In this retrospective study, the phase-contrast MR imaging of 185 patients with suspected chronic adult hydrocephalus was evaluated using the CSF Flow software package. Decision-making for shunt placement was performed in this cohort on the basis of clinical assessment alone without the availability of quantitative phase-contrast MRI results. The authors evaluated the response to lumbar puncture or lumbar drainage and shunt surgery using quantitative tests such as the Tinetti Test, the Timed Up and Go, and the Mini-Mental State Examination and qualitative measures of gait, urinary, and cognitive symptom improvement before and after lumbar puncture/lumbar drainage and shunt surgery. This study suggests that the results of phase-contrast MR imaging through the cerebral aqueduct alone should not be used to select patients for diagnostic or therapeutic CSF diversion.
Abstract
BACKGROUND AND PURPOSE:
Radiologic imaging plays a key role in diagnosing chronic adult hydrocephalus, but its role in predicting prognosis is still controversial. We sought to evaluate the effectiveness of cardiac-gated phase-contrast MR imaging through the cerebral aqueduct in predicting the clinical response to diagnostic lumbar puncture/lumbar drainage and shunt surgery in suspected adult hydrocephalus.
MATERIALS AND METHODS:
In this retrospective study, the phase-contrast MR imaging of 185 patients with suspected chronic adult hydrocephalus was evaluated using the CSF Flow software package. Decision-making for shunt placement was performed in this cohort on the basis of clinical assessment alone without the availability of quantitative phase-contrast MR imaging results. We recorded the response to lumbar puncture or lumbar drainage and shunt surgery using quantitative tests such as the Tinetti Test, the Timed Up and Go, and the Mini-Mental State Examination and qualitative measures of gait, urinary, and cognitive symptom improvement before and after lumbar puncture/lumbar drainage and shunt surgery. Quantitative analysis of phase-contrast MR imaging was compared with clinical outcome measures.
RESULTS:
Both CSF stroke volume and flow rate overlapped between lumbar puncture/lumbar drainage responders and nonresponders. There was also a significant overlap between shunt responders and nonresponders. Aqueductal stroke volume or flow rate alone was a poor predictor of lumbar puncture/lumbar drainage and shunt surgery response. Quantitative clinical measures after lumbar puncture/lumbar drainage were better predictors of shunt response.
CONCLUSIONS:
This study suggests that the results of phase-contrast MR imaging through the cerebral aqueduct alone should not be used to select patients for diagnostic or therapeutic CSF diversion.
Chronic adult hydrocephalus (CAH) is a stereotyped clinical disorder in which elderly patients present with components of a triad of gait instability, cognitive disturbance, and urinary incontinence and are found to have dilated ventricles on imaging. The syndrome encompasses a variety of conditions and includes normal pressure hydrocephalus as defined by Adams et al. in 1965,1 along with other sources of hydrocephalus such as idiopathic hydrocephalus, hydrocephalus secondary to subarachnoid hemorrhage or trauma, and communicating hydrocephalus attributable to compromised CSF dynamics, aqueductal stenosis, and compensated arrested hydrocephalus.2,3 CAH is one of the few reversible causes of dementia and is likely underdiagnosed, estimated to occur in up to 14% of patients in the nursing home setting.4 Surgical interventions such as ventriculoperitoneal or ventriculoatrial shunt can dramatically improve the symptoms from CAH.5 However, the clinical or radiologic diagnosis of CAH is challenging because gait, cognitive, and urinary abnormalities are common in the elderly population, and ventricular enlargement may alternatively be the result of atrophy or normal aging.6 Moreover, symptoms may become irreversible in long-standing CAH.7 Therefore, methods of predicting shunt outcomes have been the primary focus of research in this field.
