Abstract
BACKGROUND:
Several studies have demonstrated that 11C-methionine positron-emission tomography provides information on prognosis.
PURPOSE:
We performed a systematic review and meta-analysis of the prognostic value of the metabolic and volumetric parameters of 11C-methionine-PET for gliomas.
DATA SOURCES:
A systematic search was performed using the following combination of keywords: “methionine,” “PET,” “glioma,” and “prognosis.”
STUDY SELECTION:
The inclusion criteria were the use of 11C-methionine-PET as an imaging tool, studies limited to gliomas, studies including metabolic parameters (tumor-to-normal ratio) and/or volumetric parameters (metabolic tumor volume), and studies reporting survival data. The electronic search first identified 181 records, and 14 studies were selected.
DATA ANALYSIS:
Event-free survival and overall survival were the outcome measures of interest. The effect of the tumor-to-normal ratio and metabolic tumor volume on survival was determined by the effect size of the hazard ratio. Hazard ratios were extracted directly from each study when provided or determined by analyzing the Kaplan-Meier curves.
DATA SYNTHESIS:
The combined hazard ratios of the tumor-to-normal ratio for event-free survival was 1.74 with no significance and that of the tumor-to-normal ratio for overall survival was 2.02 with significance. The combined hazard ratio of the metabolic tumor volume for event-free survival was 2.72 with significance and that of the metabolic tumor volume for overall survival was 3.50 with significance.
LIMITATIONS:
The studies selected were all retrospective, and there were only 4 studies involving the metabolic tumor volume.
CONCLUSIONS:
The present meta-analysis of 11C-methionine-PET suggests that the tumor-to-normal ratio for overall survival and the metabolic tumor volume for event-free survival and overall survival are significant prognostic factors for patients with gliomas.
Primary brain tumors are a heterogeneous tumor group with its own biology, prognosis, and treatment approach. Gliomas constitute the most frequent pathology and account for approximately 50% of primary brain tumors.1 Glioblastomas are the most common of all malignant central nervous system tumors (46.6%), and their relative survival estimates are rather low: Only 5.5% of patients have been reported to survive 5 years postdiagnosis.2
Among various imaging modalities, MR imaging has been found to be the most effective tool for characterizing gliomas.3 However, the limitations of MR imaging have encouraged the development of other imaging modalities for the clinical management of gliomas. Not only the MR imaging enhancement patterns of local treatment-related changes but also the T2- or fluid-attenuated inversion recovery MR imaging after antiangiogenic treatment has limited value in differentiating disease progression from post-therapy changes.4 To overcome these drawbacks, advanced imaging techniques such as perfusion MR imaging, MR spectroscopy, and positron-emission tomography have been developed and used for the accurate characterization of tumors. Among them, amino acid PET has additive value compared with MR imaging when assessing the response to antiangiogenic treatments because amino acid uptake occurs independent of regional tumor perfusion and blood-brain barrier permeability.5,6 Several amino acid radiotracers for PET, such as 11C-methionine, [18F]fluoroethyl tyrosine, and 6-[18F]-fluoro-L-dopa, have been used for the metabolic imaging of brain tumors.7 Various studies have demonstrated the advantages of 11C-methionine-PET in diagnosis, grading, and the differential diagnosis between tumor recurrence and radiation necrosis.8,9 In addition, 11C-methionine-PET also provides information on patient prognosis because a high uptake in the glioma indicates a high chance of tumor progression and a poor survival rate.10
Most previous studies with 11C-methionine-PET have used a semiquantitative tumor-to-normal ratio (TNR, metabolic parameter) to identify the uptake and evaluate the prognosis of brain tumors. The TNR is usually defined as the maximum standard uptake value (SUVmax) of the brain tumor divided by the mean SUV (SUVmean) of the contralateral normal cerebral cortex.11,12 However, because the TNR reflects only the single voxel with the highest SUV in the tumor, this parameter does not reflect the total tumor uptake.13 In recent years, volumetric parameters, including the metabolic tumor volume (MTV), have been reported to have prognostic significance for several types of tumors.14–16 The MTV is defined as the tumor volume within the boundary determined by a certain threshold and theoretically reflects the total tumor amount or tumor burden.17,18 Currently, there is a lack of comprehensive and detailed reviews on the prognostic value of the MTV and/or TNR of 11C-methionine-PET for gliomas, which could guide physicians in the management of the tumor.
