Abstract
Eusocial societies are characterized by a clear division of labour between non-breeding workers and breeding queens, and queens often do not contribute to foraging, defence and other maintenance tasks. It has been suggested that the structure and organization of social mole-rat groups resembles that of eusocial insect societies. However, the division of labour has rarely been investigated in wild mole-rats, and it is unknown whether breeders show decreased foraging activity compared with non-breeding helpers in natural groups. Here, we show that, in wild Damaraland mole-rats (Fukomys damarensis), breeders show lower activity in foraging areas than non-breeding group members. Both breeders and non-breeders displayed variation in activity across the different seasons. Our results suggest that group living allows social mole-rat breeders to reduce their investment in energetically costly behaviour, or alternatively, that the high cost of reproduction in this species forces a behavioural trade-off against foraging investment.
Keywords: division of labour, cooperative breeding, social behaviour, helping, reproductive skew, bio-logging
1. Introduction
Eusocial insects show pronounced division of labour, where breeders are limited to the nest and reproduce, whereas workers are sterile and engage in foraging, brood care and defence of the colony [1]. It has been argued that two species of social mole-rats in the family Bathyergidae share some of the characteristics of termites and other eusocial insects [2–5]. In particular, previous studies have suggested that mole-rat groups consist of distinct castes of infrequent workers, frequent workers and breeders that differ in their behaviour, body size, growth and longevity [2–7]. In captive groups of both naked and Damaraland mole-rats, breeders move substrate and food through their tunnels less frequently than non-breeders and spend less time digging than non-breeding helpers [8,9], and recent studies of both species show that behavioural variation among non-breeders is continuously distributed and that non-breeders do not specialize in certain activities [10–12].
While captive studies provide detailed records of individual activity, food is usually provided ad libitum and animals are maintained in artificial tunnel systems that are far shorter than those in the natural habitat, where mole-rats dig vast tunnel systems through sandy soils in order to access the tubers of desert plants that they feed on [3,13]. Once accessed, most tubers are too large to be eaten by a single individual and are therefore left in situ and eaten when needed [3], though smaller bulbs and items are often moved to a common food store in close proximity to the nest (electronic supplementary material, figure S1). As a result, the reduced need for group members to dig and maintain tunnel systems in captive groups could decrease the difference in activity patterns between breeders and non-breeders, which may be substantially larger in natural groups. It is now important to compare the activity patterns of breeders and non-breeders in the wild to determine whether or not there are contrasts in the involvement of breeders and non-breeders in different cooperative activities, as there are in termites, primitively eusocial insects and other cooperatively breeding vertebrates.
In this study, we developed and used a bio-logging approach to record the activity of foraging Damaraland mole-rats in their natural habitat. While others have used telemetry-based methods to investigate general activity patterns of mole-rats in the wild [14–16], we deployed a radio-frequency identification (RFID) reader array to record the activity of transpondered individuals in foraging areas of the burrow system (electronic supplementary material, figures S2 and S3). Activity in these areas is likely closely linked to individual foraging behaviour because the reader array was always placed above tunnels showing recent digging activity in the form of fresh mounds (electronic supplementary material, figure S1 and table S1). Subsequently, we investigated whether breeders and non-breeders of wild Damaraland mole-rats differed in their investment in foraging activity, after having controlled for other individual characteristics and environmental drivers of activity.
2. Materials and methods
(a). Study animals
The Damaraland mole-rat is a cooperatively breeding species occurring in southern Africa in groups of up to 41 individuals, with a single reproductive female and usually a small number of reproductive males, while non-reproductive individuals engage in foraging behaviour, pup care and group defence [3]. The study was carried out at the Kalahari Research Centre in the Northern Cape of South Africa (26°58′ S, 21°49′ E), between January 2014 and September 2015. Mole-rats at our study site have been monitored from October 2013 to the present and all groups were recaptured approximately every 6 months. All individuals were implanted with a subcutaneous passive integrated transponder microchip at first capture (Trovan, DorsetID, The Netherlands; hereafter ‘transponder') and sexed by their genitals. Breeding females were identified from their perforated vagina and prominent teats [17]. Breeding males were identified through longitudinal capture records because these are usually the only large males remaining in breeding groups for a period of more than 1–2 years ([18], electronic supplementary material, figure S4]). On every capture, individuals were weighed to the nearest gram on an electronic balance.
(b). Activity recording
To measure mole-rat activity, we used a RFID reader array consisting of a stationary decoder (LID650/608, DorsetID, The Netherlands) connected to a panel antenna (Trovan ANT 612, 47.5 × 40 × 4 cm; electronic supplementary material, figures S2 and S3), which detected transponders passing within 30 cm of the panel. Mole-rat burrow tunnels run directly underneath a line of mounds. To place the array, we (i) identified an area of the burrow system with fresh mounds (indicating active foraging, see electronic supplementary material table S1), (ii) dug a shallow hole between two mounds, (iii) placed the panel antenna over the top of the tunnel and (iv) covered the panel with sand. The antenna was powered by a solar panel and a car battery to allow continuous activity recordings of up to 7 days. The decoder scanned continuously and recorded every transponder passing within range in 3 s intervals. If individuals remained in the read range for longer periods, they would, therefore, be recorded multiple times at 3 s intervals.
