Abstract
BACKGROUND AND PURPOSE:
Cortical lesions are common in multiple sclerosis and are included in the latest diagnostic criteria. The limited sensitivity of cortical MS lesions on conventional MR imaging can be improved by phase-sensitive inversion recovery. Synthetic MR imaging could provide phase-sensitive inversion recovery without additional scanning, but the use of synthetic phase-sensitive inversion recovery remains to be validated. We aimed to compare the ability and clinical value of detecting leukocortical lesions with conventional and synthetic phase-sensitive inversion recovery in MS.
MATERIALS AND METHODS:
Twenty-one patients with MS prospectively underwent conventional and synthetic phase-sensitive inversion recovery, 3D T1-weighted, and T2 FLAIR imaging. Two neuroradiologists independently performed blinded phase-sensitive inversion recovery lesion assessments; a consensus rating with all sequences was considered the criterion standard. Lesion volumes were segmented. All participants underwent standardized cognitive and physical examinations and Fatigue Severity Scale assessment. Results were analyzed with multiple linear regressions.
RESULTS:
Interrater and criterion standard agreement for leukocortical lesions was excellent for both conventional and synthetic phase-sensitive inversion recovery (intraclass correlation coefficient = 0.79–0.97). Leukocortical lesion volumes for both sequences were associated with lower information-processing speed (P ≤ .01) and verbal fluency (P ≤ .02). Both phase-sensitive inversion recovery sequences showed a positive effect on the association when combining volumes of leukocortical lesions and white matter lesions with information-processing speed (P ≤ .005) and verbal fluency (P ≤ .03). No associations were found between leukocortical lesion volumes and physical disability or fatigue.
CONCLUSIONS:
Synthetic and conventional phase-sensitive inversion recovery have a sensitivity similar to that of leukocortical MS lesions. The detected leukocortical lesions are associated with cognitive dysfunction and thus provide clinically relevant information, which encourages assessment of cortical MS involvement at conventional field strengths.
Multiple sclerosis is a chronic inflammatory and degenerative disease affecting the central nervous system and is the leading nontraumatic cause of neurologic disability in young adults.1 In recent years, there has been an increased awareness of the gray matter involvement in MS. Cortical MS lesions are closely associated with cognitive impairment2,3 and contribute to cognitive deficits independent of white matter lesions.4–6 Cortical lesions are also an independent predictor of conversion from clinically isolated syndrome to MS.7 Thus, there is a need for feasible imaging techniques that can also monitor disease evolution and treatment response in the cerebral gray matter.8,9
While MS lesions in white matter are readily visualized with MR imaging, conventional MR imaging techniques have a low sensitivity for the detection of gray matter MS pathology, which hinders accurate assessment of the total lesion burden.5 Newer MR imaging sequences such as double inversion recovery (DIR) and phase-sensitive inversion recovery (PSIR) are 1.5–5 times more sensitive than conventional MR imaging sequences in the detection of cortical lesions.4 Leukocortical lesions (LCL) are located at the interface between the white matter and the cortex. LCL have the highest detection rate among cortical lesions and are thus a feasible potential imaging biomarker for cognitive deficits that could be readily available for clinical practice.10,11
Synthetic MR imaging is a time-efficient MR imaging technique that provides simultaneous quantitative measurements of the longitudinal relaxation rate (R1), the transverse relaxation rate (R2), and proton-density with correction for field inhomogeneities.12 The technique is based on a double-echo saturation-recovery turbo spin-echo sequence applied with 4 repetitions in which the slice acquisition order is changed for each repetition. In practice, this provides 2 different TEs and 4 different TIs for each voxel. Both the magnitude and phase data are saved, providing a total of 16 complex images that are used to fit the T1- and T2-relaxation curves with a computationally efficient least-squares approach. From this simultaneous relaxometry, synthetic MR imaging can provide synthesized images with a wide range of TEs, TRs, and TIs. Thus, it is possible to obtain multiple spin-echo MR imaging weightings from a single acquisition. The technique has been shown to provide proton-density-, T1-, and T2-weighted images with diagnostic quality in MS.13–15 Furthermore, it is also possible to synthesize images with inversion pulses and by specifying the TRs, TEs, and TIs as a T1-weighted inversion recovery with phase-sensitive reconstruction; the technique makes it possible to obtain PSIR images from the same sequence without additional scanning time.14 This feature makes the technique attractive to apply in the monitoring of pathologies such as MS, in which the detection of cortical lesions on PSIR may be especially clinically important.
