Abstract
Background:
Although sex disparities in hepatocellular carcinoma (HCC) incidence have been well described, there are limited data examining sex disparities in HCC prognosis.
Aim:
To characterize sex differences in HCC presentation and prognosis
Methods:
We performed a retrospective study of consecutive patients (n=1110, 23.5% women) diagnosed with HCC between 2008–2017 at two U.S. health systems. We used Cox proportional hazard and multivariable logistic regression models to identify factors associated with overall survival, early tumor detection, and response to HCC treatment (per the modified Response Evaluation Criteria in Solid Tumors [mRECIST] criteria).
Results:
Women were older at HCC diagnosis (mean 62.5 vs 59.2 years, p<0.001) and had a higher proportion of early stage tumors (53.1% vs 43.7% Barcelona Clinic Liver Cancer [BCLC] stage 0/A, p=0.04), but similar liver function compared to men (49.2% vs 47.1% Child Pugh A, p=0.27). In univariable analysis, women had significantly better overall survival than men (median 17.1 vs 12.0 months, p=0.02). When stratified by age, younger (<65 years) women had better overall survival than men (18.3 vs 11.2 months, p=0.02); however, older (≥65 years) women and men had similar overall survival (15.5 vs 15.7 months, p=0.45). In multivariable analysis, female sex was independently associated with lower mortality after adjusting for age, race/ethnicity, alpha-fetoprotein, BCLC stage, Albumin-Bilirubin (ALBI) grade and Child Pugh score (hazard ratio [HR] 0.82, 95% confidence interval [CI] 0.68–0.98). In secondary analyses, female sex was independently associated with early tumor detection (odds ratio [OR] 1.46, 95% CI 1.05–2.02) and response to first HCC treatment (OR 1.72, 95% CI 1.18–2.53) after adjusting for the same covariates.
Conclusion:
In a large cohort of patients with HCC, women had significantly better prognosis than men.
Keywords: Prognosis, liver cancer, gender, disparities, HCC treatment
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide and a leading cause of death among patients with cirrhosis.1–3 Sex disparities in HCC incidence have been described across the globe, with men disproportionately affected compared to women in a 2:1 – 4:1 ratio, depending on region.3–5 This disparity is partly driven by differences in the prevalence of HCC risk factors, including viral hepatitis, alcohol use, and metabolic syndrome between men and women6; however, sex hormones (e.g. estrogen, androgens) and other biological factors, such as adiponectin, have also been implicated.7, 8,9
Despite well-established sex differences in HCC incidence, data conflict regarding if survival differs between men and women with HCC.10–12 Studies from East Asia, including Thailand13, Taiwan14, China15, and Japan,16 have demonstrated women with HCC have better overall survival than men, while Western single-center retrospective cohort studies from California17 and Hawaii18 report no difference in survival by sex. Further, sex differences in survival may differ based on race/ethnicity and age at HCC diagnosis. Using cancer registry data, Yang et al found women aged <55 years had better survival compared to men (hazard ratio [HR] 0.83, 95% CI 0.77 – 0.88), but there were no sex differences in patients age ≥65 years.19 Most single-center studies have been limited by small sample sizes, lacking diversity in race/ethnicity and liver disease etiology13, and prior studies using administrative datasets lack granular information on confounders such as liver dysfunction and tumor burden.12 Evaluating the magnitude of sex disparities in HCC prognosis is the first necessary step to identify mechanisms underlying these disparities, as well as modifiable intervention targets to improve care for all patients. Therefore, we conducted a retrospective cohort study to evaluate sex differences in presentation and prognosis among a large, racially/ethnically diverse population of patients with HCC.
METHODS
Study Population
We conducted a retrospective cohort study of consecutive patients diagnosed with treatment-naïve HCC between January 2008 and July 2017 at two large health systems (Parkland Health & Hospital System and UT Southwestern Medical Center). Parkland is the safety-net health system for Dallas County and UT Southwestern is an academic, tertiary care referral center with a liver transplant program. As previously described, both health systems have a multi-disciplinary liver tumor conference and clinic.20 We identified eligible patients using a prospectively maintained database of patients seen in the liver tumor clinics at each site, as previously reported.21 HCC cases were adjudicated to confirm they met diagnostic criteria, per American Association for the Study of Liver Disease (AASLD) guidelines.22 We excluded patients if they 1) did not have characteristic imaging or histology confirming HCC diagnosis; or 2) received HCC treatment at an outside facility prior to presentation at Parkland or UT Southwestern. This study was approved by the Institutional Review Board at UT Southwestern Medical Center.
