Abstract
Objectives:
Bleeding and thromboembolism are common during venovenous extracorporeal membrane oxygenation. The relative frequency of these complications and their impact on clinical outcomes have not been described, and no randomized trials exist to guide anticoagulation strategies in extracorporeal membrane oxygenation. Our objective was to examine the relative frequencies of bleeding and thromboembolic events and their associations with survival among a cohort of consecutive patients receiving venovenous extracorporeal membrane oxygenation.
Design:
Retrospective cohort study.
Setting:
A single academic medical center.
Patients:
Adult patients receiving venovenous extracorporeal membrane oxygenation and anticoagulation. Eligibility criteria for this analysis were selected to emulate the population that would be recruited for a randomized trial of anticoagulation strategies during venovenous extracorporeal membrane oxygenation. Patients were excluded if they had active bleeding or thromboembolism prior to extracorporeal membrane oxygenation initiation, a history of trauma or surgery in the 7 days prior to extracorporeal membrane oxygenation initiation, an arterial extracorporeal membrane oxygenation cannula, or if they received greater than 48 hours of extracorporeal membrane oxygenation support at another institution
Interventions:
None.
Measurements and Main Results:
Outcomes included bleeding and thromboembolic events, duration of extracorporeal membrane oxygenation support, hospital length of stay, and in-hospital survival among 55 patients receiving venovenous extracorporeal membrane oxygenation. Bleeding events occurred in 25 patients (45.5%), and thromboembolism occurred in eight patients (14.5%). Bleeding events were associated with longer duration of extracorporeal membrane oxygenation support (p = 0.007) and worse in-hospital survival (p = 0.02). Thromboembolic events did not appear to be associated with clinical outcomes.
Conclusions:
In this cohort of patients receiving venovenous extracorporeal membrane oxygenation and anticoagulation, bleeding occurred more frequently than thromboembolism and was associated with worse survival. These results highlight the need for randomized trials to evaluate the safety and efficacy of continuous IV anticoagulation among patients receiving venovenous extracorporeal membrane oxygenation.
Keywords: adult, critical care, extracorporeal membrane oxygenation, hemorrhage, respiratory distress syndrome, thromboembolism
Bleeding and thromboembolism are common during venovenous extracorporeal membrane oxygenation (ECMO) (1). Reported frequencies and associations with clinical outcomes vary and available data are limited by heterogenous study populations (1–3). Multiple anticoagulation strategies have been proposed to balance the risks of bleeding and thromboembolism during venovenous ECMO (4, 5), but which strategy is most effective remains unknown. Data on the relative frequencies of bleeding and thromboembolism during venovenous ECMO, and their respective associations with survival, are needed to provide preliminary data and inform equipoise for future randomized trials.
Our objective was to evaluate the frequency and clinical significance of bleeding and thromboembolic events during venovenous ECMO. We hypothesized that bleeding events, but not thromboembolic events, would be associated with worse in-hospital survival.
MATERIALS AND METHODS
We performed a retrospective cohort study examining data from all patients who received venovenous ECMO at the adult hospital at Vanderbilt University Medical Center between January 1, 2016, and May 10, 2020. Prespecified exclusion criteria were used with the goal of including a patient population similar to those who would be included in a randomized trial comparing anticoagulation strategies, a technique known as “target trial emulation” (6). We excluded patients who had active bleeding or thromboembolism prior to ECMO initiation, experienced trauma or surgery in the 7 days prior to ECMO initiation, received greater than 48 hours of ECMO support at another institution, or received arterial cannulation. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB no 200158).
We collected the following data from the electronic health record: patient characteristics in the 24 hours prior to ECMO initiation; bleeding and thromboembolic events during venovenous ECMO as previously defined (5); and clinical outcomes, including in-hospital survival, ECMO duration, and hospital length of stay. Bleeding events were defined as overt bleeding associated with either a drop in hemoglobin concentration by 2 g/dL or a transfusion of at least two units of packed RBCs in 24 hours, bleeding at any critical site (e.g. intracranial bleeding), or bleeding requiring a procedural intervention (5). Thromboembolic events were defined as cerebral stroke, intracardiac thrombus, acute pump head thrombosis, acute oxygenator failure, pulmonary emboli, or deep vein thrombosis (DVT) (5). Cannula-associated DVTs following decannulation did not meet the composite definition for thromboembolic event and were omitted from the survival analysis to limit immortal time bias.
