SUMMARY:
Blunt cerebrovascular injury is a relatively uncommon but sometimes life-threatening injury, particularly in patients presenting with ischemic symptoms in that vascular territory. The decision to pursue vascular imaging (generally CT angiography) is based on clinical and imaging findings. Several grading scales or screening criteria have been developed to guide the decision to pursue vascular imaging, as well as to recommend different treatment options for various injuries. The data supporting many of these guidelines and options are limited however. The purpose of this article is to review and compare these scales and criteria and the data supporting clinical efficacy and to make recommendations for future research in this area.
Blunt injury of the carotid and vertebral arteries (collectively termed blunt cerebrovascular injury [BCVI]) is an injury in patients caused by blunt trauma. Early studies (in the absence of screening) showed an incidence as low as 0.08%,1 but with the increased screening and use of CT angiography as the diagnostic test, the incidence is now estimated to be between 1.2% and 2.99% of trauma admissions.2–4 These injuries can have high morbidity and mortality. Many patients with BCVI ultimately die from other injuries; nonetheless, earlier studies (in which BCVI diagnosis was based on symptoms and not on screening) showed that isolated BCVI-related death could be as high as 38%,5,6 with permanent neurologic deficits in most patients.7 Stroke is one of the most feared outcomes of BCVI, with a reported overall incidence of 10%–13% in recent literature.4,8 Nearly half of the patients with stroke have symptoms before reaching the hospital.4,8 Among asymptomatic patients with BCVI, aggressive screening and timely treatment have been shown to decrease the incidence of stroke to <1%.9,10
Initially, digital subtraction angiography was used for diagnosis, but with advances in technology, CTA is considered the imaging technique of choice, with a sensitivity and specificity of nearly 98% and 100%, respectively.11,12 Various screening criteria have been proposed for patients with blunt trauma, with the Modified Denver Criteria being the most accepted13–15; however, other criteria (such as the Memphis Criteria16 and Boston Criteria17) may make implementation of protocol confusing and challenging for practitioners. Also, there are no specific guidelines for screening these injuries in the pediatric population. Studies have postulated that the relatively low incidence of BCVI in children with trauma (0.03%–0.9% reported incidence18–20) may be due to poor understanding of risk factors.21,22 Although scientific evidence may be lacking, commonly used BCVI screening criteria, which are based on studies in the adult population, may not be appropriate for children and may be leading to increased use of CTA, and hence more radiation to children.22
In addition to the identification of high-risk patients for screening, BCVI diagnosis may also be impacted by the appropriate use of imaging techniques and the familiarity of the radiologist with the imaging findings. The need for greater awareness of BCVI was also highlighted in a recent survey23 of radiologists based in large academic institutes, which showed that only 14.2% (5/35) of respondents identified commonly used screening criteria and none of them used injury grading in their reports. This review describes the injury mechanisms, various screening criteria and associations, imaging protocols, CT appearance and grading, and management of BCVI.
BCVI Mechanism and Pathophysiology
The major mechanisms of injury causing BCVI are extreme rotation and hyperextension, with contralateral head rotation, direct blunt injury to the vessel, injury from the fracture fragment, and direct intraoral trauma.24,25 BCVI may occur anywhere along the vessel, with the cervical carotid vessel near the skull base and the vertebral artery segments along the transverse foramina being the most common locations. The extracranial segments are more vulnerable to injury because they are more superficial and mobile and run near bony structures. Carotid artery injuries are most common at the distal extracranial segment, with injuries relating to stretching over the lateral masses of the C1–C3 vertebrae from hyperextension and contralateral head rotation.15,24,26,27 The vertebral artery is most commonly injured in the pars transversaria (V2) or the atlas loop (V3) due to laceration from fracture fragments or stretching.24,28 The BCVI typically begins at the intima or media as an intimal tear or intramural hematoma. Postmortem series have shown complete arterial transection in approximately one-third of cases,29 but this is not as common in clinical practice.6,30,31 If the blood from arterial transection is contained by periarterial soft tissue or decompresses into the adjacent injured vein, it forms a pseudoaneurysm or arteriovenous fistula, respectively.6
The eventual neurologic damage is most commonly from thromboembolism of platelet aggregates that form at the site of intimal injury. Platelet aggregates or intramural hematoma can also lead to luminal narrowing or occlusion that can cause low-flow infarcts.32,33 Typically, these ischemic infarcts occur between 10 and 72 hours after the injury.7,34 Arterial transection can cause exsanguination and AVF. Patients with AVFs can have symptoms related to increased venous pressure or steal phenomenon.
BCVI Screening
As with any entity, the utility of screening for BCVI is based on early diagnosis and treatment in a relatively asymptomatic stage, possibly preventing adverse outcomes. If injuries are not recognized and treated early, patients can have irreversible neurologic symptoms. Many screening criteria have been proposed to identify patients at risk of BCVI. Initial studies on BCVI were performed at the Denver Health Medical Center and the University of Tennessee Health Science Center, and the criteria proposed by them were named the Denver Criteria31,35 and the Memphis Criteria (Tables 1 and 2), respectively.16,36 Among the cervical spine injuries, the Denver Criteria initially suggested screening in severe cervical hyperextension/rotation or hyperflexion (particularly if associated with a displaced midface or complex mandibular fracture) or with cervical body fractures,35 while the Memphis Criteria suggested screening in all cervical spine fractures.16 In 2007, the Denver group15 suggested a modification to include only specific cervical spine fracture patterns (complex cervical spine fractures such as subluxation, extension into the foramen transversarium, or upper C1–C3 fractures) for prompt screening.