Data Collection
We abstracted demographic, clinical, laboratory, and imaging data from the electronic medical record (EMR) at both sites using standardized forms21. Data were stored in RedCap23, and discrepancies in data abstraction were resolved by consensus among authors. Demographics included age at diagnosis, self-reported sex and race/ethnicity (Supplemental methods).21 Liver disease etiology was classified as chronic hepatitis C (HCV), hepatitis B (HBV), alcohol-related, non-alcoholic fatty liver disease (NAFLD), or other.24 Cirrhosis severity was assessed using the Child Pugh score and Model for End-Stage Liver Disease (MELD) score. Laboratory data at HCC diagnosis included platelet count, creatinine, albumin, bilirubin, international normalized ratio (INR), and alpha-fetoprotein (AFP). Albumin-Bilirubin (ALBI grade) was calculated for all patients. The presence of comorbidities including diabetes, hypertension, dyslipidemia and history of prior cancer were ascertained from the electronic health record by manual review of physician clinic notes and medication lists. Performance status was assessed by Eastern Cooperative Oncology Group (ECOG) score per clinic notes.
The presentation leading to HCC diagnosis was categorized as detected via 1) surveillance, 2) incidentally, or 3) symptomatic presentation.25 Tumor characteristics (including number of nodules, maximum tumor diameter, lymph node involvement, vascular invasion, and/or metastatic disease) were determined by imaging studies, which had been interpreted by abdominal fellowship-trained radiologists. Tumors were staged according to the Barcelona Clinic Liver Cancer (BCLC) staging system.26 We abstracted all HCC treatments received, including the first treatment and the most definitive treatment. Treatments were ranked as most definitive as follows: liver transplantation > surgical resection > ablation > stereotactic body radiation therapy (SBRT) > transarterial chemoembolization (TACE) or transarterial radioembolization (TARE) > systemic therapy > best supportive care (BSC). We assessed treatment response to first treatment based on contrast-enhanced CT or MRI (typically performed 4–6 weeks post-treatment), with response classified per the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.27
Statistical Analysis
Sociodemographic, clinical, and tumor characteristics were reported using descriptive statistics, stratified by sex. Continuous variables were compared using the Kruskal-Wallis test and small- and large-sample categorical variables were compared using Fisher’s exact and chi-squared tests, respectively. The a priori independent variable of interest was sex (alone and stratified by age). Patients were stratified by age into two subgroups: older (age ≥65 years) and younger (age <65 years) adults based on the cut-off from geriatric oncology literature28.
Our primary outcome was overall survival, and secondary outcomes included early tumor detection, as defined by the Milan Criteria, and response to first HCC treatment, per mRECIST. We estimated median overall survival from date of HCC diagnosis to last known date of follow-up, liver transplantation, death, or the end of the study period (May 20, 2019) using Kaplan-Meier analysis and compared survival between groups using the log-rank test. We used univariate and multivariable Cox proportional hazard models to identify factors associated with overall survival. We then used multivariable ordinal logistic regression to identify correlates of early tumor detection and response to first HCC treatment. Treatment response was defined as an ordinal outcome (complete response, partial response, stable disease, progressive disease). All multivariable models were adjusted for factors known to be associated with HCC prognosis (e.g., Child Pugh score), and those significant (p <0.10) in univariate analyses. All multivariable tests were two-sided and performed at the 5% significance level. Statistical analysis was performed using Stata 14.0 (College Station, TX).
RESULTS
Patient and Tumor Characteristics
We identified 1110 patients with HCC who met inclusion criteria, of whom 258 (23.5%) were women and 852 (77.5%) were men. Baseline characteristics of the study population are summarized in Table 1. The proportion of women with HCC in our cohort increased annually, from 15.5% in 2008 to 30.4% in 2017 (Supplemental Figure 1). The cohort was racially/ethnically diverse (33.5% non-Hispanic white, 32.7% non-Hispanic black and 27.8% Hispanic), and the most common liver disease etiologies were HCV (64.6%), alcohol-related (13.3%) and NAFLD (11.9%). There was a wide range of tumor stages: 45.9% BCLC stage 0/A, 11.9% BCLC stage B, 24.2% BCLC stage C, and 18.0% BCLC stage D. The median follow-up from HCC diagnosis was 9.3 months (3.1 – 23.1) for all patients -- 11.4 months (4.4 – 27.8) for women and 8.6 months (2.9 – 21.8) for men.
Table 1.
