Abstract
Extreme climate events are predicted to increase in frequency and severity due to contemporary climate change. Recent studies have documented the evolutionary impacts of extreme events on single species, but no studies have yet investigated whether such events can drive community-wide patterns of trait shifts. On 22 January 2020, subtropical south Florida experienced an extreme cold episode during which air temperatures dropped below the lower thermal limit of resident lizard populations. In the week immediately after the cold event, we documented decreased lower thermal limits (CTmin) of six co-occurring lizard species that vary widely in ecology, body size and thermal physiology. Although cold tolerance of these species differed significantly before the cold snap, lizards sampled immediately after had converged on the same new, lower limit of thermal tolerance. Here, we demonstrate that extreme climate events can drive substantial and synchronous community-wide trait changes and provide evidence that tropical and subtropical ectotherms—often characterized as unable to withstand rapid changes in climatic conditions—can endure climatic conditions that exceed their physiological limits. Future studies investigating the mechanisms driving these trait shifts will prove valuable in understanding the ability of ectotherm communities to mitigate climate change.
Keywords: extreme event, climate change, thermal physiology, thermal limits, ectotherm, convergence
1. Introduction
Extreme climate events can be powerful agents of change [1]. Sudden shifts in temperature and rainfall regimes, or extreme weather events such as tropical cyclones or cold spells [2–7], can lead to dramatic changes in population-level traits [2,8]. However, the extent to which co-occurring species respond similarly to the same exceptional climate conditions is unclear. Do species-specific differences in ecology, morphology or physiology lead to differential phenotypic responses to shared extreme conditions, or can such events drive community-wide trait shifts, with responses comparable among species? While the evolutionary consequences of extreme climate events on populations of single species is a burgeoning area of research [1], no studies have yet explored the community-wide responses of multiple co-occurring species exposed to the same extreme climate event.
Under contemporary climate change, temperature fluctuations are expected to increase in frequency and magnitude leading to exceptional heat waves and cold snaps [9]. Two non-mutually exclusive classes of biodiversity are considered highly vulnerable to increased temperature variation: (i) ectotherms, those species without internal physiological temperature regulation [10], and (ii) tropical species, which typically have narrow thermal tolerances owing to low climate variability [11,12]. Tropical lizards, therefore, present a valuable model to understand responses of thermally vulnerable species to rapid changes in climate.
On 22 January 2020, south Florida experienced an extreme short-term cold event, with temperatures dropping overnight to 4.4°C—the lowest recorded temperature in the previous decade. Prior to this severe cold snap, we had measured the lower limits of thermal tolerance (critical thermal minima, CTmin) of six co-occurring tropical and subtropical lizard species in Miami, south Florida, USA [13]. Previous studies have suggested that exposure to exceptionally high or low temperatures can lead to changes in physiological traits associated with thermal biology in lizards, including CTmin [14–16]. It is possible that such changes could result from adaptation via natural selection [15,16]—only those individuals in the population able to tolerate the extreme conditions survive—or acclimation via phenotypic plasticity [17], in which individual physiologies may be labile and shift in concert with abiotic conditions.
In the week following the extreme cold event, we tested whether the surviving lizards were able to tolerate colder temperatures than those measured previously. We also investigated whether post-cold snap thermal tolerance limits were comparable across species despite substantial interspecific differences in thermal physiology, body size and ecology. Finally, we re-sampled thermal limits 10 weeks after the cold event to test whether differences in thermal tolerance observed directly after the event represented a short-term plastic response to cold temperature exposure, or if the shifts remained consistent, more indicative of an extreme selection event.
2. Methods
(a). Species sampling and field collection
We sampled the lower thermal tolerance (CTmin) of six lizard species in Miami, south Florida, USA, between 21 October and 29 November 2016 [13]. Following the extreme cold event on 22 January 2020, we re-sampled all species from 29 January to 6 February 2020. The lizard species in this community are ecologically (figure 1b) and morphologically diverse (electronic supplementary material, table S1) and live 1–2 years in nature ([18,19]; J. T. Stroud 2015–2020, unpublished data). Hemidactylus mabouia were not sampled in the second post-cold snap sampling session owing to nocturnal curfews imposed in the City of Miami in response to the spread of SARS-CoV-2 (COVID-19 coronavirus; in place from 26 March 2020).