Lumbar puncture (LP) or lumbar drainage (LD) trials are widely used in selecting patients with reversible CAH8 but are costly, invasive, and uncomfortable for patients. Furthermore, while LP and LD demonstrate a >90% positive predictive value, they are limited by a <20% negative predictive value, thereby posing a risk of excluding patients who might benefit from an operation.9 These challenges spurred attempts to develop noninvasive diagnostic techniques to better predict the effect of shunt surgery. One of the most widely used noninvasive techniques is cardiac-gated phase-contrast MR imaging through the cerebral aqueduct (AQ-PCMR). AQ-PCMR was initially reported as demonstrating 100% positive predictive value and 50% negative predictive value.10 Although subsequent studies reported overlap of the aqueductal CSF flow rate between shunt responders and nonresponders, they reported that patients with high aqueductal flow tended to improve after shunt surgery.8,11–13 This discrepancy between the initial report and subsequent reports may be driven by differences in scan processing and calibration.14 The symptoms of aqueductal stenosis have been shown to substantially overlap those of communicating CAH.15 At our institution (Johns Hopkins Hospital), patients with potential hydrocephalus undergo a standardized MR imaging study, including AQ-PCMR and high-spatial-resolution heavily T2-weighted (CISS) imaging. This protocol is performed primarily to exclude ventricular dilation due to aqueductal stenosis or other intraventricular obstructions that would contraindicate LP/LD due to risk of brain stem herniation.
Surgical decisions for the remaining cases (cases of nonobstructive CAH) at other centers are often based, at least in part, on the CSF flow rates or stroke volume through the cerebral aqueduct on AQ-PCMR, with the claim that the technique aids in distinguishing patients who will respond to shunting. Yet, there have been suggestions that AQ-PCMR is simply an epiphenomenon of ventricular dilation,16 and there have also been reports that AQ-PCMR does not aid in establishing a diagnosis or prognosis to the extent originally reported.11,17–19 Current practice guidelines from the American Academy of Neurology acknowledge the limitations of AQ-PCMR but suggest that the patients with higher aqueductal flow are more likely to respond to shunting.8
Due to controversy regarding the role of AQ-PCMR, quantitation of the results of this technique was not performed at initial imaging and has not been used in decision-making regarding shunt surgery at our institution. The aim of this study was to retrospectively perform quantitation of AQ-PCMR and to determine whether it would have predicted LP or shunt outcomes.
Materials and Methods
Selecting Patients with Chronic Adult Hydrocephalus
A total of 185 patients (83 women and 102 men; median age, 73 years; age range, 41–89 years) who had undergone high-resolution MR imaging and AQ-PCMR to evaluate the patency of the cerebral aqueduct before diagnostic lumbar CSF removal and who were clinically evaluated at our center between March 2010 and August 2014 were included in this retrospective institutional review board–approved study.
Clinical Assessment and Diagnosis of Adult Hydrocephalus
All subjects had ventriculomegaly with an Evans index of ≥0.3 and had gait symptoms or exhibited Tinetti score deficits at the time of imaging. Patients with a visible obstruction within the ventricular system (24 subjects) were excluded. For each eligible study, the clinical chart was reviewed to collect the duration of symptoms and responses to LP/LD and shunt surgery measured by the Tinetti, Timed Up and Go (TUG), Mini-Mental State Examination (MMSE) scores and subjective reports. Overall symptom improvement after LP/LD was graded in 4 levels (significant improvement, marginal improvement, no improvement, and worsening). For patients who had undergone shunt surgeries, the Tinetti, MMSE, and TUG scores and subjective symptom improvement and changes in 3 categories (gait, urinary, and cognitive symptoms) were assessed up to 1 year after ventriculoatrial or ventriculoperitoneal shunt surgeries. Patients with continuous postsurgical complications such as shunt blockage or infection within the period of inspection were excluded. To account for possible shunt malfunctions or sub-optimal shunt settings, we selected the best outcome measures reported within a year of the operation. The preprocedural aqueductal stroke volume and flow rate measured by AQ-PCMR and Flow software (http://www.tidam.fr/documentation)20 were compared among LP/LD, or shunt surgery responders and nonresponders.
MR Imaging Technique
All patients were examined with a 1.5T (Magnetom Avanto and Magnetom Espree; Siemens, Erlangen, Germany) or 3T (Magnetom Trio and Magnetom Verio; Siemens) MR imaging scanners with a 12-channel head coil. Flow images were acquired at the center of the cerebral aqueduct perpendicular to the CSF flow direction with a 2D fast cine phase-contrast MR imaging pulse sequence with retrospective peripheral gating. In the cine phase-contrast MR imaging, the through-plane velocity-encoding was in the craniocaudal direction. The sequence parameters were as follows: TR/TE, 51/6.4 ms; NEX, 2; slice thickness, 4 mm; FOV, 120 × 120 mm; pixel spacing, 0.375 mm; flip angle, 20°; and velocity encoding, 8–30 cm/s for the axial-oblique plane. Images were re-acquired with higher velocity-encoding parameters if they had aliasing artifacts. The cardiac cycle was partitioned into 32 segments. Depending on the patient's heart rate, the acquisition time was approximately 4–6 minutes for AQ-PCMR. Thin-section sagittal imaging with a CISS sequence used to evaluate possible aqueductal stenosis was obtained for all the samples. The sequence parameters were as follows: TR/TE, 6.16/2.85 ms; NEX, 2; slice thickness, 0.5 mm; FOV, 320 × 320 × 15 mm; pixel spacing, 0.5 mm; flip angle, 40°.
