Abstract
Objectives:
To assess the prevalence and prognostic value of right ventricular dysfunction as measured by echocardiography in patients treated with venovenous extracorporeal membrane oxygenation.
Design:
Retrospective cohort study. The primary endpoint was survival to discharge. Survival to extracorporeal membrane oxygenation decannulation was the secondary endpoint.
Setting:
ICU at an academic quaternary medical center.
Subjects:
Sixty-four consecutive patients treated with venovenous extracorporeal membrane oxygenation between January 2013 and December 2018 with an echocardiogram performed after cannulation.
Interventions:
Transthoracic or transesophageal echocardiography was used to assess several standard right and left ventricular characteristics after cannulation with venovenous extracorporeal membrane oxygenation.
Measurements and Main Results:
No single echo variable was predictive of outcomes. Composite markers such as right ventricular dysfunction (right ventricular dilation and abnormal septal motion) or a small dynamic left ventricle (left ventricle internal diastolic diameter < 4.0 cm and left ventricular ejection fraction > 60%) were associated with significantly decreased survival to decannulation (45% vs 83%; p < 0.01) and survival to hospital discharge (32% vs 64%; p = 0.02). Regression models confirmed the absence of both right ventricular dysfunction, and small left ventricle was highly predictive of increased survival to decannulation (odds ratio, 6; 95% CI, 1.87–19.28; p < 0.01) and discharge (odds ratio, 3.86; 95% CI, 1.29–11.55; p = 0.02).
Conclusions:
Echocardiographic variables consistent with right ventricular dysfunction or a small dynamic left ventricle were associated with decreased survival to decannulation and hospital discharge. These results enhance prognostic capabilities while implicating right ventricular dysfunction in the high mortality observed in this patient population.
Keywords: acute respiratory distress syndrome, cor pulmonale, echocardiography, extracorporeal membrane oxygenation, mortality, right ventricle
Acute right ventricular (RV) dysfunction is a known complication of severe acute respiratory distress syndrome (ARDS), occurring in 22–50% of cases (1). The exquisite sensitivity of the RV to increases in afterload makes it particularly sensitive to the hypoxia-induced pulmonary vasoconstriction and ventilator-induced increases in intrathoracic pressure incurred in the setting of ARDS. The development of RV dysfunction in ARDS has been associated with increased mortality: up to 68% in one prospective study (2).
Over the last decade, venovenous extracorporeal membrane oxygenation (VV-ECMO) has emerged as a reliable therapy for severe refractory ARDS (3, 4). Despite increased utilization of this technology, mortality in ARDS requiring VV-ECMO remains approximately 35–45% (4–6). Many of the factors leading to RV dysfunction in ARDS can be corrected with improved oxygenation and implementation of VV-ECMO, yet the presence of RV dysfunction prior to VV-ECMO cannulation has been shown to be a marker for increased mortality (7). Less is known regarding the prognostic value of RV dysfunction post VV-ECMO cannulation.
The purpose of our study was to describe the prevalence and prognostic value of postcannulation RV dysfunction as measured by echocardiography in patients treated with VV-ECMO for severe ARDS. We hypothesize that RV dysfunction in the setting of normalized oxygenation with VV-ECMO is associated with worse outcomes.
MATERIALS AND METHODS
A retrospective cohort study design was used including consecutive patients treated with VV-ECMO between January 2013 and December 2018 at a single quaternary medical center. The institutional electronic medical record was used to identify clinical variables, comorbidities, clinical test results, and outcomes. The primary endpoint was survival to discharge. Survival to extracorporeal membrane oxygenation (ECMO) decannulation was the secondary endpoint. The Institutional Review Board (IRB) at the University of Minnesota approved this study and protocol (IRB Number 00002818) with waiver of informed consent.
Study Population
All patients treated with VV-ECMO between January 2013 and December 2018 were identified. All patients with diagnostic quality postcannulation echocardiograms were included. Patients were excluded if they were less than 16 years old, had previously documented right or left ventricular dysfunction, a left ventricular assist device, or prior heart transplant. All patients were cannulated for VV-ECMO using percutaneous access with a single vessel dual-lumen cannula inserted in the right internal jugular vein.