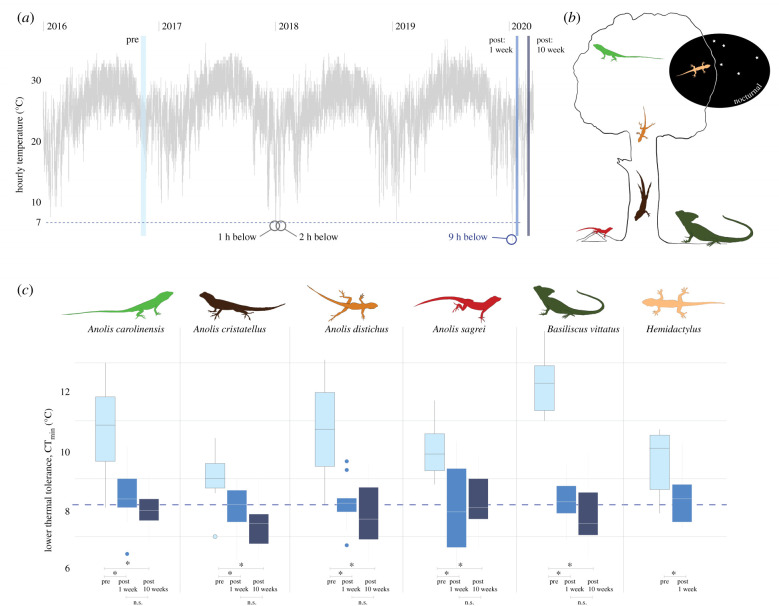
Figure 1.
Following an extreme cold event in south Florida, air temperatures (a) hit a decade-low of 4.4°C and six lizard species experienced short-term exposure to temperatures below their lower thermal tolerance limits. (b) Representation of the lizard community and their ecological niches drawn to scale with respect to relative size differences between species and not tree size. (c) Comparing lower thermal limits (CTmin) of species before (pre) and after (post) the extreme cold event, we observed shifts towards tolerance of lower temperatures. New, lower thermal limits remained 10 weeks after the extreme cold event. Horizontal blue-dashed line at 7.2°C illustrates the immediate post-cold event community-wide mean CTmin: the most recent cold event experienced temperatures below this limit for a substantially longer period of time than previous events (circled in (a)). *p < 0.05; n.s., not significant.
(b). Assessing lower thermal tolerance limits (CTmin)
Lower critical thermal limits (CTmin) were measured as previously in [13]. In short, lizards were acclimated overnight to room temperature before all thermal trials. A digital thermocouple was placed in the cloaca of each lizard and internal body temperature was monitored during gradual cooling until a righting response was lost (see electronic supplementary material).
(c). Statistical analyses and calculating selection differentials
We tested for a significant difference in CTmin between pre- and post-event populations (hereafter treatment) using analysis of covariance (ANCOVA), with species, treatment (and their interaction) and sex as model factors, and log-transformed body length (snout–vent length, SVL), body mass and starting body temperature as covariates. As the interaction between treatment and species in the full term was significant, we ran independent models per species to obtain p-values, including treatment as a model factor and log-transformed SVL as a covariate. To test if the variance in CTmin was greater in populations before or after the cold event, we conducted upper one-tailed F-tests for each species independently. A community-wide p-value was calculated via a Fisher's test of combined probability. Selection differentials were calculated by standardizing pre-cold and (plus 1 week) post-cold event samples combined to have a zero mean and unit variance for each species independently. Post-cold event means were then subtracted from pre-cold event means to calculate independent standardized differentials for each species [20]. Finally, we then tested for convergence in CTmin by fitting full models independently on pre- and post-cold event lizards; species, treatment (and their interaction) and sex were model factors, with log-transformed body length, mass and starting body temperature as covariates.
