Retrospective review of high-resolution MRIs was performed in patients without prior microvascular decompression. 3D-CISS imaging without and with contrast for 81 patients with trigeminal neuralgia and 15 controls was intermixed and independently reviewed in a blinded fashion. Cisternal segments of both trigeminal nerves were assessed for the grade of neurovascular conflict, cross-sectional area, and degree of flattening. Contrast-enhanced CISS more than doubled the prevalence of the highest grade of neurovascular conflict (14.8% versus 33.3%) and yielded significantly lower cross-sectional area and greater degree of flattening for advanced-grade neurovascular conflict on the symptomatic side compared with non-contrast-enhanced CISS.
Abstract
BACKGROUND AND PURPOSE:
Thin-section MR imaging through the posterior fossa is frequently used for trigeminal neuralgia. Typical heavily T2-weighted imaging methods yield high anatomic detail and contrast between CSF and neurovascular structures, but poor contrast between vessels and nerves. We hypothesized that the addition of gadolinium-based contrast material to 3D-constructive interference in steady-state imaging would improve the characterization of trigeminal compression.
MATERIALS AND METHODS:
Retrospective review of high-resolution MRIs was performed in patients without prior microvascular decompression. 3D-CISS imaging without contrast and with contrast for 81 patients with trigeminal neuralgia and 15 controls was intermixed and independently reviewed in a blinded fashion. Cisternal segments of both trigeminal nerves were assessed for the grade of neurovascular conflict, cross-sectional area, and degree of flattening. Data were correlated with symptom side and pain relief after microvascular decompression using the Fisher exact test, receiver operating curve analysis, and a paired t test.
RESULTS:
Contrast-enhanced CISS more than doubled the prevalence of the highest grade of neurovascular conflict (14.8% versus 33.3%, P = .001) and yielded significantly lower cross-sectional area (P = 8.6 × 10−6) and greater degree of flattening (P = .02) for advanced-grade neurovascular conflict on the symptoms side compared with non-contrast-enhanced CISS. Patients with complete pain relief after microvascular decompression had significantly lower cross-sectional area on contrast-enhanced CISS compared with non-contrast-enhanced CISS on preoperative imaging (P = 2.0 × 10−7). Performance based on receiver operating curve analysis was significantly improved for contrast-enhanced CISS compared with non-contrast-enhanced CISS.
CONCLUSIONS:
The addition of contrast material to 3D-CISS imaging improves the performance of identifying unilateral neurovascular compression for symptomatic trigeminal neuralgia and predicting outcomes after microvascular decompression.
It is generally accepted that vascular compression of the trigeminal nerve (CN V) is an etiologic factor in trigeminal neuralgia (TN).1 Lingering controversy stems mainly from 2 frequently made observations: First, neurovascular contact of the cisternal segment of CN V is not uncommon in asymptomatic individuals, ranging in prevalence from 14% to 88%.2–5 Second, it is not uncommon to find patients with TN who have no neurovascular contact at all.5 However, more severe neurovascular conflict (eg, resulting in deformity or displacement of the nerve) is commonly seen in patients with TN, but not in patients without TN.4,6,7 Moreover, both the presence and severity of trigeminal nerve root compression have been shown intraoperatively4,8–10 and by imaging11–13 to be predictive of favorable outcomes after microvascular decompression (MVD). Thus, from these studies, the concept emerges that it is the degree of compression of the CN V root, rather than simply whether there is any neurovascular contact, that is most predictive of symptoms of TN and favorable outcomes after MVD.
MVD is one of the most commonly performed surgical interventions for TN14 and has shown high effectiveness in relieving pain.9 MVD is an invasive procedure, however, and careful preoperative patient selection is important. Although rare, complications like meningitis, CSF leak, wound infections, and short- or long-term deficits such as hearing loss may occur.14
High-resolution 3D T2-weighted steady-state free precession sequences, including 3D-CISS or FIESTA, yield high spatial resolution and high contrast between CSF and neurovascular structures and have become the standard sequence for preoperative imaging in TN.15,16 However, these sequences have poor contrast between vessels and nerves because both are low in intensity, potentially limiting detection and characterization of higher grade neurovascular conflict when there is little or no intervening CSF. We hypothesized that the addition of intravenous contrast material to 3D-CISS sequences, which appear to be T2-weighted but, in fact, have both T2- and T1-weighting17 and therefore demonstrate enhancement,18–20 would improve the contrast between enhancing vessels and adjacent unenhanced trigeminal nerve roots. The aim of this study was to evaluate whether contrast-enhanced 3D-CISS (CE-CISS) improves our ability to predict the symptom side in patients with TN and further to predict which patients are most likely to have favorable outcomes after MVD, compared with 3D-CISS without contrast (NE-CISS).
Materials and Methods
Study Design and Patient Sample
A retrospective review of 310 consecutive high-resolution 3D TN protocol MR imaging studies acquired between 2011 and 2014 at our institution was performed. The study was institutional review board–approved and Health Insurance Portability and Accountability Act–compliant. Of the 261 patients imaged for TN (49 studies belonged to subjects imaged more than once) with this protocol during this period, we selected all 116 patients with a history of TN who had imaging performed before undergoing MVD. Of those, 26 patients were excluded because they had had a previous intervention for TN (rhizotomy or gamma knife treatment). Nine studies were excluded because a full set of clinical characteristics was not available or the imaging data were incomplete. A total of 81 studies qualified for the pre-MVD TN group. Separately, 51 patients without the diagnosis of TN were imaged with the same parameters performed as a component of an evaluation of the skull base. Of these, 15 studies were chosen at random as non-TN controls. An equal number of cases and asymptomatic controls would bias the reader to overestimate the asymptomatic controls as TN cases because most asymptomatic individuals do not undergo the high-resolution trigeminal neuralgia imaging protocol. Assuming a statistical power of 80% and exposure defined by those undergoing the high-resolution trigeminal neuralgia imaging protocol, 1-β is 20% exposure in controls (chance of type II error). Considering that the number of symptomatic cases was 81, fifteen (18.5%) were selected as the number of asymptomatic controls to remain below 20%.21,22
The mean age for the pre-MVD TN group was 49.2 years (range, 27–71 years), with 52 female and 29 male patients. The mean age for the control group was 44.6 years (range, 15–71 years), with 10 female and 5 male patients. There was no statistical difference in the age of the 2 groups (P = .2, two-tailed Student t test).