Echocardiographic Assessment
Postcannulation transthoracic and transesophageal echocardiograms (TEEs) were used. If a patient had more than one postcannulation study, the earliest complete study was used. All measurements were collected using standard methods in accordance with American Society of Echocardiography (ASE) guidelines (8, 9). Echocardiographic 2D images were reviewed and interpreted by two independent blinded readers for each patient. Quantitative measurements were averaged. When disagreement occurred with qualitative assessment, a third reader was used.
Left Ventricular Assessment.
Left ventricular ejection fraction (LVEF) was assessed using all available images for all patients. Left ventricular internal diameters were obtained in the parasternal long-axis view. The left ventricular internal area was obtained in the apical four-chamber view. All TEE measurements were made in a mid-esophageal four-chamber view.
RV Assessment.
RV measurements were obtained in an apical modified RV view using transthoracic echocardiography. For transesophageal studies, measurements were made in a mid-esophageal four-chamber view. RV size and function were visually assessed using all available views and quantified as normal or mildly, moderately, or severely abnormal. RV fractional area change was also calculated. Abnormal septal motion was defined as evidence of flattening during systole and/or diastole representing RV pressure and/or volume overload.
RV dysfunction was defined by the echocardiographic presence of both a dilated RV (visual assessment) and abnormal septal motion. A small dynamic left ventricle (SLV) was defined as the combination of a left ventricle internal end-diastolic diameter of less than 4.0 cm and an LVEF of greater than 60% (8). The left ventricle internal diastolic diameter (LVIDd) threshold represents an averaged value of the lower limits of normal for men and women. Examples of these echocardiographic findings are shown in Figure 1.
Figure 1.
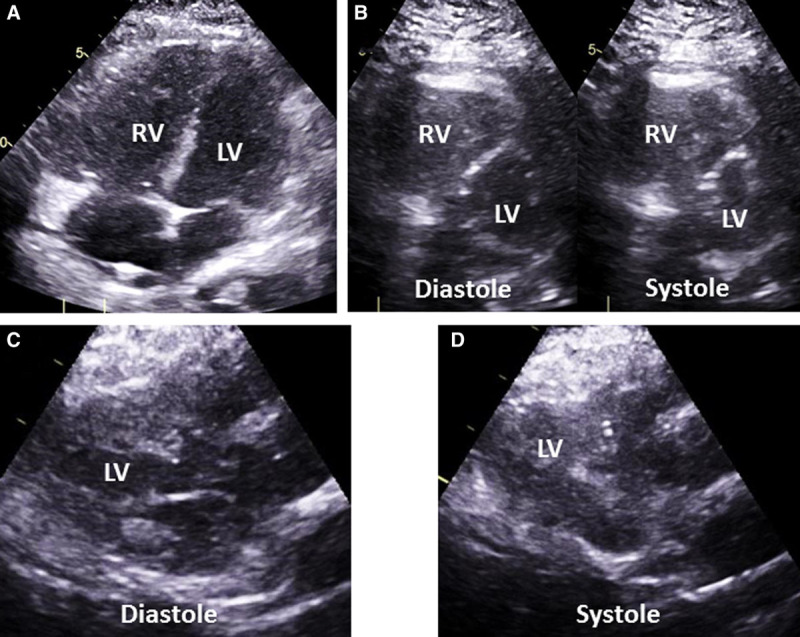
Examples of right ventricular (RV) and left ventricular (LV) echocardiographic findings. Transthoracic echocardiographic images are shown for each. A, RV dilation is demonstrated by a diastolic area equal to, or larger than, the LV diastolic area. B, Abnormal septal motion including flattening of the interventricular septum and displacement of the septum throughout the cardiac cycle demonstrating elevated RV pressures. C, Underfilled LV with a LV internal end-diastolic diameter of less than 4.0 cm. D, Hyperdynamic LV with ejection fraction greater than 60% seen here with near complete obliteration of the LV cavity.
Statistical Analysis
Categorical variables are reported as frequencies and percentages. Continuous variables are reported as mean with sd or as median with interquartile range (IQR) when appropriate. Missing variables were censored from the analysis unless specified. For continuous variables, between-group comparisons were performed with Student t test. Categorical variables were compared with Fisher exact test. Backward stepwise logistic regression was performed to identify multivariable predictors of outcomes. Statistical analysis was performed using STATA Version 15 (StataCorp. 2017, StataCorp LLC, College Station, TX). A two-tailed p value of less than 0.05 was considered statistically significant.
