Abstract
BACKGROUND AND PURPOSE:
Previous studies indicated that ischemic lesion volume might be a useful surrogate marker for functional outcome in ischemic stroke but should be considered in the context of lesion location. In contrast to previous studies using the ROI approach, which has several drawbacks, the present study aimed to measure the impact of ischemic lesion location on functional outcome using a more precise voxelwise approach.
MATERIALS AND METHODS:
Datasets of patients with acute ischemic strokes from the Multicenter Randomized Clinical Trial of Endovascular Therapy for Acute Ischemic Stroke in the Netherlands (MR CLEAN) were used. Primary outcome was functional outcome as assessed by the modified Rankin Scale 3 months after stroke. Ischemic lesion volume was determined on CT scans 3–9 days after stroke. Voxel-based lesion-symptom mapping techniques, including covariates that are known to be associated with functional outcome, were used to determine the impact of ischemic lesion location for outcome.
RESULTS:
Of the 500 patients in the MR CLEAN trial, 216 were included for analysis. The mean age was 63 years. Lesion-symptom mapping with inclusion of covariates revealed that especially left-hemispheric lesions in the deep periventricular white matter and adjacent internal capsule showed a great influence on functional outcome.
CONCLUSIONS:
Our study confirms that infarct location has an important impact on functional outcome of patients with stroke and should be considered in prediction models. After we adjusted for covariates, the left-hemispheric corticosubcortical fiber tracts seemed to be of higher functional importance compared with cortical lesions.
It is very desirable to improve prediction of functional outcome after an ischemic stroke to rapidly inform patients and their relatives and to optimize patient management, care, and rehabilitation strategies.1,2 Several studies aimed to forecast functional outcome of patients at an early stage after acute ischemic stroke using clinical and imaging data.3,4 With regard to stroke studies, it would be of major interest to replace clinical study end points with a validated surrogate end point. Within this context, image-based surrogate end points would be easier and more reliable to assess and would allow performing phase II stroke studies with a smaller sample size. Previous studies indicated that ischemic lesion volume (ILV) as a surrogate marker for functional outcome might be useful but should be considered in the context of lesion location.5–8
Voxel-based lesion-symptom mapping (VLSM) techniques have been frequently used to investigate the relationship between lesion topography and functional outcome.5,7 VLSM compares functional outcome scores between patients with and without lesions on a voxelwise basis. In this approach, patients are divided for each voxel into 2 groups according to whether they have a lesion at that voxel location.9 VLSM methods have been used to examine motor recovery,10 spatial neglect,11 and aphasia9,12 in patients with chronic stroke, and the 1-month modified Rankin Scale score was used in patients with subacute stroke (2–3 days).5 These studies have provided insight into clinical deficits linked to lesions in particular brain regions but did not include important factors known to be associated with functional outcome after stroke such as age, sex, ILV, recanalization status, and treatment technique.
We aimed to investigate the impact of stroke lesion topography on functional outcome (3-month mRS) with and without accounting for factors known to be associated with functional outcome after stroke using a large data base of well-characterized patients with acute ischemic stroke.
Materials and Methods
Study Population
The analyses are based on data from the Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands (MR CLEAN), which evaluated the effect of intra-arterial treatment versus usual care alone in patients with stroke with acute large-vessel occlusion in the anterior circulation. The main results and design of the study have been reported before.13,14
Inclusion and Exclusion Criteria
We included all patients with a follow-up noncontrast CT (FU-NCCT) scan 3–9 days after stroke. Patients with FU-NCCT scans of poor quality due to motion or beam-hardening artifacts or technical errors precluding automatic registration or segmentation were excluded. Patients with decompressive hemicraniectomy and considerable brain shift secondary to mass effect were excluded as well. Moreover, we excluded patients with previous stroke, an mRS score of >0 at baseline, or mRS = 6 three months after stroke to remove potential confounds from death unrelated to the stroke. As a result, the patient population of this substudy differed from the total MR CLEAN population.
Ethics Statement
The MR CLEAN study protocol was approved by the Medical and Ethical Review Committee (Medisch Ethische Toetsings Commissie of Erasmus MC, Rotterdam, the Netherlands) and the research board of each participating center. All patient records and images were anonymized before analysis, and written informed consent was obtained from all patients or their legal representatives as a part of the original trial protocol.