Therefore, we conducted a comprehensive systematic review of the literature on metabolic and volumetric parameters and designed a meta-analysis to assess the prognostic value of the TNR and MTV of 11C-methionine-PET for patients with gliomas.
Materials and Methods
Data Search and Selection
We performed a systematic search of MEDLINE and EMBASE and a manual search on July 31, 2017, to identify publications using the following combination of keywords: “methionine,” “PET,” “glioma,” and “prognosis.” All searches were limited to human studies. The inclusion criteria were the use of 11C-methionine-PET as an imaging tool, studies limited to gliomas, studies that reported survival data, and studies that included metabolic parameters (TNR) and/or volumetric parameters (MTV). Reviews, abstracts, case reports, and editorials were excluded. Two authors independently conducted the search and screening, and they selected eligible studies for inclusion. Any discrepancies were resolved by consensus.
Data Extraction and Quality Assessment
Two reviewers independently extracted data from the selected publications and recorded the following information: study design, first author, year of publication, country of origin, number of patients, treatment, end point, and evaluated PET parameters. The 2 reviewers scored each publication according to a quality scale used in previous studies.19 This quality scale was divided into 4 categories: scientific design, generalizability, result analysis, and PET report (On-line Table 1). A value between 0 and 2 was assigned to each item, and each category had a maximum score of 10 points. Scores were expressed as a percentage of the maximum, which was 40 points. All data were extracted, and scores were graded by 2 reviewers who performed comparisons at each step. Any discrepancies were resolved by consensus.
Statistical Analysis
The primary outcome was event-free survival (EFS). Disease-free survival and progression-free survival were defined as EFS, which was measured from the date of the initiation of therapy to the date of recurrence or metastasis.20 The secondary end point was overall survival (OS), defined as the time from the initiation of therapy until death. The effect of the TNR or MTV on survival was measured by the effect size of the hazard ratio (HR). Survival data were extracted using a methodology proposed by Parmar et al.21 We extracted the univariate HR estimate and 95% confidence interval directly from each study when provided by the authors. Otherwise, the P values of the log-rank test, 95% CI, number of events, and at-risk numbers were extracted to estimate the HR indirectly. We determined the survival rates from the Kaplan-Meier curves using the Engauge Digitizer (http://markummitchell.github.io/engauge-digitizer/) to reconstruct the HR estimate and its variance, assuming that patients were censored at a constant rate during follow-up. An HR of >1 implied worse survival for patients with a high TNR or