RESULTS
A total of 97 patients were treated with VV-ECMO at the University of Minnesota Medical Center (UMMC) during the years of 2013–2018; 64 patients (66%) met criteria for inclusion in the study (Fig. 2). Of these, 34 were cannulated at UMMC, and 30 were transferred after cannulation at a referring hospital. The study’s population was predominately male (69%) with a mean age of 44 years (range 17–70 yr). As shown in Table 1, the cohort had a low burden of comorbid disease with a mean Charlson comorbidity index of 1.4. However, they were severely acutely ill with mean Simplified Acute Physiology Score (SAPS) II and Sequential Organ Failure Assessment (SOFA) scores of 37.8 and 8.3, respectively, prior to ECMO cannulation. SAPS II and SOFA scores were not associated with survival to hospital discharge in this population. Overall survival to hospital discharge for the cohort was 53%, whereas 70% survived to decannulation. The median time on ECMO was 11 days (IQR, 6.75–17 d). The median blood flow through the VV-ECMO system was 4.1 L/min (IQR 3.5–4.5). Complications occurred in 15% of patients including four patients with malposition of the cannula requiring a repeat procedure for repositioning and six patients with life-threatening bleeding defined as an intracranial bleed or a single bleeding episode requiring more than three units of RBCs. Increased age and higher Charlson Comorbidity Score were the only baseline variables associated with an increased mortality.
Figure 2.
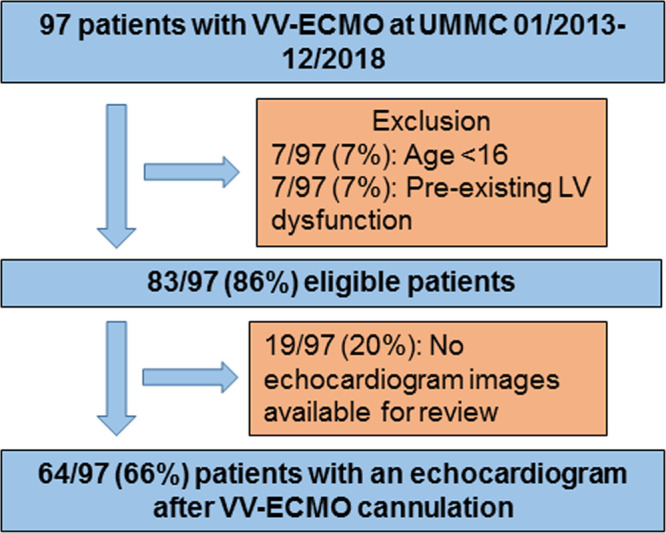
Patient flow diagram illustrating the derivation of the study population with relevant inclusion and exclusion criteria. UMMC = University of Minnesota Medical Center, VV-ECMO = venovenous extracorporeal membrane oxygenation.
TABLE 1.
Baseline Characteristics
| Baseline Characteristics | Entire Cohort n = 64 | Survived to Discharge n = 34 | Death Prior to Discharge n = 30 | p |
|---|---|---|---|---|
| Age, yr, mean (sd) | 44 (14) | 39 (14) | 51 (13) | 0.0007 |
| Gender, n (%), male | 44 (69) | 23 (68) | 21 (70) | 1.0 |
| Body mass index, mean (sd) | 30.7 (7.2) | 32.1 (8.1) | 29.2 (5.7) | 0.1 |
| Hypertension, n (%) | 11 (17.1) | 7 (20.6) | 4 (13.3) | 0.5 |
| Diabetes, n (%) | 11 (17) | 5 (14.7) | 6 (20) | 0.4 |
| Prior myocardial infarction, n (%) | 2 (3.1) | 0 (0) | 2 (6.7) | 0.2 |
| Congestive heart failure, n (%) | 4 (6.3) | 2 (5.9) | 2 (6.7) | 1 |
| Coronary artery disease, n (%) | 3 (4.7) | 2 (5.8) | 1 (3.3) | 1 |
| Peripheral vascular disease, n (%) | 2 (3.1) | 1 (2.9) | 1 (3.3) | 1 |
| Cerebral vascular accident, n (%) | 0 (0) | 0 (0) | 0 (0) | n/a |
| Chronic obstructive pulmonary disease, n (%) | 5 (7.8) | 2 (5.8) | 3 (10) | 0.4 |
| Reactive airway disease, n (%) | 3 (4.7) | 2 (5.9) | 1 (3.3) | 1 |
| Interstitial lung disease, n (%) | 16 (25) | 9 (26) | 7 (23) | 1 |
| Chronic kidney disease, n (%) | 3 (4.7) | 1 (2.9) | 2 (6.7) | 0.6 |
| Liver disease, n (%) | 3 (4.7) | 2 (5.9) | 1 (3.3) | 1 |