We aimed to compare the sensitivity of conventional and synthetic PSIR in detecting leukocortical MS lesions and to evaluate its clinical value in terms of their associations with clinical disability. We hypothesized that synthetic PSIR would have a sensitivity comparable with that of conventional PSIR and that the volume of LCL detected with synthetic PSIR would correlate with cognitive and physical disability.
Materials and Methods
Study Population
We prospectively recruited a sample of 21 patients at the MS outpatient clinic at the Department of Neurology, Karolinska University Hospital in Huddinge, Stockholm, Sweden. The inclusion criterion was a diagnosis of MS according to the concurrent diagnostic criteria,16 and the exclusion criteria were contraindications for MR imaging, neurologic comorbidities, or a history of head trauma. The cohort was representative of the MS population in our region, represented by all clinical subtypes: 13 relapsing-remitting, 7 secondary-progressive, and 1 primary-progressive.17 The demography of the study population is further detailed in Table 1.
Table 1:
Demography of the study populationc
| Patients with MS | |
|---|---|
| Female/male | 14:7 |
| Age (yr) | 44.5 ± 12 |
| Disease duration (yr) | 14.5 ± 9.7 |
| MS subtype (RR/SP/PP) (No.) | 13/7/1 |
| Disease-modifying therapy (No.) (%) | 14 (67%) |
| EDSS score (median) (interquartile range) | 2.0 (2.0) |
| Symbol Digit Modalities Test, z scores (median) (interquartile range) | −0.48 (1.46) |
| Verbal Fluency Test z scores | −0.37 ± 1.37 |
| Fatigue Severity Scale score | 4.53 ± 1.79 |
Note:—RR indicates relapsing-remitting; SP, secondary-progressive; PP, primary-progressive.
Values reported as mean ± SD unless otherwise specified.
Image Acquisition
All participants were scanned on the same Magnetom Trio 3T MR imaging scanner (Siemens, Erlangen, Germany) using a 12-channel head coil. The imaging protocol included a multidynamic multiecho turbo spin-echo sequence for synthetic MR imaging, conventional PSIR images, and additionally a 3D T1-weighted MPRAGE and T2-weighted FLAIR images. Synthetic PSIR images were achieved by applying a phase-sensitive reconstruction on the T1 inversion recovery parameters from synthetic MR imaging as specified in Table 2. The PSIR reconstruction is performed instantaneously in the synthetic MR imaging software after reading the DICOM images and fitting the quantitative maps (which takes <20 seconds on a standard workstation). All acquisition parameters are detailed in Table 2. None of the sequences were acquired with motion correction to accurately reflect clinical image acquisitions.
Table 2:
Image-acquisition parameters
| Sequence Type | Synthetic PSIR | Conventional PSIR | MPRAGE | FLAIR |
|---|---|---|---|---|
| Acquisition plane | 2D axial | 2D axial | 3D sagittal | 3D sagittal |
| Matrix | 256 × 204 | 256 × 204 | 256 × 256 | 256 × 256 |
| In-plane resolution (mm) | 0.9 × 0.9 | 0.9 × 0.9 | 1.0 × 1.0 | 1.0 × 1.0 |
| Slices (No.) | 34 | 34 | 176 | 160 |
| Slice thickness (mm) | 3.0 | 3.0 | 1.0 | 1.0 |
| Distance factor | 0.5 | 0.5 | – | – |
| Flip angle | 120° | 120° | 9° | 120°, T2 variable |
| TR (ms) | 4820a (6000b) | 6000 | 2300 | 6000 |
| TE (ms) | 22/100a (10b) | 10 | 2.98 | 388 |
| TI (ms) | 150/580/2000/4130a (500b) | 500 | 900 | 2100 |
| Acquisition time (min:sec) | 7:47 | 3:32 | 5:15 | 7:02 |
Note:—TR indicates repetition time; TE, echo time; TI, inversion time.