3. Results
(a). Species shift to tolerate colder temperatures
Following the extreme cold event in south Florida, we observed shifts to lower thermal tolerance (CTmin, i.e. increased tolerance of colder temperatures) in all species of lizard tested (table 1 and figure 1). Species-level decreases in CTmin ranged from 1.09°C (Anolis cristatellus) to 4.04°C (Basiliscus vittatus). Variances in CTmin were smaller for all species after the cold event, except B. vittatus (table 2). Standardized selection differentials ranged from 0.92 to 1.80 (table 2).
Table 1.
(a) Following an extreme cold climate event in Miami, south Florida USA, we observed shifts to lower thermal tolerance limits (CTmin) in six lizard species, which remained consistent 10 weeks after the cold episode. Mean CTmin values per species are shown (±1 s.e.); sample sizes are given in parentheses. (b) Full community model indicates that CTmin of all species decreased following the cold event, including body size, mass, sex and starting temperature of CTmin trial as covariates. (c) Final model drops all non-significant covariates. **p < 0.01, ***p < 0.001.
| pre-cold event CTmin (°C) | 1 week post-cold event CTmin (°C) | F-value (d.f.) | p-value | 10 weeks post-cold event CTmin (°C) | F-value (d.f.) | p-value | ecological niche: structural microhabitat | |
|---|---|---|---|---|---|---|---|---|
| (a) pre-/post-cold event differences in CTmin | ||||||||
| Anolis carolinensis | 9.75 ± 0.4 (12) | 7.43 ± 0.3 (11) | 18.63 (1,21) | <0.001*** | 6.92 ± 0.2 (11) | 32.96 (1,21) | <0.001*** | canopy branches, leaves, trunks |
| Anolis cristatellus | 8.04 ± 0.3 (10) | 6.95 ± 0.2 (13) | 8.85 (1,21) | 0.007** | 6.43 ± 0.2 (16) | 19.05 (1,24) | <0.001*** | low tree trunks and ground |
| Anolis distichus | 9.6 ± 0.5 (10) | 7.15 ± 0.2 (12) | 19.96 (1,20) | <0.001*** | 6.75 ± 0.4 (11) | 42.41 (1,19) | <0.001*** | broad tree trunks |
| Anolis sagrei | 9.05 ± 0.3 (12) | 6.91 ± 0.5 (10) | 14.17 (1,20) | 0.001** | 7.25 ± 0.3 (15) | 23.18 (1,25) | <0.001*** | low tree trunks and ground |
| Basiliscus vittatus | 11.29 ± 0.3 (11) | 7.25 ± 0.2 (11) | 111.20 (1,20) | <0.001*** | 6.81 ± 0.4 (10) | 85.84 (1,19) | <0.001*** | ground and riparian areas |
| Hemidactylus mabouia | 8.57 ± 0.5 (6) | 7.38 ± 0.5 (6) | 3.03 (1,10) | 0.113 | — | — | — | trees and branches; nocturnal |
| (b) full community: CTmin shifts (full model) | ||||||||
| cold event | 20.79 (1,88) | <0.001*** | ||||||
| species | 5.05 (5,88) | <0.001*** | ||||||
| log(body size, SVL) | 1.06 (1,88) | 0.307 | ||||||
| log(mass) | 0.78 (1,88) | 0.379 | ||||||
| sex | 1.33 (3,88) | 0.269 | ||||||
| log(starting temp.°C) | 1.52 (1,88) | 0.221 | ||||||
| cold event × species | 3.49 (5,88) | 0.006** | ||||||
| (c) full community: CTmin shifts (reduced model) | ||||||||
| cold event | 23.27 (1,112) | <0.001*** | ||||||
| species | 9.77 (5,112) | <0.001*** | ||||||
| cold event × species | 4.33 (5,112) | 0.001** | ||||||
Table 2.