Continuous variables are presented as median with interquartile range (IQR). Categorical variables are summarized as frequencies and percentages. Differences between groups were compared using a chi-square test, Fisher exact test, or Wilcoxon rank-sum test as appropriate. Log-rank tests were used to compare time with hospital discharge between groups. All analyses were performed using STATA 16.1 (StataCorp, College Station, TX), and a two-sided p value of 0.05 was considered to be statistically significant. No adjustments were made for multiple testing.
RESULTS
Of the 156 patients who received venovenous ECMO during the study period, 101 met exclusion criteria. A total of 69 patients were excluded for recent trauma or surgery, 13 patients were excluded for active bleeding, 11 patients were excluded for recent thromboembolism, five patients were excluded for receiving ECMO at an another institution for greater than 48 hours, and three patients were excluded for arterial cannula placement while receiving venovenous ECMO. A total of 55 patients were included in the analysis. The median age was 50 years (IQR, 40–60 yr), and 38% were women. According to institutional protocols, all patients received a continuous infusion of unfractionated heparin following ECMO cannulation, titrated to either antifactor Xa levels of 0.2–0.4 U/mL or a partial thromboplastin time of 40–60 seconds.
A total of 30 bleeding events occurred among 25 patients (45.5%), including eight gastrointestinal bleeds, seven intracranial hemorrhages, four cannula site bleeds, four episodes of hemoptysis, three tracheostomy bleeds, two hemothoraces, and two episodes of epistaxis. Of these, six (5 intracranial hemorrhages and 1 gastrointestinal bleed) were considered the primary cause of death. The median time from ECMO cannulation to first bleeding event was 5 days (IQR, 2–7 d).
Eight patients (14.5%) experienced a thromboembolic event during ECMO, including five deep venous thromboses (DVT), two acute circuit thromboses requiring circuit exchange, and one brachial artery thrombosis. The median time from ECMO cannulation to first thromboembolic event was 6 days (IQR, 2–18 d). No thromboembolic events were considered the primary cause of death. A total of 14 additional cannula-associated DVTs were identified on protocolized ultrasound screening following decannulation.
Baseline characteristics and serum markers of coagulation and thrombocytopenia were similar between groups (Table 1). Anticoagulation monitoring did not vary between groups. RBC transfusion requirement was greater among patients with a bleeding event than patients without a bleeding event (p = 0.002). In univariate analysis, patients who experienced a bleeding event had a longer duration of ECMO support (p = 0.007) and worse in-hospital survival compared with patients who did not experience a bleeding event (p = 0.02) (Table 1 and Fig. 1). Thromboembolic events did not appear to be associated with any differences in duration of ECMO support, hospital length of stay, or in-hospital survival (Table 1 and Fig. 1).
TABLE 1.