Table 1:
Published screening criteria for BCVI
| Name of Screening Criteria | Patients to Be Screened |
|
|---|---|---|
| Signs/Symptoms of BCVI | Risk Factors of BCVI | |
| Modifieda Denver Criteria14 | Arterial hemorrhage (from neck, nose, or mouth) | High-energy transfer mechanism with the following: |
| Cervical bruit (in younger than 50 yr of age) | LeFort II or III fracture | |
| Expanding cervical hematoma | Basilar skull fracture involving carotid canal | |
| Focal neurologic defect: TIA, hemiparesis, vertebrobasilar symptoms, Horner syndrome | Cervical vertebral body or transverse foramen fracture, subluxation, or ligamentous injury at any level; any fracture at C1–C3 | |
| Stroke on CT or MRI | Closed head injury consistent with DAI and GCS <6 | |
| Neurologic deficit inconsistent with head CT | Near-hanging with anoxia | |
| Clothesline-type injury or seat belt abrasion with significant swelling, pain, or altered mental status | ||
| Memphis Criteria16 | Cervical spine fracture | |
| Neurologic exam not explained by brain imaging | ||
| Horner syndrome | ||
| LeFort II or III facial fractures | ||
| Skull base fractures involving the foramen lacerum | ||
| Neck soft-tissue injury (eg, seat belt injury or hanging) | ||
Note:—DAI indicates diffuse axonal injury; GCS, Glasgow Coma Scale.
Suggested expansion: occipital condyle fractures, mandibular fractures, traumatic brain injuries with thoracic injuries, scalp degloving, thoracic vascular injuries, and blunt cardiac rupture.9
Table 2:
Boston Criteria17 for BCVI
| First Tiera | Second Tierb |
|---|---|
| Skull base fractures: petrous and basilar fractures | DAI |
| Any cervical spine fractures | Complex facial fractures with midface instability |
| Cervical spine injury (cord, vertebral body, or ligaments) | Combined significant head and chest trauma |
| Soft-tissue injury to anterior neck with swelling/ecchymosis/hematoma/or bruit | Near-hanging |
| Significant neurologic deficit: lateralizing neurologic deficit, TIA, Horner syndrome | Seat belt abrasions on neck |
| Evidence of brain infarct on CT | Other unexplained neurologic deficits: vertigo, tinnitus, or GCS ≤6 |
Note:—DAI indicates diffuse axonal injury; GCS, Glasgow Coma Scale.
First tier criteria: CTA screening on presentation.
Second tier criteria: CTA screening within 24–48 hours of presentation.
Both the Western Trauma Association14 and the Eastern Association for the Surgery of Trauma13 (EAST) published their most recent guidelines in 2009 and 2010, respectively, and suggested that though scientific evidence may be lacking (level III recommendation), it may be most justifiable to screen asymptomatic patients based on the Modified Denver Criteria. Later studies showed that nearly 20% of cases of BCVI were missed if the patients were screened on the basis of the Modified Denver Criteria.10,37,38 These “missed” injuries were revisited, and in 2012, the Denver group suggested including mandible fractures, complex skull fractures, traumatic brain injury with thoracic injuries, scalp degloving, and thoracic vascular injuries in addition to previously described screening criteria.9 In 2016, the Denver group4 showed that the expanded Denver Criteria (On-line Figure) are effective in the detection of previously missed injuries and should now be used for screening. In their study, they found that the overall incidence of BCVI increased to 2.99% from 2.36% with expansion of screening criteria. At the same time, the BCVI-related neurologic event incidence decreased from 12% (18/150 patients) to 9.3% (22/236 patients). Adding to the number of screening criteria, additional Boston Criteria (Tables 1 and 2) have been recently proposed for detection of BCVI, based on a study at Boston Medical Center.17 The Modified Denver Criteria are most studied, are endorsed by the EAST and Western Trauma Association, and are most commonly used. Because the current literature suggests that BCVI may be missed on the Modified Denver Criteria, further expansion as suggested by the Denver group may be appropriate. However, additional multicenter validation studies are needed to identify the best screening criteria.
BCVI in the Pediatric Population
The EAST guidelines recommend that the adult screening guidelines be applied to the pediatric population.13 A study by Kopelman et al19 in 2011 also supported this recommendation; however, in 2012, a study by Jones et al21 showed that more than two-thirds of pediatric patients with BCVI and stroke-like symptoms did not have the adult screening risk indications. Overall, the incidence of BCVI is less in the pediatric population compared with adults, which could, in part, be due to less screening in children, as highlighted in the multicenter Arizona-Texas-Oklahoma-Memphis-Arkansas Consortium study.18 Also, the pattern of intracranial injury in the pediatric population may be different from that in adults.39 BCVI in pediatric patients was retrospectively analyzed by Ravindra et al22 from the University of Utah School of Medicine in 2015, and they suggested the Utah Score (Table 3) for estimated risk of BCVI in children. A recent multicenter validation study of the Utah Score also showed that compared with the Denver Criteria, the Utah Score correlated better with the risk of BCVI in the pediatric population and the Denver Criteria would have led to overscreening and unnecessary radiation exposure.40 The initial study showed that a score of ≤2 had a 7.9%22 risk of BCVI, whereas the validation study showed an even lower number of 2.7%40 BCVI risk. A score of ≥3 had a BCVI risk of 39.3% per the initial study22 and 18.1% per the validation study.40 For high-risk patients, CTA is the imaging test of choice. For low-risk (Utah Score ≤2), the authors suggested that delayed MR angiography should be considered if there is clinical suspicion. This may be an area of further research because currently no pediatric population studies exist to support MRA in low-risk patients.