Synthetic MRI is based on a single quantitative acquisition that is then used to generate synthetic images post hoc.
Settings for the generation of synthetic PSIR are in parentheses.
Radiologic Evaluation
The radiologic lesion assessments were performed independently by 2 neuroradiologists (F.H. and J.M.), blinded to all clinical information to avoid biased assessments. Using conventional and synthetic PSIR, the neuroradiologists identified juxtacortical lesions and assessed any adjacent cortical involvement, thus reclassifying the lesions as LCL. To compare the performance of conventional and synthetic PSIR and to avoid bias by the influence of other MR imaging sequences, the neuroradiologists initially assessed only these 2 sequences. For each patient, the conventional and synthetic PSIR images were assessed at 2 separate sessions separated by 12 weeks. For half of the participants (randomly assigned), the conventional PSIR image was presented in the first session, and the synthetic PSIR image, in the second session, and vice versa for the other half of the participants. A consensus agreement, considered to be the ground truth, was performed an additional 12 weeks later jointly by the 2 raters. For the consensus rating, both conventional and synthetic PSIR images were available, together with 3D T1-weighted MPRAGE and T2-weighted FLAIR images.
Lesion Segmentations
WM lesion volumes were segmented on conventional FLAIR images using the lesion probability algorithm in the Lesion Segmentation Toolbox 2.0.12 (Technische Universität München, Munich, Germany) for Statistical Parametric Mapping 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12).18 The resulting WM lesion probability masks were binarized in the FMRIB Software Library 5.0.9 (FSL; (http://www.fmrib.ox.ac.uk/fsl) using a binarization threshold of 0.1.19 A resident in radiology (Y.F.) then performed manual corrections of the automatic WM lesion segmentations using ITK-SNAP, Version 3.4.0 (www.itksnap.org).20 On the basis of the identified LCL in the consensus agreement assessment, a neuroradiologist (F.H.) manually segmented the LCL in ITK-SNAP on both conventional and synthetic PSIR images separately.
Clinical Assessments
Physical disability was assessed with the Expanded Disability Status Scale (EDSS) by an experienced MS neurologist (S.F.). Cognitive testing was performed by an experienced neuropsychologist (Å.B.) with the Symbol Digit Modalities Test, the F-A-S Verbal Fluency Test, and the Fatigue Severity Scale. The testing was performed on the same day as the MR imaging. All cognitive scores were converted into z scores normalized to age and sex.
Statistics