| Malignancy, n (%) | 5 (7.8) | 1 (2.9) | 4 (13.3) | 0.2 |
| Hepatitis C virus, n (%) | 3 (4.7) | 2 (5.8) | 1 (3.3) | 1 |
| Charlson Comorbidity Score, mean (sd) | 1.3 (1.4) | 0.8 (1.0) | 1.9 (1.5) | 0.0008 |
| Simplified Acute Physiology Score II score, mean (sd) | 37.8 (9.5) | 36.3 (10.2) | 39.5 (8.5) | 0.18 |
| Sequential Organ Failure Assessment score, mean (sd) | 8.3 (3.2) | 9 (3.5) | 7.6 (2.8) | 0.08 |
| Number of patients on vasopressora, n (%) | 38 (60) | 22 (65) | 21 (70) | 0.6 |
| Number of patients on inotropesa, n (%) | 14 (22) | 9 (26) | 5 (17) | 0.5 |
| Prone positioning, n (%) | 30 (47) | 15 (44) | 15 (50) | 0.8 |
| Days intubated prior to cannulation, mean (sd) | 4.3 (0.6) | 4.4 (0.9) | 4.2 (0.7) | 0.8 |
| Serum creatininea, mean (sd) | 1.7 (1.2) | 1.7 (1.3) | 1.6 (1.2) | 0.5 |
| Serum albumina, mean (sd) | 2.3 (0.8) | 2.5 (0.9) | 2.1 (0.6) | 0.04 |
| Hemoglobina, mean (sd) | 9.9 (2.0) | 10.1 (1.9) | 9.7 (2.3) | 0.4 |
| pHa, mean (sd) | 7.2 (0.1) | 7.2 (0.2) | 7.3 (0.1) | 0.6 |
| Po2a, mean (sd) | 61 (12.7) | 60 (12.3) | 61 (13.1) | 0.9 |
| Pco2a, mean (sd) | 63 (23.9) | 60 (23.5) | 66 (24.4) | 0.3 |
| Po2/Fio2a, mean (sd) | 63 (22.8) | 58 (20.8) | 69 (23.9) | 0.05 |
aLast known value prior to cannulation.
Echocardiograms were performed post cannulation for 64 of 83 eligible VV-ECMO patients (77%). Repeated echocardiograms were performed in 35 of 64 patients (55%). However, only the first echocardiogram after VV-ECMO cannulation was used in this analysis. Echocardiograms were obtained at a median time of 1 day (IQR 0–3 d) post cannulation. Most echocardiograms were transthoracic (70%). There were no significant pericardial effusions noted by echocardiography. However, three of 64 patients (5%) had trivial or small pericardial effusions noted after VV-ECMO cannulation. Although more than half of the patients were receiving inhaled pulmonary vasodilators at the time of the echocardiogram (52%), this was not significantly associated with increased survival or RV dysfunction. Of those receiving pulmonary vasodilators, 45% survived to hospital discharge, whereas 61% of those without pulmonary vasodilators survived (p = 0.22). RV dysfunction was present in 21% of patients receiving pulmonary vasodilators and 13% of patients without pulmonary vasodilators (p = 0.51).
The central venous pressure and ventilator settings at the time of the echocardiogram were similar between survivors and nonsurvivors. Central venous pressure was 15.1 ± 5.7 and 13.3 ± 2.5 mm Hg in survivors and nonsurvivors, respectively (p = 0.13). Ventilator plateau pressures were 31.5 ± 4.1 and 31.9 ± 5.4 cm H2O in survivors and nonsurvivors, respectively (p = 0.78). Positive end-expiratory pressure was also similar with 13.2 ± 3.7 and 13.4 ± 4.0 cm H2O for survivors and nonsurvivors, respectively (p = 0.83). The Fio2 on the ventilator was 97.3% ± 7.1% and 91.8% ± 13.9% for survivors and nonsurvivors, respectively (p = 0.85).