Table 3:
Utah Score22 for probability of BCVI in the pediatric population
| Variable | Scorea |
|---|---|
| GCS score ≤8 | 1 |
| Focal neurologic deficit | 2 |
| Carotid canal fracture | 2 |
| Petrous temporal bone fracture | 3 |
| Cerebral infarct on CT | 3 |
| All | 11 |
Imaging Modalities for BCVI
Evaluation of the BCVI may be performed with CTA, MR imaging/MRA, or DSA. Despite its portable nature and bedside availability, duplex sonography assessment of BCVI is not preferred because about 90% of lesions are in sonographically nonaccessible segments. Additionally, duplex sonography is operator-dependent, with higher chances of missing dissecting aneurysms24 and lower sensitivity (38.6% for both the carotid and vertebral injuries, 86% for carotid injuries alone).13 The EAST guidelines13 recommend against the use of duplex sonography for screening. CTA, on the other hand, is fast, noninvasive, readily available, and has high spatial resolution, which is preferred for diagnosing injuries in smaller vessels. It also evaluates coexisting bony and soft-tissue injuries. The initial studies on single- and 4-section scanners showed a sensitivity of 45%–70% for BCVI compared with DSA.16,41 However, subsequent studies with 16-section or higher CT scanners have shown that the sensitivity and specificity of BCVI diagnosis approaches 100%.5,12,13 Hence, CTA is considered the screening technique of choice for BCVI.6,13,24,42 The EAST guidelines13 recommend against the use of ≤4-section multidetector array CTA for BCVI screening. With newer generation CT technology (enabling ultrafast imaging with even higher spatial resolution, metal artifact reduction, and low contrast dose), the role of CT continues to expand.43
DSA is regarded as the criterion standard for the diagnosis of BCVI but has certain limitations. It is not readily available and may not be feasible to perform in a critically injured patient. Transportation of the patient to the angiography suite and the procedural time add to the delay in diagnosis. Also, it is invasive and has a complication rate of 1%–3%, which includes vascular dissection and thromboembolism. Furthermore, DSA provides no information about the vessel wall and is therefore limited in characterizing vessel wall hematomas.24,42 With the universal use of CT for diagnosis, DSA is mostly performed only when an intervention is planned.
MRA also offers comprehensive vessel imaging with the added benefit of non-nephrotoxic contrast, concurrent assessment of ligamentous/spinal injuries, and simultaneous detection of infarction. However, the current literature is limited on the use of MR imaging for BCVI, with a reported sensitivity of approximately 50%.16 As per the EAST guidelines,13 it is not recommended as a single technique for diagnosis. MR imaging has a complementary role, with improved differentiation of intramural hematoma, atherosclerotic plaques, and intraluminal thrombus in carotid or vertebrobasilar dissections.44,45 The limitations of MR imaging include longer scanning times, lack of widespread availability, the need for MR imaging–compatible lines and tubes in an acutely injured patient, inferior spatial resolution, and low sensitivity in characterizing acute intramural hematoma (that is isointense in the acute stage).24,42 MRA is mostly used complementary to CTA in selected cases and for follow-up (in children or patients with kidney disease).
CT and MR Imaging Protocol for BCVI Screening
Almost universally, nearly all patients with suspected BCVI are screened by CTA. The trauma imaging protocol pan scan starts as noncontrast CT of the head and cervical spine, which are reviewed by the trauma surgery team or the radiologist at the CT scanner before the administration of IV contrast. If any injury necessitating BCVI screening is noted, CTA of the head and neck is also performed along with contrast-enhanced CT of the chest, abdomen, and pelvis (and reconstructions of the thoracolumbar spine) with a single intravenous contrast injection. If the injury requiring BCVI screening is noted later, CTA is performed within 24–36 hours of the injury. MR imaging is rarely used for screening. If used, it is mostly in pediatric patients or in patients with absolute contraindications to CT contrast. The CTA and MRA protocol at our institution is highlighted in On-line Tables 1 and 2, respectively. Multiplanar reconstructions and 3D postprocessing are always done. The images are reconstructed as volume-rendered images, which are helpful for better detection of stenosis and the relation of the abnormality to the adjacent bony and soft-tissue structures.46 Low-grade BCVIs are also better detected on MPR and volume-rendered images.6 The 3D images are always interpreted in conjunction with the primary dataset and are never interpreted alone.
Imaging Findings and Grading of BCVI
On the basis of the appearance of these injuries, Biffl et al31 from the Denver Health Medical Center proposed an injury grading scale in 1999, with increasing risk of stroke and worse prognosis with increasing grade. This grading system was based on carotid injuries visualized with DSA, but it has been adopted for CTA and MRA with the same grading system for both carotid and vertebral injuries.14,30,31 The Denver grading scale, corresponding CTA findings, and grade-based stroke risk are highlighted in Table 4. In 2015, a study by Crawford et al47 reported that many of the injuries on CTA could not be initially graded per the Denver grading scale and were labeled as “indeterminate BCVI.” Follow-up of these injuries in 48 hours was suggested to rule in a true BCVI. Approximately 25% of these injuries were reclassified as true injuries, and 5% of patients with indeterminate BCVI developed cerebrovascular symptoms. In our experience, BCVI can always be graded on the Denver scale.
Table 4:
Denver grading system, corresponding CTA findings, and grade-based stroke incidence
| Grade of Injury | Denver Grading System31 | CTA Findings | Stroke Incidence (%)9,48 (CAI/VAI) |
|---|---|---|---|
| I | Irregularity of vessel wall | Nonstenotic luminal irregularity | |
| Dissection or IMH with <25% narrowing | Intimal flap or wall thickening with <25% stenosis | 3/6 | |
| II | Intraluminal thrombus | Luminal hypodensity | |
| Dissection or IMH with >25% narrowing | Intimal flap or wall thickening with >25% stenosis | 14/38 | |
| III | Pseudoaneurysm | Eccentric contrast-filled outpouching limited by periarterial tissue | 26/27 |
| IV | Occlusion | Lack of any intraluminal enhancement | 50/28 |
| Carotid occlusions: abrupt or tapered | |||
| Vertebral occlusion: usually abrupt | |||
| V | Transection | Irregular extravascular collection of contrast, not limited by periarterial tissue | 100/100 |
| Increases in density on delayed images, if obtained |
Note:—CAI indicates carotid artery injury; VAI, vertebral artery injury; IMH, intramural hematoma.