Normality of data was assessed using the Shapiro-Wilk test. Lesion counts and volumes were positively skewed. Differences in lesion count/volume on conventional and synthetic PSIR images were therefore compared using the Wilcoxon signed rank test. Interrater agreement was evaluated using the intraclass coefficient (ICC); ICC ratings of <0.40, 0.40–0.59, 0.60–0.74, and 0.75–1.0 were considered weak, fair, good, or excellent according to statistical convention.21 Standard multiple linear regression was used to evaluate associations between the EDSS, Fatigue Severity Scale, Symbol Digit Modalities Test, and Verbal Fluency Test z scores (dependent variables) and LCL volume (independent variable). Fatigue and verbal fluency z scores were normally distributed, while Symbol Digit Modalities Test z scores were negatively skewed and therefore underwent a reflect and logarithmic transformation [Lg10 (largest score in data +1) − data] to obtain a normal distribution for the regression analysis; EDSS scores were positively skewed and underwent logarithmic transformation to achieve normal distribution. In a second step, WM lesion volumes were added to the analyses to look for any positive interaction between the 2 lesion metrics. P < .05 was considered statistically significant, which after correction for the false discovery rate according to the Benjamini-Hochberg method, corresponded to an adjusted level of P < .030.22
Results
Lesion Counts and Volumes
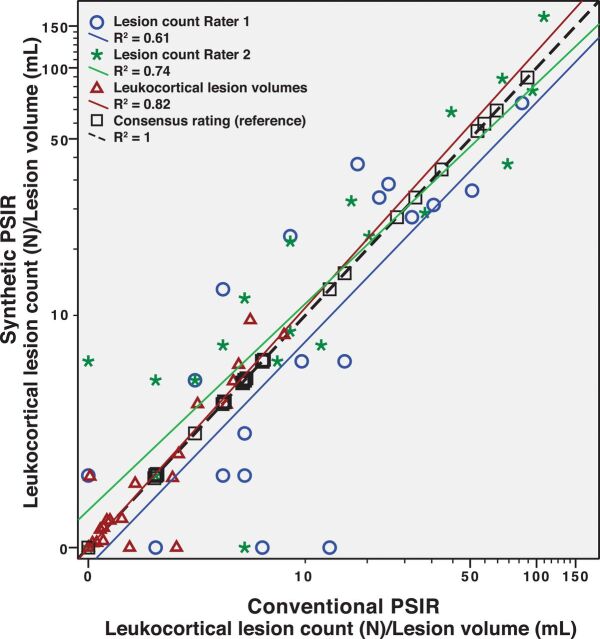
The ICC between the 2 raters was excellent for LCL for both conventional PSIR (0.79, P < .001) and synthetic PSIR (0.87, P < .001). Both raters also had excellent agreement with the consensus rating on both conventional (ICC = 0.91 and 0.97, respectively, for each rater, P < .001) and synthetic PSIR (ICC = 0.92 and 0.94, P < .001). There was no significant difference in the number of detected LCL between conventional and synthetic PSIR (P = .47 and P = .08, respectively, for each rater). Figure 1 illustrates the relation of the individual lesion ratings and the consensus rating as well as the relation between conventional and synthetic PSIR. The 2 raters seemed to have relatively larger differences in their LCL counts in patients with fewer lesions. When we compared each individual rating with the consensus rating, one of the raters showed a small-but-significant difference between the individual and consensus rating in the LCL count on conventional PSIR (P = .008, by the Wilcoxon signed rank test). There was no significant difference between the manually segmented LCL volumes on conventional and synthetic PSIR (P = .17). A detailed comparison of the lesion counts and volumes is presented in Table 3. Figure 2 illustrates the appearance of 2 leukocortical lesions on conventional and synthetic PSIR.
Fig 1.
Leukocortical lesion count and volume on conventional and synthetic PSIR.
Table 3:
Comparison of leukocortical lesion counts and volumes on conventional and synthetic PSIRa
| Conventional PSIR | Synthetic PSIR | P Value Conventional vs Synthetic PSIRb | Consensus Rating | P Value Conventional/Synthetic PSIR vs Consensus Ratingb | |
|---|---|---|---|---|---|
| Leukocortical lesion count, Rater 1 (No.) | 7 ± 17 | 5 ± 26 | .47 | 5 ± 30 | .14/.06 |
| Leukocortical lesion count, Rater 2 (No.) | 7 ± 34 | 7 ± 29 | .08 | 5 ± 30 | .008/.96 |
| Leukocortical lesion volume (mL) | 0.53 ± 2.46 | 0.32 ± 2.89 | .17 |
All values are given as median ± interquartile range.
P value by the Wilcoxon signed rank test.
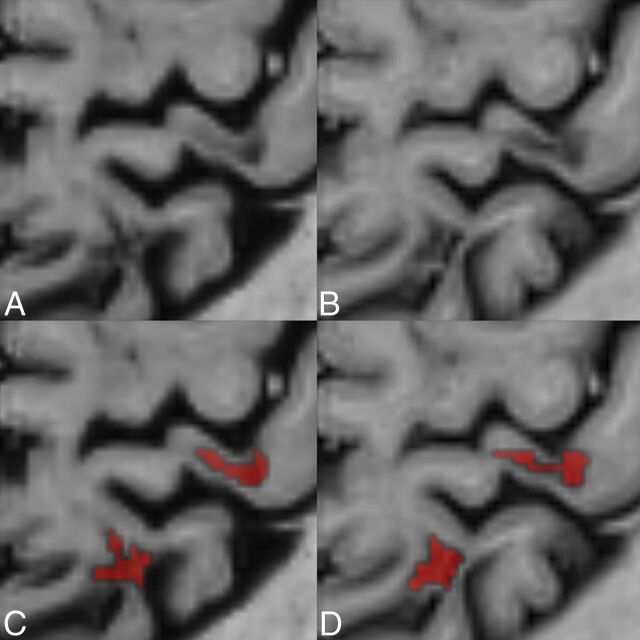
Fig 2.