MTV, whereas an HR <1 implied better survival for patients with a high TNR or MTV. Heterogeneity between the studies was assessed by a χ2 test and I2 statistics as described by Higgins et al.22 A fixed-effects model was used with Higgins I2 ≤ 50% and Cochran Q at P ≥ .1, and a random-effects model was used with Higgins I2 > 50% or Cochran Q at P < .1. Subgroup analyses were performed according to the tumor grade, tumor stage, calculation methods of the TNR, and references for the MTV. Funnel plots were used to assess publication bias graphically.23 P < .05 was considered statistically significant, and .05 ≤ P ≤ .1 indicated a significant trend. Data from each study were analyzed using Review Manager (RevMan, Version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Characteristics of the Study
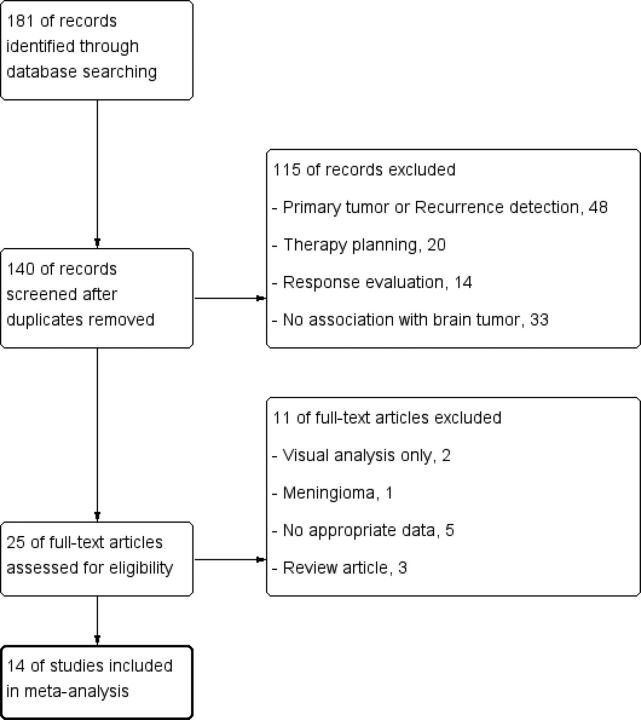
The results of the data search and selection are summarized in Fig 1. A total of 14 studies involving 735 patients were included in our meta-analysis. All 14 studies were of a retrospective design.24–37 The grade of glioma was low in 3 studies,25,29,31 high in 4 studies,30,32,35,37 and mixed in 7 studies.24,26–28,33,34,36 The prognostic value of the TNR was determined in all 14 studies,24–37 and the prognostic value of the MTV was determined in 4 studies.32,34,35,37 The tumor parameters used were SUVmax in 13 studies24–32,34–37 and SUVmean in 1 study.33 The reference parameters were contralateral cortex SUVmean in 10 studies,24–27,30–35 SUVmax in 2 studies,36,37 and undefined in 2 studies.28,29 The cutoff values of the TNR ranged from 1.51 to 3.42, and those of the MTV ranged from 35 to 60 cm3 (On-line Table 2). The mean quality score of the selected studies was 58.0%, with a range of 41.9%–71.3% (On-line Table 3).
Fig 1.
A flow diagram of the study.
Prognostic Value of the TNR and MTV
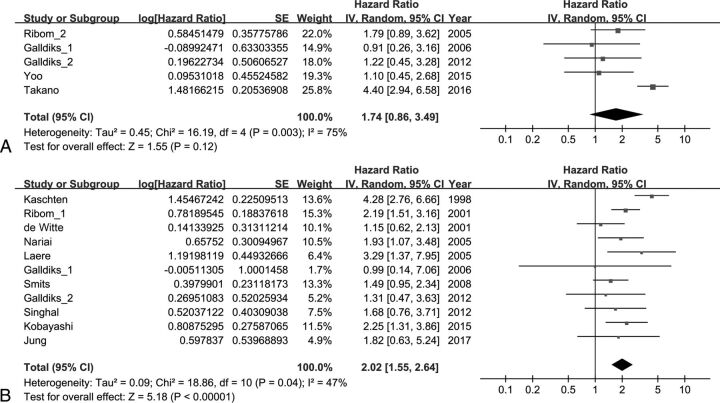
The effect of the TNR on EFS was analyzed using 5 studies. The combined HR of 1.74 for adverse events was not statistically significant (95% CI, 0.86–3.49; P = .12). Heterogeneity was high with statistical significance (χ2 = 16.19, P = .003; I2 = 75%). The effect of the TNR on OS was analyzed using 11 studies. The combined HR of 2.02 for death was statistically significant (95% CI, 1.55–2.64; P < .001). Heterogeneity was moderate with statistical significance (χ2 = 18.86, P = .04; I2 = 47%) (Fig 2).
Fig 2.
Forest plot results of the EFS (A) and OS (B) based on the TNR.
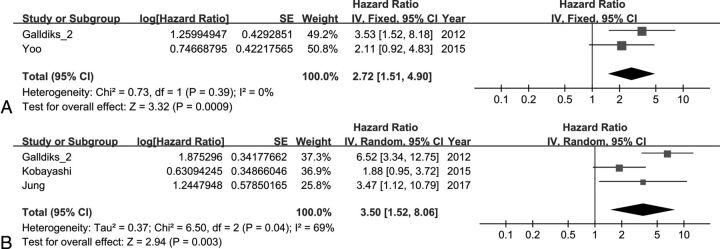
The effect of the MTV on EFS was analyzed using 2 studies. The combined HR of 2.72 for adverse events was statistically significant (95% CI, 1.51–4.90; P < .001). Heterogeneity was not statistically significant (χ2 = 0.73, P = .39; I2 = 0%). The effect of the MTV on OS was analyzed using 3 studies. The combined HR of 3.50 for death was statistically significant (95% CI, 1.52–8.06; P < .003). Heterogeneity was moderate with statistical significance (χ2 = 6.50, P = .04; I2 = 69%) (Fig 3).
