019-04), Construction of GCP Platform for Pulmonary Tuberculosis Drugs (2020ZX09201001).
Availability of data and materials
The corresponding authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data are available from the corresponding authors upon reasonable request.
Ethics approval and consent to participate
The Jin Yin-tan Hospital Ethics Committee approved the study (No. KY-2020-44.01) and granted a waiver of informed consent from study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chaomin Wu, Dongni Hou, Chunling Du, Yanping Cai and Junhua Zheng have contributed equally to this manuscript
Contributor Information
Yuanlin Song, Email: ylsong70@163.com.
Fengyun Gong, Email: gfy.yuyingzi@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13054-020-03340-4.
References
- 1.WHO. Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020.
- 2.Coronavirus disease 2019 (COVID-19) Situation report-156. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200624-covid-19-sitrep-156.pdf?sfvrsn=af42e480_2.
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2019;2020:181. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;S0140–6736(0120):30317–30312. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JW, Yang L, Luo RG, Xu JF. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.3390/ijerph17186845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020 doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva M, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Safe IP, Borba MGS, Abreu-Netto RL, Maciel ABS, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020;1:6. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan SY, Lin HH, Huang CT, Kuo PH, Wu HD, Yu CJ. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2014;18(2):R63. doi: 10.1186/cc13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications/i/item/10665-332299.
- 19.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. JAMA. 2001;286(10):1187–1194. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]
- 21.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 23.Pastores SM, Annane D, Rochwerg B. Corticosteroid guideline task force of S, Esicm: guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Inten Care Med. 2018;44(4):474–477. doi: 10.1007/s00134-017-4951-5. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto S, Sanui M, Egi M, Ohshimo S, Shiotsuka J, Seo R, Tanaka R, Tanaka Y, Norisue Y, Hayashi Y, et al. The clinical practice guideline for the management of ARDS in Japan. J Inten Care. 2017;5:50. doi: 10.1186/s40560-017-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri GU, Bridges L, Shih MC, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Inten Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 26.Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez-Higueras E, Conesa LA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee DT, Wing YK, Leung HC, Sung JJ, Ng YK, Yiu GC, Chen RY, Chiu HF. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case–control study. Clin Infect Dis. 2004;39(8):1247–1249. doi: 10.1086/424016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan MH, Chan PK, Griffith JF, Chan IH, Lit LC, Wong CK, Antonio GE, Liu EY, Hui DS, Suen MW, et al. Steroid-induced osteonecrosis in severe acute respiratory syndrome: a retrospective analysis of biochemical markers of bone metabolism and corticosteroid therapy. Pathology. 2006;38(3):229–235. doi: 10.1080/00313020600696231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao JZ, Ma L, Gao J, Yang ZJ, Xing XY, Zhao HC, Jiao JS, Li GW. Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi. 2004;43(3):179–182. [PubMed] [Google Scholar]
- 30.Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, et al. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 32.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, Lui GC, Wong BC, Wong RY, Lam WY, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosco JL, Silliman RA, Thwin SS, Geiger AM, Buist DS, Prout MN, Yood MU, Haque R, Wei F, Lash TL. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC, Mamdani MM, van Walraven C, Tu JV. Quantifying the impact of survivor treatment bias in observational studies. J Eval Clin Pract. 2006;12(6):601–612. doi: 10.1111/j.1365-2753.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 36.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 37.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. National Institutes of Health NHL, Blood Institute AN: comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: eMethods, eTable 1 and 2, and eFigure 1 were included.
Data Availability Statement
The corresponding authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data are available from the corresponding authors upon reasonable request.