Comparison between conventional and synthetic phase-sensitive inversion recovery. A comparison between conventional (B and D) and synthetic phase-sensitive inversion recovery (A and C) illustrates 2 leukocortical MS lesions in a 40-year-old female patient with MS. Lower row illustrates the manual segmentation of the lesions by a neuroradiologist.
Associations with Dysfunction
Multiple linear regression showed that higher volumes of LCL were associated with lower Symbol Digit Modalities Test z scores, reflecting information-processing speed, both with measurements from conventional (β = −0.62, P = .003, adjusted R2 = 0.35) and synthetic PSIR (β = −0.55, P = .010, adjusted R2 = 0.26). Similarly, higher volumes of LCL on conventional PSIR (β = −0.51, P = .019, adjusted R2 = 0.22) were associated with lower Verbal Fluency Test z scores, and a similar trend was seen for synthetic PSIR (β = −0.43, P = .054, adjusted R2 = 0.14). Using both the LCL and WM lesion volumes from conventional MR imaging, we saw a positive effect on the association for both the Symbol Digit Modalities Test (β = −0.66, P = .001, adjusted R2 = 0.41) and the Verbal Fluency Test (β = −0.52, P = .015, adjusted R2 = 0.24). An increased association was similarly observed for synthetic PSIR with the Symbol Digit Modalities Test (β = −0.58, P = .005, adjusted R2 = 0.31) and the Verbal Fluency Test (β = −0.47, P = .030, adjusted R2 = 0.18).
There were no statistically significant associations between EDSS scores and conventional PSIR (β = 0.45, P = .18) or synthetic PSIR (β = 0.60, P = .12). Neither were there any associations between fatigue and volumes of LCL on conventional PSIR (β = 0.04, P = .88) or synthetic PSIR (β = −0.03, P = .89).
Discussion
In this prospective cohort of 21 patients with MS, we show that synthetic PSIR based on the multiparametric synthetic MR imaging technique shows a performance comparable with that of conventional PSIR in detecting leukocortical MS lesions. We further show that larger volumes of LCL on both synthetic and conventional PSIR are associated with lower cognitive performance, thus suggesting that the finding of LCL on PSIR is clinically valuable.
Visualization of cortical pathology in vivo improves the diagnostic accuracy in MS and its differential diagnoses.23 A single-center study and a larger multicenter study have demonstrated that including cortical lesions in the criteria for dissemination in space in clinically isolated syndrome increases the specificity in the prediction of those who later convert to MS.7,24 Our results support the potential clinical feasibility of including the combined term “cortical/juxtacortical lesions” in the evaluation of dissemination in space in the latest MAGNIMS criteria for MS diagnostics and the latest revision of the diagnostic criteria for MS.9,23
Including cortical lesions in the diagnostic algorithms for MS has also been previously proposed,7 though a histopathologic validation study later showed a fairly low cortical lesion detection rate of merely 18% with double inversion recovery.25 However, PSIR has been suggested to be superior to DIR in detecting cortical MS lesions.26 The excellent agreement of LCL on both conventional and synthetic PSIR in the current study indicates that the proposed rating of LCL on PSIR may give a robust assessment of cortical disease involvement in MS.27,28 Nevertheless, we found that there was less difference in the LCL count between the 2 different PSIR sequences than between the raters and the consensus rating. This might be because the consensus rating generated a larger total lesion burden when the overall sensitivity and specificity increased with the combination of all available sequences. Combining different sequences, as performed for the criterion standard, was subjectively the preferred approach by the raters in the current study to accurately delineate cortical involvement, which supports previously proposed multimodal reading protocol approaches.4,29–31
Synthetic MR imaging has previously been shown to provide proton-density-, T1-, and T2-weightings in diagnostic quality (as illustrated in the On-line Figure)13–15 as well as automatic volumetrics,13 with a single acquisition. The image quality of synthetic FLAIR images has, however, been shown to be hampered by artifacts.13 We here show that it is possible to obtain diagnostic synthetic PSIR images from the same acquisition without additional scanning, thus providing a clinically feasible way to visualize leukocortical MS pathology, relevant for the latest revision of the MS criteria.23 Nevertheless, if the purpose would be to solely acquire a PSIR contrast, the conventional PSIR would be a faster approach (3 minutes and 32 seconds versus 7 minutes and 47 seconds) but without the additional imaging information provided with synthetic MR imaging.