The diagnosis of TN was based on clinical history and physical examination performed by the neurosurgery team (C.R.G. and M.L.). Demographic and baseline disease characteristics were collected during the presurgical work-up for MVD. Patients were classified as having either type 1 TN (classic TN, purely paroxysmal) or type 2 TN (TN with persistent facial pain). The clinical outcome of pain relief following MVD was assessed during follow-up clinic visits and classified as type 1: complete pain relief without need for medication; type 2: complete pain relief with continued need for medication; type 3: partial pain relief with continued need for medication; and type 4: no pain relief despite medication. The range of clinical follow-up was 3 days to 1228 days, with an average of 275 days. On-line Tables 1 and 2 list the patients and clinical outcomes. For one of the patients, clinical outcome data were not available because the patient was lost to follow up.
Imaging Technique
All studies were conducted on Verio or Magnetom Trio 3T scanners (Siemens, Erlangen, Germany) (n = 78) or Magnetom Espree or Avanto 1.5T scanners (Siemens) (n = 3) using a standardized high-resolution TN protocol. The protocol included triplanar pre- and postcontrast 3D-CISS (slice thickness, 0.6 mm; matrix, 256 × 256; FOV, 16.9 × 24.6) and 3D time-of-flight MR angiography.
Image and Data Analysis
The studies of patients with TN and controls were intermixed and reviewed independently by 2 neuroradiologists (D.S. and B.N., each with >5 years of experience) blinded to patient history, including the presence or absence and side of symptoms. The cisternal segment of CN V was assessed on both sides for the presence or absence of neurovascular conflict. The following types of data were acquired for each side: type of vessel involved (vein, artery, or both), nerve surface involved (superior, inferior, medial, or lateral), the grade of neurovascular conflict (0 = none, 1 = simple contact without displacement or distortion of the nerve, 2 = displacement and/or mild distortion, 3 = severe distortion or decrease in cross-sectional area of the nerve), the cross-sectional area (CSA) of CN V at the site of neurovascular conflict, orthogonal dimensions of the nerve root at the site of neurovascular conflict (degree of flattening [DOF]), and the distance of neurovascular conflict from the apparent origin of the nerve at the surface of the pons.
Orthogonal dimensions were obtained by first measuring the greatest possible dimension of the nerve in the coronal plane and then taking the measurement of the nerve orthogonal to and at the midportion of the first measurement (see illustrative drawing in On-line Fig 1) from multiplanar reformats. For statistical analysis, we took the mean of the CSA, the distance of neurovascular conflict from the pons, and DOF between the 2 observers. For the type of vessel in conflict, grading of neurovascular conflict, and the surface of the nerve involved, if there was discrepancy between the 2 readers, those cases were re-reviewed jointly, blinded to study type, and a consensus was reached. 3D TOF MRA was used to help in the assessment of the type of vessel in conflict with the nerve (the artery is bright).
Statistical Analysis
We calculated the sensitivity and specificity of characteristics of neurovascular conflict (grade and type of vessel) comparing the symptomatic (diseased) with the asymptomatic side or controls (healthy). Statistical comparisons for CSA and DOF were performed by a paired t test with the Holm-Bonferroni method of P value adjustment for multiple comparisons after performing Shapiro-Wilk normality tests. Statistical comparisons between the area under the curves were performed as described in DeLong et al.23 The Fisher exact test was applied when assessing associations of categoric variables such as grades of neurovascular conflicts or types of vascular involvement. The κ statistic for interobserver agreement was calculated. All analyses were performed using R statistical and computing software, Version 3.2.2 (http://www.r-project.org) with packages caroline, plotrix, beeswarm, and pROC. A P value of <.05 was considered significant.
Results
Comparison of Neurovascular Conflict on the Symptomatic and Asymptomatic Sides and in Controls on NE-CISS and CE-CISS Imaging
Examples of the different grades of neurovascular conflict (0–3) and examples illustrating improvement in contrast between trigeminal nerve roots and adjacent vessels in advanced grades of neurovascular conflict are shown in Figs 1–4. On NE-CISS, neurovascular conflict in general (grade 1, 2, or 3) was common on the asymptomatic side and in controls (54/81 [66.7%] and 18/30 [60%], respectively), and it was even more common on the symptomatic side (71/81 [87.7%]; Table 1). On the asymptomatic side and in controls, this consisted mostly of grade 1 neurovascular conflict (48/54 [88.9%] and 18/18 [100%] cases, respectively). Advanced-grade neurovascular conflict (grade 2 or 3) was encountered in 38/81 (46.9%; n = 26 for grade 2 and n = 12 for grade 3) cases on the symptomatic side versus in only 6/81 (7.4%, all grade 2) on the asymptomatic side and not at all in controls. Most of the advanced-grade cases of neurovascular conflict (grade 2 or 3) had involvement of an artery (33/38 [86.8%]), but venous involvement was found in more than half of these cases as well (22/38 [57.9%]).
Fig 1.