Baseline Characteristics and Clinical Outcomes
| Variable | Overall (n = 55) | Bleeding Event (n = 25) | No Bleeding Event (n = 30) | p | Thromboembolic Event (n = 8) | No Thromboembolic Event (n = 47) | p |
|---|---|---|---|---|---|---|---|
| Baseline characteristics | |||||||
| Age (yr), median (interquartile range) | 50.0 (40.0–60.0) | 53.0 (42.0–60.0) | 48.5 (39.0–60.0) | 0.45 | 53.5 (45.5–65) | 48.0 (39.0–60.0) | 0.21 |
| Female, n (%) | 21 (38.2) | 11 (44.0) | 10 (33.3) | 0.42 | 5 (62.5) | 16 (34.0) | 0.24 |
| Simplified Acute Physiology Score-II, median (interquartile range) | 33.0 (24.0–41.0) | 33.0 (28.0–37.0) | 34.5 (21.0–43.0) | 0.98 | 44.5 (35.0–55.5) | 33.0 (22.0–38.0) | 0.01 |
| Body mass index (kg/m2), median (interquartile range) | 30.5 (25.9–37.2) | 32.9 (29.0–36.7) | 29.1 (25.7–37.2) | 0.30 | 29.3 (26.2–36.7) | 31.7 (25.9–37.2) | 0.93 |
| Renal failure requiring continuous renal replacement therapy on ECMO, n (%) | 20 (36.4) | 12 (48.0) | 8 (26.7) | 0.10 | 3 (37.5) | 17 (36.2) | 0.10 |
| Indication for ECMO, n (%) | 0.56 | 0.49 | |||||
| Acute respiratory distress syndrome | 46 (83.6) | 22 (88.0) | 24 (80.0) | 6 (75.0) | 40 (85.1) | ||
| Acute respiratory distress syndrome due to coronavirus disease 2019 | 1 (2.0) | 1 (4.0) | 0 (0) | 0 (0) | 1 (2.1) | ||
| Postlung transplantation | 5 (9.1) | 2 (8.0) | 4 (13.3) | 1 (12.5) | 4 (8.5) | ||
| Asthma or chronic obstructive pulmonary disease exacerbation | 4 (7.3) | 1 (4.0) | 2 (6.7) | 1 (12.5) | 3 (6.4) | ||
| Initial ECMO settings, median (interquartile range) | |||||||
| Blood flow rate (L/m) | 4.5 (4.0–5.1) | 4.8 (3.9–5.2) | 4.5 (4.0–4.9) | 0.43 | 4.3 (3.8–4.8) | 4.5 (4.0–5.2) | 0.29 |
| Sweep gas flow rate (L/m) | 5.0 (3.0–7.0) | 5.0 (3.5–6.0) | 5.0 (2.5–7.0) | 0.64 | 3.8 (2.5–5.5) | 5.0 (3.0–8.0) | 0.25 |
| Fraction of delivered O2 (%) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 0.35 | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | 0.30 |
| Initial configuration, n (%) | 0.90 | 1.00 | |||||
| Single-site internal jugular | 13 (23.6) | 5 (20.0) | 8 (26.7) | 2 (25.0) | 11 (23.4) | ||
| Dual-site femoral to internal jugular | 39 (70.9) | 19 (76) | 20 (66.7) | 6 (75.0) | 33 (70.2) | ||
| Dual-site femoral to femoral | 3 (5.5) | 1 (4.0) | 2 (6.7) | 0 (0.0) | 3 (6.4) | ||
| Anticoagulation monitoring goals, n (%) | 0.16 | 0.45 | |||||
| Partial thromboplastin time 40–60 s | 32 (58.2) | 12 (48.0) | 20 (66.7) | 6 (75.0) | 26 (55.3) | ||
| Antifactor Xa 0.2–0.4 U/mL | 23 (41.8) | 13 (52.0) | 10 (33.3) | 2 (25.0) | 21 (44.7) | ||
| Laboratory values 24 hr prior to ECMO, median (interquartile range) | |||||||
| Platelets (uL) | 195.0 (129.0–262.0) | 168.5 (108.5–249.0) | 206.0 (154.0–268.0) | 0.23 | 208 (105–286) | 192 (129–262) | 0.97 |
| Hemoglobin (g/dL) | 11.5 (9.7–13.0) | 11.1 (9.3–13.0) | 11.9 (10.9–13.6) | 0.27 | 11.4 (8.1–12.8) | 11.6 (9.7–13.4) | 0.66 |
| Hematocrit (%) | 36 (30.0–39.2) | 34.2 (27.5–39.1) | 37.0 (34.0–40.0) | 0.36 | 34.9 (24.0–39.0) | 36.0 (30.0–40) | 0.60 |
| International normalized ratio | 1.2 (1.1–1.4) | 1.3 (1.2–1.5) | 1.2 (1.0–1.3) | 0.10 | 1.3 (1.2–1.6) | 1.2 (1.1–1.4) | 0.43 |
| Partial thromboplastin time (s) | 37.0 (34.5–62.8) | 36.9 (35.0–63.4) | 38.4 (31.7–48.6) | 0.64 | 35.0 (34.5–35.6) | 38.3 (34.9–63.1) | 0.37 |
| Prothrombin time (s) | 15.4 (13.8–17.2) | 16.1 (14.8–18.0) | 14.8 (13.5–16.3) | 0.10 | 15.1 (14.7–18.5) | 15.4 (13.8–17.2) | 0.69 |
| RBCs transfused per ECMO day (mL) | 62.3 (0.0–140.0) | 140.0 (58.3–185.8) | 43.8 (0.0–87.5) | 0.002 | 108.5 (6.0–336.5) | 62.3 (0.0–139.3) | 0.40 |
| Outcomes | |||||||
| ECMO duration (d), median (interquartile range) | 7.0 (5.0–15.0) | 11.0 (6.0–26.0) | 6.0 (5.0–9.0) | 0.007 | 8.5 (4.0–22.0) | 7.0 (5.0–14.0) | 0.90 |
| Length of hospital stay (d), median (interquartile range) | 21.0 (12.0–36.0) | 22.0 (11.0–36.0) | 20.0 (13.0–28.0) | 0.59 | 21.5 (13.0–33.5) | 21.0 (12.0–36.0) | 0.98 |
| In-hospital survival, n (%) | 37 (67.3) | 11 (44.0) | 26 (86.7) | 0.02 | 5 (62.5) | 32 (68.1) | 0.73a |
ECMO = extracorporeal membrane oxygenation.