Patient and tumor characteristics at HCC diagnosis, stratified by sex (n=1110)
| Variable | Women (n = 258) | Men (n = 852) | P value |
|---|---|---|---|
| Age, mean (SD) | 62.5 (11.2) | 59.2 (8.3) | <0.001 |
| Age | <0.001 | ||
| <65 | 156 (60.5) | 693 (81.3) | |
| ≥65 | 102 (39.5) | 159 (18.7) | |
| BMI, mean (SD) | 28.9 (7.0) | 27.1 (5.7) | <0.001 |
| Race/ethnicity, n (%) | 0.003 | ||
| White | 63 (24.4) | 306 (35.9) | |
| Black | 92 (35.7) | 268 (31.5) | |
| Hispanic | 89 (34.5) | 217 (25.5) | |
| Asian | 10 (3.9) | 48 (5.6) | |
| Other | 4 (1.5) | 13 (1.5) | |
| Liver Disease Etiology, n (%) | <0.001 | ||
| HCV | 96 (37.9) | 274 (32.5) | |
| HCV + Alcohol | 39 (15.4) | 281 (33.0) | |
| Alcohol | 14 (5.4) | 145 (17.0) | |
| HBV | 9 (3.5) | 48 (5.6) | |
| HBV + HCV | 2 (0.8) | 8 (0.9) | |
| NAFLD | 69 (26.7) | 65 (7.6) | |
| Other/unknown | 17 (6.6) | 8 (0.9) | |
| None | 6 (2.3) | 8 (0.9) | |
| Diabetes mellitus, n (%) | 0.03 | ||
| None | 160 (62.0) | 581 (68.2) | |
| Yes, not on insulin | 35 (13.6) | 127 (14.9) | |
| Yes, on insulin | 63 (24.4) | 144 (16.9) | |
| Hypertension, n (%) | 180 (69.8) | 518 (60.8) | 0.01 |
| Dyslipidemia, n (%) | 62 (25.3) | 155 (18.7) | 0.02 |
| History of prior cancer, n (%) | 20 (7.8) | 68 (8.1) | 0.91 |
| Child Pugh, n (%) | 0.27 | ||
| A | 127 (49.2) | 400 (47.1) | |
| B | 103 (39.9) | 324 (38.1) | |
| C | 28 (10.9) | 126 (14.8) | |
| Ascites, n (%) | 0.10 | ||
| None | 148 (57.4) | 465 (54.6) | |
| Mild/controlled | 95 (36.8) | 300 (35.2) | |
| Severe/uncontrolled | 15 (5.8) | 87 (10.2) | |
| Hepatic encephalopathy, n (%) | 0.37 | ||
| None | 201 (77.9) | 695 (81.7) | |
| Mild/controlled | 54 (20.9) | 145 (17.1) | |
| Severe/uncontrolled | 3 (1.2) | 10 (1.2) | |
| Platelet count (109/L), median (IQR) | 108 (68 – 179) | 129 (82 – 191) | 0.003 |
| Creatinine (mg/dL), median (IQR) | 0.76 (0.62 – 0.98) | 0.87 (0.72 – 1.06) | <0.001 |
| MELD score, median (IQR) | 9 (7 – 13) | 10 (8 – 13) | 0.006 |
| ALBI grade | 0.10 | ||
| 1 | 60 (23.3) | 157 (18.4) | |
| 2 | 142 (55.0) | 465 (54.6) | |
| 3 | 56 (21.7) | 230 (27.0) | |
| AFP (ng/mL), median (IQR) | 26 (7 – 778) | 53 (8 – 1172) | 0.045 |
| AFP (ng/mL), median (IQR) | 0.045 | ||
| <20 | 121 (46.9) | 326 (38.3) | |
| 20–200 | 56 (21.7) | 206 (24.2) | |
| >200 | 81 (31.4) | 319 (37.5) | |
| HCC detected, n (%) | 0.08 | ||
| Surveillance | 119 (46.1) | 328 (38.5) | |
| Incidental | 88 (34.1) | 317 (37.2) | |
| Symptomatic | 49 (19.0) | 198 (23.2) | |
| Unknown/not reported | 2 (0.8) | 9 (1.1) | |
| Number of tumors at diagnosis, n (%) | 0.002 | ||
| 1 | 157 (60.8) | 418 (49.1) | |
| 2 | 36 (14.0) | 124 (14.5) | |
| 3 or more | 65 (25.2) | 310 (36.4) | |
| Largest tumor diameter (cm), median (IQR) | 3.7 (2.2 – 7.2) | 4.4 (2.5 – 9.8) | 0.004 |
| Infiltrative-type tumor, n (%) | 46 (17.9) | 217 (25.6) | 0.02 |
| Macrovascular invasion, n (%) | 50 (20.3) | 236 (28.5) | 0.04 |
| Metastases, n (%) | 23 (8.9) | 114 (13.4) | 0.16 |
| BCLC stage, n (%) | 0.04 | ||
| 0/A | 137 (53.1) | 372 (43.7) | |
| B | 30 (11.6) | 102 (12.0) | |
| C | 52 (20.2) | 217 (25.5) | |
| D | 39 (15.1) | 161 (18.9) | |
| Most definitive treatment, n (%) | 0.29 | ||
| Resection | 34 (13.2) | 105 (12.3) | |
| Ablation | 34 (13.2) | 74 (8.7) | |
| OLT | 17 (6.6) | 65 (7.6) | |
| TACE/TARE | 72 (27.9) | 203 (23.8) | |
| SBRT | 4 (1.6) | 20 (2.4) | |
| Systemic therapy | 24 (2.3) | 98 (11.5) | |
| None/BSC | 73 (28.3) | 287 (33.7) | |
| Response most definitive treatment, n (%) | 0.04 | ||
| Complete response | 100 (54.1) | 240 (42.4) | |
| Partial response | 32 (17.3) | 91 (16.1) | |
| Stable disease | 5 (2.7) | 16 (2.8) | |
| Progressive disease | 31 (16.7) | 142 (25.1) | |
| Unknown | 17 (9.2) | 76 (13.5) |
<5% missing data for all variables unless otherwise specified
AFP – alpha-fetoprotein; ALBI grade – Albumin-Bilirubin grade; BCLC - Barcelona Clinic Liver Cancer; BMI – body mass index; HBV – hepatitis B virus; HCC - hepatocellular carcinoma; HCV – hepatitis C virus; INR – International Normalized Ratio; MELD – Model for End-Stage Liver Disease; NAFLD – nonalcoholic fatty liver disease; NLR – neutrophil-lymphocyte ratio; SD – standard deviation
Women were significantly older than men at diagnosis (mean 62.5 vs 59.2 years), and a higher proportion of women had non-viral liver disease (39.5% vs 25.3%; p <0.001 for both). Liver function was similar between women and men, with a similar proportion having Child Pugh A cirrhosis (49.2% vs 47.1%; p=0.27). Compared to men, women had a higher proportion of all components of the metabolic syndrome, including diabetes (38.0% vs 31.8%, p=0.03), hypertension (69.8% vs 60.8%, p=0.01), dyslipidemia (25.3% vs 18.7%, p=0.02) as well as higher median BMI (28.9 vs 27.1, p<0.001).