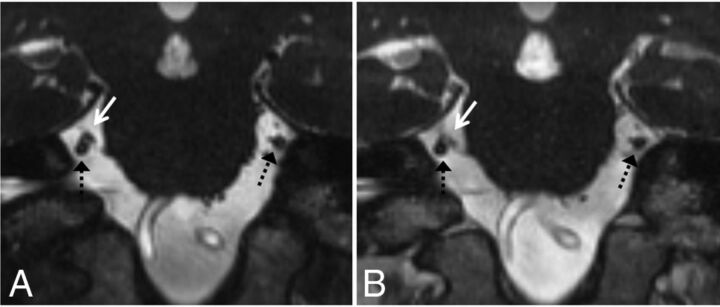
Grades 0 and 1 neurovascular conflict. Coronal NE-CISS (A) and CE-CISS (B) images show grade 1 (simple contact) on the patient's right side with a branch from the superior cerebellar artery (white solid arrow) contacting the cisternal segment of the trigeminal nerve root (dashed black arrow) from above. Note enhancement of the artery on the CE-CISS image. On the patient's left, the cisternal trigeminal nerve root (dashed black arrow) has no neurovascular conflict (grade 0).
Fig 2.
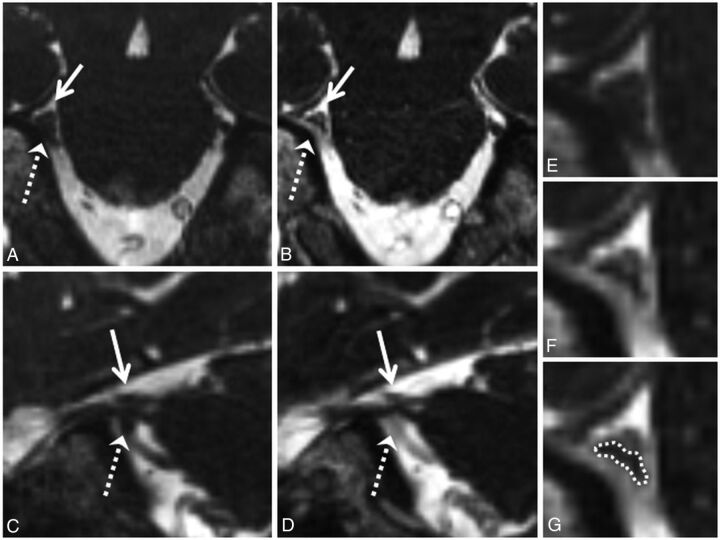
Grade 2 neurovascular conflict. Coronal NE-CISS (A) and CE-CISS (B) and sagittal NE-CISS (C) and CE-CISS (D) images show neurovascular conflict of the cisternal segment of the patient's right trigeminal nerve with a branch of the superior cerebellar artery from above (solid white arrow) and the superior petrosal vein from below (dashed white arrow), resulting in flattening of the nerve near the porus trigeminus. On the NE-CISS images (A and C), the nerve is not well-delineated from the adjacent vascular structures. On the CE-CISS images (B and D), the vessels enhance, outlining the compressed nerve between them. Zoomed-in images of the site of neurovascular conflict in the coronal plane (E and F) illustrate the poor contrast between vessels and nerve on the NE-CISS image (E) and the improved contrast after administration of gadolinium contrast material (F), allowing more confident delineation of the compressed nerve from the adjacent vessels (G). Both NE-CISS and CE-CISS images were interpreted as grade 2 compression. On the patient's left, grade 0 was given for both the NE-CISS and CE-CISS images.
Fig 3.
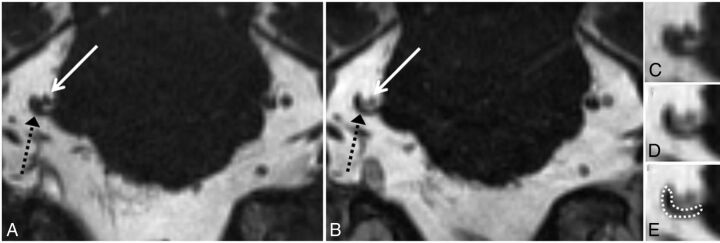
Grade 3 neurovascular conflict. Coronal NE-CISS (A) and CE-CISS (B) images show compression of the cisternal segment of the patient's right trigeminal nerve (black dashed arrow) by branches of the superior cerebellar artery from above (solid white arrow). On the unenhanced image (A), the nerve root is not well-distinguished from the compressing arterial branches. After contrast administration (B), at least 2 arterial branches enhance, allowing more confident delineation of the markedly compressed nerve. Zoomed-in images of the site of neurovascular conflict in the coronal plane (C–E) illustrate the poor contrast between vessels and nerve on the NE-CISS image (C) and the improved contrast after administration of gadolinium contrast material (D), allowing more confident delineation of the compressed nerve from the adjacent vessels (E). In this patient, grade 3 was given for both the NE-CISS and CE-CISS images, but the measured CSA was lower and the degree of flattening was more pronounced on the CE-CISS images. On the patient's left, grade 0 was given for both the NE-CISS and CE-CISS images.
Fig 4.
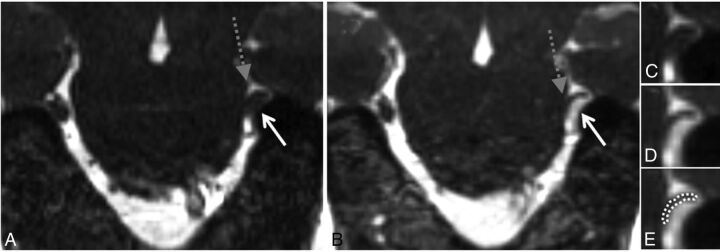
High-grade neurovascular conflict. Coronal NE-CISS (A) and CE-CISS (B) images show compression of the distal cisternal segment of the patient's left trigeminal nerve (gray dashed arrow) by a branch of the superior petrosal venous complex from below (solid white arrow). On the unenhanced image (A), the nerve root is not well-distinguished from the compressing venous branch. After contrast administration (B), the venous branch fills with contrast, showing a severely compressed unenhanced adjacent trigeminal nerve root at the level of the porus trigeminus. Zoomed-in images of the site of neurovascular conflict in the coronal plane (C–E) illustrate the poor contrast between vessels and nerve on the NE-CISS image (C) and the improved contrast after administration of gadolinium contrast material (D), allowing more confident delineation of the compressed nerve from the adjacent vessel (E). In this patient, grade 2 was given on the NE-CISS and grade 3 was given on CE-CISS images. On the patient's right, grade 1 was given on both the NE-CISS and CE-CISS images.
