Abstract
BACKGROUND AND PURPOSE:
Patients with multiple sclerosis routinely have MR imaging with contrast every 6–12 months to assess response to medication. Multiple recent studies provide evidence of tissue deposition of MR imaging contrast agents, questioning the long-term safety of these agents. The goal of this retrospective image-analysis study was to determine whether contrast could be reserved for only those patients who show new MS lesions on follow-up examinations.
MATERIALS AND METHODS:
We retrospectively reviewed brain MRIs of 138 patients. To increase our sensitivity, we used a previously described computerized image-comparison software to evaluate the stability or progression of multiple sclerosis white matter lesions in noncontrast FLAIR sequences. We correlated these findings with evidence of contrast-enhancing lesions on the enhanced T1 sequence from the same scan.
RESULTS:
Thirty-three scans showed an increase in white matter lesion burden. Among those 33 patients, 14 examinations also demonstrated enhancing new lesions. While we found a single example of enhancement of a pre-existing white matter lesion that appeared unchanged in size, that same examination showed an overall increase in lesion burden with enhancement of other, new lesions. Thus, we found that all patients with enhancing lesions had evidence of progression on their noncontrast imaging.
CONCLUSIONS:
Because all enhancing lesions were associated with new lesions on unenhanced imaging and progression was only evident in 24% of patients, in patients with relapsing-remitting MS, it is reasonable to consider reserving contrast for only those patients with evidence of progression on noncontrast MR images.
Multiple sclerosis is a nervous system disease caused by immune-mediated myelin loss with an unpredictable course, usually characterized by clinical remission, relapse, and progression. Symptoms frequently correlate with a change in the number or volume of MS lesions in the central nervous system.1 Serial clinical assessments have underestimated disease activity and lesion burden when correlated with findings on serial brain MR imaging.2 According to the guidelines outlined in the Standardized MR Imaging Protocol for Multiple Sclerosis,3 MR imaging using standard FLAIR, T1-weighted, and T2-weighted pulse sequences with and without injection of gadolinium-based contrast agents (GBCA) is currently the preferred technique for the long-term monitoring of patients with MS.
Contrast-enhancing lesions indicate that there is acute active inflammatory disease and subsequent blood-brain barrier disruption,4 while noncontrast images identify progression of lesion burden. It has already been shown that new lesions typically enhance after administration of gadolinium-based contrast agents for approximately 3–4 weeks after their development,5 and after the resolution of enhancement, these lesions are radiologically defined as chronic.6 Although pathologic specimens have also shown evidence of perivascular inflammatory infiltrates in chronic lesions in patients with MS, the blood-brain barrier either remains intact or is only minimally damaged; therefore, the lesion no longer enhances on MR imaging.7 There has been recent evidence that noncontrast, susceptibility-based imaging can be used to detect a paramagnetic rim representing the presence of macrophages and other paramagnetic inflammatory species indicative of ongoing inflammation in some of these chronic lesions. However, these studies all used high-resolution imaging with a 7T scanner, which currently is primarily limited to research protocols.8–15
Considerable histopathologic evidence has linked leptomeningeal inflammation with subpial cortical demyelination in patients with MS.16–21 While cortical lesions have been difficult to detect on in vivo MR imaging,22 recent studies have shown foci of leptomeningeal enhancement in patients with MS using specific delayed-acquisition postcontrast T2 FLAIR sequences.23,24 These findings have also been correlated with cortical volume loss.25 However, leptomeningeal enhancement has been shown to be unrelated to white matter lesion load and enhancement,24 and the same study showed that 85% of foci of leptomeningeal enhancement persisted on follow-up with no change in morphology, shape, or size, despite the patient receiving disease-modifying therapy. While cortical volume loss and leptomeningeal enhancement are promising new biomarkers in understanding the progression and pathophysiology of this disease, they do not provide immediate information on response to currently available treatments, and MR imaging pulse sequences used for their detection and quantification are not routinely used in clinical practice.