Aqueductal Flow Analysis
Data were analyzed using an image-processing software Flow with an optimized CSF flows segmentation algorithm, which automatically extracts the ROI at the level of the cerebral aqueduct.20 The CSF flow profiles were generated versus the 32 segments of the cardiac cycle, and the integration of this curve provided the CSF cephalic and caudal stroke volume (in microliters) and flow rate (in milliliters/minute), which represented the CSF volume displaced in both directions through the considered ROI at the level of cerebral aqueduct. We averaged the cephalic and caudal flow rate and stroke volume.
Statistical Analysis
Statistical comparisons for all the analyses were performed by using R statistical and computing software (http://www.r-project.org/). Comparisons among Tinetti, TUG, and MMSE scores before and after LP/LD and/or shunt surgery were made using the Wilcoxon rank sum test. When comparing the improvement after LP/LD and the improvement after a shunt, we also used the Wilcoxon rank sum test. We used Pearson correlation tests to assess the relationship between improvements in hydrocephalus symptoms after LP/LD and after shunt. To analyze whether the aqueductal flow rate and stroke volume had prognostic or diagnostic value, we first divided patients into quartiles with an equal number of patients based on the Tinetti, TUG, or MMSE scores collected at baseline. We then compared the aqueductal flow rate (in milliliters/minute) and aqueductal stroke volume (in microliters) between each pair of these quartiles using ANOVA along with the Tukey Honest Significant Difference test. We repeated the same analysis using quartiles defined by the Tinetti, TUG, or MMSE score changes after LP/LD or shunt surgery. To compare LP/LD or shunt responders and nonresponders, we defined “shunt response” by 3 criteria: subjective improvement, >20% improvement in TUG score, or >20% improvement in Tinetti score. The Wilcoxon rank sum test was used when comparing flow rate and stroke volume between LP/LD or shunt responders and nonresponders. We tested the likelihood of improvement after shunt surgery given improvement after LP/LD using the Fisher exact test. Finally, we used receiver operating characteristic analysis to find the cutoff values for the aqueductal flow rate and stroke volume that maximized sensitivity and specificity with respect to shunt outcomes.
Results
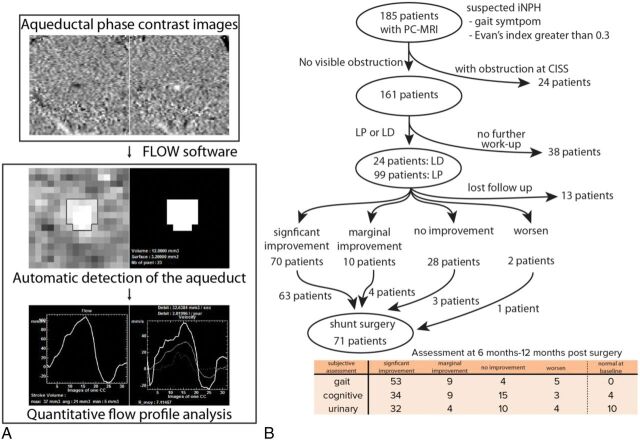
The process of Flow software to survey the flow rate from AQ-PCMR is shown in Fig 1A.20 Clinical assessment and AQ-PCMR results post-LP/LD and postshunt response work flow are shown in Fig 1B. One hundred eighty-five patients with suspected CAH symptoms with ventricular enlargement determined by the Evans index were evaluated for potential obstruction in the aqueductal system determined with high-resolution CISS images and AQ-PCMR. After we removed 24 patients with obstructions in the ventricular system, 123 patients underwent LP/LD. Seventy and 10 patients showed significant and moderate symptom improvement after LP/LD, whereas 28 and 2 patients reported no improvement or worsening of symptoms, respectively. Most patients with significant improvement (88.2% of patients with significant improvement) but also some patients without symptom improvement (6% of patients with no improvement) after LP/LD underwent shunt surgery (Fig 1B). Both subjective and objective assessments after shunt surgery were performed. After the operation, 75%, 53%, and 52% of patients who had gait, urinary, or cognitive symptoms, respectively, reported significant improvement.