Minimum and maximum lower thermal limit (CTmin) values (range in parentheses), upper one-tailed F-tests for equality of variance and standardized selection differentials for observed shifts in CTmin in six lizard species sampled before and one week after an extreme cold event in Miami, south Florida, USA. The F-value represents an F : 1 ratio of variances between pre-cold event versus post-cold event samples. Community-wide shift in CTmin variance, p = 0.047 (Fisher's combined probability test).
| species | pre-event CTmin range (°C) | post-event CTmin range (°C) | F | p-value | selection differential, s |
|---|---|---|---|---|---|
| Anolis carolinensis | 7.0–12.0 (5.0) | 5.4–9.1 (3.7) | 2.16 | 0.118 | 1.34 |
| Anolis cristatellus | 6.0–9.4 (3.4) | 5.2–8.0 (2.8) | 1.33 | 0.315 | 1.07 |
| Anolis distichus | 7.1–12.1 (5.0) | 5.7–8.6 (2.9) | 4.67 | 0.010 | 1.39 |
| Anolis sagrei | 7.8–10.7 (2.9) | 4.9–9.3 (4.4) | 0.38 | 0.935 | 1.26 |
| Basiliscus vittatus | 10.0–13.1 (3.1) | 5.9–8.5 (2.6) | 1.81 | 0.177 | 1.80 |
| Hemidactylus mabouia | 6.8–9.7 (2.9) | 6.3–9.2 (2.9) | 1.27 | 0.401 | 0.92 |
(b). Community-wide convergence on lower thermal limits
Prior to the cold event, there was a significant difference in CTmin between species (F5,55 = 8.14, p < 0.001; figure 1). Interspecific differences also existed when excluding B. vittatus, which had the highest CTmin (F4,45 = 2.98, p = 0.029). By contrast, there were no interspecific differences in CTmin after the January 2020 cold event (F5,57 = 0.45, p = 0.813). Full model output is available in the electronic supplementary material.
(c). Lower thermal limits maintained after the extreme cold event
Re-sampling ca 10 weeks after the cold event revealed that CTmin did not differ from those observed immediately after the cold event (plus 1 week versus plus 10 weeks post-cold event: F1,110 = 1.42, p = 0.236), but significantly differed from those prior to the cold event (pre-cold event versus plus 10 weeks post-cold event: F1,108 = 36.67, p < 0.001; individual species differences in table 1). There also remained no interspecific differences in CTmin after 10 weeks (F4,56 = 1.90, p = 0.123; community-wide CTmin average = 6.28 ± 0.12°C (s.e.), mean CTmin range per species from 6.43 to 7.25°C).
4. Discussion
Extreme climate events such as unusual cold snaps, heat waves, droughts or exceptional rainfall are expected to increase in frequency and severity due to human-mediated climate change [21–24]. Such atypical conditions can drastically affect populations and may drive long-lasting and large-scale effects on species and communities [3,25]. Following an extreme cold episode in subtropical south Florida, we observed shifts to increased tolerance of lower temperatures in six species of ecologically, physiologically and morphologically diverse lizards, resulting in community-wide convergence of lower thermal limits.
Two non-exclusive mechanisms may have driven this observed community-wide shift in thermal tolerance: directional selection and physiological plasticity. A previous study demonstrated the role selection may play in driving the evolution of lizard cold tolerance [16]. During the winter of 2013–14, the south-central United States—north-west of south Florida—experienced record-setting extreme cold weather during the so-called ‘polar vortex.’ Similar to our observations, populations of the American green anole (Anolis carolinensis) had significantly lower thermal tolerance limits (CTmin) immediately after this extreme cold event [16]. Resampling the same A. carolinensis populations five months later revealed that the lower thermal tolerance limits of the populations following the cold event were maintained [16]. In concert with evidence of evolutionary change in genes relevant to thermal physiology, the maintenance of lower thermal limits suggested a plastic response was unlikely and so the observed genetic and phenotypic trait shifts most likely reflected the operation of natural selection [25].