Currently, the consensus is that follow-up scans can be ordered when clinically indicated3; however, the standard of care followed in many large MS centers is to acquire regular, scheduled enhanced MR imaging. The intent is not only to analyze treatment effectiveness but also provide evidence for treatment decisions because these scans may reveal contrast-enhancing lesions in the absence of clinical symptoms.26 These routine contrast-enhanced studies are performed as often as every 6 months.5
MS typically presents in young adults between 20 and 50 years of age, with a peak onset around 30 years of age27 and the median time to death of approximately 30 years from disease onset.28 This means that in their lifetimes, patients with MS on a 6-month imaging schedule will have ≥60 contrast-enhanced MR images.
While there exists an accepted literature on gadolinium risk for patients with severely impaired renal function, there is now emerging evidence that retained intracranial gadolinium-related deposits appear after intravenous administration of GBCA in patients with normal renal function after contrast-enhanced MR imaging. This finding suggests that we explore avoiding unnecessary the use of MR imaging contrast agents. In light of recent studies that have shown that gadolinium tissue deposition takes place even in those without intracranial abnormalities and with normal renal function,29–33 the FDA has recently issued a warning precaution on all GBCA.34 Although we await the results of systematic studies to better characterize the nature of this tissue deposition from the use of GBCA and, more importantly, to determine whether it results in adverse effects, it seems prudent, in the meantime, to limit their use and develop new strategies to monitor disease progression in this potentially vulnerable population of patients with MS. One such proposed strategy is the use of additional noncontrast MR imaging techniques, such as DTI-based fractional anisotropy, which has been shown to be helpful in detection of MS lesion acuity without gadolinium.35
To a similar end, we designed the current study to determine whether the nonenhanced MR imaging is reliable for selecting only those patients who need additional imaging with contrast when characterizing progression of MS. As previously mentioned, new lesions typically only enhance for approximately 3–4 weeks after their development. Therefore, even with imaging intervals as short as every 6 months, all new, enhancing lesions that develop in that timeframe are not captured.5 Also, enhancing lesions are not sufficiently sensitive for a sole measure of disease activity.36
We wondered, then, whether the findings on nonenhanced MS follow-up MR images could be used as a reliable predictor for the presence of enhancing lesions. If this were established with enough certainty, it would offer patients an option of forgoing gadolinium-based contrast agent administration in specific situations. While both enhanced and unenhanced sequences have proved useful together in maximizing the sensitivity and reliability in detecting a change in the stable disease state versus progression, we designed this study to examine whether there could be enhancing white matter lesions in patients when the unenhanced FLAIR sequence is stable—that is, no evidence of new or enlarging existing lesions. While we recognize that standard FLAIR imaging is imperfect for the detection of lesions, we re-examined this question using a 3D-FLAIR sequence along with computer-aided detection software, which we find increases the sensitivity of FLAIR to new lesions.37
MATERIALS and Methods
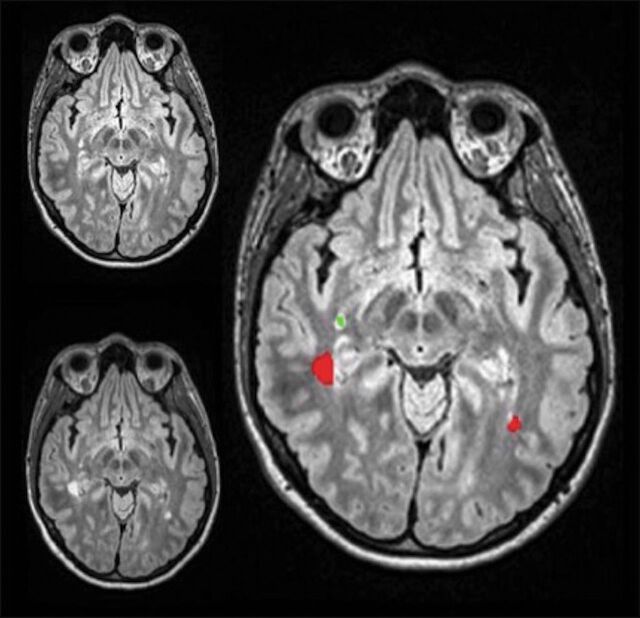
After receiving institutional review board approval for the study, a list of 197 sequential, unique identifying numbers of follow-up brain MR images was generated from September 19, 2017, to October 31, 2017. All cases were available on our PACS for review, and the list contained cases that were imaged with MR imaging using a specific, proprietary multisequence MS protocol that was performed on one of several of our 3T scanners, Siemens Verio, Skyra, and Trio (Siemens, Erlangen, Germany). Our department 3D lab then processed the MR images obtained in this fashion using an in-house computer-assisted detection software system.17 This program takes the 3D FLAIR images from the current and prior studies and applies an imaging-processing pipeline, including coregistration, skull stripping, and inhomogeneity correction. Then the program computes both forward and backward differences between the 2 time point datasets and subsequently creates composite images that display the coregistered current and prior studies, as well as a third image that shows new MS lesions in red and resolving lesions in green (Fig 1). The software is routinely run by our departmental 3D lab, and this program then sends the DICOM image files directly back to the PACS.