We placed the reader array repeatedly at 19 different groups (mean group size ± s.e. = 6.6 ± 0.53) in 39 different reading sessions (electronic supplementary material, figure S5). In most cases, the complete group was captured, as indicated by the absence of any trapping activity for 48 h at the end of the trapping session. Across the study, 128 unique individuals were trapped and recorded. The number of reading sessions on each group ranged from 1 to 8, (mean ± s.e. = 2.21 ± 0.40), and the number of reading sessions per month ranged from 1 to 7 (mean ± s.e. = 3.55 ± 0.56). The mean duration of reader placement was 41.84 h (s.e. = 4.20).
(c). Statistical analysis
To analyse individual variation in total daily activity, we calculated a daily activity score for each individual in a group that was detected at least once during a recording session and included individuals with zero values if they were recaptured after the recording session with the group but were not recorded by the RFID reader array. For each recording session, we removed data from the first 4 h after reader placement because visual inspection of the activity plots suggested that the mole-rat activity in this period may be affected by the disturbance of the group brought about by placement of the reader array and associated tunnel repair behaviour. Each time an individual was detected it gained a point of activity. Because previous radio tracking of Damaraland mole-rats suggested a mean duration of activity bouts of approximately 1 h [13], an individual was assumed active for the hour following each reading of its transponder. It would then not gain a further activity point until it was detected beyond the time of this 1 h window.
We analysed the variation in daily activity by fitting a generalized linear mixed model (GLMM) with Poisson error using the glmmTMB package [19] in R 3.6.2 [20]. As predictors of daily activity, we included individual characteristics (sex, body mass, breeding status), group characteristics (group size), the day of the reading (first day, second day, etc. …), the time of year (cosine and sine wave expressed in radians, where 0 is 1 January, 2π is 31 December) and rainfall. Rainfall was measured by an on-site weather station, and because data from a soil moisture probe indicated that rainfall increased soil moisture for a leading period of 20 days, rainfall represented the log-transformed total rainfall 20 days prior to an activity recording (electronic supplementary material, figure S6). We also added two-way interactions between the sex and reproductive status factors, as well as between sex and body mass. Individual identity and the unique reading session ID were specified as random intercepts to control for non-independence among observations. All continuous predictors were scaled to the mean and unit variance prior to model fitting, and because males are generally larger than females, the weight term was scaled within each sex. Inspection of the scaled model residuals indicated that assumptions of independence and normality of errors were met, and the dispersion parameter did not suggest any over- or under-dispersion. The proportion of explained variance was estimated using marginal and conditional R2 [21].
3. Results
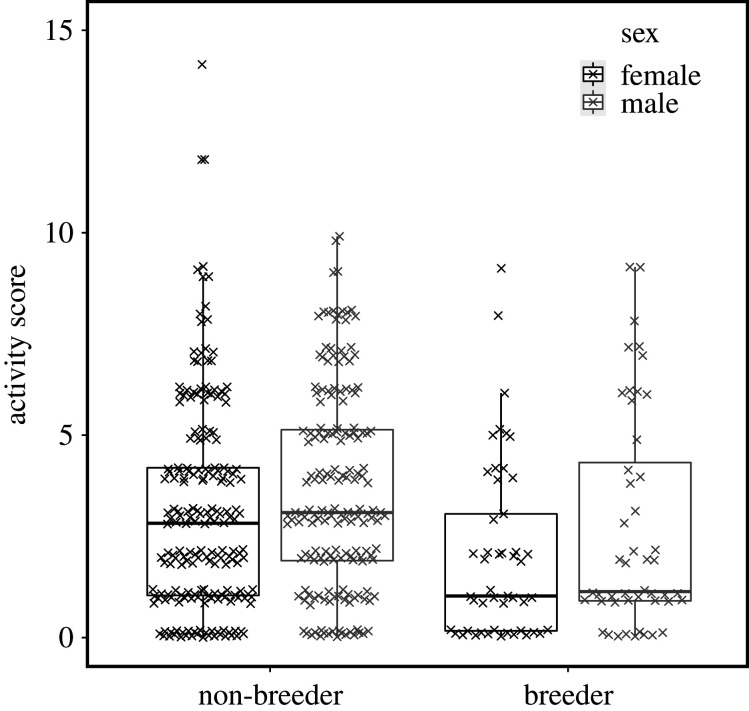