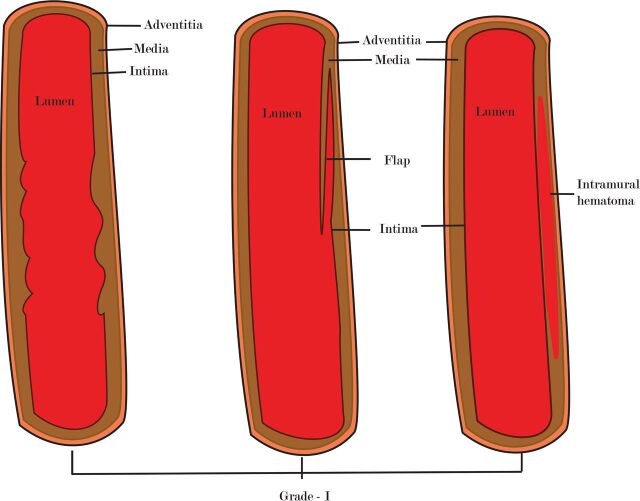
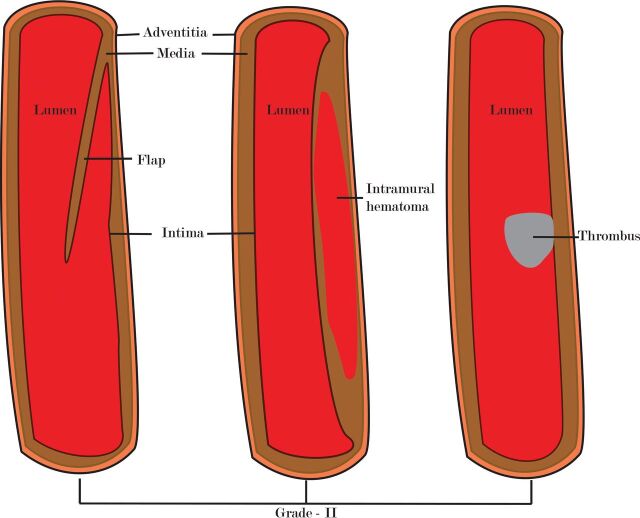
Grade I injury (luminal irregularity with <25% narrowing, Figs 1 and 2) is often subtle, non-flow-limiting, and better appreciated with multiplanar or volume-rendered images.6,24 CTA in patients with grade II injury (intraluminal thrombus, raised intimal flap, or dissection/intramural hematoma with >25% narrowing, Figs 3 and 4) may reveal crescentic wall thickening of variable length, luminal narrowing, or the presence of a dissection flap. On MR imaging, the visualization of an intramural hematoma depends on the stage of hemoglobin degradation, most apparent in the subacute stage when it has high signal on T1 images. Because the hematomas may be isointense in the acute (<7 days) and chronic (>2 months) phase, they may be missed.24 Grade III injury (pseudoaneurysm, Fig 5) is seen as saccular outpouchings of variable size. The imaging appearance of grade IV injury (occlusion of the vessel) may vary. Occlusion is often abrupt in the vertebral circulation (Fig 6), whereas they are often long segments with gradual tapering in the carotid arteries (Fig 7).6,42 Patients with grade V injury (vessel transection, Fig 8) may have contrast extravasation into the surrounding soft tissues or adjacent vein (giving rise to an arteriovenous fistula). Although the exact site of the fistula may not be apparent on imaging, the presence of early draining veins or an increase in the size of the draining veins is useful for detecting its presence.6
Fig 1.
A diagram showing grade I injury patterns.
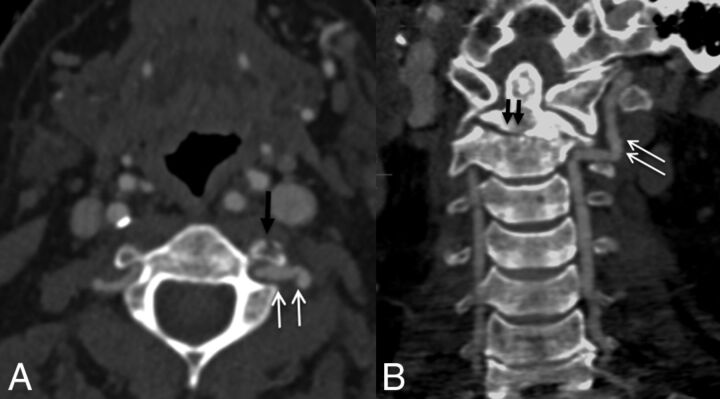
Fig 2.
A 42-year-old man with a fall from a height. CTA shows a C2 transverse foramina fracture (black arrow in A) and type 3 dens fracture (black arrows in B) with irregularity of the left vertebral artery (white arrows), grade I injury.
Fig 3.
A diagram showing grade II injury patterns.
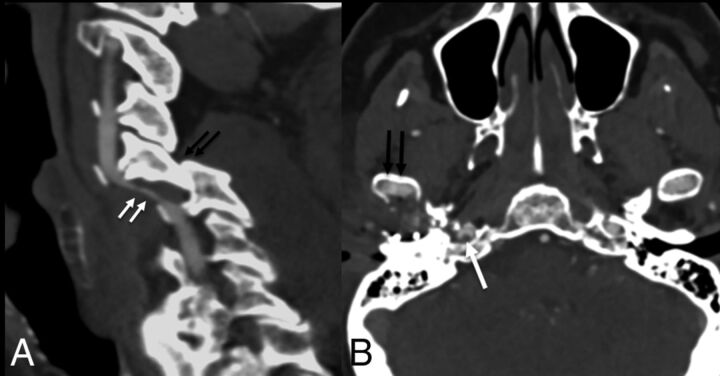
Fig 4.