Here, we also observed that lower thermal limits recorded immediately after the cold event remain consistent over two months later in all species tested, suggesting there may have been community-wide directional selection towards lower thermal tolerance. Examination of the pre- and post-cold snap distributions and selection differentials suggests that, at least for some species, mortality would have had to be very high for directional selection to explain these patterns (table 2). While local reports of both perished and cold-stunned lizards following the cold event are well documented (see electronic supplementary material; albeit, all larger Iguana spp.), it is difficult to determine from our field observations whether sufficient mortality occurred for such extreme selection to alone explain these patterns. Another signature of selection would be a decrease in trait variance as only a subset of the initial population would have survived [26]. While we observed less variation in CTmin in post- versus pre-cold event sampling in all species except Anolis sagrei (table 2), we only observed a statistically significant decrease in one species (Anolis distichus; table 2). It is unclear, therefore, whether directional selection favouring the survival of more cold-tolerant individuals is responsible for the replicated CTmin shifts we observed across this community.
Thermal trait shifts can also arise through phenotypic plasticity (i.e. acclimation; [17,27–29]). For example, seasonal acclimation can lead to lower thermal limits during colder winter temperatures relative to hotter summers in some ectotherms (insects [30], freshwater and marine invertebrates [31], freshwater fish [32]), including temperate lizards [33–36]. Tropical lizards, however, have a much weaker ability to acclimate to colder temperature regimes [37–39]. In Miami specifically, species which exhibit seasonal variation in CTmin also acclimate during experimental exposure to weeks-long colder ambient temperatures (e.g. A. carolinensis and A. sagrei; [40,41]), while those with no seasonal variation do not (e.g. A. cristatellus; [14,40,42]). While some seasonal variation may exist, it remains unclear whether any tropical lizards are able to acclimate in response to short-term, rapid and extreme dips to low temperatures, as observed here. Plastic responses to such ‘cold shocks' have been observed in a range of insects [43,44], although the physiological mechanisms which underlie these responses remain unknown [30].
It is also possible that directional selection and physiological plasticity have both operated in our study. If species within this lizard community varied in their acclimation ability, as indicated by previous studies [14,40,42], then it is possible that plasticity may underlie extreme shifts in trait distributions of CTmin in some species, whereas selection is more plausible for others. For example, the post-cold event CTmin levels of brown basilisks (B. vittatus) were substantially below those of any individual measured prior to the cold event. Such a pattern suggests that it was highly unlikely that natural selection was responsible, and plasticity may have played a greater role than in other species where selective mortality of existing variation could explain the shift (e.g. Anolis spp.). We also observed high interspecific variation in CTmin prior to the cold event, as well as differences in habitat use and body size (figure 1), suggesting that species may have been expected to respond differently to an extreme event. However, we observed convergence among all species on a new, lower CTmin. While differences in ecology and behaviour translate into different thermal ecologies of diurnal species during the day [45], species differ little in nocturnal behaviour: all species in this community sleep perched among above-ground vegetation ([18,46,47]; figure 2). As such, extreme cold temperatures which occurred in the middle of the night were likely experienced similarly across species.
Figure 2.

All diurnal lizards in this study sleep exposed on above-ground vegetation during periods of nocturnal inactivity. For example, here are typical sleeping site locations of Anolis sagrei in Miami, FL. Photo: J. T. Stroud, taken at 21.30 on 15 October 2019.
Here, we observed that six co-occurring species of lizards converged on a new, lower limit of thermal tolerance following an extreme cold climate event. Multi-species field studies of thermal physiology remain rare, yet are likely to provide valuable insights into both our forecasting of the community-wide effects of contemporary climate change, and in developing our understanding of how climate and physiology interact in community assembly and associated dynamics. It is clear that extreme climate events pose particular risks to those species or life stages with limited ability to regulate internal body temperatures (e.g. ectotherms; [48–51]) or those adapted to a narrow range of climatic conditions (e.g. thermal niche specialists; [12,52]); future physiological, genetic and demographic studies are needed to clarify the mechanisms that underlie both the immediate and long-term effects on the physiology of extreme climate events on thermally vulnerable species.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank S. Clements, H. Howell, A. Messerman, C. Searcy and L. Stemle for field assistance. We would like to thank Sean Tomlinson and two anonymous reviewers for comments that improved the manuscript.