Fig 1.
An overview of the AQ-PCMR image-processing pipeline and treatment flowchart of patients suspected of having CAH in this study. A, The phase-contrast MR imaging data acquired at the level of cerebral aqueduct are analyzed by Flow software to calculate the stroke volume and flow rate of each patient. B, One hundred eighty-five patients with gait abnormality and ventricular enlargement determined by the Evans index are included in this study. Depending on quantitative and subjective response to LP/LD, patients undergo shunt surgery. The shunt outcomes are determined within 12 months after surgery using both subjective assessment in 3 symptom categories and quantitative measurements such as the Tinetti, TUG, or MMSE scores.
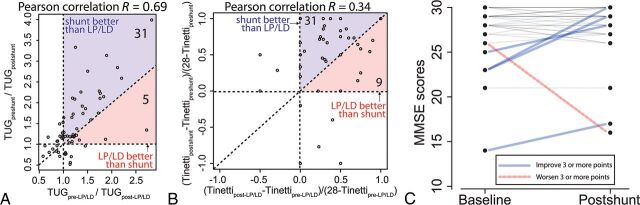
In addition to patients' subjective reports, we surveyed quantitative assessments: Tinetti and TUG scores (before and after LP/LD and shunt surgery) and MMSE scores (before and after shunt surgery) (Fig 2). In our patient population, both LP/LD and shunt surgery significantly resulted in objective improvement in the TUG score (Fig 2A, paired Wilcoxon rank sum test; P values < .001 for LP/LD and < .001 for shunt surgery) and Tinetti score (Fig 2B, paired Wilcoxon rank sum test; P values < .001 for LP/LD and < .001 for shunt surgery). In contrast, MMSE scores did not show significant improvement after shunt surgery (Fig 2C, paired Wilcoxon rank sum test; P value = .74 for shunt surgery).
Fig 2.
Quantitative assessment for LP/LD and shunt response. A, Comparison between the improvement in TUG scores after LP/LD (x-axis) and after shunt surgery (y-axis). Thirty-one patients show better improvement after shunt surgery than after LP/LD, whereas 5 patients show better improvement after LP/LD than after shunt surgery. B, Comparison between the improvement in the Tinetti score “deficit” after LP/LD (x-axis) and after shunt surgery (y-axis). Patients with a Tinetti score of 28 at baseline and 2 outliers (−1.4, 0.6) and (−1, −2) are not shown on this graph. Thirty-one patients show better improvement after shunt surgery than after LP/LD, whereas 9 patients show better improvement after LP/LD than after shunt surgery. C, Changes in the MMSE score after shunt surgery. Blue lines represent patients who improved, and red lines represent patients who worsened by ≥3 points in the MMSE test after shunt surgery.
When we compared the improvement after LP/LD and the improvement after shunt surgery, shunt surgery resulted in significantly greater improvement than LP/LD in both Tinetti and TUG scores (Fig 2A, -B, paired Wilcoxon rank sum test; P values = .007 for the Tinetti Test and .001 for the TUG test). Nonetheless, both TUG and Tinetti scores showed positive correlations (Fig 2A, -B, Pearson correlation R = 0.69 and 0.34) between LP/LD response and shunt surgery, suggesting that the LP/LD could predict the responses to the shunt surgery quantitatively.
We next tested whether aqueductal CSF flow has a diagnostic or prognostic value. When they were divided into 4 quartiles based on Tinetti, TUG, and MMSE scores collected at baseline, there was no significant difference in the aqueductal flow rate and aqueductal stroke volume among quartiles (On-line Tables 1 and 2, upper 3 rows of each table). These quartiles did not demonstrate any significant differences in Tinetti, TUG, and MMSE score changes after LP/LD or shunt surgery (On-line Tables 1 and 2, lower 5 rows of each table). When we divided subjects into LP/LD responders and nonresponders based on their subjective improvement, the flow rate and stroke volume of the responders and nonresponders overlapped (Fig 3A). Most interesting, a small number of patients within the LP nonresponder group had very high aqueductal flow rates. These patients exhibited significantly higher flow rates than the LP responder group (P value = .04). Likewise, TUG score improvement after LP/LD and shunt surgery showed a weak negative correlation with respect to stroke volume and flow rate (Fig 3B and On-line Fig 1B). We performed the same analyses for shunt surgery. Patients were divided into shunt responders and nonresponders based on the symptom relief after shunt surgery, and their aqueductal flow parameters were compared (On-line Fig 1A). Similar to the LP/LD response, the flow rate and stroke volume of the shunt responders and nonresponders overlapped. The difference between the 2 groups was not significantly different for both the aqueductal flow rate and stroke volume (P value = .35 and .38, respectively). There were 4 patients within the shunt responder group exhibiting higher aqueductal flow than other study participants.