Table 1:
Prevalence of various grades of neurovascular conflict and types of vascular involvement in patients with TN and in controls
| Contrast Enhancement | Symptomatic Side (No.) (%) of 81 Cases | Asymptomatic Side (No.) (%) of 81 Cases | Control (No.) (%) of 30 Cases | |
|---|---|---|---|---|
| Grades of neurovascular conflict | ||||
| 0 | Contrast− | 10 (12.3) | 27 (33.3) | 12 (40.0) |
| Contrast+ | 10 (12.3) | 27 (33.3) | 12 (40.0) | |
| 1 | Contrast− | 33 (40.7) | 48 (59.3) | 18 (60.0) |
| Contrast+ | 29 (35.8) | 48 (59.3) | 18 (60.0) | |
| 2 | Contrast− | 26 (32.1) | 6 (7.4) | 0 (0.0) |
| Contrast+ | 15 (18.5) | 6 (7.4) | 0 (0.0) | |
| 3 | Contrast− | 12 (14.8) | 0 (0) | 0 (0.0) |
| Contrast+ | 27 (33.3) | 0 (0) | 0 (0.0) | |
| 1, 2, or 3 | Contrast− | 71 (87.7) | 54 (66.7) | 18 (60.0) |
| Contrast+ | 71 (87.7) | 54 (66.7) | 18 (60.0) | |
| 2 and 3 | Contrast− | 38 (46.9) | 6 (7.4) | 0 (0.0) |
| Contrast+ | 42 (51.9) | 6 (7.4) | 0 (0.0) | |
| Artery alone | 27 (33.3) | 17 (21.0) | 4 (13.3) | |
| Artery involved | 49 (60.5) | 30 (37.0) | 5 (16.7) | |
| Vein alone | 22 (27.2) | 24 (29.6) | 12 (40.0) | |
| Vein involved | 44 (54.3) | 37 (45.7) | 13 (43.3) | |
| Mixed | 22 (27.2) | 13 (16.0) | 1 (3.3) |
Note:—Contrast+ indicates contrast enhancement; Contrast−, no contrast enhancement.
Grade 3 neurovascular conflict was encountered only on the symptomatic side, so it was 100% specific for that group. Its sensitivity for the symptomatic side was 14.8% on NE-CISS imaging, which more than doubled to 33.3% on CE-CISS (P = 9.6 × 10−3, Tables 2 and 3). Advanced-grade neurovascular conflict (grade 2 or 3) had a specificity for the symptomatic side of 94.6% on both NE-CISS and CE-CISS and a sensitivity of 46.9% and 51.9% on NE-CISS and CE-CISS, respectively (P = .64). The κ statistic for interobserver agreement in grading of neurovascular conflict was 0.68 for NE-CISS, which increased to 0.72 for CE-CISS.
Table 2:
Statistical analysis
| Grades of Neurovascular Conflict | Contrast Enhancement |
P Values |
Contrast vs Noncontrast |
Sensitivity | Specificity | |||
|---|---|---|---|---|---|---|---|---|
| Sym vs Asym | Sym vs Cntrl | Asym vs Cntrl | Sym | Asym | ||||
| 0 | Contrast− | .002 | .002 | .51 | 1 | 1 | 12.3% | 66.7% |
| Contrast+ | .002 | .003 | .51 | 12.3% | 66.7% | |||
| 1 | Contrast− | .03 | .09 | 1 | 0.63 | 1 | 40.7% | 40.5% |
| Contrast+ | .004 | .03 | 1 | 35.8% | 40.5% | |||
| 2 | Contrast− | <.001 | <.001 | .19 | 0.07 | 1 | 32.1% | 94.6% |
| Contrast+ | .06 | .01 | .19 | 18.5% | 94.6% | |||
| 3 | Contrast− | <.001 | .03 | 1 | 0.001 | 1 | 14.8% | 100.0% |
| Contrast+ | <.001 | <.001 | 1 | 33.3% | 100.0% | |||
| 1, 2, or 3 | Contrast− | .002 | .003 | .51 | 1 | 1 | 87.7% | 35.1% |
| Contrast+ | .002 | .002 | .51 | 87.7% | 35.1% | |||
| 2 and 3 | Contrast− | <.001 | <.001 | .19 | 0.64 | 1 | 46.9% | 94.6% |
| Contrast+ | <.001 | <.001 | .19 | 51.9% | 94.6% | |||
Note:—Sym indicates symptomatic; Asym, asymptomatic; Cntrl, control; Contrast +, contrast enhancement; Contrast −, no contrast enhancement.