Ethics
Research was conducted under Florida Fish and Wildlife permit LSSC 16-00013 and University of Miami IACUC protocol 17-061.
Data accessibility
All raw data and code are included as electronic supplementary material.
Authors' contributions
J.T.S. conceived and designed the study. J.T.S., C.C.M. and W.B. acquired lizards and thermal trait data. J.T.S. analysed the data. All authors contributed to the interpretation of the data. J.T.S. drafted the manuscript and all authors provided critical revisions. All authors approved the final version of the manuscript and agreed to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Grant PR, et al. 2017. Evolution caused by extreme events. Phil. Trans. R. Soc. B 372, 20160146 ( 10.1098/rstb.2016.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donihue CM, Herrel A, Fabre AC, Kamath A, Geneva AJ, Schoener TW, Kolbe JJ, Losos JB. 2018. Hurricane-induced selection on the morphology of an island lizard. Nature 560, 88–91. ( 10.1038/s41586-018-0352-3) [DOI] [PubMed] [Google Scholar]
- 3.Donihue CM, et al. 2020. Hurricane effects on neotropical lizards span geographic and phylogenetic scales. Proc. Natl Acad. Sci. USA 117, 10 429–10 434. ( 10.1073/pnas.2000801117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton I. 2007. Weather-related mass-mortality events in migrants. Ibis 149, 453–467. ( 10.1111/j.1474-919X.2007.00704.x) [DOI] [Google Scholar]
- 5.Lirman D, et al. 2011. Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida reef tract and reversed previous survivorship patterns. PLoS ONE 6, e0023047 ( 10.1371/journal.pone.0023047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colella MA, Ruzicka RR, Kidney JA, Morrison JM, Brinkhuis VB. 2012. Cold-water event of January 2010 results in catastrophic benthic mortality on patch reefs in the Florida Keys. Coral Reefs 31, 621–632. ( 10.1007/s00338-012-0880-5) [DOI] [Google Scholar]
- 7.Fey SB, Siepielski AM, Nusslé S, Cervantes-Yoshida K, Hwan JL, Huber ER, Fey MJ, Catenazzi A, Carlson SM. 2015. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proc. Natl Acad. Sci. USA 112, 1083–1088. ( 10.1073/pnas.1414894112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant BR, Grant PR. 1993. Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. Lond. B 251, 111–117. ( 10.1098/rspb.1993.0016) [DOI] [Google Scholar]
- 9.Easterling DR, Evans JL, Groisman PY, Karl TR, Kunkel KE, Ambenje P. 2000. Observed variability and trends in extreme climate events: a brief review. Bull. Am. Meteorol. Soc. 81, 417–426. () [DOI] [Google Scholar]
- 10.Angilletta MJ Jr, Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 12.Perez TM, Stroud JT, Feeley KJ. 2016. Thermal trouble in the tropics. Science 351, 1392–1393. ( 10.1126/science.aaf3343) [DOI] [PubMed] [Google Scholar]
- 13.Mothes CC, Stroud JT, Clements SL, Searcy CA. 2019. Evaluating ecological niche model accuracy in predicting biotic invasions using south Florida's exotic lizard community. J. Biogeogr. 46, 432–441. ( 10.1111/jbi.13511) [DOI] [Google Scholar]
- 14.Leal M, Gunderson AR. 2012. Rapid change in the thermal tolerance of a tropical lizard. Am. Nat. 180, 815–822. ( 10.1086/668077) [DOI] [PubMed] [Google Scholar]
- 15.Logan ML, Cox RM, Calsbeek R. 2014. Natural selection on thermal performance in a novel thermal environment. Proc. Natl Acad. Sci. USA 111, 14 165–14 169. ( 10.1073/pnas.1404885111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell-Staton SC, Cheviron ZA, Rochette N, Catchen J, Losos JB, Edwards SV. 2017. Winter storms drive rapid phenotypic, regulatory, and genomic shifts in the green anole lizard. Science 357, 495–498. ( 10.1126/science.aam5512) [DOI] [PubMed] [Google Scholar]