In terms of the clinical importance of LCL, we found a significant association between higher LCL volume (measured on both synthetic and conventional PSIR) and lower cognitive scores. The good correspondence of both PSIR methods with the cognitive scores is expected because both sequences had similar detection rates and volumes of LCL. This association was increased when adding WM lesion volume to the analyses for both sequences, showing the clinical importance of also detecting LCL with the PSIR methods used here. However, no associations with physical disability or fatigue were found, suggesting that the LCL burden is more related to cognitive disabilities. To further expand our understanding of the pathologic meaning of the imaging findings on conventional/synthetic MR imaging, future studies may investigate the association with biofluid markers of interest in MS.
This study has some limitations: The sample size is relatively small, making it unfeasible to perform additional analyses within the different MS subtypes. The sparse number of raters makes the interrater assessment less robust. Furthermore, a histopathologic validation was not possible in this in vivo study. A comparison with an ultra-high-field strength MR imaging scanner for the ground truth would have been a more optimal validation, but that was, unfortunately, not available for the purpose of the study and synthetic MR imaging has yet to be applied at 7T. A slice distance factor of 0.5 was used to avoid interslice talk. A complementing 3D acquisition approach, as used for the consensus agreement, could be valuable to further increase the detection of smaller lesions such as purely intracortical lesions. With this in mind, we harmonized the spatial resolutions for conventional and synthetic PSIR so that the comparability of the LCL detection rate was not confounded by partial volume effects.
Conclusions
Synthetic MR imaging provides PSIR with a sensitivity similar to that of conventional PSIR in terms of the detection of leukocortical MS lesions. The leukocortical burden detected with synthetic PSIR is associated with cognitive deficits and, therefore, is of clinical relevance in MS. Our results highlight the value of evaluating leukocortical MS lesions, even without the use of ultra-high-field scanners and suggest that either synthetic or conventional PSIR could be a part of a multimodal approach with additional 3D-based sequences, applied to meet the new demands of the latest revision of the MS diagnostic criteria.
Supplementary Material
Acknowledgments
We thank Russell Ouellette for valuable comments on the manuscript.
ABBREVIATIONS:
- DIR
double inversion recovery
- EDSS
Expanded Disability Status Scale
- ICC
intraclass correlation coefficient
- LCL
leukocortical lesions
- PSIR
phase-sensitive inversion recovery
- R1
longitudinal relaxation rate
- R2
transverse relaxation rate
Footnotes
Disclosures: Yngve Forslin, Åsa Bergendal, Farouk Hashim, Juha Martola, Sara Shams, Tobias Granberg, Maria Kristoffersen-Wiberg—RELATED: Grant: ALF Grant* from Karolinska Institutet and Stockholm City Council. *Money paid to the institution. Sten Fredrikson—UNRELATED: Board Membership: Merck, Sanofi Genzyme, Teva Pharmaceutical Industries, Novartis, Roche; Consultancy: Merck, Sanofi Genzyme, Teva Pharmaceutical Industries, Novartis, Roche; Payment for Lectures Including Service on Speakers Bureaus: Merck, Sanofi Genzyme, Teva Pharmaceutical Industries, Novartis, Roche; Payment for Development of Educational Presentations: Merck, Sanofi Genzyme, Teva Pharmaceutical Industries, Novartis, Roche.
This work was supported by Karolinska Institutet and Stockholm County Council through an ALF grant.