Table 3:
Characteristics of neurovascular involvement
| Involvement of Neurovascular Conflict |
P Values |
Contrast vs | Noncontrast | ||
|---|---|---|---|---|---|
| Sym vs Asym | Sym vs Cntrl | Asym vs Cntrl | |||
| Artery alone | 0.11 | 0.06 | 0.43 | 33.3% | 81.1% |
| Artery involved | .005 | <.001 | .06 | 60.5% | 68.5% |
| Vein alone | 0.86 | 0.25 | 0.36 | 27.2% | 67.6% |
| Vein involved | 0.35 | 0.39 | 1 | 54.3% | 55.0% |
| Mixed | 0.13 | 0.007 | 0.11 | 27.2% | 87.4% |
Grade 1 neurovascular conflict occurred with similar frequency along the course of the cisternal segment of the trigeminal nerve root, with 8/29 (27.6%) occurrences between 0 and 3 mm from the surface of the pons, 11/29 (37.9%) between 3 and 6 mm, and 9/29 (31.0%) distal to 6 mm (On-line Fig 2). Advanced-grade neurovascular conflict (grade 2 or 3), on the other hand, was most prevalent between 3 and 6 mm from the surface of the pons (32/42 [76.2%]), with 6/42 (14.3%) cases occurring between 0 and 3 mm, and 4/42 (9.5%) cases occurring distal to 6 mm.
Comparison between NE-CISS and CE-CISS in the Evaluation of CSA and DOF
When we compared CSA and DOF between the symptomatic and asymptomatic sides on either NE-CISS or CE-CISS, there was no significant difference in the setting of grade 1 neurovascular conflict (On-line Fig 1). However, with higher grades of neurovascular conflict (grade 2 or 3), there was a significant difference in CSA and DOF between the symptomatic and asymptomatic sides on both NE-CISS and on CE-CISS. Moreover, when we compared the CSA and DOF on the symptomatic side between NE-CISS and CE-CISS, there was a significant decrease in CSA and an increase in DOF on CE-CISS for higher grades of neurovascular conflict, but no significant difference in the setting of low-grade neurovascular conflict. There was also no significant difference when comparing the CSA or DOF on the asymptomatic side between NE-CISS and CE-CISS.
Correlation between Postsurgical Symptomatic Relief and the Metrics of Preoperative Neurovascular Conflict and Comparison of NE-CISS versus CE-CISS in Predicting the Symptomatic Side and Symptomatic Relief after MVD in Patients with TN
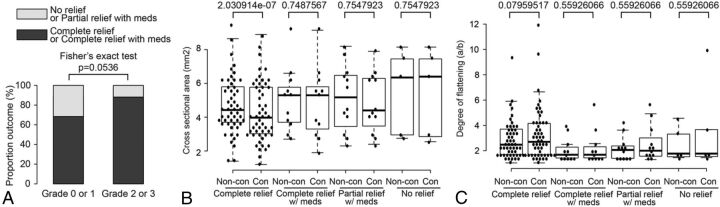
On-line Table 3 shows the correlation between the grade of neurovascular conflict as determined on CE-CISS and NE-CISS with the type of postoperative outcome after MVD. There was a significant difference between the grades of compression found in patients with type 1 postsurgical outcome when comparing NE-CISS with CE-CISS images (P = .031), but not in patients with type 2, 3, or 4 outcome. The proportion of patients with complete relief from pain postoperatively with or without a continued need for analgesics (type 1 and 2 outcomes, respectively) was 26/38 (68%) in patients with grade 0 or 1 neurovascular conflict and 37/42 (88%) in patients with grade 2 or 3 neurovascular conflict, a difference that did not quite reach statistical significance (P = .054, Fig 5A).
Fig 5.
Correlation of the metrics of neurovascular conflict with postsurgical outcomes after MVD. Grades of neurovascular conflict (A), CSA (B), and DOF (C) were correlated with different postsurgical outcomes after MVD, as described in the individual graphs. Non-con indicates non-contrast; Con, contrast-enhanced.
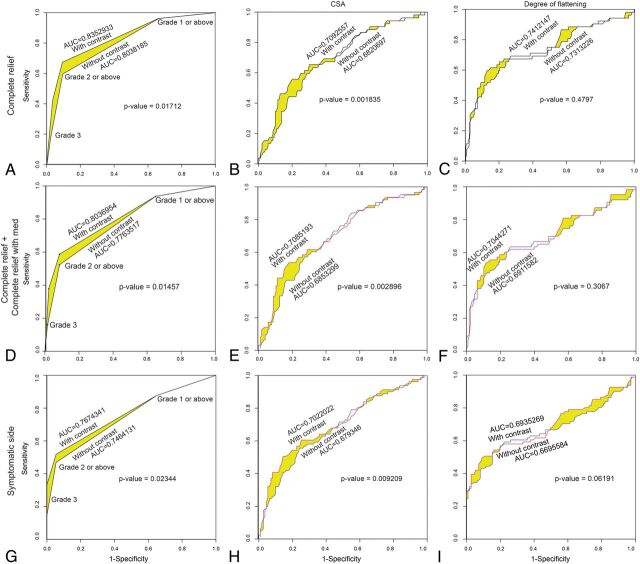
The CSA in patients with type 1 outcome was significantly lower on CE-CISS compared with NE-CISS (P = 2.03 × 10−7, Fig 5B). With the other types of outcome,2–4 there was no significant difference between the CSA on NE-CISS and CE-CISS. Using receiver operating curve analysis, we compared the performance of NE-CISS versus CE-CISS in predicting the symptomatic side or relief of symptoms after MVD based on the grade of neurovascular conflict, CSA, and DOF (Fig 6). All tests were found to be useful in predicting the outcome in question (area under the curve [AUC] > 0.65). Optimum cutoffs to maximize sensitivity and specificity are given in Table 4. Using the grade of neurovascular conflict to predict complete relief after MVD yielded an AUC of 0.804 on NE-CISS and a significantly higher AUC of 0.835 on CE-CISS (P = .017, Fig 6A). The grade of neurovascular conflict was also the best predictor of the side of symptoms, with an AUC of 0.746 on NE-CISS and a significantly higher AUC of 0.767 on CE-CISS (P = 1.7 × 10−14, Fig 6G).
Fig 6.