Overall Survival
Median overall survival was 13.3 months (IQR 3.9 – 37.6 months) for all patients, with significantly longer survival in women compared to men (median 17.1 vs 12.0 months, p=0.02) (Figure 1A). Among younger patients (<65 years), women had longer survival compared to men (18.3 vs 11.2 months, p=0.02); however, there was not a significant difference in survival between older women and men (15.5 vs 15.7 months respectively, p=0.45) (Figure 1B & Figure 1C). In multivariable analyses adjusting for age, race/ethnicity, liver disease etiology, Albumin-Bilirubin (ALBI) grade, Child Pugh score, and BCLC tumor stage, female sex was associated with lower mortality compared to male sex (HR 0.82, 95%CI 0.68 – 0.98) (Table 2).
Figure 1.
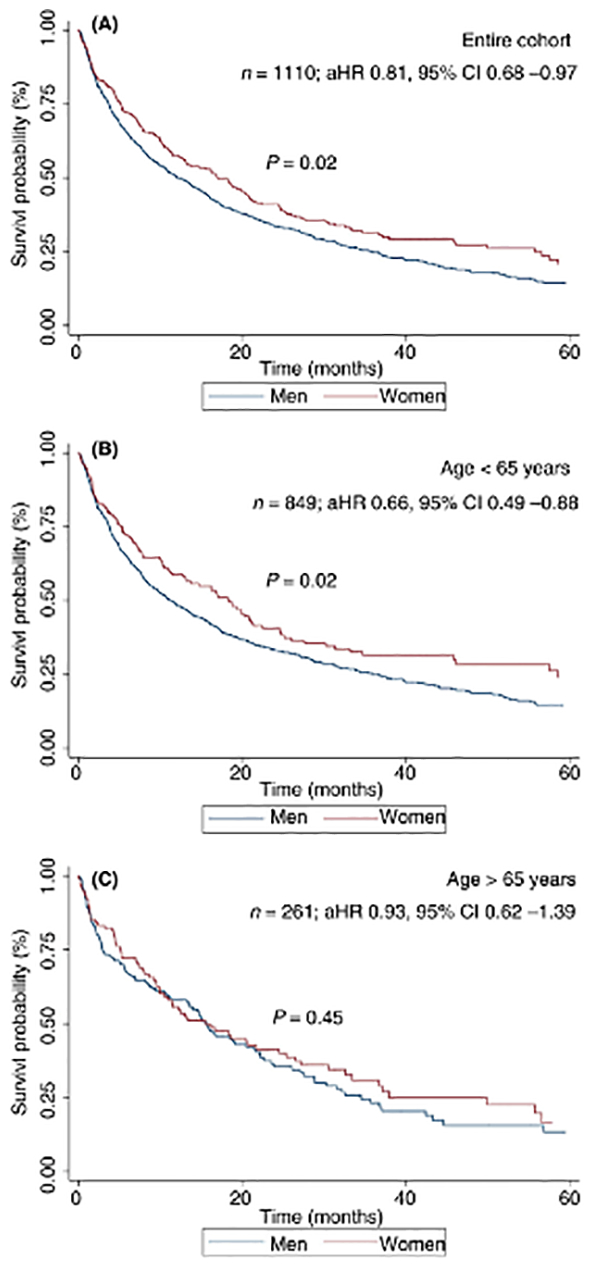
Overall survival, stratified by sex, in (A) the entire cohort, (B) patients aged <65 years and (C) patients aged ≥65 years
Table 2.