Ischemic Lesion Segmentation
Binary masks of the ischemic lesions on 3- to 9-day FU-NCCT were created using a validated automatic method.15 An intensity-based region-growing algorithm implemented in Matlab (MathWorks, Natick, Massachusetts) iteratively examined all neighboring voxels of a segmented volume to determine whether these neighboring voxels should be included in the segmentation. A voxel was included if the difference in its intensity and the average intensity of the segmented volume was smaller than a predefined threshold. The region-growing was repeated for multiple thresholds. The range of thresholds was 1.5–4.5 HU with steps of 0.5 HU, resulting in 7 repeated segmentations. If needed, the segmentations were adjusted by an experienced observer with >5 years' experience in neuroradiology (M.E.), blinded to clinical information and treatment as well as further imaging. Subsequently, the ILV was calculated.
Functional Outcome
Functional outcome was assessed by the mRS at 3 months after stroke onset. The mRS is one of the most widely used end points for stroke severity in clinical trials in acute stroke, shown to be valid and reliable.16 It captures the patient's functional outcome on an ordinal scale that ranges from 0 to 6, where zero indicates no symptoms and 6 denotes a patient's death.
Statistical Analysis
Two-tailed Wilcoxon rank sum tests were used to evaluate differences in continuous variables between patients with left-versus-right hemispheric strokes. Categoric variables were compared using the Pearson χ2 test. Backward stepwise regression was performed to investigate the relation among age, sex, ILV, admission National Institutes of Health Stroke Scale score, occlusion site, collateral score, pretreatment Alberta Stroke Program Early CT Score, time to treatment, treatment technique (intra-arterial treatment, intravenous administration of alteplase, or conservative treatment), and recanalization status with functional outcome. Successful recanalization was defined as a modified Arterial Occlusive Lesion score ≥2 or, if missing, as a modified Thrombolysis in Cerebral Infarction score of 2b–3.17 All analyses were performed in SPSS 21 (IBM, Armonk, New York).
Voxel-Based Lesion-Symptom Mapping
Voxelwise analysis requires that all images be in the same coordinate space to allow comparison of voxels between groups. Therefore, after ischemic lesion segmentation, each of the FU-NCCT images was coregistered to the standard Montreal Neurological Institute18 atlas space using an affine transformation and subsequent nonlinear transformation implemented in the NiftyReg toolkit (https://www.nitrc.org/projects/niftyreg/).19 The average CT atlas template described by Rorden et al20 served as a reference image. This template was based on healthy individuals with ages similar to those commonly seen in stroke (mean, 65 years of age). All coregistration results were visually checked for quality and adjusted if needed. Statistical maps of lesion contribution related to functional outcome were generated using VLSM, Version 2.55 (http://160.129.198.244/resources.html). For each voxel, patients were divided into 2 groups according to whether they had a lesion at that voxel location. In VLSM maps, high t-scores indicate that a lesion in that specific voxel has a very significant effect on functional outcome. We limited our analysis to voxels that were affected in ≥10 individuals (10%). Resulting t-score maps were thresholded (P < .01) on the basis of cluster size and permutation method (2000 permutations; P < .05) to correct for multiple comparisons. To evaluate the impact of predefined brain regions on mRS, we calculated median, mean, minimum, and maximum t-score values for each anatomic ROI provided by the Harvard-Oxford cortical and subcortical structural atlas21 and the Johns Hopkins University International Consortium of Brain Mapping Diffusion Tensor-81 white matter labels,22 distributed as part of the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl).23 In a second step, we repeated the VLSM and recalculated the t-score maps, adjusting for the variables found to be significantly associated with functional outcome in a backward stepwise regression analysis.
Results
Patient Characteristics
Of the 500 patients included in MR CLEAN, 353 patients had a 3- to 9-day FU-NCCT scan (On-line Fig 1). Sixty-nine patients were excluded because of technical errors and insufficient scan quality precluding automatic registration or segmentation. Moreover, patients were excluded because of hemicraniectomy (21 patients), severe swelling (3 patients), previous stroke or mRS of >0 at baseline (22 patients), or mRS = 6 three months after stroke (22 patients).
All analyses were based on the final dataset comprising 216 patients.
On-line Table 1 shows the baseline and follow-up characteristics of patients included in this study and of the original MR CLEAN population for comparison. The patients in this study had a mean age of 63 years, and 63% were men. The overall mean ILV was 73 mL.