Receiver operating curves assessing the performance of the grade of neurovascular conflict (A, D, and G), CSA (B, E, and H), and DOF (C, F, and I) in predicting complete relief without further need for analgesic medication (type 1 outcome) after MVD (A–C), complete relief with or without further need for analgesic medication (type 1 or 2 outcome) after MVD (D–F), and symptom side in patients with TN (G–I) for both NE-CISS and CE-CISS images, as delineated in the graphs. The area under the curve and P values comparing the NE-CISS and CE-CISS curves are provided in the individual graphs.
Table 4:
Cutoff values for grade of neurovascular conflict, CSA, and DOF to obtain the highest sum of sensitivity and specificity in predicting complete relief of pain after MVD (type 1 outcome), complete relief of pain with or without need for analgesic medication (type 1 or 2 outcome), or in predicting the side of symptoms in patients with TN
| Outcome | Cutoff | Contrast | Sensitivity | Specificity |
|---|---|---|---|---|
| Type 1 | ||||
| Grade | ≥2 | − | 59.6 | 90.7 |
| ≥2 | + | 67.3 | 90.7 | |
| CSA | ≤5.1 | − | 63.5 | 67.6 |
| ≤4.15 | + | 55.8 | 79.9 | |
| DOF | ≥2.0 | − | 69.2 | 73.4 |
| ≥2.5 | + | 59.6 | 87.1 | |
| Type 2 | ||||
| Grade | ≥2 | − | 52.4 | 91.4 |
| ≥2 | + | 58.7 | 91.4 | |
| CSA | ≤4.7 | − | 57.1 | 72.7 |
| ≤4.15 | + | 54 | 82 | |
| DOF | ≥2.0 | − | 63.5 | 74.3 |
| ≥2.5 | + | 54 | 88.3 | |
| Predicting side of sym in patients with TN | ||||
| Grade | ≥2 | − | 46.9 | 94.6 |
| ≥2 | + | 51.9 | 94.6 | |
| CSA | ≤4.7 | − | 55.6 | 75.7 |
| ≤4.15 | + | 50.6 | 85.6 | |
| DOF | ≥2.2 | − | 53.1 | 84.7 |
| ≥2.5 | + | 49.4 | 91 |
Note:—sym indicates symptoms; −, absent; +, present.
Discussion
We found that while neurovascular conflict in general was prevalent in both patients with TN and controls, higher grade (grade 2 or 3) neurovascular conflict was specific to the symptomatic side in patients with TN, but only about 50% sensitive, like findings in previous studies.6,7 These findings imply that neurovascular compression is sufficient but not necessary to induce symptoms of TN.1 Patients with little or no neurovascular conflict were less likely to experience pain relief after MVD compared with patients with advanced-grade neurovascular conflict; these resultscorroborate previous reports from preoperative imaging and intraoperative findings.4,9–14 In these patients, other etiologic considerations, including pathology in or near the trigeminal root ganglion, may be in play1,24 and an initial trial with rhizotomy of the trigeminal ganglion could be a rational alternative to MVD, especially if the patient is not an ideal surgical candidate.1,12
Most higher grade neurovascular conflict was found between 3 and 6 mm from the surface of the pons, corresponding to the location of the root entry zone, in line with findings in previous reports.6,7,25,26 This relates to the proposed pathophysiologic mechanism (ignition hypothesis) of vascular compression resulting in microstructural damage to the nerve root at this vulnerable transition zone between central and peripheral myelin, making the axons hyperexcitable and giving rise to pain paroxysms as a result of synchronization after discharge activity.1
Like findings published by Zhou et al,27 we found evidence of the known somatotopic organization of the trigeminal nerve,28 which relates the surface of the compressed nerve to distribution of symptoms. Advanced-grade neurovascular conflict had a venous component in approximately 58% of the cases, most commonly along the inferior or lateral surface of the trigeminal nerve root, corresponding to the most common drainage pattern of the superior petrosal venous complex into the superior petrosal sinus between the porus trigeminus and porus acusticus.29 Venous contribution to neurovascular compression has been reported previously, though typically as constituting less than half of the cases of neurovascular conflict.6,7,11,26,28,30 The differences may be due to differences in technique, for instance related to increased sensitivity for venous detection after contrast administration. The reporting of a venous component in neurovascular conflict is important for presurgical planning because these cases may have a higher recurrence rate31 and different surgical techniques have been proposed for decompression of culprit veins.32 In a notable study of healthy subjects by Yousry et al,33 the enhanced 3D-CISS sequence was determined to be superior for visualization of the trigeminal ganglion, sinus ganglion, and sinus lips. The current study, however, is unique in assessing symptomatic trigeminal compression.
Metrics (CSA, DOF, grading) of neurovascular conflict between NE-CISS and CE-CISS sequences in cases of little or no neurovascular conflict demonstrate little difference, which is expected because the nerve is mostly outlined by CSF in those cases and is thus well-characterized on NE-CISS. However, in cases of advanced-grade nerve root compression, significant differences in the metrics of neurovascular conflict between NE-CISS and CE-CISS emerged. These differences, in turn, resulted in significantly improved performance based on receiver operating curve analysis for CE-CISS compared with NE-CISS in predicting the symptom side and the degree of relief of symptoms after MVD. Notably, a cutoff of grade 2 neurovascular conflict or higher on CE-CISS predicted complete relief of symptoms after MVD, with a specificity of 90.7% and a sensitivity of 67.3% (AUC on the receiver operating curve analysis = 0.835), and the side of symptoms, with a specificity of 94.6% and a sensitivity 51.9% (AUC on the receiver operating curve analysis = 0.767). Given the invasiveness of MVD and potential risks to the patient, such advances in the optimization of preoperative imaging, allowing improved preoperative planning and patient selection, are of potential importance for clinical care.14
Limitations of our study include inherent biases associated with the retrospective study design and those of a single-institution cohort. In addition, although the reviewers were blinded to the type of subject (control versus patient with TN), it is not possible to blind the reviewers to the presence or absence of contrast in the grading of neurovascular conflict, CSA, and degree of flattening. Another limitation in this retrospective study was the range in duration of clinical follow-up for postoperative patients, raising the possibility that some patients might have had a different pain-relief outcome if they were followed for a shorter or longer time. Finally, our measurements of CSA and DOF were performed at the submillimeter level, which is at the limits of the spatial resolution of even high-resolution 3D-CISS volumetric imaging. However, spatial resolution for acquisition and measurement techniques during evaluation was consistent across all conditions.