Fig 3.
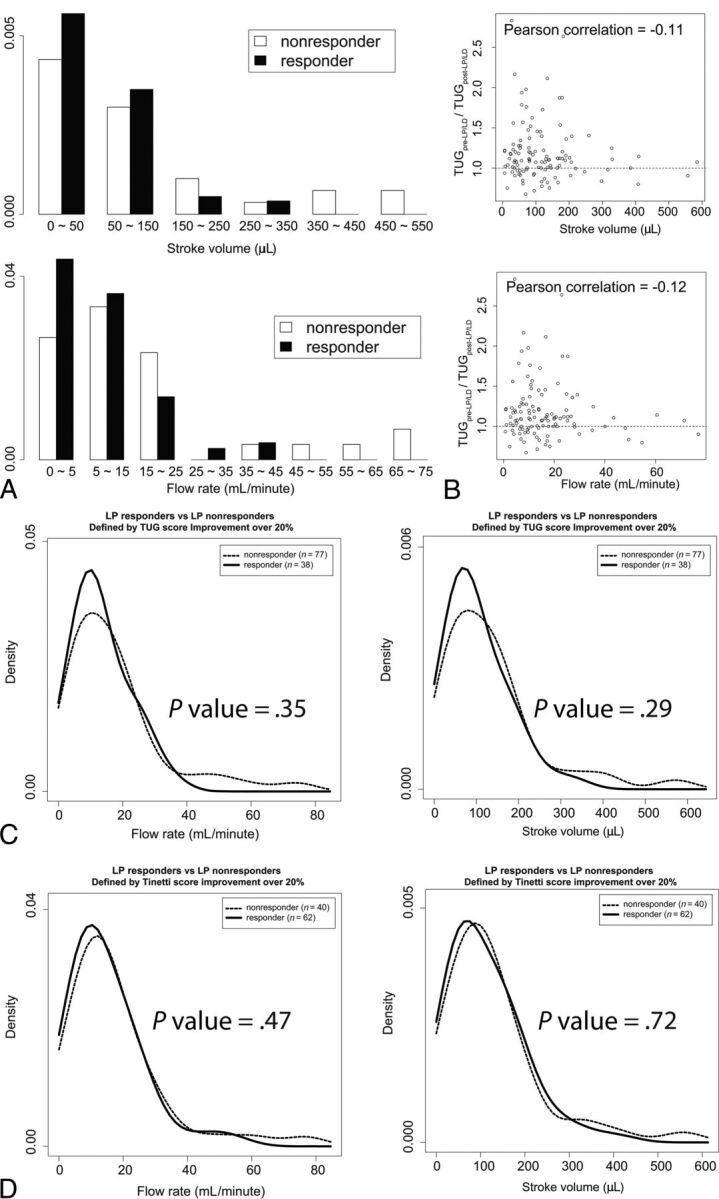
The aqueductal CSF flow and LP/LD response measured by subjective improvement and Tinetti and TUG score improvement. A, The distributions of aqueductal stroke volume (upper) and flow rate (lower) are compared between LP/LD responders and nonresponders. The aqueductal flow rate is significantly higher in LP/LD nonresponders than in responders (Wilcoxon rank sum test, P = .03 for stroke volume and .028 for flow rate). B, The aqueductal stroke volume (upper) and flow rate (lower) are plotted against the quantitative improvement of the TUG score (y-axis) after LP/LD. The Pearson correlation between the TUG score improvement and the stroke volume and flow rate are −0.113 and −0.116, respectively. C, Twenty percent or greater improvement in the TUG score after LP/LD was used to define LP/LD responders, and <20% improvement in the TUG score after LP/LD was used to define LP/LD nonresponders. The number of patients in each group is shown as a number on the graph. The 2 groups exhibit an overlapping distribution of aqueductal flow rate and stroke volumes. D, “Tinetti score deficit” is defined as the difference between a perfect Tinetti score and the patient's Tinetti score. Twenty percent or greater improvement in the Tinetti score deficit after LP/LD was used to define LP/LD responders, and < 20% improvement in the Tinetti score deficit after LP/LD was used to define LP/LD nonresponders. Again, the 2 groups exhibit overlapping distribution of aqueductal flow rate and stroke volumes.