Fig 1.
Prior FLAIR MR imaging in the upper left-hand corner, current FLAIR MR imaging in the lower left-hand corner, and the coregistered composite image on the right displaying new lesions in red, while lesions that regressed are green.
While this program has not received a formal FDA certification, the paired, matching source multiplanar reformats from each of the 3D-FLAIR scans are available to imagers at the time of interpretation along with the computer-processed composite images indicating change. These composite images together with the source images have been found helpful in clinical practice for identifying new lesions, particularly among confluent lesions, which are then confirmed or refuted on the source imaging. However, these synthetic composite images are not used in isolation for establishing the diagnosis.
Of the 197 patients imaged with our MS protocol, 53 were excluded because they did not have any prior studies or the prior examination was not acquired with the necessary 3D-FLAIR sequence used for image analysis, usually because the previous examinations were performed at an outside institution. An additional 2 patients were excluded because they did not receive or declined contrast for their scan. Another 2 were excluded due to nondiagnostic imaging or the absence of critical sequences. We eliminated, in total, 57 cases from consideration for these reasons.
The composite images of the remaining 140 patients were then reviewed independently by 2 investigators to determine the lesion burden of white matter lesions on the most recent scan compared with the prior one. This was characterized in terms of increase, decrease, or stability on the basis of the nonenhanced FLAIR sequence, often supported by proton-density sequences. The source images used to create the composite images were reviewed at the same time by the investigators to validate the computer-generated markings because artifactual lesions in the posterior fossa and parasellar region are commonplace. Then each of the investigators recorded their decision regarding lesion enhancement based on the contrast-enhanced T1-weighted sequence. The presence of any parenchymal enhancing lesions, with or without changes on the FLAIR scan, were recorded. The imaging findings of each of the study observers were then correlated with the report generated by the attending radiologist at the time of the initial interpretation, and agreement or differences with the final report were recorded.
The independent assessments of the 2 investigators were recorded on an Excel (Microsoft, Redmond, Washington) spreadsheet, and their findings for all variables were compared to determine whether there were any differences. Sixteen cases were identified with differences between the observers' findings or that were discordant with the initial report. These were then reviewed by a third more senior investigator (a neurologist with a Certificate of Added Qualification with 27 years' experience) to resolve any differences. The senior investigator agreed 14 times with investigator A and 2 times with investigator B.
Among these 140 cases, 3 were identified in which there was enhancement of a pre-existing lesion. All of these were also reviewed by the third, more senior investigator. Two of the 3 cases were excluded from the study because the findings indicated a pathologic process other than multiple sclerosis. In one case, the findings were most consistent with progressive multifocal leukoencephalopathy, and in the second, the findings were consistent with a low-grade brain tumor based on prolonged mass effect and persistent, stable enhancement.