Conclusions
Low-grade neurovascular conflict is common in control patients and on the asymptomatic side in patients with TN, while the presence of higher grade neurovascular conflict is highly specific for the symptomatic side in TN and in predicting symptomatic relief after MVD. The addition of contrast material to 3D-CISS imaging improves characterization of higher grade neurovascular conflict and improves performance in predicting both the side of symptoms in patients with TN and favorable outcomes after MVD. These improvements in TN imaging may be helpful in optimizing patient selection and preoperative planning.
Supplementary Material
ABBREVIATIONS:
- AUC
area under the curve
- CSA
cross-sectional area
- CE
contrast-enhanced
- DOF
degree of flattening
- MVD
microvascular decompression
- NE
non-contrast-enhanced
- TN
trigeminal neuralgia
Footnotes
Disclosures: Ari M. Blitz—UNRELATED: Board Membership: Guerbet, Comments: Medical Advisory Board; Grants/Grants Pending: Aesculab, Siemens, National Institutes of Health, Comments: Aesculab, research funding on hydrocephalus; Siemens, in-kind support for a pulse programming course for graduate students; National Institutes of Health, support unrelated to this submission: R21 NS096497 (coinvestigator), FAIN U01DC013778 (lead radiologist)*; Payment for Lectures Including Service on Speakers Bureaus: Siemens, Comments: payment for an educational talk at a symposium. C. Rory Goodwin—UNRELATED: Grants/Grants Pending: Johns Hopkins Neurosurgery Pain Research Institute, Burroughs Wellcome Fund, United Negro College Fund-Merck Science Initiative.* Michael Lim—UNRELATED: Consultancy: Stryker; Grants/Grants Pending: Stryker*; Payment for Lectures Including Service on Speakers Bureaus: Stryker, Comments: consulting and speaking; Payment for Development of Educational Presentations: Stryker, Comments: speaker for education; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Stryker, Comments: pay for travel to consulting and speaking engagements. *Money paid to the Institution.
Paper preciously presented at: Annual Meeting of the American Society of Head and Neck Radiology, September 9–13, 2015; Naples, Florida, by Seeburg et al; and Annual Meeting of the Congress of Neurological Surgeons, September 26–30, 2015; New Orleans, Louisiana, by Goodwin et al.
References
- 1. Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 2002;18:4–13 10.1097/00002508-200201000-00002 [DOI] [PubMed] [Google Scholar]
- 2. Peker S, Dinçer A, Necmettin Pamir M. Vascular compression of the trigeminal nerve is a frequent finding in asymptomatic individuals: 3-T MR imaging of 200 trigeminal nerves using 3D CISS sequences. Acta Neurochir (Wien) 2009;151:1081–88 10.1007/s00701-009-0329-y [DOI] [PubMed] [Google Scholar]
- 3. Miller JP, Acar F, Hamilton BE, et al. Radiographic evaluation of trigeminal neurovascular compression in patients with and without trigeminal neuralgia. J Neurosurg 2009;110:627–32 10.3171/2008.6.17620 [DOI] [PubMed] [Google Scholar]
- 4. Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia, 2: neurovascular compression of the trigeminal nerve in cadaveric controls and patients with trigeminal neuralgia—quantification and influence of method. Clin Anat 1997;10:380–88 [DOI] [PubMed] [Google Scholar]
- 5. Adams CB. Microvascular compression: an alternative view and hypothesis. J Neurosurg 1989;70:1–12 10.3171/jns.1989.70.1.0001 [DOI] [PubMed] [Google Scholar]
- 6. Maarbjerg S, Wolfram F, Gozalov A, et al. Significance of neurovascular contact in classical trigeminal neuralgia. Brain 2015;138:311–19 10.1093/brain/awu349 [DOI] [PubMed] [Google Scholar]
- 7. Antonini G, Di Pasquale A, Cruccu G, et al. Magnetic resonance imaging contribution for diagnosing symptomatic neurovascular contact in classical trigeminal neuralgia: a blinded case-control study and meta-analysis. Pain 2014;155:1464–71 10.1016/j.pain.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 8. Sindou M, Leston J, Decullier E, et al. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg 2007;107:1144–53 10.3171/JNS-07/12/1144 [DOI] [PubMed] [Google Scholar]
- 9. Sarsam Z, Garcia-Fiñana M, Nurmikko TJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. Br J Neurosurg 2010;24:18–25, 2010 10.3109/02688690903370289 [DOI] [PubMed] [Google Scholar]
- 10. Szapiro J Jr, Sindou M, Szapiro J. Prognostic factors in microvascular decompression for trigeminal neuralgia. Neurosurgery 1985;17:920–29 10.1227/00006123-198512000-00009 [DOI] [PubMed] [Google Scholar]
- 11. Leal PR, Barbier C, Hermier M, et al. Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes. J Neurosurg 2014;120:1484–95 10.3171/2014.2.JNS131288 [DOI] [PubMed] [Google Scholar]
- 12. Han-Bing S, Wei-Guo Z, Jun Z, et al. Predicting the outcome of microvascular decompression for trigeminal neuralgia using magnetic resonance tomographic angiography. J Neuroimaging 2010;20:345–49 10.1111/j.1552-6569.2009.00378.x [DOI] [PubMed] [Google Scholar]