- 17.Pintor AF, Schwarzkopf L, Krockenberger AK. 2016. Extensive acclimation in ectotherms conceals interspecific variation in thermal tolerance limits. PLoS ONE 11, e0150408 ( 10.1371/journal.pone.0150408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirth HF. 1963. The ecology of two lizards on a tropical beach. Ecol. Monogr. 33, 83–112. ( 10.2307/1948557) [DOI] [Google Scholar]
- 19.Schoener TW, Schoener A. 1982. The ecological correlates of survival in some Bahamian anolis lizards. Oikos 39, 1–16. ( 10.2307/3544525) [DOI] [Google Scholar]
- 20.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.1111/j.1558-5646.1983.tb00236.x) [DOI] [PubMed] [Google Scholar]
- 21.Alexander LV, et al. 2006. Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res. Atmos. 111, D05109 ( 10.1029/2005JD006290) [DOI] [Google Scholar]
- 22.Drijfhout S, Bathiany S, Beaulieu C, Brovkin V, Claussen M, Huntingford C, Scheffer M, Sgubin G, Swingedouw D. 2015. Catalogue of abrupt shifts in Intergovernmental Panel on Climate Change climate models. Proc. Natl Acad. Sci. USA 112, E5777–E5786. ( 10.1073/pnas.1511451112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stott P. 2016. How climate change affects extreme weather events. Science 352, 1517–1518. ( 10.1126/science.aaf7271) [DOI] [PubMed] [Google Scholar]
- 24.Johnson NC, Xie SP, Kosaka Y, Li X. 2018. Increasing occurrence of cold and warm extremes during the recent global warming slowdown. Nat. Commun. 9, 1724 ( 10.1038/s41467-018-04040-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant PR. 2017. Evolution, climate change, and extreme events. Science 357, 451–452. ( 10.1126/science.aao2067) [DOI] [PubMed] [Google Scholar]
- 26.Endler JA. 1986. Natural selection in the wild, vol. 21. Princeton, NJ: Princeton University Press. [Google Scholar]
- 27.Patterson JW. 1984. Thermal acclimation in two subspecies of the tropical lizard Mabuya striata. Physiol. Zool. 57, 301–306. ( 10.1086/physzool.57.3.30163718) [DOI] [Google Scholar]
- 28.Clusella-Trullas S, Chown SL. 2014. Lizard thermal trait variation at multiple scales: a review. J. Comp. Physiol. B 184, 5–21. ( 10.1007/s00360-013-0776-x) [DOI] [PubMed] [Google Scholar]
- 29.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 30.Teets NM, Denlinger DL. 2013. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 38, 105–116. ( 10.1111/phen.12019) [DOI] [Google Scholar]
- 31.Layne JR Jr, Manis ML, Claussen DL. 1985. Seasonal variation in the time course of thermal acclimation in the crayfish Orconectes rusticus. Freshw. Invertebr. Biol. 4, 98–104. ( 10.2307/1467181) [DOI] [Google Scholar]
- 32.Sharma NK, Akhtar MS, Pandey N, Singh R, Singh AK. 2015. Seasonal variation in thermal tolerance, oxygen consumption, antioxidative enzymes and non-specific immune indices of Indian hill trout, Barilius bendelisis (Hamilton, 1807) from central Himalaya, India. J. Therm. Biol 52, 166–176. ( 10.1016/j.jtherbio.2015.07.005) [DOI] [PubMed] [Google Scholar]
- 33.Kour EL, Hutchison VH. 1970. Critical thermal tolerances and heating and cooling rates of lizards from diverse habitats. Copeia 1970, 219–229. ( 10.2307/1441644) [DOI] [Google Scholar]
- 34.Patterson JW, Davies PM. 1978. Thermal acclimation in temperate lizards. Nature 275, 646–647. ( 10.1038/275646a0) [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Sun YY, An H, Ji X. 2008. Northern grass lizards (Takydromus septentrionalis) from different populations do not differ in thermal preference and thermal tolerance when acclimated under identical thermal conditions. J. Comp. Physiol. B 178, 343–349. ( 10.1007/s00360-007-0227-7) [DOI] [PubMed] [Google Scholar]