To test the hypothesis that aqueductal CSF flow may reveal shunt responders among LP/LD nonresponders, we evaluated the aqueductal flow rate and stroke volume of LP/LD responders and nonresponders depending on their shunt responses (On-line Fig 2). As expected, the LP/LD responders had significantly higher likelihood of having a favorable shunt surgery outcome than LP/LD nonresponders (90% versus 50%; Fisher exact test, P value = .03). Patients with a high flow rate or stroke volume, regardless of their LP/LD results, showed improvement after shunt surgery. A small number of patients with a 25 mL/min or higher flow rate or all patients with 180 μL or higher stroke volume had favorable shunt surgery outcomes.
We next evaluated the diagnostic and prognostic values of low aqueductal stroke volume or low flow rate alone in predicting a shunt outcome. Receiver operating characteristic analysis revealed that the diagnostic value of the AQ-PCMR-derived flow rate or stroke volume in predicting shunt outcome is minimal (On-line Fig 3).
Discussion
In this study, we retrospectively evaluated the relationship between quantitative AQ-PCMR measures to LP/LD responsiveness and shunt responsiveness. We selected subjects with potentially treatable communicating CAH based on their responses to LP/LD. The patients' response to shunt surgery was determined by patients' subjective reports of gait, urinary, and cognitive symptom improvement. At our institution, surgical decisions are made on the basis of radiologic evaluation of the patency of the ventricular system on high-resolution MR imaging and AQ-PCMR and the patients' clinical symptom improvement after LP or LD.
The efficiency of shunt surgery in our study (87%) is comparable with that in previous studies.9 The positive and negative predictive values of LP/LD for shunt success (90% and 50%, respectively) are higher than those in most other studies.8 This result is potentially due to the stringent criteria we used to select patients with CAH. We used high-resolution imaging to exclude stenosis in the ventricular system by carefully examining any signs of obstruction at the foramen of Monro, cerebral aqueduct, and fourth ventricular outflow, findings that can be difficult to visualize using conventional 2D MR images or 3D acquisitions with larger voxel sizes. The patients with obstructive hydrocephalus who were excluded from our study are not expected to improve after LP/LD but would improve after shunt surgery. Including patients with obstructive hydrocephalus would cause underestimation of the specificity and negative predictive value of LP/LD.
Most prior research used either stroke volume or flow rate, leaving the possibility of the conclusion changing with other variables.5,10,11,21 Although related, these 2 variables are affected differentially by the heart rate. We used both stroke volume and flow rate to evaluate AQ-PCMR and found that the results were largely consistent between the 2 parameters.
We found that the CSF flow rate and stroke volume mostly overlapped between LP/LD nonresponders and responders. There are, however, a few patients with very high flow rates among LP/LD nonresponders, making the aqueductal flow parameters of LP/LD nonresponders significantly higher than those of LP/LD responders. Because the LP/LD responder group has a greater number of patients compared with LP/LD nonresponder group, the wider dispersion of LP/LD nonresponders is rather unexpected and implies underlying clinical significance. Most interesting, patients with the highest aqueductal flow do not to respond to LP/LD yet respond to shunt surgery. This result is indeed consistent with that of Dixon et al,11 who showed that patients with high flow rates (>33 mL/min) were LP/LD nonresponders but shunt responders. Although there are not enough patients who did not respond to LP/LD but responded to shunt surgery in our study, our data suggest that LP/LD and shunt surgery results may be inconsistent for patients with high aqueductal flow.
Aqueductal stroke volume is a dynamic variable that increases at the early phase but decreases after a peak at the later disease course, potentially due to brain ischemia (On-line Fig 4).22–24 Because there are also significant heterogeneities in baseline aqueductal flow in the normal state, it is impossible to infer the extent of disease progression based on a single “snapshot” of aqueductal flow characteristics.5,18,19,23,25 LP and LD have high positive predictive values but low negative predictive values; thus, they may miss patients who would derive benefit from shunt surgery.9 If this model were true, patients with moderately progressed disease, who do not demonstrate response to LP/LD but still benefit from the shunt surgery (middle group in the On-line Fig 4) would have high aqueductal flow. In this setting, we can use AQ-PCMR as an adjunct tool in determining patients who can benefit from shunt surgery among LP/LD nonresponders. Additional studies targeting such patient populations should follow.