No single echocardiographic variable was associated with the primary outcome (Table 2). LVIDd was associated with failure to decannulate from VV-ECMO. No other variable was associated with the secondary endpoint. The presence of a SLV, defined as a combination of LVIDd less than 4.0 cm and LVEF greater than 60%, was observed in 14 patients (22%). A SLV was associated with reduced odds of survival to discharge (odds ratio [OR] 0.27; 95% CI, 0.07–0.97; p = 0.05) (Table 3). Although there was also a trend toward reduced odds of decannulation (OR 0.32; 95% CI 0.09–1.08; p = 0.07), this was not statistically significant. Similarly, presence of RV dysfunction, defined as the combination of RV dilation and abnormal septal motion, was observed in 11 patients (17%). RV dysfunction was associated with reduced odds of decannulation (OR, 0.17; 95% CI, 0.04–0.67; p = 0.01). There was a trend toward reduced survival to discharge (OR, 0.44; 95% CI, 0.11–1.68; p = 0.23) that did not reach statistical significance.
TABLE 2.
Echocardiographic Findings
| Clinical Variables | Entire Cohort n = 64 | Survived to Discharge n = 34 | Death Prior to Discharge n = 30 | p | Survived to Decannulation n = 45 | Death Prior to Decannulation n = 19 | p |
|---|---|---|---|---|---|---|---|
| Left ventricular ejection fraction %, mean (sd) | 59 (8.8) | 58 (10.5) | 61 (6.1) | 0.19 | 58 (9.7) | 61 (5.7) | 0.2 |
| Left ventricle internal diastolic diameter cm, mean (sd) | 4.4 (0.6) | 4.5 (0.6) | 4.3 (0.5) | 0.06 | 4.5 (0.6) | 4.2 (0.4) | 0.04 |
| RV fractional area change %, mean (sd) | 38 (10) | 38 (11) | 38 (9.9) | 0.8 | 38 (10) | 38 (11) | 0.8 |
| RVEDA cm2, mean (sd) | 24.6 (6.3) | 25.1 (6.6) | 24.1 (6.0) | 0.53 | 25.2 (6.5) | 23.3 (5.7) | 0.3 |
| RVEDA/left ventricle end diastolic area, mean (sd) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.5 | 0.97 (0.2) | 1.0 (0.2) | 0.4 |
| TAPSEa mm, mean (sd) | 21.0 (3.5)a | 21.9 (2.9)a | 20.3 (3.9)a | 0.13 | 21.3 (3.5)a | 20.6 (3.7)a | 0.6 |
| TAPSE ≤ 18 mma, n (%) | 8 (18)a | 3 (13)a | 5 (24)a | 0.44 | 5 (16)a | 2 (15)a | 1.0 |
| Abnormal septal motion, n (%) | 13 (20) | 6 (18) | 7 (23) | 0.8 | 6 (13) | 7 (37) | 0.05 |
| Qualitative RV dilation, n (%) | 19 (30) | 8 (23) | 11 (37) | 0.3 | 11 (24) | 8 (42) | 0.2 |
| Qualitative reduction RV ejection fraction, n (%) | 19 (30) | 8 (24) | 11 (37) | 0.3 | 11 (24) | 6 (32) | 0.6 |
RV = right ventricle, RVEDA = right ventricle end diastolic area, TAPSE = tricuspid annular plane systolic excursion.
aTAPSE only measured from transthoracic echocardiographic studies (cohort n = 45).
TABLE 3.
Adjusted and Unadjusted Survival for Patients With Echocardiographic Findings
| Predictive Models | Survival to Discharge | Survival to Decannulation | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Univariate predictive models | ||||
| SLV | 0.27 (0.07–0.97) | 0.05 | 0.32 (0.0–1.08) | 0.07 |
| RV dysfunction | 0.44 (0.11–1.68) | 0.23 | 0.17 (0.04–0.67) | 0.01 |
| No RV dysfunction or SLV | 3.86 (1.29–11.55) | 0.02 | 6 (1.87–19.28) | 0.01 |
| Multivariate predictive modelsa | ||||
| SLV | 0.14 (0.03–0.63) | 0.03 | 0.28 (0.08–1.03) | 0.06 |
| RV dysfunction | 0.23 (0.05–1.18) | 0.08 | 0.09 (0.02–0.47) | < 0.01 |
| No RV dysfunction or SLVb | 7.59 (1.88–30.59) | 0.004 | 7.68 (2.16–27.23) | 0.004 |
OR = odds ratio, RV = right ventricle, SLV = small dynamic left ventricle.
aMultivariate logistic regression model using age, Charlson Comorbidity Score, SLV, and RV dysfunction.
bMultivariate logistic regression model using age and Charlson Comorbidity Score.