References
- 1. Peterson JW, Trapp BD. Neuropathobiology of multiple sclerosis. Neurol Clin 2005;23:107–29, vi–vii [DOI] [PubMed] [Google Scholar]
- 2. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–51 10.1016/S1474-4422(08)70259-X [DOI] [PubMed] [Google Scholar]
- 3. Odenthal C, Coulthard A. The prognostic utility of MRI in clinically isolated syndrome: a literature review. AJNR Am J Neuroradiol 2015;36:425–31 10.3174/ajnr.A3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson F, Poonawalla AH, Hou P, et al. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol 2007;28:1645–49 10.3174/ajnr.A0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geurts JJ, Bö L, Pouwels PJ, et al. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 2005;26:572–77 [PMC free article] [PubMed] [Google Scholar]
- 6. Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 2009;66:1144–50 [DOI] [PubMed] [Google Scholar]
- 7. Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology 2010;75:1988–94 10.1212/WNL.0b013e3181ff96f6 [DOI] [PubMed] [Google Scholar]
- 8. Rocca MA, Amato MP, De Stefano N, et al. ; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 2015;14:302–17 10.1016/S1474-4422(14)70250-9 [DOI] [PubMed] [Google Scholar]
- 9. Filippi M, Rocca MA, Ciccarelli O, et al. ; MAGNIMS Study Group. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016;15:292–303 10.1016/S1474-4422(15)00393-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson F, Datta S, Garcia N, et al. Intracortical lesions by 3T magnetic resonance imaging and correlation with cognitive impairment in multiple sclerosis. Mult Scler 2011;17:1122–29 10.1177/1352458511405561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen AS, Kinkel RP, Madigan N, et al. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology 2013;81:641–49 10.1212/WNL.0b013e3182a08ce8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warntjes JB, Leinhard OD, West J, et al. Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med 2008;60:320–29 10.1002/mrm.21635 [DOI] [PubMed] [Google Scholar]
- 13. Granberg T, Uppman M, Hashim F, et al. Clinical feasibility of synthetic MRI in multiple sclerosis: a diagnostic and volumetric validation study. AJNR Am J Neuroradiol 2016;37:1023–29 10.3174/ajnr.A4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagiwara A, Hori M, Yokoyama K, et al. Synthetic MRI in the detection of multiple sclerosis plaques. AJNR Am J Neuroradiol 2017;38:257–63 10.3174/ajnr.A5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krauss W, Gunnarsson M, Nilsson M, et al. Conventional and synthetic MRI in multiple sclerosis: a comparative study. Eur Radiol 2018;28:1692–1700 10.1007/s00330-017-5100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278–86 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 2012;59:3774–83 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- 19. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–19 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 20. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116–28 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 21. Cicchetti D. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instrument in psychology. Psychological Assessment 1994;6:284–90 10.1037/1040-3590.6.4.284 [DOI] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300 [Google Scholar]
- 23. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 24. Preziosa P, Rocca MA, Mesaros S, et al. Diagnosis of multiple sclerosis: a multicentre study to compare revised McDonald-2010 and Filippi-2010 criteria. J Neurol Neurosurg Psychiatry 2018;89:316–18 10.1136/jnnp-2017-315863 [DOI] [PubMed] [Google Scholar]
- 25. Seewann A, Kooi EJ, Roosendaal SD, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 2012;78:302–08 10.1212/WNL.0b013e31824528a0 [DOI] [PubMed] [Google Scholar]
- 26. Sethi V, Yousry TA, Muhlert N, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry 2012;83:877–82 10.1136/jnnp-2012-303023 [DOI] [PubMed] [Google Scholar]
- 27. Nielsen AS, Kinkel RP, Tinelli E, et al. Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J Magn Reson Imaging 2012;35:537–42 10.1002/jmri.22847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology 2009;73:941–48 10.1212/WNL.0b013e3181b64bf7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Favaretto A, Poggiali D, Lazzarotto A, et al. The parallel analysis of phase sensitive inversion recovery (PSIR) and double inversion recovery (DIR) images significantly improves the detection of cortical lesions in multiple sclerosis (MS) since clinical onset. PLoS One 2015;10:e0127805 10.1371/journal.pone.0127805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson F, Poonawalla A, Datta S, et al. Is 3D MPRAGE better than the combination DIR/PSIR for cortical lesion detection at 3T MRI? Mult Scler Relat Disord 2014;3:253–57 10.1016/j.msard.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 31. Maranzano J, Rudko DA, Arnold DL, et al. Manual segmentation of MS cortical lesions using MRI: a comparison of 3 MRI reading protocols. AJNR Am J Neuroradiol 2016;37:1623–28 10.3174/ajnr.A4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.