Of the final 138 patients included in the data analysis, 39 were men and 99 were women, and the entire cohort had a mean age of 49.1 ± 13.3 years. Forty-one patients had specific mention of a clinical diagnosis of a certain type of MS in the electronic medical record at some point in their history. Four had secondary-progressive MS, 3 had possible primary-progressive MS, 33 had relapsing-remitting MS (RRMS), and one was thought to have secondary-progressive MS or RRMS. For the remaining 97 patients, we could not find a specific classification of MS mentioned in the clinical record. We assume that the overwhelming majority of these remaining patients had RRMS, in keeping with the expectation that 85% of patients will meet the criteria for this diagnosis.38
Data on 133 patients regarding whether they were on immune-modulating therapy was collected. Of these patients, 82 were on immune-modulating therapy at the time of their most recent scan (Table).
Patients on IMT at the time of their most recent scan
| Type of IMT | No. of Patients |
|---|---|
| Fingolimod | 11 |
| Glatiramer acetate | 36 |
| Interferon β-1a | 23 |
| Ocrelizumab | 1 |
| Dimethyl fumarate | 1 |
| Teriflunomide | 9 |
| Natalizumab | 1 |
Note:—IMT indicates immune-modulating therapy.
Results
Thirty-three scans (24%) showed an increase in white matter lesion burden, 102 scans (74%) showed stable lesion burden, and 3 scans (2%) showed a decrease in lesion burden. Of the scans that showed an increased lesion burden, 31 demonstrated new discrete lesions, while 2 demonstrated only enlargement of pre-existing lesions.
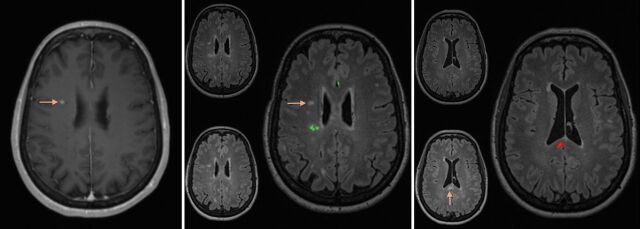
Fourteen scans (10%) demonstrated enhancing new lesions, all of which also demonstrated an increase in lesion burden. In 1 patient, there was enhancement of a single pre-existing lesion that appeared unchanged in size, but the same scan showed an overall increase in lesion burden with enhancement of other, new lesions (Fig 2). In short, we could not find, among the 138 follow-up studies reviewed, a single case that demonstrated abnormal parenchymal enhancement without increased lesion burden.
Fig 2.
Left: Contrast-enhanced T1 MR imaging shows enhancement of a pre-existing lesion in the right centrum semiovale (arrow). Middle: The composite image from the coregistering software shows no growth of this corresponding lesion on FLAIR between the prior and current study (arrow). Additionally, 1 lesion had slightly regressed in the right posterior corona radiata (green on the composite image). Right: The composite image using the coregistering software at a more inferior level shows evidence of a new lesion in the splenium of the corpus callosum (arrow and red on composite image on the right).
Of note, in the 1 instance of enhancement of a pre-existing lesion, no specific phenotype of MS was indicated in the clinical history.
Discussion
While prudent use of contrast is a cornerstone of practice for every responsible radiology department, this still allows considerable latitude when risk is perceived as nearly nonexistent and the expected small gains in sensitivity support its use. With the emerging evidence of T1 shortening in brain tissue linked to contrast administration and recent FDA warnings regarding the use of MR imaging contrast, it is an appropriate time to consider an evidence-based approach to the use of MR imaging contrast in common clinical situations. In addition, there is also a responsibility to minimize cost because contrast agents add to the examination cost due to the price of the agent itself and the added time for administration, image generation, and interpretation.
We decided to focus on the imaging of patients with multiple sclerosis because their exposure to contrast agents can be substantial because their imaging often starts at a young age and continues for decades. While there have been a few other studies examining FLAIR signal characteristics of specific, individual lesions in patients with MS and their prediction of lesion enhancement, none of these studies examine our specific question regarding the relationship of enhancement based on each patient's change in the entire white matter lesion load.39,40
On the basis of the results in our patient group reported here, enhancing lesions were found in 10% of the follow-up scans. This is well within the expected range for our patients based on clinical experience. Lesion burden progression was evident in 24% of our subjects, also not surprising on the basis of clinical experience, and all patients with enhancing lesions also had evidence of progression.