Correlates of overall survival
| Univariate (n = 1106) | Multivariable (n = 1103) | |||
|---|---|---|---|---|
| Variable | HR | 95% CI | aHR | 95% CI |
| Female Sex | 0.81 | 0.68 – 0.96 | 0.82 | 0.68 – 0.98 |
| Age | 1.00 | 0.99 – 1.01 | 1.01 | 1.00 – 1.02 |
| Race/ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 1.07 | 0.89 – 1.27 | 0.92 | 0.77 – 1.10 |
| Hispanic | 0.96 | 0.79 – 1.16 | 0.75 | 0.62 – 0.91 |
| Asian | 1.11 | 0.79 – 1.55 | 1.31 | 0.93 – 1.84 |
| Liver disease etiology | ||||
| Viral | Ref | Ref | ||
| Non-viral | 0.92 | 0.78 – 1.08 | ||
| AFP (ng/mL), n (%) | ||||
| <20 | Ref | Ref | Ref | Ref |
| 20–200 | 1.34 | 1.10 – 1.64 | 1.32 | 1.08 – 1.62 |
| >200 | 3.32 | 2.80 – 3.92 | 2.17 | 1.80 – 2.61 |
| Child Pugh | ||||
| A | Ref | Ref | Ref | Ref |
| B/C | 2.37 | 2.05 – 2.75 | 1.62 | 1.33 – 1.97 |
| ALBI grade | ||||
| 1 | Ref | Ref | Ref | Ref |
| 2 | 2.33 | 1.87 – 2.90 | 1.55 | 1.23 – 1.98 |
| 3 | 4.29 | 3.38 – 5.44 | 1.56 | 1.12 – 2.18 |
| BCLC stage | ||||
| 0/A | Ref | Ref | Ref | Ref |
| B | 2.25 | 1.78 – 2.85 | 1.81 | 1.42 – 2.29 |
| C | 6.18 | 5.11 – 7.48 | 4.27 | 3.47 – 5.26 |
| D | 7.73 | 6.30 – 9.50 | 5.04 | 3.88 – 6.57 |
AFP – alpha-fetoprotein; ALBI – Albumin-Bilirubin grade; BCLC - Barcelona Clinic Liver Cancer; HR – hazard ratio; NLR – neutrophil-lymphocyte ratio; OLT – orthotopic liver transplantation; SBRT – stereotactic body radiation therapy; TACE – transarterial chemoembolization; TARE – transarterial radioembolization
In exploratory analyses, we evaluated sex differences in survival when stratified by liver disease etiology, tumor stage, and HCC treatment receipt. We found a consistent association between sex and survival across subgroups, although none were statistically significant given limited statistical power (Supplemental Figure 2, Supplemental Table 1).
Early Tumor Detection
Compared to men, a higher proportion of women had HCC detected by surveillance (46.5% vs 38.9%), and fewer presented symptomatically (19.1% vs. 23.5%), although this did not reach statistical significance (p=0.08). A similar proportion of women and men required biopsy to confirm the diagnosis of HCC (8.9% vs 6.6%, p=0.35). Fewer women had multifocal tumors (39.2% vs 50.8%), macrovascular invasion (20.3% vs 28.5%) or infiltrative disease (17.9% vs 25.6%) (p<0.05 for all). Overall, a higher proportion of women had HCC detected at an early stage (50.8% vs 42.1% within Milan Criteria and 53.1% vs 43.7% BCLC 0/A, respectively). After adjusting for age, race/ethnicity, liver disease etiology, and Child Pugh score, women were more likely to be diagnosed within Milan criteria compared to men (OR 1.55, 95%CI 1.16 – 2.08). Results were consistent when early stage was defined as BCLC stage 0/A (OR 1.55, 95%CI 1.15 – 2.09).
Response to HCC treatment
Female sex was associated with treatment response in univariate analysis (OR 1.57, 95%CI 1.13 – 2.18). In multivariable analysis, after adjusting for age, race/ethnicity, BCLC tumor stage, Child Pugh score, AFP, and treatment received, female sex remained associated with response to first HCC treatment (OR 1.72, 95%CI 1.18 – 2.53) (Table 3). In an exploratory analysis, we found a consistent association between female sex and improved response to treatment across treatment types.
Table 3.