There are a few limitations to our study. First, it is a retrospective study. However, all surgery and assessments were performed in a standardized fashion under the guidance of a single surgeon (D.R.). When measuring aqueductal CSF flow, we minimized measurement variability using automated software to measure stroke volume and flow rate. Second, because we used LP/LD response to select patients to undergo an operation, the number of patients in the LP/LD non-responder group was smaller than LP/LD responder group. Most patients with high aqueductal flow among LP/LD nonresponders were excluded from shunt surgery. Third, the number of patients who failed to have improvement after shunt surgery was smaller than patients with improvement. Unlike patients with high aqueductal CSF flow in the smaller LP/LD negative group, patients with high aqueductal CSF flow in the larger shunt-positive group could simply be a result of random distribution. Finally, the relationship between aqueduct radius and stroke volume was not assessed in this study. Aqueductal radius plays an important role in the relationship between flow and pressure26; thus, variation in the aqueductal radius may impact our findings. Additional studies will be required to determine the significance of the aqueductal radius as it relates to LP/LD response and shunt success.
In summary, although it has been previously suggested that the utility of phase-contrast imaging may lie in excluding those patients with low aqueductal CSF flow from surgery, in fact, shunt responders can also be found among those patients with the lowest aqueductal CSF flow rates. The proportion of shunt responders to nonresponders was relatively high at each cerebral aqueductal flow value as a result of the prevalence of shunt responsiveness in the population studied. AQ-PCMR added little further benefit beyond clinical evaluation with suspicion of disease, though it may be useful as an adjunct tool to support low negative predictive values of LP/LD tests.
Conclusions
Shunt surgery can significantly improve symptoms and potentially stop the progression of CAH, but the response to the surgery is heterogeneous. The field is searching for less invasive and more accurate clinical assays to select surgical candidates. The evidence to support AQ-PCMR as a diagnostic and prognostic assay for CAH has been discordant. In this retrospective study, we show that AQ-PCMR alone has little diagnostic and prognostic value in determining shunt outcomes.
Supplementary Material
ABBREVIATIONS:
- AQ-PCMR
aqueductal phase-contrast MRI
- CAH
chronic adult hydrocephalus
- LD
lumbar drainage
- LP
lumbar puncture
- MMSE
Mini-Mental State Examination
- TUG
Timed Up and Go
Footnotes
Disclosures: Ari M. Blitz—UNRELATED: Expert Testimony: medical legal consultation; Grants/Grants Pending: FAIN U01DC013778, R21 NS096497*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: International Society for Hydrocephalus and CSF Disorders, International Society for Magnetic Resonance in Medicine, French-Israeli Course in Radiology. *Money paid to the institution.
References
- 1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with normal cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med 1965;273:117–26 10.1056/NEJM196507152730301 [DOI] [PubMed] [Google Scholar]
- 2. Edwards RJ, Dombrowski SM, Luciano MG, et al. Chronic hydrocephalus in adults. Brain Pathol 2004;14:325–36 10.1111/j.1750-3639.2004.tb00072.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarnaris A, Watkins LD, Kitchen ND. Biomarkers in chronic adult hydrocephalus. Cerebrospinal Fluid Res 2006;3:11 10.1186/1743-8454-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marmarou A, Young HF, Aygok GA. Estimated incidence of normal-pressure hydrocephalus and shunt outcome in patients residing in assisted-living and extended-care facilities. Neurosurg Focus 2007;22:E1 [DOI] [PubMed] [Google Scholar]
- 5. Kahlon B, Sjunnesson J, Rehncrona S. Long-term outcome in patients with suspected normal pressure hydrocephalus. Neurosurgery 2007;60:327–32; discussion 332 10.1227/01.NEU.0000249273.41569.6E [DOI] [PubMed] [Google Scholar]
- 6. Resnick SM, Pham DL, Kraut MA, et al. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 2003;23:3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vakili S, Moran D, Hung A, et al. Timing of surgical treatment for idiopathic normal pressure hydrocephalus: association between treatment delay and reduced short-term benefit. Neurosurg Focus 2016;41:E2 10.3171/2016.6.FOCUS16146 [DOI] [PubMed] [Google Scholar]