When considered from the perspective of minimizing contrast use, one could argue that contrast administration could be withheld in those patients with stable lesions on the basis of our results. Contrast could also be selectively administered to only those patients with evidence of progression on their noncontrast imaging. Additionally, this decision to administer contrast in those patients with progression may also be influenced by whether the additional findings of enhancing lesions would then alter the clinical management of the patient. In our experience, it is not currently feasible to complete the interpretation of the imaging with the processed imaging components while the patient waits in the scanner for a decision, so with this approach, patients would need to come back for their enhanced imaging.
Because our patient population consists almost entirely of patients with RRMS and there may be differences among the different phenotypes of MS, our findings may not be generalized to all types of MS. However, this approach could be reserved for patients with known RRMS, and that would still include >80% of patients with MS.
Adoption of this approach would then avoid unnecessary use of contrast. While we can only suggest this as a probability not proved by this study, it is likely that this proposed change in practice would provide a substantial cost savings to the health system and patients by shortening examination time, reducing examination complexity, and minimizing patients' risks. However, to make a more definitive claim regarding cost savings, it would be necessary to systematically quantify the true cost and complexity required for the remaining one-quarter of patients with evidence of lesion progression returning for contrast administration.
While this proposed approach may be somewhat novel, we believe these findings at least offer patients with RRMS a choice: completing the MR imaging examination with contrast in 1 session or imaging without contrast with the awareness that additional imaging may be necessary at a later date. This choice may be influenced by personal concern regarding receiving contrast, difficulty in returning for contrast imaging based on factors such as distance and work schedule, as well as obtaining insurance approval. However, one would think that some agreement could be reached with insurers based on the assurance that the overall number of enhanced studies and accompanying additional costs would be reduced.
While a larger study with more patients and a larger proportion of patients with progressive MS would be ideal, this study population, we believe, provides evidence that supports a larger-but-anecdotal clinical experience that enhancement is seen only with progression. The number of cases reviewed (i.e. >100) is in keeping with other studies published in the literature.41 Furthermore, there are precedents for the use of imaging with marginally lower sensitivity to minimize overall risk and cost such as the use of MRA rather than CTA for aneurysm screening in patients with a family history of aneurysms.
There were 2 cases in the study group that appeared to have a disease process in addition to MS. One had imaging findings of a brain stem tumor; the other had findings of progressive multifocal leukoencephalopathy, which rarely occurs in patients with MS treated with immune modulators. Although excluded from the analysis, with our proposed approach, both patients would have received contrast had they been imaged initially with noncontrast MR imaging because of the new white matter findings in one and unresolving mass effect in the second.
One additional limitation of this study is the nature of our postcontrast imaging. We do not routinely use pulse sequences that maximize visualization of contrast enhancement, such as magnetization transfer or fat-suppressed T1-weighted postcontrast imaging mainly due to our choice of imaging all these patients at 3T, because that allows generation of high-quality FLAIR volume images for the postprocessing software to improve detection of progression. We also recognize that this type of software is not available at most imaging centers, but there are other, similar products available,42 and we were intent on maximizing detection of progression for this study. We believe that this approach improves detection of new white matter lesions and may have contributed to our overall finding that enhancement of lesions was only evident when there was progression. However, we cannot say that careful review of FLAIR, proton-density, and T2-weighted scans performed in a consistent manner using landmarks determined from sagittal scans such as the anterior/posterior commissure line without computer processing would not have provided identical results.
Finally, we did not include spine imaging in this study and recognize that many sites, including our own, include cervical and thoracic imaging on routine follow-up. On the basis of our experience that enhancing cord lesions are much less common than brain lesions and that there is considerable variability in the quality of spinal cord imaging between examinations, we elected to focus on brain imaging to evaluate a substantial number of cases with enhancing lesions. It seems very likely that this same approach (ie, enhancement only for patients with progression) could be used for spinal cord imaging because the underlying biology of MS in the brain and cord should be comparable. Nevertheless, we did not directly examine that question in this study. We also did not include findings of leptomeningeal enhancement or cortical lesions because these require specialized imaging sequences that are not routine at our institution and their significance is still being evaluated.