Correlates of response to first HCC treatment
| Univariate (n=594)† | Multivariable (n=591) | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | aOR | 95% CI |
| Female sex | 1.57 | 1.13 – 2.18 | 1.72 | 1.18 – 2.53 |
| Age | 1.01 | 0.99 – 1.03 | 0.99 | 0.97 – 1.01 |
| Race/ethnicity | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 0.75 | 0.53 – 1.08 | 0.86 | 0.57 – 1.33 |
| Hispanic | 0.82 | 0.57 – 1.19 | 0.89 | 0.58 – 1.35 |
| Asian | 0.91 | 0.44 – 3.45 | 1.07 | 0.46 – 2.45 |
| Liver disease etiology | ||||
| Viral | Ref | Ref | ||
| Non-viral | 1.23 | 0.89 – 1.70 | ||
| AFP (ng/mL), n (%) | ||||
| <20 | Ref | Ref | Ref | Ref |
| 20–200 | 0.51 | 0.36 – 0.73 | 0.56 | 0.38 – 0.83 |
| >200 | 0.20 | 0.13 – 0.29 | 0.34 | 0.21 – 0.54 |
| Child Pugh | ||||
| A | Ref | Ref | Ref | Ref |
| B/C | 0.59 | 0.44 – 0.80 | 0.76 | 0.52 – 1.10 |
| BCLC stage | ||||
| 0/A | Ref | Ref | Ref | Ref |
| B | 0.30 | 0.19 – 0.47 | 0.29 | 0.19 – 0.45 |
| C | 0.05 | 0.03 – 0.09 | 0.15 | 0.07 – 0.32 |
| D | 0.49 | 0.25 – 0.95 | 0.65 | 0.30 – 1.43 |
| First HCC treatment | ||||
| Surgical | Ref | Ref | Ref | Ref |
| Locoregional treatment | 0.08 | 0.05 – 0.14 | 0.08 | 0.05 – 0.15 |
| Systemic therapy | 0.01 | 0.003 – 0.01 | 0.02 | 0.01 – 0.05 |
Results from ordinal logistic regression model comparing complete response vs partial response vs stable disease vs progressive disease, in the subset of patients with available imaging assessment to 1st HCC treatment (n=597 of 752 treated patients). AFP – alpha-fetoprotein; BCLC - Barcelona Clinic Liver Cancer; HCC – hepatocellular carcinoma; OR – odds ratio
DISCUSSION
Although sex differences in HCC incidence have been described, data are sparse and conflicting on sex differences in HCC prognosis.12, 15, 29 In this study, using clinically granular data from a large, diverse cohort of patients, we found women with HCC had significantly better overall survival compared to men. This survival benefit persisted after adjusting for confounders including liver disease etiology, degree of liver dysfunction, and BCLC tumor stage. The survival benefit in women differed by age at HCC diagnosis; whereas younger women had a survival benefit compared to younger men, we did not find a difference in survival between older men and women.
Our findings highlight the importance of studying sex differences in HCC prognosis. Although traditional HCC risk factors (e.g., viral hepatitis, alcohol consumption, smoking) have traditionally been more common in men than women, the incidence of chronic hepatitis C infection and its related complications continue to rise in women, including women of childbearing age30, women veterans,31 and those who use injection drugs.32 Further, the epidemiology of HCC is shifting worldwide from viral hepatitis to NASH-related, and some studies suggest women may be more prone to NASH than men.33 Therefore, an increasing proportion of HCC diagnoses may occur in women in the future. Studying sex differences in HCC risk and prognosis can provide insight into future trends in HCC burden and mortality, as well as further our understanding of mechanisms underlying HCC pathogenesis in both sexes.
Our data add to existing literature demonstrating women have better survival than men in other cancers, including colon, gastric, lung, and melanoma.34, 35 Various potential factors may drive these striking disparities in cancer prognosis – including sex-related biologic factors (e.g. tumor biology, sex hormones) and gender-related environmental and behavioral factors (e.g. alcohol and smoking history, health-seeking behaviors). The interplay between biologic and environmental determinants has been demonstrated for racial/ethnic disparities in incidence36 and mortality37 for several cancers, and similarly likely contributes to sex disparities.
Sex hormones have been implicated as possible biological factors in HCC pathogenesis, driven by the observation that sex disparities in HCC incidence persist across time and region. We and others found women were significantly older than men at time of HCC diagnosis, suggesting a potential protective role of estrogen against incident HCC.10, 11, 29However, the role of estrogen in HCC pathogenesis remains controversial.38, 39
Our study extends this literature by suggesting a potential role of sex hormones in HCC prognosis. We found younger women had significantly better survival than younger men; however, this survival difference was not observed in older men and women. Given limitations inherent to retrospective analyses, we used age as a proxy of menopause and did not have data on factors such as oral contraceptives and hormonal therapy. However, our findings are consistent with the existing data evaluating the role of sex hormones in HCC prognosis.40 A study with over 3000 HCC patients from China found that female sex and use of oral contraceptives were associated with improved survival41, and a case-control study of 234 women with HCC found hormonal therapy was associated with improved survival.42
Potential sex-related biological drivers of HCC prognosis are also suggested by data showing sex differences in response to HCC treatment. Our results are consistent with prior studies from Asia demonstrating better survival43 and lower post-operative recurrence in women compared to men undergoing surgical resection, although it is unclear if this effect was related to tumor recurrence or de novo HCC. Our study is one of the first to highlight better HCC prognosis in women compared to men in a non-hepatitis B virus infected population with a wider range of tumor stages. Overall, these data highlight the need for prospective studies in patients with HCC, including granular data on oral contraceptives and hormonal therapy, to better evaluate the association between sex-related biologic factors and HCC prognosis.