Importantly, patients without a SLV or RV dysfunction had a six-fold increase in the odds of surviving to decannulation (OR, 6; 95% CI, 1.87–19.28; p < 0.01) and nearly four-fold increase in survival to discharge (OR, 3.86; 95% CI, 1.29–11.55; p = 0.02). These findings were persistent in multivariate models adjusting for age and comorbidities (Table 3). As demonstrated in Figure 3, 64% of patients without either finding survived to discharge compared with 29% of patients with SLV and 36% of patients with RV dysfunction (p = 0.02).
Figure 3.
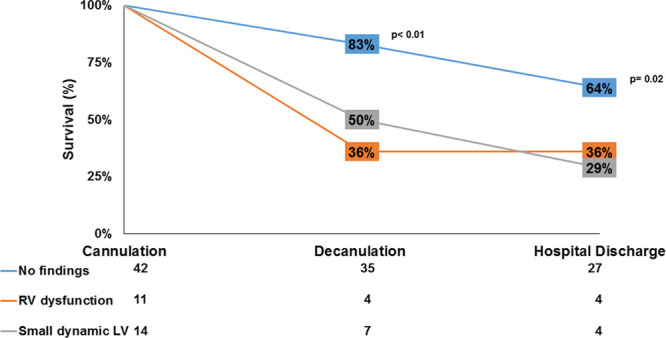
Patient survival to decannulation and hospital discharge in relation to echocardiographic findings. Two key survival benchmarks (decannulation and hospital discharge) are shown with percent survival in patients with the relevant echocardiographic findings obtained after cannulation with venovenous extracorporeal membrane oxygenation. The displayed p values represent comparisons between patients without right ventricle (RV) dysfunction or a small dynamic left ventricle (LV) and those with either finding.
DISCUSSION
The RV is sensitive to changes in afterload with acute dysfunction arising when pulmonary vascular resistance increases. Both pulmonary vasoconstriction and increased intrathoracic pressures caused by mechanical ventilation increase pulmonary vascular resistance; both are increased in patients treated for ARDS. Pulmonary vascular resistance can be improved with the implementation of low volume ventilation and VV-ECMO (10). VV-ECMO oxygenates blood in the right atrium which directly alleviates hypoxia-induced pulmonary vasoconstriction. In addition, the oxygenation support allows use of low pressure ventilation strategies reducing the intrathoracic pressures that promote RV dysfunction. Despite these benefits, VV-ECMO may not fully reverse the cardiac manifestations of ARDS which develop prior to VV-ECMO initiation. The current study demonstrates the prevalence and prognostic impact of post VV-ECMO cardiac dysfunction. Postcannulation echocardiographic evidence of RV dysfunction or SLV was present in 34% of patients cannulated with VV-ECMO. Evidence of RV dysfunction or a SLV post VV-ECMO cannulation was highly predictive of failure to decannulate from VV-ECMO and failure to survive to hospital discharge. Conversely, a post-VV-ECMO echocardiogram showing no RV dysfunction or SLV was highly predictive of successful ECMO decannulation and survival to hospital discharge. To our knowledge, this is the first observation describing RV dysfunction post VV-ECMO cannulation and its association with mortality.
Cor pulmonale or RV dysfunction has been well described in patients with severe ARDS where it has been associated with increased mortality (2). RV dysfunction in ARDS is thought to be a consequence of multiple factors including microvascular obstruction, metabolic changes, hypoxemia-induced pulmonary vasoconstriction, mechanical ventilation, pulmonary vascular endothelial dysregulation, and vascular architecture changes secondary to underlying lung disease (11–14). Many of these factors can be corrected with the initiation of VV-ECMO. In fact, Reis et al (10) showed a significant improvement in RV hemodynamics in the first 6 hours after starting VV-ECMO in patients with acute cor pulmonale from ARDS. The authors speculated that these improvements were driven by correction of hypoxia and hypercapnia. However, the increased mortality associated with RV dysfunction pre VV-ECMO persists despite these potentially favorable hemodynamic changes (7). We have now observed similar results in ARDS patients with RV dysfunction after VV-ECMO cannulation.