Fig 3.
Forest plot results of the EFS (A) and OS (B) based on the MTV.
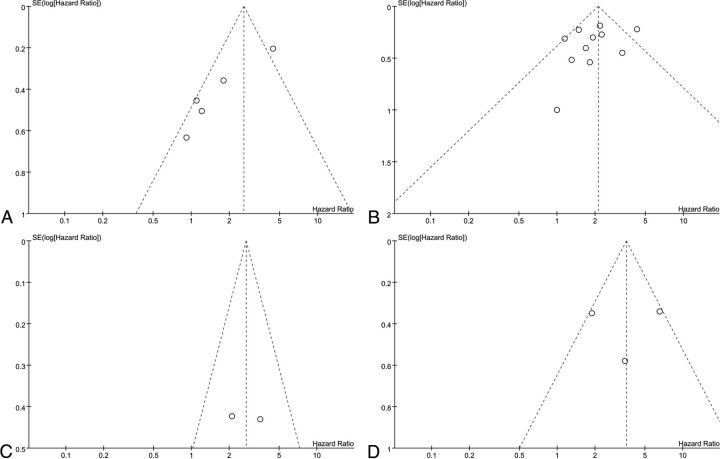
The results of the meta-analysis are summarized in Table 1, and a visual inspection of the funnel plot suggests no evidence of publication bias, as shown in Fig 4.
Table 1:
Summary of the meta-analysis results
| Parameters | End Point | No. of Studies | HR | 95% CI of HR | P Value | I2 (%) | Model |
|---|---|---|---|---|---|---|---|
| TNR | EFS | 5 | 1.74 | 0.86–3.49 | .12 | 75 | Random |
| TNR | OS | 11 | 2.02 | 1.55–2.64 | <.001a | 47 | Random |
| MTV | EFS | 2 | 2.72 | 1.51–4.90 | <.001a | 0 | Fixed |
| MTV | OS | 3 | 3.50 | 1.52–8.06 | .003a | 69 | Random |
Statistically significant (P < .05).
Fig 4.
Funnel plot results of the EFS based on the TNR (A), OS based on the TNR (B), EFS based on the MTV (C), and OS based on the MTV (D).
Subgroup Analysis
Subgroup analysis was performed in relation to the tumor grade, tumor stage, methods of TNR calculation, and references for the MTV (Table 2). According to the variables, eligible studies were divided into 2 subgroups. Among studies of OS in terms of the TNR, high-grade glioma had a significant HR of 1.76 (95% CI, 1.36–2.28; P < .001), and low-grade glioma had an HR of 2.19 with a significant trend (95% CI, 0.98–4.86; P = .05). Studies of primary tumors had a significant HR of 1.95 (95% CI, 1.45–2.63; P < .001), and those of recurrent tumors had a significant HR of 2.58 (95% CI, 1.31–5.08; P = .006). Studies of TNR calculation methods (tumor SUVmax divided by normal contralateral cortex SUVmean) had a significant HR of 1.97 (95% CI, 1.42–2.74; P < .001), and those of other calculation methods had a significant HR of 2.15 (95% CI, 1.29–3.60; P = .003). Among studies that included the OS in terms of the MTV, high-grade glioma (HR = 5.54; 95% CI, 3.11–9.86; P < .001), primary tumor (HR = 3.51; 95% CI, 1.04–11.88; P = .04), and references for the MTV (1.3 × SUVmean of the normal contralateral cortex; HR = 3.51; 95% CI, 1.04–11.88; P = .04) showed significant results.