Backward Stepwise Regression Analysis
The odds for a good outcome (lower mRS) were significantly higher given male sex and successful recanalization status (OR = 2.58; 95% CI, 1.16–6.01; P = .023; OR = 2.03; 95% CI, 1.05–4.04; P = .038, respectively) and significantly lower given a high ILV, older age, long time to treatment, and a high NIHSS score (OR = 0.71; 95% CI, 0.62–0.79; P < .001; OR = 0.64; 95% CI, 0.46–0.88; P = .007; OR = 0.78; 95% CI, 0.60–0.98; P = .043; OR = 0.79; 95% CI, 0.70–0.88; P < .001, respectively). Odds ratios and confidence intervals of the variables in the final model are shown in On-line Fig 2. These variables were used as covariates in subsequent VLSM analysis. The discriminative power of the final regression model was very good (area under the curve = 0.890; 95% CI, 0.846–0.933) (On-line Fig 3).
Voxel-Based Lesion-Symptom Mapping
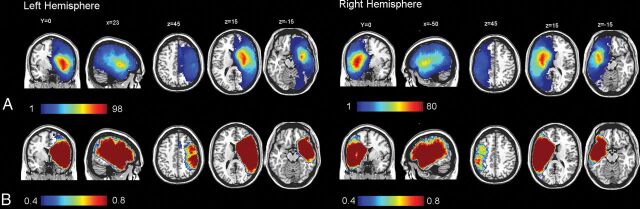
Figure 1 shows the incidence of lesions within each voxel for all patients with stroke as well as the statistical power for α = 0.01 by color-coded overlays. The distribution of stroke lesions was comparable for the left and right hemispheres, with a slightly higher incidence and higher power in the left hemisphere (maximum incidences, n = 98) than the right hemisphere (maximum incidences, n = 80). Table 1 shows the demographic differences between patients with left and right ischemic strokes. VLSM revealed a significant contribution of brain regions comprising the motor pathway, such as the left and right corticospinal tracts. Moreover, injury involving the opercular cortex, the left superior longitudinal fasciculus, and anterior thalamic radiation was associated strongly with mRS (Table 2). A full list of mean, median, minimal, and maximal t-score values of all template ROIs is provided in On-line Table 2.
Fig 1.
Incidence of lesions within each voxel for all patients with right and left hemispheric stroke (A) and a statistical power map between 40% and 80% for α = 0.01 for 3-month mRS (B).
Table 1:
Demographic differences between patients with left-versus-right hemispheric strokesa
| Left (n = 122) | Right (n = 94) | P Value | |
|---|---|---|---|
| Age (yr) | 62.4 ± 11.8 | 63.3 ± 14.1 | .57 |
| Male sex (%) | 80 (66) | 55 (59) | .23 |
| Admission NIHSS | 20 [14–23] | 16 [14–19] | .44 |
| ILV (cm3) | 65.9 ± 62.9 | 81.5 ± 71.5 | .13 |
| 3-Month mRS | 3 [2–4] | 3 [2–4] | .81 |
| Treated with tPA (%) | 115 (94) | 86 (92) | .43 |
| Treated with EVT (%) | 58 (62) | 45 (48) | .96 |
| Recanalized | 64 (53) | 49 (52) | .51 |
| Time to treatment (min) | 97.7 ± 52.0 | 107.7 ± 71.2 | .44 |
| SICH (%) | 1 (1) | 4 (4) | .11 |
| ASPECTS | 9 [7–10] | 9 [8–10] | .16 |
Note:—EVT indicates endovascular thrombectomy; SICH symptomatic intracranial hemorrhage.
Values are means or median [25th–75th percentiles].
Table 2:
VLSM results of regions with mean t-values of >4.00a
| Region | Mean | Median | SD | Max | Min |
|---|---|---|---|---|---|
| Left superior longitudinal fasciculus | 4.85 | 4.86 | 0.50 | 6.21 | 3.06 |
| Left corticospinal tract | 4.45 | 4.42 | 1.53 | 8.06 | 2.36 |
| Left uncinate fasciculus | 4.44 | 4.19 | 1.12 | 7.04 | 2.93 |
| Left central opercular cortex | 4.37 | 4.48 | 0.97 | 6.81 | 2.43 |
| Right corticospinal tract | 4.32 | 4.45 | 0.86 | 6.23 | 2.39 |
| Left inferior fronto-occipital fasciculus | 4.26 | 4.04 | 1.23 | 7.14 | 2.38 |
| Left anterior thalamic radiation | 4.09 | 4.10 | 0.71 | 6.58 | 2.36 |
| Right frontal operculum cortex | 4.08 | 4.06 | 0.39 | 5.06 | 2.66 |
| Left planum temporale | 4.03 | 4.05 | 0.41 | 5.30 | 2.52 |
Note:—Min indicates minimum; Max, maximum.