On the other hand, there is also rationale to believe that gender-related environmental and behavioral factors may drive observed differences in survival. Gender differences in healthcare utilization45, including men underusing preventative services (e.g., cancer screening)46 have been well described47. This is particularly relevant given underuse of HCC surveillance and treatment in clinical practice.48–51 Our results were consistent with this phenomenon. We found women were more likely than men to have HCC detected by surveillance. The differences in surveillance detection could also be related to improving surveillance use over time, in parallel with HCC increasingly being diagnosed in women. However, we noted sex disparities in survival persisted after adjusting for BCLC stage, suggesting this difference is not entirely explained by health care utilization. Alternatively, this finding could also suggest women are more likely to have biologically indolent tumors, although we recently found no difference in tumor growth patterns by sex.44 Second, there may be gender differences in receipt of HCC treatment. Women are less likely than men to undergo liver transplantation in the US52 and are more likely to die on the waitlist.53 However, women may be more likely than men to undergo surgical resection or ablation for HCC.54
Our findings contrast with those of Ladenheim10 and Wu18, who found female sex was not associated with mortality in HCC patients. There are differences between these study populations and ours that may explain this discrepancy, including demographics, liver disease etiologies, and degree of liver dysfunction. Most notably, the Ladenheim and Wu studies were predominately Asian and white populations, with few (<2%) non-Hispanic blacks and Hispanics (~15%), and our cohort comprised more than 60% racial/ethnic minorities. This is particularly important in light of recent data highlighting racial/ethnic disparities in HCC prognosis.21
To our knowledge, this is one of the largest Western studies to examine sex disparities in HCC prognosis in a diverse patient population. We used granular electronic health record data, including detailed information on risk factors, tumor stage, and liver function, that are not available in administrative datasets, and all HCC cases were adjudicated to ensure they met AASLD diagnostic criteria. Though our study has several strengths, there are a few limitations. First, this was a retrospective cohort study so there is the potential for selection bias and unmeasured confounders. However, our study included consecutive patients with HCC, mitigating concerns about selection bias, and variables were manually extracted from the electronic health record resulting in minimal missing data. Second, although we included more than 1100 patients over a 10-year period, we were underpowered to detect sex differences across subgroups and to evaluate potential interaction effects with factors such as race/ethnicity and tumor stage. Third, we had limited data on non-liver comorbidities, such as frailty, which may differ by sex and impact overall survival. Finally, we had no detailed information on endogenous (e.g., age of menarche and/or menopause) or exogenous hormone use, and these factors may partially explain differences in survival by sex.
In summary, we found significant differences in overall survival and treatment response among men and women with HCC, independent of tumor stage or liver disease severity. Further studies evaluating the mechanisms underlying sex disparities in HCC are needed to identify modifiable factors to promote equity in HCC outcomes for all patients.
Supplementary Material
Supplemental Figure 1. Relative proportion of women and men diagnosed with HCC, by year (2008–2017)
Supplemental Figure 2. Forest plot with adjusted hazard ratios for risk of death in women with HCC (compared to men) for various subgroups.
Grant Support:
Dr. Singal’s research is supported by National Cancer Institute R01 CA222900 and R01 MD12565. This research was supported in part by NIH UL-1TR001105 and CTSA NIH UL-1 RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- ECOG
Eastern Cooperative Oncology Group
- EMR
electronic medical record
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- MELD
Model for End-Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- OS
overall survival
Footnotes
Conflicts of Interest: Jorge Marrero has served as a consultant for Glycotest and received research funding from AstraZeneca. Amit Singal has been on advisory boards and served as a consultant for Wako Diagnostics, Roche, Exact Sciences, Glycotest, Bayer, Eisai, Exelixis BMS, Merck and TARGET-Pharmasolutions. The other authors have no relevant conflicts of interest.