Table 2:
Results of subgroup analysis
| Parameters | End Point | Factor | No. of Studies | HR | 95% CI of HR | P Value | I2 (%) | Model |
|---|---|---|---|---|---|---|---|---|
| TNR | OS | Tumor grade (high) | 7 | 1.76 | 1.36–2.28 | <.001a | 22 | Fixed |
| Tumor grade (low) | 6 | 2.19 | 0.98–4.86 | .05b | 97 | Random | ||
| TNR | OS | Tumor stage (primary) | 9 | 1.95 | 1.45–2.63 | <.001a | 55 | Random |
| Tumor stage (recurrence) | 2 | 2.58 | 1.31–5.08 | .006a | 0 | Fixed | ||
| TNR | OS | Calculation method of TNR (tumor SUVmax/normal contralateral SUVmean) | 8 | 1.97 | 1.42–2.74 | <.001a | 60 | Random |
| Calculation method of TNR (others) | 3 | 2.15 | 1.29–3.60 | .003a | 0 | Fixed | ||
| MTV | OS | Tumor grade (high) | 2 | 5.54 | 3.11–9.86 | <.001a | 0 | Fixed |
| MTV | OS | Tumor stage (primary) | 2 | 3.51 | 1.04–11.88 | .04a | 85 | Random |
| MTV | OS | Reference for MTV (1.3 × SUVmean of normal contralateral cortex) | 2 | 3.51 | 1.04–11.88 | .04a | 85 | Random |
Statistically significant (P < .05).
Significant trend (.05 ≤ P ≤ .10).
Discussion
In the current study, the prognostic value of the TNR and MTV of 11C-methionine-PET for patients with gliomas was evaluated through a meta-analysis of published studies. The TNR for OS and the MTV for EFS and OS were useful in predicting the prognosis of patients. In addition, subgroup analysis demonstrated that tumor grade may affect the prognosis. To our knowledge, this is the first meta-analysis that has investigated the prognostic value of metabolic and volumetric parameters for patients with gliomas.
Most of the previous studies have used the TNR to quantify the intensity of 11C-methionine uptake to determine the prognosis.24–31,33,36 Our meta-analysis indicated that the TNR for OS (but not the TNR for EFS) of 11C-methionine-PET could be a significant prognostic parameter. A previous study compared 11C-methionine uptake with the pathologic features of tumors and showed that the malignant portions of lesions were coincident with the areas with higher 11C-methionine uptake.28 In subgroup analysis, the TNR showed significant prognostic value for OS in high-grade tumors; however, only a significant trend was found in low-grade tumors. Regarding the tumor stage for OS, the TNR demonstrated significant prognostic value for both primary and recurrent tumors. Regarding the calculation methods of the TNR for OS, the SUVmax of the tumor divided by the SUVmean of the normal contralateral cortex and other TNR calculation methods revealed significant prognostic values.
The TNR represents the high metabolic activity of the tumor, and the MTV reflects the size of the metabolically active tumor. In theory, volumetric parameters should be more useful than metabolic parameters in the prediction of tumor behavior because both metabolic activity and tumor burden are taken into consideration.17,18 Our meta-analysis indicated that the MTV of 11C-methionine-PET could reflect patient prognosis. The results revealed its significance for both EFS and OS. In comparison with the HR of the TNR, the HR of the MTV for EFS was significant, whereas the HR of the TNR for EFS was not significant. The HR of the MTV for OS was higher than the HR of the TNR for OS; however, it was not statistically significant (P = .19; data not shown). Furthermore, previous direct comparison studies reported that the MTV has a better prognostic value than the TNR.32,34,35,37 The direct comparison results are summarized in On-line Table 4. In subgroup analysis, the MTV had significant prognostic value for OS in high-grade tumors, and its statistical significance was compared with that of the TNR for OS in high-grade tumors (P < .001; data not shown). With respect to the tumor stage for OS, the MTV demonstrated significant prognostic value for primary gliomas with higher HRs than those of the TNR. MTV defined by normal contralateral cortex SUVmean × 1.3, was prognostic and showed higher HRs than those of TNR calculation methods.
According to our systematic review and meta-analysis, the TNR could be used for the prognosis of OS, especially in cases of high-grade gliomas. In addition, the MTV could be used for the prognosis of both EFS and OS. Furthermore, the MTV could be superior to the TNR for the prognosis of high-grade gliomas.
There are some limitations to this study. First, the studies selected were all retrospective. There were only 4 studies involving the MTV, and the number of patients in each study was relatively small. In addition, a possible publication bias was not excluded; nevertheless, the funnel plot did not clearly show this. Furthermore, we were unable to determine an optimal cutoff value to categorize the TNR and MTV as high or low due to the lack of individual data. Last, a comparison between the MTV and total lesion glycolysis (TLG = SUVmean multiplied by the MTV, a frequently used parameter in FDG-PET studies) should be performed in the future.34
Conclusions
The TNR and MTV of 11C-methionine-PET are significant prognostic parameters for patients with gliomas. Patients with a high TNR have a higher risk of death, and patients with a high MTV have a higher risk of adverse events or death. The MTV could be used as an incremental predictor of prognosis instead of the TNR.