Two patients after motor vehicle collisions and grade II injuries. Sagittal CTA (A) shows locked C5–6 facets (black arrows), with intramural hematoma of the left vertebral artery causing >50% narrowing (white arrows). Axial CTA (B) shows a right mandibular condyle fracture (black arrows) with nonocclusive thrombus (white arrow) in the right internal carotid artery.
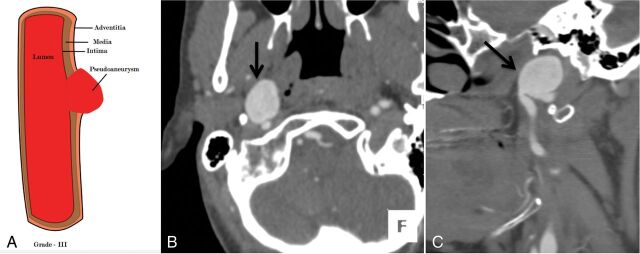
Fig 5.
A 36-year-old man with a fall from a height and a C6 fracture (not shown). An illustrative diagram (A) shows grade III injury. CTA axial (B) and sagittal (C) images show a focal outpouching of the anterior wall of the distal cervical right ICA (black arrow), suggesting a pseudoaneurysm, grade III injury. Case courtesy Sachin S. Saboo, MD, UT Southwestern Medical Center, Dallas, Texas.
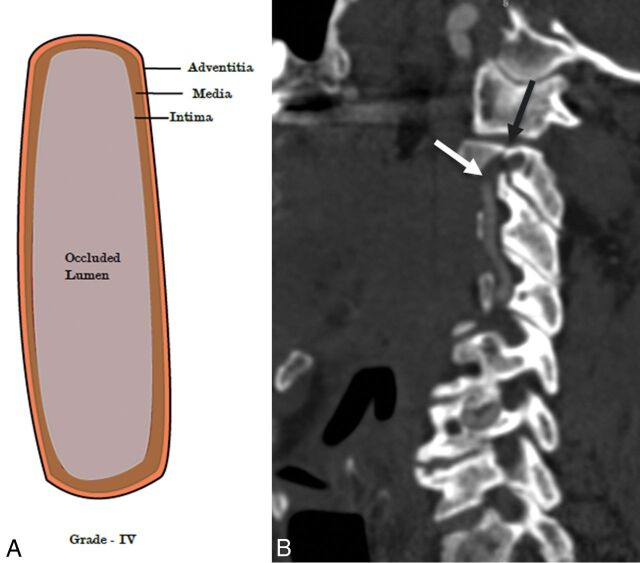
Fig 6.
A 64-year-old man with blunt trauma to the neck. An illustrative diagram (A) shows grade IV injury. CTA sagittal image (B) shows a fracture of the C2 transverse foramina (black arrow) with abrupt occlusion of the right vertebral artery (white arrow), grade IV injury.
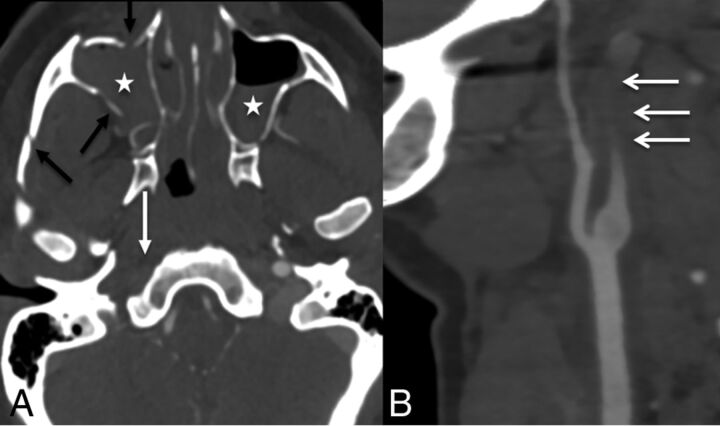
Fig 7.
A 36-year-old woman after a motor vehicle collision with facial fractures. CTA axial (A) and sagittal (B) images show fractures of the zygomatic arch and maxilla (black arrows) with hemosinus (stars) and tapered occlusion of the right ICA (white arrows), grade IV injury.
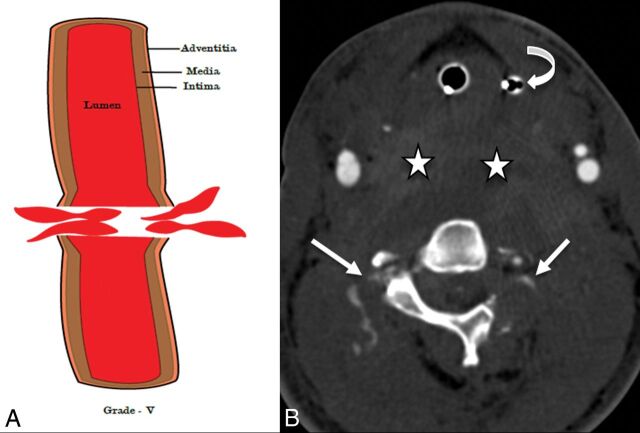
Fig 8.
A 28-year-old man after a motor vehicle collision. An illustrative diagram (A) shows a grade V injury. CTA axial image (B) shows transection of the bilateral vertebral arteries with active contrast extravasation (white arrows), grade V injury. Also, note the large prevertebral neck hematoma (stars) displacing the orogastric tube (curved arrow) anteriorly. The patient also sustained C1 and C2 fractures (not shown).