The progressive development of RV dysfunction even after VV-ECMO cannulation suggests the involvement of mechanisms beyond hypoxia and hypercapnia-induced pulmonary vasoconstriction. Myocardial stunning, incomplete reversal of metabolic abnormalities, or pulmonary vascular remodeling are potential persistent etiologies. Although VV-ECMO readily reverses hypoxia-induced pulmonary vasoconstriction, endothelial dysfunction, and vascular dysregulation related to an imbalance in the vascular tone mediators eNOS, endothelins and prostanoids may persist (15). Given the high on-pump mortality of patients with RV dysfunction (64% vs 23%; p = 0.01) in our cohort, further investigation into the mechanisms of worsened outcomes and the potential benefits of RV support (pharmacologic or mechanical) is important. Of note in our cohort, 52% of patients were receiving inhaled pulmonary vasodilators at the time of their echocardiogram. This did not correlate with survival (p = 0.22), although it remains unknown whether the continuation of pulmonary vasodilators in a selected population would alter outcomes.
Although other studies have shown a weak correlation between simple markers of RV dysfunction, such as tricuspid annular plane systolic excursion, and mortality, our study highlights the difficulties of assessing RV dysfunction in clinical practice. No single echocardiographic variable measured was able to predict the primary or secondary outcomes, but instead an integrated approach was needed, a practice that is consistent with current ASE recommendations (9). We found that composite measurements of RV dysfunction and a SLV predicted outcomes. Although the RV dysfunction measure is intuitive based on the proposed effects of ARDS on RV afterload, the SLV measure is most likely an indirect measure of poor RV function. The finding of a SLV is secondary to left ventricle underfilling due to poor forward flow from the RV. Hence, this is hypothesized to be another marker for severe RV dysfunction. Alternatively, it could reflect dehydration, a small native LV cavity, or severely reduced systemic vascular resistance. However, this effect is similar to that observed in other clinical scenarios of acute RV failure such as massive pulmonary embolism (16).
Given the strong association between in-hospital mortality and echocardiographic findings of RV failure, further investigation is needed to explore the potential role for RV hemodynamic support. Mechanical hemodynamic support devices such as temporary RV assist devices or pharmacologic therapy with inotropes or pulmonary vasodilators may provide an opportunity for improved systemic perfusion, RV recovery, and improved survival. Further, mortality associated with RV dysfunction but related to systemic hypoperfusion may be more fully treated with venoarterial ECMO which bypasses the heart and lungs to provide enhanced systemic perfusion. However, in the setting of ARDS, traditional femoral VA-ECMO cannulation increases the risk of North-South Syndrome. North-South Syndrome occurs when deoxygenated blood pumped antegrade through the failing lungs competes with the retrograde flow of well-oxygenated blood from the VA-ECMO circuit. This can lead to poor oxygen delivery to the upper body with delivery of well-oxygenated ECMO circuit blood to the abdomen and lower body. This can be avoided through various methods including central or upper extremity arterial VA-ECMO cannulation resulting in delivery of oxygenated ECMO blood to the proximal aorta. Alternatively, a venous return cannula can be added to create a VV-ECMO circuit with splitting of the oxygenated blood returning to the patient through the existing arterial cannula and the additional venous return cannula. This hybrid of venoarterial and VV-ECMO can provide partial oxygenation support of the native antegrade cardiac blood flow and partial hemodynamic support with blood return directly to the arterial circulation. Alternatively, ARDS patients can be supported with right atrial to pulmonary artery cannulation, wherein RV function is supported, and blood is oxygenated prior to ejection into the pulmonary artery. This can be accomplished with either an open surgical or percutaneous dual lumen cannula approach (17). Traditional VV-ECMO with creation of an atrial septostomy has also been performed although evidence of success with this strategy remains limited (18). Further study is necessary to assess the potential benefits of these techniques.