Supplementary Material
ABBREVIATIONS:
- EFS
event-free survival
- HR
hazard ratio
- MTV
metabolic tumor volume
- OS
overall survival
- SUVmax
maximum standardized uptake value
- SUVmean
mean standardized uptake value
- TNR
tumor-to-normal ratio
Footnotes
Disclosures: Yong-il Kim—RELATED: Grant: Research Driven Hospital R&D project, funded by the CHA Bundang Medical Center, Comments: grant No. BDCHA R&D 2017-018.
This work was supported by a grant of the Research Driven Hospital R&D project, funded by the CHA Bundang Medical Center (grant No. BDCHA R&D 2017-018).
References
- 1. Rigau V, Zouaoui S, Mathieu-Daudé H, et al. ; Société Française de Neuropathologie (SFNP), Société Française de Neurochirurgie (SFNC), Club de Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC), Association des Neuro-Oncologues d'Expression Française (ANOCEF). French brain tumor database: 5-year histological results on 25 756 cases. Brain Pathol 2011;21:633–44 10.1111/j.1750-3639.2011.00491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2014;16(Suppl 4):iv1–63 10.1093/neuonc/nou223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behin A, Hoang-Xuan K, Carpentier AF, et al. . Primary brain tumours in adults. Lancet 2003;361:323–31 10.1016/S0140-6736(03)12328-8 [DOI] [PubMed] [Google Scholar]
- 4. van den Bent MJ, Vogelbaum MA, Wen PY, et al. . End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol 2009;27:2905–08 10.1200/JCO.2009.22.4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hutterer M, Hattingen E, Palm C, et al. . Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol 2015;17:784–800 10.1093/neuonc/nou322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galldiks N, Rapp M, Stoffels G, et al. . Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging 2013;40:22–33 10.1007/s00259-012-2251-4 [DOI] [PubMed] [Google Scholar]
- 7. Galldiks N, Langen K. Applications of PET imaging of neurological tumors with radiolabeled amino acids. Q J Nucl Med Mol Imaging 2015;59:70–82 [PubMed] [Google Scholar]
- 8. Glaudemans AW, Enting RH, Heesters MA, et al. . Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging 2013;40:615–35 10.1007/s00259-012-2295-5 [DOI] [PubMed] [Google Scholar]
- 9. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. . Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008;49:694–99 10.2967/jnumed.107.048082 [DOI] [PubMed] [Google Scholar]
- 10. Kim S, Chung JK, Im SH, et al. . 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2005;32:52–59 10.1007/s00259-004-1598-6 [DOI] [PubMed] [Google Scholar]
- 11. Cicuendez M, Lorenzo-Bosquet C, Cuberas-Borrós G, et al. . Role of [11C] methionine positron emission tomography in the diagnosis and prediction of survival in brain tumours. Clin Neurol Neurosurg 2015;139:328–33 10.1016/j.clineuro.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 12. Watanabe A, Muragaki Y, Maruyama T, et al. . Usefulness of 11C-methionine positron emission tomography for treatment-decision making in cases of non-enhancing glioma-like brain lesions. J Neurooncol 2016;126:577–83 10.1007/s11060-015-2004-x [DOI] [PubMed] [Google Scholar]
- 13. Boellaard R, Krak NC, Hoekstra OS, et al. . Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004;45:1519–27 [PubMed] [Google Scholar]
- 14. Kang CM, Lee SH, Hwang HK, et al. . Preoperative volume-based PET parameter, MTV2. 5, as a potential surrogate marker for tumor biology and recurrence in resected pancreatic cancer. Medicine 2016;95:e2595 10.1097/MD.0000000000002595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hyun SH, Choi JY, Shim YM, et al. . Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 2010;17:115–22 10.1245/s10434-009-0719-7 [DOI] [PubMed] [Google Scholar]