- 13. Duan Y, Sweet J, Munyon C, et al. Degree of distal trigeminal nerve atrophy predicts outcome after microvascular decompression for type 1a trigeminal neuralgia. J Neurosurg 2015;123:1512–18 10.3171/2014.12.JNS142086 [DOI] [PubMed] [Google Scholar]
- 14. Zakrzewska JM, Coakham HB. Microvascular decompression for trigeminal neuralgia: update. Curr Opin Neurol 2012;25:296–301 10.1097/WCO.0b013e328352c465 [DOI] [PubMed] [Google Scholar]
- 15. Casselman JW, Kuhweide R, Deimling M, et al. Constructive interference in steady state-3DFT MR imaging of the inner ear and cerebellopontine angle. AJNR Am J Neuroradiol 1993;14:47–57 [PMC free article] [PubMed] [Google Scholar]
- 16. Blitz AM, Macedo LL, Chonka ZD, et al. High-resolution CISS MR imaging with and without contrast for evaluation of the upper cranial nerves: segmental anatomy and selected pathologic conditions of the cisternal through extraforaminal segments. Neuroimaging Clin N Am 2014;24:17–34 10.1016/j.nic.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 17. Chavhan GB, Babyn PS, Jankharia BG, et al. Steady-state MR imaging sequences: physics, classification, and clinical applications. Radiographics 2008;28:1147–60 10.1148/rg.284075031 [DOI] [PubMed] [Google Scholar]
- 18. Shigematsu Y, Korogi Y, Hirai T, et al. Contrast-enhanced CISS MRI of vestibular schwannomas: phantom and clinical studies. J Comput Assist Tomogr 1999;23:224–31 10.1097/00004728-199903000-00010 [DOI] [PubMed] [Google Scholar]
- 19. Blitz AM, Choudhri AF, Chonka ZD, et al. Anatomic considerations, nomenclature, and advanced cross-sectional imaging techniques for visualization of the cranial nerve segments by MR imaging. Neuroimaging Clin N Am 2014;24:1–15 10.1016/j.nic.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 20. Amemiya S, Aoki S, Ohtomo K. Cranial nerve assessment in cavernous sinus tumors with contrast-enhanced 3D fast-imaging employing steady-state acquisition MR imaging. Neuroradiology 2009;51:467–70 10.1007/s00234-009-0513-z [DOI] [PubMed] [Google Scholar]
- 21. Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol 1982;116:547–53 10.1093/oxfordjournals.aje.a113439 [DOI] [PubMed] [Google Scholar]
- 22. Noordzij M, Tripepi G, Dekker FW, et al. Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transpl 2010;25:1388–93 10.1093/ndt/gfp732 [DOI] [PubMed] [Google Scholar]
- 23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 24. Beaver DL. Electron microscopy of the gasserian ganglion in trigeminal neuralgia. J Neurosurg 1967;26(Suppl):138–50 10.3171/jns.1967.26.1part2.0138 [DOI] [PubMed] [Google Scholar]
- 25. Yousry I, Moriggl B, Holtmannspoetter M, et al. Detailed anatomy of the motor and sensory roots of the trigeminal nerve and their neurovascular relationships: a magnetic resonance imaging study. J Neurosurg 2004;101:427–34 10.3171/jns.2004.101.3.0427 [DOI] [PubMed] [Google Scholar]
- 26. Leal PR, Hermier M, Souza MA, et al. Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: a prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery 2011;69:15–25; discussion 26 10.1227/NEU.0b013e318212bafa [DOI] [PubMed] [Google Scholar]
- 27. Zhou Q, Liu ZL, Qu CC, et al. Preoperative demonstration of neurovascular relationship in trigeminal neuralgia by using 3D FIESTA sequence. Magn Reson Imaging 2012;30:666–71 10.1016/j.mri.2011.12.022 [DOI] [PubMed] [Google Scholar]
- 28. Gudmundsson K, Rhoton AL Jr, Rushton JG. Detailed anatomy of the intracranial portion of the trigeminal nerve. J Neurosurg 1971;35:592–600 10.3171/jns.1971.35.5.0592 [DOI] [PubMed] [Google Scholar]
- 29. Tanriover N, Abe H, Rhoton AL Jr, et al. Microsurgical anatomy of the superior petrosal venous complex: new classifications and implications for subtemporal transtentorial and retrosigmoid suprameatal approaches. J Neurosurg 2007;106:1041–50 10.3171/jns.2007.106.6.1041 [DOI] [PubMed] [Google Scholar]
- 30. Matsushima T, Huynh-Le P, Miyazono M. Trigeminal neuralgia caused by venous compression. Neurosurgery 2004;55:334–37; discussion 338–39 10.1227/01.NEU.0000129552.87291.87 [DOI] [PubMed] [Google Scholar]
- 31. Lee SH, Levy EI, Scarrow AM, et al. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery 2000;46:356–61; discussion 361–62 10.1097/00006123-200002000-00019 [DOI] [PubMed] [Google Scholar]
- 32. Hong W, Zheng X, Wu Z, et al. Clinical features and surgical treatment of trigeminal neuralgia caused solely by venous compression. Acta Neurochir (Wien) 2011;153:1037–42 10.1007/s00701-011-0957-x [DOI] [PubMed] [Google Scholar]
- 33. Yousry I, Moriggl B, Schmid UD, et al. Trigeminal ganglion and its divisions: detailed anatomic MR imaging with contrast-enhanced 3D constructive interference in the steady state sequences. AJNR Am J Neuroradiol 2005;26:1128–35 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.