Conclusions
In our study of patients with MS undergoing routine MR imaging using a 3D-FLAIR acquisition and processed images that highlight new lesions, we found that all cases with enhancing brain lesions had evidence of progression on the noncontrast imaging. Because progression was only evident in 24% of our cases, approximately three-quarters of the patients having follow-up scans did not benefit from contrast enhancement. We believe that this has implications regarding the need for contrast enhancement in all follow-up scans in this patient population.
ABBREVIATIONS:
- GBCA
gadolinium-based contrast agents
- RRMS
relapsing-remitting MS
References
- 1. Rovira À, Wattjes MP, Tintoré M, et al. ; MAGNIMS study group. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol 2015;11:471–82 10.1038/nrneurol.2015.106 [DOI] [PubMed] [Google Scholar]
- 2. Traboulsee A, Simon JH, Stone L, et al. . Revised recommendations of the Consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 2016;37:394–401 10.3174/ajnr.A4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simon JH, Li D, Traboulsee A, et al. . Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol 2006;27:455–61 [PMC free article] [PubMed] [Google Scholar]
- 4. Rovira A, Auger C, Alonso J. Magnetic resonance monitoring of lesion evolution in multiple sclerosis. Ther Adv Neurol Disord 2013;6:298–310 10.1177/1756285613484079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cotton F, Weiner HL, Jolesz FA, et al. . MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 2003;60:640–46 10.1212/01.WNL.0000046587.83503.1E [DOI] [PubMed] [Google Scholar]
- 6. Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol 2016;12:358–68 10.1038/nrneurol.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frischer JM, Bramow S, Dal-Bianco A, et al. . The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009;132(Pt 5):1175–89 10.1093/brain/awp070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond KE, Metcalf M, Carvajal L, et al. . Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol 2008;64:707–13 10.1002/ana.21582 [DOI] [PubMed] [Google Scholar]
- 9. Absinta M, Sati P, Gaitan MI, et al. . Seven-Tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol 2013;74:669–78 10.1002/ana.23959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta V, Pei W, Yang G, et al. . Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS One 2013;8:e57573 10.1371/journal.pone.0057573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pitt D, Boster A, Pei W, et al. . Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol 2010;67:812–18 10.1001/archneurol.2010.148 [DOI] [PubMed] [Google Scholar]
- 12. Bagnato F, Hametner S, Yao B, et al. . Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 2011;134:3602–15 10.1093/brain/awr278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bian W, Harter K, Hammond-Rosenbluth KE, et al. . A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler 2013;19:69–75 10.1177/1352458512447870 [DOI] [PubMed] [Google Scholar]
- 14. Yao B, Bagnato F, Matsuura E, et al. . Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology 2012;262:206–15 10.1148/radiol.11110601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagemeier J, Heininen-Brown M, Poloni GU, et al. . Iron deposition in multiple sclerosis lesions measured by susceptibility-weighted imaging filtered phase: a case control study. J Magn Reson Imaging 2012;36:73–83 10.1002/jmri.23603 [DOI] [PubMed] [Google Scholar]
- 16. Choi SR, Howell OW, Carassiti D, et al. . Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 2012;135(Pt 10):2925–37 10.1093/brain/aws189 [DOI] [PubMed] [Google Scholar]
- 17. Lucchinetti CF, Popescu BF, Bunyan RF, et al. . Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011;365:2188–97 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Popescu BF, Lucchinetti CF. Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol 2012;12:11 10.1186/1471-2377-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magliozzi R, Howell OW, Reeves C, et al. . A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010;68:477–93 10.1002/ana.22230 [DOI] [PubMed] [Google Scholar]
- 20. Magliozzi R, Howell O, Vora A, et al. . Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007;130(Pt 4):1089–104 [DOI] [PubMed] [Google Scholar]