The potential mimics of BCVI on imaging include atherosclerosis (seen as wall thickening and/or stenosis), fibromuscular dysplasia (seen as wall irregularity), a hypoplastic vertebral artery (seen as long-segment narrowing), an acutely looped cervical internal carotid artery (mimics a pseudoaneurysm), or early venous enhancement (may mimic an AVF).
Management and Follow-Up of BCVI
There is a dearth of controlled trials to help guide treatment of BCVI, but current guidelines (EAST and Western Trauma Association)13,14 suggest observation, surgical repair, antithrombotic therapy (ATT), and endovascular therapy as acceptable management strategies, based on injury location and grade and patient symptoms. If there is no contraindication (such as active bleeding), ATT is indicated for all patients because it lowers the chance of stroke to <1%.4,10,14,48 The guidelines13,14 recommend the use of weight-based unfractionated heparin (10 U/Kg/h without a bolus and a low activated partial thromboplastin time goal of 40–50 seconds) because it is reversible compared with antiplatelets. The antiplatelet agents (clopidogrel, 75 mg daily, or aspirin, 325 mg daily) are equally as efficacious as heparin for stroke prevention10,49 and can be used in the acute setting if the patient has contraindications for heparin. The use of dual antiplatelets is not indicated and may increase the chance of bleeding. Earlier studies suggested that heparin could be transitioned to warfarin, but more recent studies have preferred antiplatelets for the long term due to a better safety profile, equivalent efficiency, and lower cost.10,14 Nearly one-third of patients have an initial contraindication to ATT, with concomitant traumatic brain or solid organ injury being the most common.37 A recent study50 showed that initiation of ATT at a median hospital day 3 in 119 patients with BCVI did not worsen traumatic brain or solid organ injury above baseline. A multidisciplinary team is valuable for open communication and discussion on antiplatelet and anticoagulant treatment in these patients, because these decisions can also be complex due to frequently associated intracranial and extracranial injuries.
While the ATT may suffice for grade I injuries because there is no flow-limiting potential, patients with grade II may need surgical or endovascular management if there is progression at follow-up (typically at 7–10 days). Grade III–V injuries are preferably repaired surgically or treated endovascularly (more common because most injuries are not surgically accessible). In grade V injuries (active bleeding), the guidelines13,14 suggest immediate attempts to control bleeding (direct pressure and emergent intervention/surgery). The management and follow-up of BCVI, depending on the injury grade, suggested by the Denver group4 is highlighted in Table 5. A recent multicenter study on the treatment and outcome of BCVI in the pediatric population showed a benefit of ATT with treatment approach similar to adults.51 The study, however, highlighted the lack of consensus for treatment among different institutions.
Table 5:
Grade-based treatment, follow-up, and suggested management of BCVI
| Injury Grade | Initial Treatment | Surgical or Endovascular Treatment | Follow-Upa | Long-Term Treatment |
|---|---|---|---|---|
| I | Antithrombotic therapyb (preferably unfractionated heparin) or observation (rarely used) | Not needed | 7–10 days, then 3–6 mo until healed | Antiplatelet therapy until healing |
| II | Antithrombotic therapyb | Needed if neurologic symptoms or progression of dissection | 7–10 days, then 3–6 mo (until healed or definitive management) | Long-term antiplatelet therapy until healing or definitive interventional or surgical treatment |
| III | Needed if symptomatic or size >1 cm | 7–10 days and then 3–6 mo or based on symptoms | Long-term antiplatelet therapy until healing or definitive treatment | |
| IV | Typically not beneficial | Based on symptoms | Life-long antiplatelets | |
| V | Direct pressure on actively bleeding area | Emergent intervention/surgery | Based on symptoms | No data (symptomatic) |
Follow-up for asymptomatic carotid cavernous injury is 3–4 weeks.
Unless contraindicated.
The Denver group4 and the current guidelines13,14 suggest follow-up of medically managed injuries (grades I, II, and sometimes III) at 7–10 days. If repeat imaging at this time reveals resolution of imaging findings, the therapy may be discontinued. If findings persist, the ATT may be further continued for 3–6 months, and the patients reimaged to determine a further course of action. In case of worsening symptomatology or worsening imaging findings at 7–10 days, patients may be treated with an endovascular approach. The follow-up of patients managed by surgical or endovascular treatment is symptom-based and is usually decided on a case-by-case basis. The most recent expanded Denver BCVI screening criteria and management guideline4 are highlighted in the On-line Figure.
Conclusions
BCVIs are rare, but the incidence is increasing due to increasing high-velocity trauma, screening, and universal availability of CT. If undiagnosed, these can cause adverse neurologic outcome or death. Hence, the adoption of institutional screening criteria is suggested. Multiple screening criteria are available; however, the Modified Denver Criteria are the most well-studied. The Utah Score has been suggested for the pediatric population and was recently validated in a multicenter study; however, current trauma guidelines (the most recent being the EAST guidelines published in 2010) recommend the use of adult screening criteria. There is a need for randomized trials for establishment of screening criteria and treatment for adults and the pediatric population. Also, a radiologist should be aware of not only the imaging appearance but also the high-risk injury patterns because in these cases, the recommendation for evaluation with CTA should come from the radiologist. Familiarity with the injury grading scale is also needed for standardized reporting, to help trauma teams provide uniform definitive treatment based on the current standard of care.
Supplementary Material
ABBREVIATIONS:
- ATT
antithrombotic therapy
- BCVI
blunt cerebrovascular injury
- EAST
Eastern Association for the Surgery of Trauma
Footnotes
Disclosures: Colin Derdeyn—UNRELATED: Stock/Stock Options: Pulse Therapeutics, Comments: novel stroke treatment device*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Bayer AG, Comments: stroke lectures in Japan.* Dionne Skeete—UNRELATED: Other: Iowa Department of Public Health, American College of Surgeons, Comments: trauma center reviewer for the State of Iowa and the American College of Surgeons. There is a contract with the University of Iowa with the above entities. Compensation is given directly to the University of Iowa.* *Money paid to the institution.