Mean, median, minimum, maximum, and SD of t-scores calculated for each anatomic ROI provided by the Harvard-Oxford cortical and subcortical structural atlas and Johns Hopkins University International Consortium of Brain Mapping Diffusion Tensor-81 white matter labels.
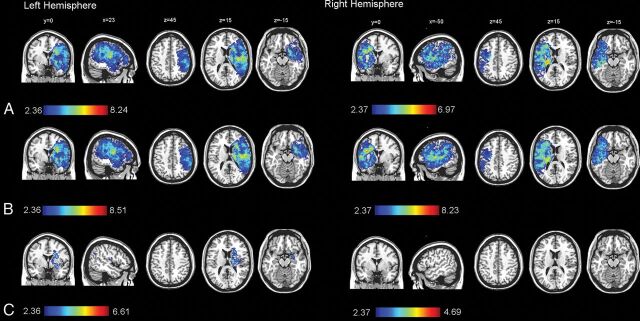
After we included sex, age, and ILV as covariates, the left deep periventricular white matter and adjacent internal capsule showed a great influence on mRS (Fig 2 and Table 3). When we included sex and age as covariates, larger regions of brain tissue in the right hemisphere (245 versus 263 cm3) and in the left hemisphere (267 versus 272 cm3) were significantly associated with mRS (Fig 2). The volume of voxels significantly associated with mRS was 250 cm3 in the right hemisphere and 247 cm3 in the left hemisphere with sex, age, and time to treatment as covariates (On-line Fig 4). When we included sex, age, and the NIHSS as covariates, the volume of voxels significantly associated with mRS was reduced to 217 cm3 in the right hemisphere and 230 cm3 in the left hemisphere. When we included sex, age, and recanalization status as covariates, the volume of voxels significantly associated with mRS was reduced to 155 cm3 in the right hemisphere and 236 cm3 in the left hemisphere. When ILV was included as a covariate, only voxels defined by injury to the left deep periventricular white matter and adjacent internal capsule (31.6 cm3) were associated with mRS.
Fig 2.
Voxel-based lesion-symptom mapping of lesion impact on the modified Rankin Scale score calculated separately for each hemisphere (right side, n = 94; left side, n = 122) using no covariates (A); sex and age as covariates (B); and sex, age, and ischemic lesion volume as covariates (C). The color range indicates t-scores thresholded at a 1% false discovery rate. Higher t-scores (red) indicate areas strongly associated with functional outcome (mRS).
Table 3:
VLSM results after inclusion of sex, age, and ischemic lesion volumea
| Region | Mean | Median | SD | Max | Min |
|---|---|---|---|---|---|
| Left corticospinal tract | 3.86 | 4.11 | 0.97 | 5.90 | 2.36 |
| Left inferior fronto-occipital fasciculus | 3.29 | 3.36 | 0.55 | 4.84 | 2.36 |
| Left uncinate fasciculus | 3.25 | 3.28 | 0.56 | 4.86 | 2.39 |
| Left anterior thalamic radiation | 3.04 | 3.01 | 0.42 | 4.62 | 2.36 |
| Left superior longitudinal fasciculus | 2.65 | 2.62 | 0.21 | 3.68 | 2.36 |
Note:—Min indicates minimum; Max, maximum.
Mean, median, minimum, maximum, and SD of t-scores calculated for each anatomic ROI provided by the Harvard-Oxford cortical and subcortical structural atlas and Johns Hopkins University International Consortium of Brain Mapping Diffusion Tensor-81 white matter labels.
Discussion
By means of a voxelwise approach, our data demonstrate the influence of ischemic lesion location on functional outcome in patients with acute large-vessel-occlusion ischemic stroke. After we included covariates in the model, the left deep periventricular white matter and adjacent internal capsule showed a definite influence on mRS 3 months after stroke.