ap value calculated using log-rank tests.
Figure 1.
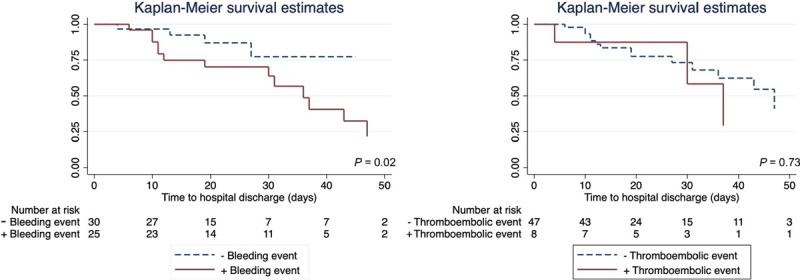
Kaplan-Meier in-hospital survival curves from time of venovenous extracorporeal membrane oxygenation for patients who did and did not experience a bleeding event and for patients who did and did not experience a thromboembolic event. p value is for the log-rank test.
DISCUSSION
In this retrospective cohort study of patients receiving venovenous ECMO for respiratory failure, all of whom received continuous anticoagulation, nearly half of patients experienced a bleeding event. Patients who experienced a bleeding event experienced worse survival than patients who did not experience a bleeding event. In contrast, thromboembolic events were less frequent and did not appear to affect survival. This is the first study to examine the relative impact of bleeding or thromboembolism during venovenous ECMO only. These results should prompt further research to evaluate the safety and efficacy of continuous IV anticoagulation in such patients.
Several factors may contribute to bleeding and thromboembolism in patients receiving ECMO. The interface of blood and nonbiologic circuit components causes activation of the coagulation system and the consumption and degradation of hemostatic factors (7, 8). Underlying critical illness compounds the risks of bleeding and thromboembolism (7). Continuous anticoagulation during ECMO may increase the risk of bleeding (1), and prior retrospective data suggest a dose-response relationship between anticoagulation and bleeding events (1, 3).
Conducting venovenous ECMO without continuous systemic anticoagulation has been proposed (9, 10). Although confounded by indication bias, recent observational studies suggest that strategies using only prophylactic doses of anticoagulation appear safe in venovenous ECMO (9, 10). Further, a recent systematic review suggested that the rates of thromboembolism and circuit thrombosis among patients who did not receive systemic anticoagulation during venovenous ECMO were comparable with the rates reported among patients treated with systemic anticoagulation (11). It is possible that avoidance of systemic anticoagulation might improve outcomes for some patients receiving venovenous ECMO.