- 16. La TH, Filion EJ, Turnbull BB, et al. . Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009;74:1335–41 10.1016/j.ijrobp.2008.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim Y-i, Paeng JC, Cheon GJ, et al. . Prediction of posttransplantation recurrence of hepatocellular carcinoma using metabolic and volumetric indices of 18F-FDG PET/CT. J Nucl Med 2016;57:1045–51 10.2967/jnumed.115.170076 [DOI] [PubMed] [Google Scholar]
- 18. Fonti R, Larobina M, Del Vecchio S, et al. . Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med 2012;53:1829–35 10.2967/jnumed.112.106500 [DOI] [PubMed] [Google Scholar]
- 19. Berghmans T, Dusart M, Paesmans M, et al. ; European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6–12 10.1097/JTO.0b013e31815e6d6b [DOI] [PubMed] [Google Scholar]
- 20. Zhao Q, Feng Y, Mao X, et al. . Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography or PET-computed tomography in cervical cancer: a meta-analysis. Int J Gynecol Cancer 2013;23:1184–90 10.1097/IGC.0b013e31829ee012 [DOI] [PubMed] [Google Scholar]
- 21. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34 [DOI] [PubMed] [Google Scholar]
- 22. Higgins J, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith GD, Schneider M, et al. . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaschten B, Stevenaert A, Sadzot B, et al. . Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 1998;39:778–85 [PubMed] [Google Scholar]
- 25. Ribom D, Eriksson A, Hartman M, et al. . Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer 2001;92:1541–49 [DOI] [PubMed] [Google Scholar]
- 26. De Witte O, Goldberg I, Wikler D, et al. . Positron emission tomography with injection of methionine as a prognostic factor in glioma. J Neurosurg 2001;95:746–50 10.3171/jns.2001.95.5.0746 [DOI] [PubMed] [Google Scholar]
- 27. Van Laere K, Ceyssens S, Van Calenbergh F, et al. . Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging 2005;32:39–51 10.1007/s00259-004-1564-3 [DOI] [PubMed] [Google Scholar]
- 28. Nariai T, Tanaka Y, Wakimoto H, et al. . Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 2005;103:498–507 10.3171/jns.2005.103.3.0498 [DOI] [PubMed] [Google Scholar]
- 29. Ribom D, Smits A. Baseline 11C-methionine PET reflects the natural course of grade 2 oligodendrogliomas. Neurol Res 2005;27:516–21 10.1179/174313213X13789811969265 [DOI] [PubMed] [Google Scholar]
- 30. Galldiks N, Kracht LW, Burghaus L, et al. . Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging 2006;33:516–24 10.1007/s00259-005-0002-5 [DOI] [PubMed] [Google Scholar]
- 31. Smits A, Westerberg E, Ribom D. Adding 11C-methionine PET to the EORTC prognostic factors in grade 2 gliomas. Eur J Nucl Med Mol Imaging 2008;35:65–71 10.1007/s00259-007-0531-1 [DOI] [PubMed] [Google Scholar]
- 32. Galldiks N, Dunkl V, Kracht LW, et al. . Volumetry of [11C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging 2012;11:516–27 [PubMed] [Google Scholar]
- 33. Singhal T, Narayanan TK, Jacobs MP, et al. . 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med 2012;53:1709–15 10.2967/jnumed.111.102533 [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi K, Hirata K, Yamaguchi S, et al. . Prognostic value of volume-based measurements on (11)C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging 2015;42:1071–80 10.1007/s00259-015-3046-1 [DOI] [PubMed] [Google Scholar]
- 35. Yoo MY, Paeng JC, Cheon GJ, et al. . Prognostic value of metabolic tumor volume on (11)C-methionine PET in predicting progression-free survival in high-grade glioma. Nucl Med Mol Imaging 2015;49:291–97 10.1007/s13139-015-0362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takano K, Kinoshita M, Arita H, et al. . Diagnostic and prognostic value of 11C-methionine PET for nonenhancing gliomas. AJNR Am J Neuroradiol 2016;37:44–50 10.3174/ajnr.A4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jung TY, Min JJ, Bom HS, et al. . Prognostic value of post-treatment metabolic tumor volume from 11C-methionine PET/CT in recurrent malignant glioma. Neurosurg Rev 2017;40:223–29 10.1007/s10143-016-0748-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.