Our findings are in line with those in previous studies mapping corticospinal tract lesions to motor impairment and motor function recovery in patients with ischemic stroke.5,7,24,25 In a previous study analyzing the ILV in motor-related brain structures (primary motor cortex, putamen, supplementary motor area, corticospinal tract, and cerebellum), patients who achieved functional independence (mRS 0–2) had a higher rate of MCA recanalization at 24 hours, a smaller final infarct volume, and less infarct growth. However, the multivariable analysis showed that the best predictor of outcome in terms of mRS was the degree of ischemic damage in the ipsilateral corticospinal tract, which classified the patients with 78% accuracy.26 In a following voxel-based analysis, the voxels of the brain linked to persistent disability were clustered in an area centered in the deep periventricular white matter and in the adjacent internal capsule.24 The strategic functional importance of this area might be because it represents an important crossroad for many cortico-subcortical and long-range intrahemispheric association fiber tracts, including the corticospinal tract. Our findings support the hypothesis of greater impact of the cortical spinal tract over cortical lesions because reorganization at the cortical level remains insufficient for the patient's recovery in case of damage to the main motor outflow tract.26,27 Although the mRS is currently the most commonly used outcome scale in stroke studies, it is mainly determined by motor disability and is relatively insensitive to cognitive dysfunction. The analysis of other functional outcome parameters such as the NIHSS, Barthel Index, or Glasgow Outcome Scale should be examined in future studies using VLSM.
The voxelwise approach allowed a more precise analysis of lesion location compared with previous studies using the ROI approach,28 which is an intrinsic limitation implying an anatomic a priori localization of the supposed relevant brain area.
In contrast to previous studies that mirrored right-sided lesions onto left-sided ones to increase statistical power,5,25 our study distinguished brain areas of the right and left hemispheres. This distinction is important given that the association between lesion location and outcome in terms of mRS is likely to be influenced by the side of the lesion.7,29 Similar to the findings of Wu et al,7 lesion location in the right hemisphere was no longer significantly associated with poor mRS scores after including ILV as a covariate in the model. In contrast to Wu et al, including age and sex into our model increased the number of voxels associated with mRS not only in the right hemisphere but also in the left hemisphere. This finding suggests that for a given sex and age, ischemic lesion location influences the risk of disability.
An important strength of our study was the adjustment for factors known to be associated with functional outcome after stroke. We showed that even after we adjusted for these predictors of functional outcome, infarcted voxels in the motor pathways remained independently associated with functional outcome. This finding highlights the importance of taking both lesion location and ILV into account to augment stroke-outcome prediction models.
In contrast to previous studies that used acute MR imaging,5,7 the current study is the first to apply the voxel-based approach in FU-NCCTs of patients with stroke. This is important because CT is still the most common and widely available diagnostic imaging technique. However, there are limitations to CT used for determining lesion size, and high-resolution MR imaging can provide more precise results.30
With regard to the acquisition time of the FU-NCCT, the current study measured ILV in imaging acquired at the subacute time point of 3–9 days after stroke onset because it shows better association with acute imaging. Thus, a recent MR CLEAN substudy reported that the growth of ILV was common 24 hours after symptom onset.31 Moreover, previous studies observed a good association between subacute and chronic ILV, as well as a similar correlation between mRS and chronic and subacute volume, and recommended assessing ILV 3–6 days after stroke onset.32–34 The exclusive use of FU-NCCTs that were acquired after stroke treatment distinguishes our approach from a study that analyzed acute DWI in an inhomogeneous group of patients with stroke undergoing MR imaging partly before and partly after revascularization therapy.7
The use of FU-NCCT 3–9 days after stroke and the exclusion of patients with hemicraniectomy and brain shift as well as death before follow-up limit the generalizability of our study. However, these patients are generally known to have unfavorable functional outcome, while the analyzed patients with stroke are those whose outcome is more difficult to predict.
Conclusions
Our study confirms that infarct location has an important impact on the outcome of patients with acute ischemic stroke and should be considered in prediction models. In the presented voxelwise approach, lesions in the periventricular white matter and internal capsule showed a strong influence on functional outcome, measured by the mRS, which underlines the importance of white matter injury to stroke pathology.