- 8. Halperin JJ, Kurlan R, Schwalb JM, et al. Practice guideline: idiopathic normal pressure hydrocephalus—response to shunting and predictors of response: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2015;85:2063–71 10.1212/WNL.0000000000002193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wikkelsø C, Hellström P, Klinge PM, et al. ; European iNPH Multicentre Study Group. The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF tap test in patients with idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2013;84:562–68 10.1136/jnnp-2012-303314 [DOI] [PubMed] [Google Scholar]
- 10. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996;198:523–29 10.1148/radiology.198.2.8596861 [DOI] [PubMed] [Google Scholar]
- 11. Dixon GR, Friedman JA, Luetmer PH, et al. Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin Proc 2002;77:509–14 10.4065/77.6.509 [DOI] [PubMed] [Google Scholar]
- 12. Sankari SE, Fichten A, Gondry-Jouet C, et al. Correlation between tap test and CSF aqueductal stroke volume in idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl 2012;113:43–46 10.1007/978-3-7091-0923-6_9 [DOI] [PubMed] [Google Scholar]
- 13. Poca MA, Sahuquillo J, Busto M, et al. Agreement between CSF flow dynamics in MRI and ICP monitoring in the diagnosis of normal pressure hydrocephalus. Sensitivity and specificity of CSF dynamics to predict outcome. Acta Neurochir Suppl 2002;81:7–10 [DOI] [PubMed] [Google Scholar]
- 14. Bradley WG. Intracranial pressure versus phase-contrast MR imaging for normal pressure hydrocephalus. AJNR Am J Neuroradiol 2015;36:1631–32 10.3174/ajnr.A4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tisell M, Tullberg M, Hellström P, et al. Neurological symptoms and signs in adult aqueductal stenosis. Acta Neurol Scand 2003;107:311–17 10.1034/j.1600-0404.2003.00124.x [DOI] [PubMed] [Google Scholar]
- 16. Chiang WW, Takoudis CG, Lee SH, et al. Relationship between ventricular morphology and aqueductal cerebrospinal fluid flow in healthy and communicating hydrocephalus. Invest Radiol 2009;44:192–99 10.1097/RLI.0b013e31819a640b [DOI] [PubMed] [Google Scholar]
- 17. Algin O, Hakyemez B, Parlak M. The efficiency of PC-MRI in diagnosis of normal pressure hydrocephalus and prediction of shunt response. Acad Radiol 2010;17:181–87 10.1016/j.acra.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 18. Kahlon B, Annertz M, Ståhlberg F, et al. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery 2007;60:124–29; discussion 129–30 10.1227/01.NEU.0000249208.04344.A3 [DOI] [PubMed] [Google Scholar]
- 19. Scollato A, Gallina P, Di Lorenzo N, et al. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery 2008;63:E1209; author reply E1209 10.1227/01.NEU.0000315863.32544.EB [DOI] [PubMed] [Google Scholar]
- 20. Balédent O, Henry-Feugeas MC, Idy-Peretti I. Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Invest Radiol 2001;36:368–77 10.1097/00004424-200107000-00003 [DOI] [PubMed] [Google Scholar]
- 21. Al-Zain FT, Rademacher G, Meier U, et al. The role of cerebrospinal fluid flow study using phase contrast MR imaging in diagnosing idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl 2008;102:119–23 [DOI] [PubMed] [Google Scholar]
- 22. Bradley WG. CSF flow in the brain in the context of normal pressure hydrocephalus. AJNR Am J Neuroradiol 2015;36:831–38 10.3174/ajnr.A4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scollato A, Tenenbaum R, Bahl G, et al. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol 2008;29:192–97 10.3174/ajnr.A0785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradley WG. MR prediction of shunt response in NPH: CSF morphology versus physiology. AJNR Am J Neuroradiol 1998;19:1285 [PMC free article] [PubMed] [Google Scholar]
- 25. Andréen K, Wikkelsø C, Tisell M, et al. Natural course of idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2014;85:806–10 10.1136/jnnp-2013-306117 [DOI] [PubMed] [Google Scholar]
- 26. Chaarani B, Capel C, Zmudka J, et al. Estimation of the lateral ventricles volumes from a 2D image and its relationship with cerebrospinal fluid flow. Biomed Res Int 2013;2013:215989 10.1155/2013/215989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.