Our study has several strengths. By including only patients without a pre-existing indication or contraindication to anticoagulation, the population in our study emulates the population that would be recruited for a randomized trial of anticoagulation strategies during venovenous ECMO. Further, we used previously published, objective criteria to define bleeding and thromboembolism. Our study has several limitations. The study was conducted at a single center using a retrospective design. Although we used structured and prespecified eligibility criteria, selection bias remains possible. The risks of bleeding and thromboembolism may be confounded by severity of illness and immortal time bias. Finally, this study was largely conducted prior to the coronavirus disease 2019 (COVID-19) pandemic. Only one patient in the study cohort experienced respiratory failure as a consequence of COVID-19. Emerging data describe both hypercoagulability (12) and a higher risk of bleeding for patients receiving venovenous ECMO for COVID-19 (13). It is unknown if the results of this analysis would be different if conducted entirely among a population of patients receiving venovenous ECMO for COVID-19.
Our data include only patients who received anticoagulation and do not inform the risks of thromboembolism among patients receiving venovenous ECMO without anticoagulation or with prophylactic-dose anticoagulation. This purely descriptive univariate analysis does not attempt to account for potential confounders and does not infer a causal relationship between bleeding or thromboembolism and survival.
CONCLUSIONS
In this cohort of patients receiving venovenous ECMO and anticoagulation, bleeding occurred more frequently than thromboembolism and was associated with worse survival. These results provide preliminary data for a randomized trial examining the safety and efficacy of systemic anticoagulation in select patients receiving venovenous ECMO.
Footnotes
Drs. Stokes and Gannon contributed equally to this work.
Drs. Stokes, Gannon, Sherrill, Bacchetta, Rice, Semler, and Casey contributed to conception and design of the study. Drs. Stokes, Gannon, Sherrill, and Armistead contributed to data acquisition. Drs. Stokes, Gannon, and Sherrill contributed to analysis of the data. Drs. Stokes and Gannon drafted the initial article. All authors contributed to the data interpretation and edited the article for important scientific content. All of the authors agree to be accountable for all aspects of the work in regards to accuracy and integrity.
Dr. Bacchetta was supported, in part, by the National Institutes of Health (NIH) (R01 HL140231) and H. William Scott, Jr Chair in Surgery no 2. Dr. Rice was supported, in part, by the NIH (UL1 RR024975). Dr. Semler was supported, in part, by the National Heart, Lung, and Blood Institute (K23HL143053). Dr. Casey was supported, in part, by the NIH (K12HL133117). The remaining authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Aubron C, DePuydt J, Belon F, et al. : Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care 2016; 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockie CJA, Gillon SA, Barrett NA, et al. : Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med 2017; 45:1642–1649 [DOI] [PubMed] [Google Scholar]
- 3.Sklar MC, Sy E, Lequier L, et al. : Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review. Ann Am Thorac Soc 2016; 13:2242–2250 [DOI] [PubMed] [Google Scholar]
- 4.Extracorporeal Life Support Organization: ELSO Anticoagulation Guideline, 2014, Ann Arbor, MI: Available at: https://www.elso.org/Portals/0/ELSO%20Guidelines%20For%20Adult%20Respiratory%20Failure%201_4.pdf. Accessed August 1, 2020 [Google Scholar]
- 5.Aubron C, McQuilten Z, Bailey M, et al. ; endorsed by the International ECMO Network (ECMONet): Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: A pilot randomized trial. Crit Care Med 2019; 47:e563–e571 [DOI] [PubMed] [Google Scholar]
- 6.Hernán MA, Robins JM: Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016; 183:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlebowski MM, Baltagi S, Carlson M, et al. : Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care 2020; 24:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle AJ, Hunt BJ: Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med (Lausanne) 2018; 5:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurihara C, Walter JM, Karim A, et al. : Feasibility of veno-venous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg 2020; 110:1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger K, Schmutz A, Zieger B, et al. : Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: An observational study in more than 60 patients. Artif Organs 2017; 41:186–192 [DOI] [PubMed] [Google Scholar]
- 11.Olson SR, Murphree CR, Zonies D, et al. : Thrombosis and bleeding in extracorporeal membrame oxygenation (ECMO) without anticoagulation: A systematic review. ASAIO J 2020. July 23 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teuwen LA, Geldhof V, Pasut A, et al. : COVID-19: The vasculature unleashed. Nat Rev Immunol 2020; 20:389–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt M, Hajage D, Lebreton G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir Med. 2020 Aug 13; doi: 10.1016/S2213-2600(20)30328-3. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