There are several limitations of this study. First, the retrospective design leads to potential selection and information bias. Although consecutive patients were included, echocardiograms were not performed in 23% of eligible VV-ECMO patients. These patients would be expected to have low incidence of cardiac dysfunction as echocardiography would have typically been performed in the setting of concern for hemodynamic compromise. However, this cannot be determined from the available data. Hemodynamic data were also limited in this study due to multiple factors including use of TEE where hemodynamic evaluation is limited, inability to measure reliable RV systolic pressures in a significant number of patients, and unknown accuracy of echocardiographic measures of hemodynamic variables in the setting of VV-ECMO. Pulmonary artery catheters were rarely used in this cohort. The use of pulmonary vasodilators in 52% of patients at the time of echocardiography may also reduce the sensitivity of the echocardiogram for dysfunction due to the potential for benefits to RV function. This may lead to underestimation of the prognostic potential in this study. This was a single-center study, which may limit generalizability due to differences in local treatment protocols. However, the patients were transferred from multiple sites where cannulation was performed, which enhances the generalizability with respect to precannulation patient selection and procedural characteristics. Although this study provides a window into “real-world” practice, prognostication in a setting where negative data are known by the bedside team and patient’s family provides the opportunity for a self-fulfilling prophecy and confirmation bias. However, the completion of the echocardiogram shortly after VV-ECMO cannulation followed by prolonged duration of ECMO support suggests that other factors were included in the decision-making. The limited size of this cohort may also limit the power to detect differences between groups. Finally, invasive hemodynamic data were not available to correlate imaging findings to hemodynamics.
CONCLUSIONS
Echocardiographic variables consistent with RV dysfunction (dilated RV and abnormal septal motion) or a SLV (small LV internal end-diastolic diameter and elevated LVEF) were associated with decreased survival to decannulation and hospital discharge. This provides important prognostic information for critically ill patients while also suggesting that RV support devices may provide an avenue for improved outcomes in the future.
Footnotes
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Zochios V, Parhar K, Tunnicliffe W, et al. The right ventricle in ARDS. Chest. 2017; 152:181–193 [DOI] [PubMed] [Google Scholar]
- 2.Osman D, Monnet X, Castelain V, et al. ; French Pulmonary Artery Catheter Study Group Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009; 35:69–76 [DOI] [PubMed] [Google Scholar]
- 3.Kon ZN, Bittle GJ, Pasrija C, et al. Venovenous versus venoarterial extracorporeal membrane oxygenation for adult patients with acute respiratory distress syndrome requiring precannulation hemodynamic support: A review of the ELSO registry. Ann Thorac Surg. 2017; 104:645–649 [DOI] [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 5.Rozencwajg S, Pilcher D, Combes A, et al. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care. 2016; 20:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018; 378:1965–1975 [DOI] [PubMed] [Google Scholar]
- 7.Lazzeri C, Bonizzoli M, Cianchi G, et al. Lactate and echocardiography before veno- venous extracorporeal membrane oxygenation support. Heart Lung Circ. 2018; 27:99. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28:1–39.e14 [DOI] [PubMed] [Google Scholar]
- 9.Rudski LG, Lai WW, Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23:685. [DOI] [PubMed] [Google Scholar]
- 10.Reis Miranda D, van Thiel R, Brodie D, et al. Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2015; 191:346–348 [DOI] [PubMed] [Google Scholar]
- 11.Vesconi S, Rossi GP, Pesenti A, et al. Pulmonary microthrombosis in severe adult respiratory distress syndrome. Crit Care Med. 1988; 16:111–113 [DOI] [PubMed] [Google Scholar]
- 12.Tomashefski JF, Jr, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983; 112:112–126 [PMC free article] [PubMed] [Google Scholar]
- 13.Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003; 21:720–727 [DOI] [PubMed] [Google Scholar]
- 14.Price LC, McAuley DF, Marino PS, et al. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L803–L815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunge JJH, Caliskan K, Gommers D, et al. Right ventricular dysfunction during acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation. J Thorac Dis. 2018; 10:S674–S682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields JM, Davis J, Girson L, et al. Transthoracic echocardiography for diagnosing pulmonary embolism: A systematic review and meta-analysis. J Am Soc Echocardiogr. 2017; 30:714–723.e4 [DOI] [PubMed] [Google Scholar]
- 17.Harjola VP, Mebazaa A, Čelutkienė J, et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016; 18:226–241 [DOI] [PubMed] [Google Scholar]
- 18.Kon ZN, Pasrija C, Shah A, et al. Venovenous extracorporeal membrane oxygenation with atrial septostomy as a bridge to lung transplantation. Ann Thorac Surg. 2016; 101:1166–1169 [DOI] [PubMed] [Google Scholar]