References
- 1. Davis JW, Holbrook TL, Hoyt DB, et al. Blunt carotid artery dissection: incidence, associated injuries, screening, and treatment. J Trauma 1990;30:1514–17 10.1097/00005373-199012000-00013 [DOI] [PubMed] [Google Scholar]
- 2. Mutze S, Rademacher G, Matthes G, et al. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology 2005;237:884–92 10.1148/radiol.2373042189 [DOI] [PubMed] [Google Scholar]
- 3. Berne JD, Reuland KS, Villarreal DH, et al. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma 2006;60:1204–09; discussion 1209–10 10.1097/01.ta.0000220435.55791.ce [DOI] [PubMed] [Google Scholar]
- 4. Geddes AE, Burlew CC, Wagenaar AE, et al. Expanded screening criteria for blunt cerebrovascular injury: a bigger impact than anticipated. Am J Surg 2016;212:1167–74 10.1016/j.amjsurg.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 5. Schneidereit NP, Simons R, Nicolaou S, et al. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma 2006;60:209–15; discussion 215–16 10.1097/01.ta.0000195651.60080.2c [DOI] [PubMed] [Google Scholar]
- 6. Sliker CW. Blunt cerebrovascular injuries: imaging with multidetector CT angiography. Radiographics 2008;28:1689–708; discussion 1709–10 10.1148/rg.286085521 [DOI] [PubMed] [Google Scholar]
- 7. Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg 1998;228:462–70 10.1097/00000658-199810000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiCocco JM, Fabian TC, Emmett KP, et al. Functional outcomes following blunt cerebrovascular injury. J Trauma Acute Care Surg 2013;74:955–60 10.1097/TA.0b013e318287800f [DOI] [PubMed] [Google Scholar]
- 9. Burlew CC, Biffl WL, Moore EE, et al. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg 2012;72:330–35; discussion 336–37, quiz 539 10.1097/TA.0b013e31823de8a0 [DOI] [PubMed] [Google Scholar]
- 10. Cothren CC, Biffl WL, Moore EE, et al. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg 2009;144:685–90 10.1001/archsurg.2009.111 [DOI] [PubMed] [Google Scholar]
- 11. Bruns BR, Tesoriero R, Kufera J, et al. Blunt cerebrovascular injury screening guidelines: what are we willing to miss? J Trauma Acute Care Surg 2014;76:691–95 10.1097/TA.0b013e3182ab1b4d [DOI] [PubMed] [Google Scholar]
- 12. Eastman AL, Chason DP, Perez CL, et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma 2006;60:925–29; discussion 929 10.1097/01.ta.0000197479.28714.62 [DOI] [PubMed] [Google Scholar]
- 13. Bromberg WJ, Collier BC, Diebel LN, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma 2010;68:471–77 10.1097/TA.0b013e3181cb43da [DOI] [PubMed] [Google Scholar]
- 14. Biffl WL, Cothren CC, Moore EE, et al. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma 2009;67:1150–53 10.1097/TA.0b013e3181c1c1d6 [DOI] [PubMed] [Google Scholar]
- 15. Cothren CC, Moore EE, Ray CE Jr, et al. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery 2007;141:76–82 10.1016/j.surg.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 16. Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 2002;236:386–93; discussion 393–95 10.1097/00000658-200209000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buch K, Nguyen T, Mahoney E, et al. Association between cervical spine and skull-base fractures and blunt cerebrovascular injury. Eur Radiol 2016;26:524–31 10.1007/s00330-015-3858-1 [DOI] [PubMed] [Google Scholar]
- 18. Azarakhsh N, Grimes S, Notrica DM, et al. Blunt cerebrovascular injury in children: underreported or underrecognized?: a multicenter ATOMAC study. J Trauma Acute Care Surg 2013;75:1006–11; discussion 1011–12 10.1097/TA.0b013e31829d3526 [DOI] [PubMed] [Google Scholar]
- 19. Kopelman TR, Berardoni NE, O'Neill PJ, et al. Risk factors for blunt cerebrovascular injury in children: do they mimic those seen in adults? J Trauma 2011;71:559–64; discussion 564 10.1097/TA.0b013e318226eadd [DOI] [PubMed] [Google Scholar]
- 20. Lew SM, Frumiento C, Wald SL. Pediatric blunt carotid injury: a review of the National Pediatric Trauma Registry. Pediatr Neurosurg 1999;30:239–44 10.1159/000028804 [DOI] [PubMed] [Google Scholar]
- 21. Jones TS, Burlew CC, Kornblith LZ, et al. Blunt cerebrovascular injuries in the child. Am J Surg 2012;204:7–10 10.1016/j.amjsurg.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 22. Ravindra VM, Riva-Cambrin J, Sivakumar W, et al. Risk factors for traumatic blunt cerebrovascular injury diagnosed by computed tomography angiography in the pediatric population: a retrospective cohort study. J Neurosurg Pediatr 2015;15:599–606 10.3171/2014.11.PEDS14397 [DOI] [PubMed] [Google Scholar]
- 23. Wu X, Malhotra A, Forman HP, et al. The use of high-risk criteria in screening patients for blunt cerebrovascular injury: a survey. Acad Radiol 2017;24:456–61 10.1016/j.acra.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 24. Nace SR, Gentry LR. Cerebrovascular trauma. Neuroimaging Clin N Am 2014;24:487–511, viii 10.1016/j.nic.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 25. Crissey MM, Bernstein EF. Delayed presentation of carotid intimal tear following blunt craniocervical trauma. Surgery 1974;75:543–49 [PubMed] [Google Scholar]
- 26. Arthurs ZM, Starnes BW. Blunt carotid and vertebral artery injuries. Injury 2008;39:1232–41 10.1016/j.injury.2008.02.042 [DOI] [PubMed] [Google Scholar]