- 21. Howell OW, Reeves CA, Nicholas R, et al. . Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011;134(Pt 9):2755–71 10.1093/brain/awr182 [DOI] [PubMed] [Google Scholar]
- 22. Daams M, Geurts JJ, Barkhof F. Cortical imaging in multiple sclerosis: recent findings and ‘grand challenges.’ Curr Opin Neurol 2013;26:345–52 10.1097/WCO.0b013e328362a864 [DOI] [PubMed] [Google Scholar]
- 23. Eisele P, Griebe M, Szabo K, et al. . Investigation of leptomeningeal enhancement in MS: a postcontrast FLAIR MRI study. Neurology 2015;84:770–75 10.1212/WNL.0000000000001286 [DOI] [PubMed] [Google Scholar]
- 24. Absinta M, Vuolo L, Rao A, et al. . Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015;85:18–28 10.1212/WNL.0000000000001587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zivadinov R, Ramasamy DP, Vaneckova M, et al. . Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: a retrospective, pilot, observational longitudinal study. Mult Scler 2017;23:1336–45 10.1177/1352458516678083 [DOI] [PubMed] [Google Scholar]
- 26. Miller DH, Barkhof F, Nauta JJ. Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain 1993;116:1077–94 10.1093/brain/116.5.1077 [DOI] [PubMed] [Google Scholar]
- 27. Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 2010;9:A387–94 10.1016/j.autrev.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 28. Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004;127(Pt 4):844–50 10.1093/brain/awh104 [DOI] [PubMed] [Google Scholar]
- 29. Kanda T, Ishii K, Kawaguchi H, et al. . High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–41 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 30. Kanda T, Fukusato T, Matsuda M, et al. . Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015;276:228–32 10.1148/radiol.2015142690 [DOI] [PubMed] [Google Scholar]
- 31. McDonald RJ, McDonald JS, Kallmes DF, et al. . Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–82 10.1148/radiol.15150025 [DOI] [PubMed] [Google Scholar]
- 32. Murata N, Gonzalez-Cuyar LF, Murata K, et al. . Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 2016;51:447–53 10.1097/RLI.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 33. McDonald RJ, McDonald JS, Kallmes DF, et al. . Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 2017;285:546–54 10.1148/radiol.2017161595 [DOI] [PubMed] [Google Scholar]
- 34. FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Accessed March 5, 2018.
- 35. Gupta A, Al-Dasuqi K, Xia F, et al. . The use of noncontrast quantitative MRI to detect gadolinium-enhancing multiple sclerosis brain lesions: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017;38:1317–22 10.3174/ajnr.A5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wattjes MP, Rovira À, Miller D, et al. ; MAGNIMS study group. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015;11:597–606 10.1038/nrneurol.2015.157 [DOI] [PubMed] [Google Scholar]
- 37. Bilello M, Arkuszewski M, Nucifora P, et al. . Multiple sclerosis: identification of temporal changes in brain lesions with computer-assisted detection software. Neuroradiol J 2013;26:143–50 10.1177/197140091302600202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol 2014;27:271–78 10.1097/WCO.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Treabă CA, Bălaşa R, Podeanu DM, et al. . Cerebral lesions of multiple sclerosis: is gadolinium always irreplaceable in assessing lesion activity? Diagn Interv Radiol 2014;20:178–84 10.5152/dir.2013.13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guranda M, Essig M, Poulin A, et al. . Fluid-attenuated inversion recovery signal intensity as a predictor of gadolinium enhancement in relapsing-remitting multiple sclerosis. Int J MS Care 2018;20:62–66 10.7224/1537-2073.2016-053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elster AD, Mirza W. MR imaging in chronic partial epilepsy: role of contrast enhancement. AJNR Am J Neuroradiol 1991;12:165–70 [PMC free article] [PubMed] [Google Scholar]
- 42. Dworkin JD, Linn KA, Oguz I, et al. ; North American Imaging in Multiple Sclerosis Cooperative. An automated statistical technique for counting distinct multiple sclerosis lesions. AJNR Am J Neuroradiol 2018;39:626–33 10.3174/ajnr.A5556 [DOI] [PMC free article] [PubMed] [Google Scholar]