Supplementary Material
ABBREVIATIONS:
- FU-NCCT
follow-up noncontrast CT
- ILV
ischemic lesion volume
- VLSM
voxel-based lesion-symptom mapping
Footnotes
Disclosures: Anna M.M. Boers—UNRELATED: Stock/Stock Options: Nico.lab. Nils D. Forkert—UNRELATED: Grants/Grants Pending: Natural Sciences and Engineering Research Council of Canada, National Institutes of Health, Heart and Stroke Foundation, MS Society.* Olvert A. Berkhemer—UNRELATED: Consultancy: Stryker, Comments: The Academic Medical Center received funds from Stryker for consultations by Olvert A. Berkhemer.* Diederik W.J. Dippel—UNRELATED: Consultancy: Stryker, Bracco Imaging, Comments: Erasmus MC received funds from Stryker and Bracco Imaging for consultations by Diederik W.J. Dippel*; Grants/Grants Pending: The MR CLEAN trial was partly funded by the Dutch Heart Foundation and by unrestricted grants from AngioCare, Medtronic/Covidien/ev3, Medac Gmbh/Lamepro, Penumbra, Stryker, and Top Medical/Concentric.* Aad van der Lugt—UNRELATED: Consultancy: Stryker*; Grants/Grants Pending: Dutch Heart Foundation, Dutch Brain Foundation, Stryker, Medtronic, Penumbra. Wim H. van Zwam—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Stryker, Cerenovus.* Jens Fiehler—UNRELATED: Consultancy: Acandis, Boehringer Ingelheim, Cerenovus, Covidien, Medtronic, MicroVention, Penumbra, Route 92 Medical, Stryker; Grants/Grants Pending: Acandis, Medtronic, MicroVention, Stryker.* Henk A. Marquering—OTHER RELATIONSHIPS: cofounder and shareholder of Nico.lab. Charles B.L.M. Majoie—RELATED: Grant: Dutch Heart Foundation*; UNRELATED: Grants/Grants Pending: European Commission, CardioVasculair Onderzoek Nederland/Dutch Heart Foundation, Stryker*. *Money paid to the institution.
The MR CLEAN trial was partly funded by the Dutch Heart Foundation and by nominal, unrestricted grants from AngioCare, Medtronic/Covidien, Medac, Lamepro, Penumbra, Stryker, and Top Medical/Concentric.
References
- 1. Kwakkel G, Veerbeek JM, Harmeling-van der Wel BC, et al. ; Early Prediction of functional Outcome after Stroke (EPOS) Investigators. Diagnostic accuracy of the Barthel Index for measuring activities of daily living outcome after ischemic hemispheric stroke: does early poststroke timing of assessment matter? Stroke 2011;42:342–46 10.1161/STROKEAHA.110.599035 [DOI] [PubMed] [Google Scholar]
- 2. Heiss WD, Kidwell CS. Imaging for prediction of functional outcome and assessment of recovery in ischemic stroke. Stroke 2014;45:1195–201 10.1161/STROKEAHA.113.003611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwakkel G, Wagenaar RC, Kollen BJ, et al. . Predicting disability in stroke–a critical review of the literature. Age Ageing 1996;25:479–89 10.1093/ageing/25.6.479 [DOI] [PubMed] [Google Scholar]
- 4. Kemmling A, Flottmann F, Forkert ND, et al. . Multivariate dynamic prediction of ischemic infarction and tissue salvage as a function of time and degree of recanalization. J Cereb Blood Flow Metab 2015;35:1397–405 10.1038/jcbfm.2015.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng B, Forkert ND, Zavaglia M, et al. . Influence of stroke infarct location on functional outcome measured by the Modified Rankin Scale. Stroke 2014;45:1695–702 10.1161/STROKEAHA.114.005152 [DOI] [PubMed] [Google Scholar]
- 6. Munsch F, Sagnier S, Asselineau J, et al. . Stroke location is an independent predictor of cognitive outcome. Stroke 2016;47:66–73 10.1161/STROKEAHA.115.011242 [DOI] [PubMed] [Google Scholar]
- 7. Wu O, Cloonan L, Mocking SJ, et al. . Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke 2015;46:2438–44 10.1161/STROKEAHA.115.009643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forkert ND, Verleger T, Cheng B, et al. . Multiclass support vector machine-based lesion mapping predicts functional outcome in ischemic stroke patients. PLoS One 2015;10:e0129569 10.1371/journal.pone.0129569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bates E, Wilson SM, Saygin AP, et al. . Voxel-based lesion-symptom mapping. Nat Neurosci 2003;6:448–50 10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- 10. Lo R, Gitelman D, Levy R, et al. . Identification of critical areas for motor function recovery in chronic stroke subjects using voxel-based lesion symptom mapping. Neuroimage 2010;49:9–18 10.1016/j.neuroimage.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 11. Karnath HO, Rennig J, Johannsen L, et al. . The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain 2011;134(Pt 3):903–12 10.1093/brain/awq355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnusdottir S, Fillmore P, den Ouden DB, et al. . Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: a lesion-symptom mapping study. Hum Brain Mapp 2013;34:2715–23 10.1002/hbm.22096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berkhemer OA, Fransen PS, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 14. Fransen PS, Beumer D, Berkhemer OA, et al. ; MR CLEAN Investigators. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials 2014;15:343 10.1186/1745-6215-15-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boers AM, Marquering HA, Jochem JJ, et al. ; MR CLEAN Investigators. Automated cerebral infarct volume measurement in follow-up noncontrast CT scans of patients with acute ischemic stroke. AJNR Am J Neuroradiol 2013;34:1522–27 10.3174/ajnr.A3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091–96 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 17. Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators, STIR Revascularization working group, STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazziotta J, Toga A, Evans A, et al. . A four-dimensional probabilistic atlas of the human brain. J Am Med Inform Assoc 2001;8:401–30 10.1136/jamia.2001.0080401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Modat M, Ridgway GR, Taylor ZA, et al. . Fast free-form deformation using graphics processing units. Comput Methods Programs Biomed 2010;98:278–84 10.1016/j.cmpb.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 20. Rorden C, Bonilha L, Fridriksson J, et al. . Age-specific CT and MRI templates for spatial normalization. Neuroimage 2012;61:957–65 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 22. Mori S, Wakana S, van Zijl PC, et al. . MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005 [Google Scholar]
- 23. Smith SM, Jenkinson M, Woolrich MW, et al. . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–19 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 24. Cuingnet R, Rosso C, Chupin M, et al. . Spatial regularization of SVM for the detection of diffusion alterations associated with stroke outcome. Med Image Anal 2011;15:729–37 10.1016/j.media.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 25. Phan TG, Demchuk A, Srikanth V, et al. . Proof of concept study: relating infarct location to stroke disability in the NINDS rt-PA trial. Cerebrovasc Dis 2013;35:560–65 10.1159/000351147 [DOI] [PubMed] [Google Scholar]
- 26. Rosso C, Colliot O, Pires C, et al. . Early ADC changes in motor structures predict outcome of acute stroke better than lesion volume. J Neuroradiol 2011;38:105–12 10.1016/j.neurad.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 27. Seitz RJ, Sondermann V, Wittsack HJ, et al. . Lesion patterns in successful and failed thrombolysis in middle cerebral artery stroke. Neuroradiology 2009;51:865–71 10.1007/s00234-009-0576-x [DOI] [PubMed] [Google Scholar]
- 28. Ernst M, Boers AM, Aigner A, et al. . Association of computed tomography ischemic lesion location with functional outcome in acute large vessel occlusion ischemic stroke. Stroke 2017;48:2426–33 10.1161/STROKEAHA.117.017513 [DOI] [PubMed] [Google Scholar]
- 29. Rangaraju S, Streib C, Aghaebrahim A, et al. . Relationship between lesion topology and clinical outcome in anterior circulation large vessel occlusions. Stroke 2015;46:1787–92 10.1161/STROKEAHA.115.009908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Price CJ, Friston KJ. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase 2002;8:345–54 10.1076/neur.8.4.345.16186 [DOI] [PubMed] [Google Scholar]
- 31. Bucker A, Boers AM, Bot JCJ, et al. ; MR CLEAN Trial Investigators (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). Associations of ischemic lesion volume with functional outcome in patients with acute ischemic stroke: 24-hour versus 1-week imaging. Stroke 2017;8:1233–40 10.1161/STROKEAHA.116.015156 [DOI] [PubMed] [Google Scholar]
- 32. Beaulieu C, de Crespigny A, Tong DC, et al. . Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol 1999;46:568–78 [DOI] [PubMed] [Google Scholar]
- 33. Ebinger M, Christensen S, De Silva DA, et al. ; Echoplanar Imaging Thrombolytic Evaluation Trial Investigators. Expediting MRI-based proof-of-concept stroke trials using an earlier imaging end point. Stroke 2009;40:1353–58 10.1161/STROKEAHA.108.532622 [DOI] [PubMed] [Google Scholar]
- 34. Tourdias T, Renou P, Sibon I, et al. . Final cerebral infarct volume is predictable by MR imaging at 1 week. AJNR Am J Neuroradiol 2011;32:352–58 10.3174/ajnr.A2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.