REFERENCES
- 1.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clinical Gastroenterology and Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular Carcinoma. New England Journal of Medicine 2011;365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47 Suppl:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich NE, Yopp AC, Singal AG, et al. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122:1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalra M, Mayes J, Assefa S, et al. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol 2008;14:5945–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol 2002;193:59–63. [DOI] [PubMed] [Google Scholar]
- 9.Manieri E, Herrera-Melle L, Mora A, et al. Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J Exp Med 2019;216:1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladenheim MR, Kim NG, Nguyen P, et al. Sex differences in disease presentation, treatment and clinical outcomes of patients with hepatocellular carcinoma: a single-centre cohort study. BMJ Open Gastroenterology 2016;3 (1) (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D, Hanna DL, Usher J, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: A Surveillance, Epidemiology, and End Results analysis. Cancer 2014;120:3707–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangkijvanich P, Mahachai V, Suwangool P, et al. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World journal of gastroenterology 2004;10:1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai M-W, Chu Y-D, Lin C-L, et al. Is there a sex difference in postoperative prognosis of hepatocellular carcinoma? BMC Cancer 2019;19:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam CM, Yong JL, Chan AO, et al. Better survival in female patients with hepatocellular carcinoma: oral contraceptive pills related? J Clin Gastroenterol 2005;39:533–9. [DOI] [PubMed] [Google Scholar]
- 16.Dohmen K, Shigematsu H, Irie K, et al. Longer survival in female than male with hepatocellular carcinoma. J Gastroenterol Hepatol 2003;18:267–72. [DOI] [PubMed] [Google Scholar]
- 17.Ladenheim MR, Kim NG, Nguyen P, et al. Sex differences in disease presentation, treatment and clinical outcomes of patients with hepatocellular carcinoma: a single-centre cohort study. BMJ Open Gastroenterology 2016;3:e000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu EM, Wong LL, Hernandez BY, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma research 2018;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang D, Hanna DL, Usher J, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer 2014;120:3707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014;21:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich NE, Hester C, Odewole M, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2019;17:551–559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hester CA, Rich NE, Singal AG, et al. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw 2019;17:322–329. [DOI] [PubMed] [Google Scholar]
- 25.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival Among Patients with Cirrhosis in the US. Am J Med 2017;130:1099–1106.e1. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification, In Seminars in liver disease, © 1999 by Thieme Medical Publishers, Inc., 1999. [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- 28.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients With Cancer. Journal of Clinical Oncology 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farinati F, Sergio A, Giacomin A, et al. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol 2009;21:1212–8. [DOI] [PubMed] [Google Scholar]
- 30.Patrick SW, Bauer AM, Warren MD, et al. Hepatitis C virus infection among women giving birth—Tennessee and United States, 2009–2014. MMWR. Morbidity and mortality weekly report 2017;66:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer JR, El-Serag HB, Taylor TJ, et al. Hepatitis C virus-related complications are increasing in women veterans: A national cohort study. J Viral Hepat 2017;24:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmaeili A, Mirzazadeh A, Carter GM, et al. Higher incidence of HCV in females compared to males who inject drugs: A systematic review and meta-analysis. J Viral Hepat 2017;24:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook MB, McGlynn KA, Devesa SS, et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev 2011;20:1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Wang G, He J, et al. Gender differences in colorectal cancer survival: A meta-analysis. International Journal of Cancer 2017;141:1942–1949. [DOI] [PubMed] [Google Scholar]
- 36.Ashktorab H, Kupfer SS, Brim H, et al. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology 2017;153:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health 2015;3:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukocheva OA. Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet? World J Gastroenterol 2018;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maheshwari S, Sarraj A, Kramer J, et al. Oral contraception and the risk of hepatocellular carcinoma. J Hepatol 2007;47:506–13. [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Xie SH, Hu S, et al. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget 2017;8:68131–68137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam CM, Yong JL, Chan AO, et al. Better Survival in Female Patients With Hepatocellular Carcinoma: Oral Contraceptive Pills Related? Journal of Clinical Gastroenterology 2005;39:533–539. [DOI] [PubMed] [Google Scholar]
- 42.Hassan MM, Botrus G, Abdel-Wahab R, et al. Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology 2017;15:1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagasue N, Galizia G, Yukaya H, et al. Better survival in women than in men after radical resection of hepatocellular carcinoma. Hepatogastroenterology 1989;36:379–83. [PubMed] [Google Scholar]
- 44.Rich NE, John BV, Parikh ND, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi-center cohort of patients with cirrhosis. Hepatology 2020;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertakis KD, Azari R, Helms LJ, et al. Gender differences in the utilization of health care services. J Fam Pract 2000;49:147–52. [PubMed] [Google Scholar]
- 46.Viera AJ, Thorpe JM, Garrett JM. Effects of sex, age, and visits on receipt of preventive healthcare services: a secondary analysis of national data. BMC Health Serv Res 2006;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis JL, Buchanan KL, Katz RV, et al. Gender differences in cancer screening beliefs, behaviors, and willingness to participate: implications for health promotion. Am J Mens Health 2012;6:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singal AG, Pillai A, Tiro J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLOS Medicine 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singal AG, Yopp A, C SS, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012;27:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singal AG, Tiro J, Li X, et al. Hepatocellular Carcinoma Surveillance Among Patients With Cirrhosis in a Population-based Integrated Health Care Delivery System. J Clin Gastroenterol 2017;51:650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan D, Yopp A, Beg MS, et al. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 2013;38:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathur AK, Schaubel DE, Gong Q, et al. Sex-based disparities in liver transplant rates in the United States. Am J Transplant 2011;11:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mindikoglu AL, Regev A, Seliger SL, et al. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 2010;16:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sobotka L, Hinton A, Conteh L. Women receive more inpatient resections and ablations for hepatocellular carcinoma than men. World J Hepatol 2017;9:1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Relative proportion of women and men diagnosed with HCC, by year (2008–2017)
Supplemental Figure 2. Forest plot with adjusted hazard ratios for risk of death in women with HCC (compared to men) for various subgroups.