- 36.Campbell-Staton SC, Bare A, Losos JB, Edwards SV, Cheviron ZA. 2018. Physiological and regulatory underpinnings of geographic variation in reptilian cold tolerance across a latitudinal cline. Mol. Ecol. 27, 2243–2255. ( 10.1111/mec.14580) [DOI] [PubMed] [Google Scholar]
- 37.Rogowitz GL. 1996. Evaluation of thermal acclimation and altitudinal variation of metabolism in the neotropical lizard, Anolis gundlachi. Copeia 1996, 535–542. ( 10.2307/1447517) [DOI] [Google Scholar]
- 38.Tsuji JS. 1988. Thermal acclimation of metabolism in Sceloporus lizards from different latitudes. Physiol. Zool. 61, 241–253. ( 10.1086/physzool.61.3.30161237) [DOI] [Google Scholar]
- 39.Patterson JW. 1991. Emergence, basking behaviour, mean selected temperature and critical thermal minimum in high and low altitude subspecies of the tropical lizard Mabuya striata. Afr. J. Ecol. 29, 330–339. ( 10.1111/j.1365-2028.1991.tb00470.x) [DOI] [Google Scholar]
- 40.Kolbe JJ, VanMiddlesworth PS, Losin N, Dappen N, Losos JB. 2012. Climatic niche shift predicts thermal trait response in one but not both introductions of the Puerto Rican lizard Anolis cristatellus to Miami, Florida, USA. Ecol. Evol. 2, 1503–1516. ( 10.1002/ece3.263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ Jr. 2014. Physiological variation among invasive populations of the brown anole (Anolis sagrei). Physiol. Biochem. Zool. 87, 92–104. ( 10.1086/672157) [DOI] [PubMed] [Google Scholar]
- 42.Rogowitz GL. 1996. Evaluation of thermal acclimation of metabolism in two eurythermal lizards, Anolis cristatellus and A. sagrei. J. Therm. Biol. 21, 11–14. [Google Scholar]
- 43.Lee RE, Chen CP, Denlinger DL. 1987. A rapid cold-hardening process in insects. Science 238, 1415–1417. ( 10.1126/science.238.4832.1415) [DOI] [PubMed] [Google Scholar]
- 44.Lee RE, Denlinger DL. 2010. Rapid cold-hardening: ecological significance and underpinning mechanisms. In Low temperature biology of insects (eds Denlinger DL, Lee RE), pp. 35–58. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Muñoz MM, Stimola MA, Algar AC, Conover A, Rodriguez AJ, Landestoy MA, Bakken GS, Losos JB. 2014. Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proc. R. Soc. B 281, 20132433 ( 10.1098/rspb.2013.2433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singhal S, Johnson M, Ladner JT. 2007. The behavioral ecology of sleep: natural sleeping site choice in three Anolis lizard species. Behaviour 144, 1033–1052. ( 10.1163/156853907781871860) [DOI] [Google Scholar]
- 47.Losos JB. 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles, vol. 10. Berkeley, CA: University of California Press. [Google Scholar]
- 48.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. 2013. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373–2380. ( 10.1111/gcb.12240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanger TJ, Kyrkos J, Lachance DJ, Czesny B, Stroud JT. 2018. The effects of thermal stress on the early development of the lizard Anolis sagrei. J. Exp. Zool. A 329, 244–251. ( 10.1002/jez.2185) [DOI] [PubMed] [Google Scholar]
- 51.Soroye P, Newbold T, Kerr J. 2020. Climate change contributes to widespread declines among bumble bees across continents. Science 367, 685–688. ( 10.1126/science.aax8591) [DOI] [PubMed] [Google Scholar]
- 52.Laurance WF, et al. 2011. Global warming, elevational ranges and the vulnerability of tropical biota. Biol. Conserv. 144, 548–557. ( 10.1016/j.biocon.2010.10.010) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data and code are included as electronic supplementary material.