- 27. Kang SY, Lin EM, Marentette LJ. Importance of complete pterygomaxillary separation in the Le Fort I osteotomy: an anatomic report. Skull Base 2009;19:273–77 10.1055/s-0029-1220198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold M, Bousser MG, Fahrni G, et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke 2006;37:2499–503 10.1161/01.STR.0000240493.88473.39 [DOI] [PubMed] [Google Scholar]
- 29. Moar JJ. Traumatic rupture of the cervical carotid arteries: an autopsy and histopathological study of 200 cases. Forensic Sci Int 1987;34:227–44 10.1016/0379-0738(87)90036-3 [DOI] [PubMed] [Google Scholar]
- 30. Biffl WL, Moore EE, Elliott JP, et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg 2000;231:672–81 10.1097/00000658-200005000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 1999;47:845–53 10.1097/00005373-199911000-00004 [DOI] [PubMed] [Google Scholar]
- 32. Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid and vertebral arterial injuries. World J Surg 2001;25:1036–43 10.1007/s00268-001-0056-x [DOI] [PubMed] [Google Scholar]
- 33. Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 2009;8:668–78 10.1016/S1474-4422(09)70084-5 [DOI] [PubMed] [Google Scholar]
- 34. Esnault P, Cardinale M, Boret H, et al. Blunt cerebrovascular injuries in severe traumatic brain injury: incidence, risk factors, and evolution. J Neurosurg 2017;127:16–22 10.3171/2016.4.JNS152600 [DOI] [PubMed] [Google Scholar]
- 35. Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg 1999;178:517–22 10.1016/S0002-9610(99)00245-7 [DOI] [PubMed] [Google Scholar]
- 36. Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma 2001;51:279–85; discussion 85–86 10.1097/00005373-200108000-00009 [DOI] [PubMed] [Google Scholar]
- 37. Stein DM, Boswell S, Sliker CW, et al. Blunt cerebrovascular injuries: does treatment always matter? J Trauma 2009;66:132–43; discussion 143–44 10.1097/TA.0b013e318142d146 [DOI] [PubMed] [Google Scholar]
- 38. Emmett KP, Fabian TC, DiCocco JM, et al. Improving the screening criteria for blunt cerebrovascular injury: the appropriate role for computed tomography angiography. J Trauma 2011;70:1058–63; discussion 1063–65 10.1097/TA.0b013e318213f849 [DOI] [PubMed] [Google Scholar]
- 39. Sarkar K, Keachie K, Nguyen U, et al. Computed tomography characteristics in pediatric versus adult traumatic brain injury. J Neurosurg Pediatr 2014;13:307–14 10.3171/2013.12.PEDS13223 [DOI] [PubMed] [Google Scholar]
- 40. Ravindra VM, Bollo RJ, Sivakumar W, et al. Predicting blunt cerebrovascular injury in pediatric trauma: validation of the “Utah Score.” J Neurotrauma 2017;34:391–99 10.1089/neu.2016.4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Biffl WL, Ray CE Jr, Moore EE, et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma 2002;53:850–56 10.1097/00005373-200211000-00008 [DOI] [PubMed] [Google Scholar]
- 42. Liang T, Plaa N, Tashakkor AY, et al. Imaging of blunt cerebrovascular injuries. Semin Roentgenol 2012;47:306–19 10.1053/j.ro.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 43. Nagpal P, Mullan BF, Sen I, et al. Advances in imaging and management trends of traumatic aortic injuries. Cardiovasc Intervent Radiol 2017;40:643–54 10.1007/s00270-017-1572-x [DOI] [PubMed] [Google Scholar]
- 44. Sakurai K, Miura T, Sagisaka T, et al. Evaluation of luminal and vessel wall abnormalities in subacute and other stages of intracranial vertebrobasilar artery dissections using the volume isotropic turbo-spin-echo acquisition (VISTA) sequence: a preliminary study. J Neuroradiol 2013;40:19–28 10.1016/j.neurad.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 45. Hunter MA, Santosh C, Teasdale E, et al. High-resolution double inversion recovery black-blood imaging of cervical artery dissection using 3T MR imaging. AJNR Am J Neuroradiol 2012;33:E133–37 10.3174/ajnr.A2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagpal P, Khandelwal A, Saboo SS, et al. Modern imaging techniques: applications in the management of acute aortic pathologies. Postgrad Med J 2015;91:449–62 10.1136/postgradmedj-2014-133178 [DOI] [PubMed] [Google Scholar]
- 47. Crawford JD, Allan KM, Patel KU, et al. The natural history of indeterminate blunt cerebrovascular injury. JAMA Surg 2015;150:841–47 10.1001/jamasurg.2015.1692 [DOI] [PubMed] [Google Scholar]
- 48. Biffl WL, Ray CE Jr, Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg 2002;235:699–706; discussion 706–07 10.1097/00000658-200205000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Edwards NM, Fabian TC, Claridge JA, et al. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg 2007;204:1007–13; discussion 1014–15 10.1016/j.jamcollsurg.2006.12.041 [DOI] [PubMed] [Google Scholar]
- 50. Shahan CP, Magnotti LJ, McBeth PB, et al. Early antithrombotic therapy is safe and effective in patients with blunt cerebrovascular injury and solid organ injury or traumatic brain injury. J Trauma Acute Care Surg 2016;81:173–77 10.1097/TA.0000000000001058 [DOI] [PubMed] [Google Scholar]
- 51. Dewan MC, Ravindra VM, Gannon S, et al. Treatment practices and outcomes after blunt cerebrovascular injury in children. Neurosurgery 2016;79:872–78 10.1227/NEU.0000000000001352 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.