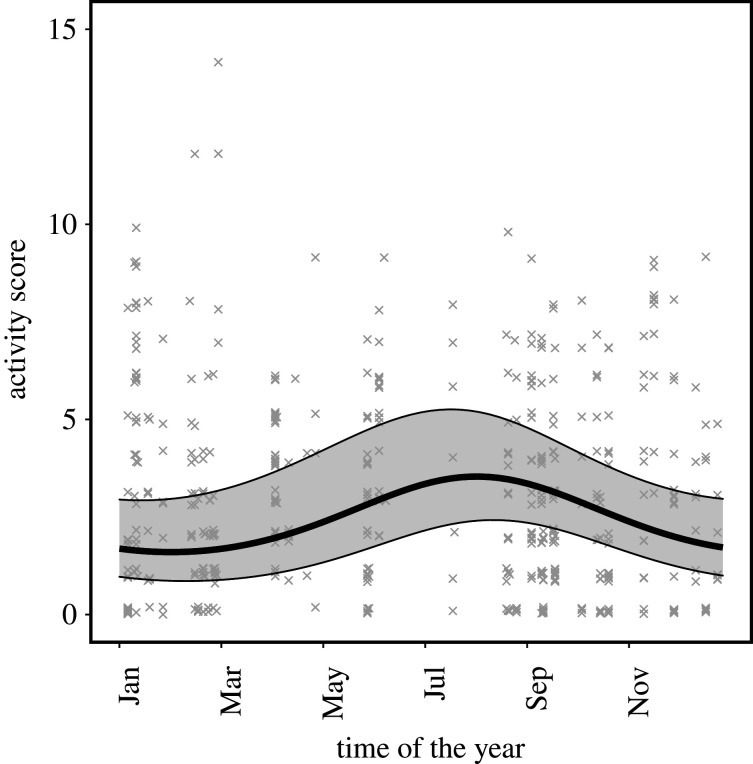
Our results show that breeders were less active than non-breeding group members in foraging areas of their group's tunnel system (table 1, figure 1 and electronic supplementary material, figure S7). Male and female breeders did not differ significantly from each other (interaction term sex ∗ breeder status, table 1, figure 1 and electronic supplementary material, figure S7). Foraging activity was independent of body mass (table 1). Across the seasons, rainfall increased mole-rat activity and highest activity occurred between April and October (table 1, figure 2).
Table 1.
Predictors of daily activity of Damaraland mole-rats, model output. Estimates, standard errors and p-values taken from a generalized linear mixed model assuming Poisson error structure. Estimates provided on the log scale (log link). Conditional R2 of the model: R2 = 0.61. Marginal R2 of the model: R2 = 0.21. All continuous predictors were scaled to the mean and unit variance for model fitting. See electronic supplementary material, table S2 for a corresponding minimal model.
| fixed effect | estimate | s.e | p-value |
|---|---|---|---|
| (intercept) | 1.42 | 0.12 | <0.001 |
| sex: male | 0.15 | 0.11 | 0.18 |
| breeding status: breeder | −0.52 | 0.20 | 0.01 |
| sex ∗ breeder status | 0.12 | 0.26 | 0.64 |
| sex ∗ body mass | 0.002 | 0.08 | 0.98 |
| body mass | 0.07 | 0.07 | 0.34 |
| group size | −0.17 | 0.11 | 0.14 |
| rainfall (log) | 0.41 | 0.15 | 0.006 |
| cos(annual variation) | −0.34 | 0.13 | 0.01 |
| sin(annual variation) | −0.20 | 0.14 | 0.15 |
| day | −0.24 | 0.04 | <0.001 |
| breeder status ∗ group size | 0.00 | 0.10 | 1.00 |
Figure 1.
Foraging activity of male (grey) and female (black) breeders and non-breeders in Damaraland mole-rats. Daily activity score represents the total number of unique 1 h windows in which an individual was detected in the foraging tunnel. The box and whiskers represent the median, 25/75% quantiles (hinges) and the smallest/largest observation less/more than lower/upper hinge ±1.5 × inter-quantile range. The raw data points are jittered behind the boxes (table 1).
Figure 2.
Foraging activity of Damaraland mole-rats across different seasons. Daily activity score represents the total number of unique 1 h windows in which an individual was detected in the foraging tunnel. Predictions were estimated from the GLMMs for a non-breeding female at the mean group size, with error bars indicating the 95% confidence intervals conditional on the fixed effects (table 1). The raw data points are plotted in the background.
4. Discussion
Our analysis of activity frequencies of wild Damaraland mole-rats suggests that male and female breeders spend about half as much time in foraging areas as do non-breeding individuals. Because activity in foraging areas is likely to be closely associated with levels of foraging [12], our results indicate that the contrasts in cooperative foraging may be more pronounced in the wild than in captive groups. In captivity, pregnant breeder females show about a 25% reduction across the population, and breeder males show similar investment to non-breeding helpers [2,8,9]. Given that the energetic costs of foraging are high in mole-rats [22], our results suggest that group living might allow social mole-rat breeders to reduce their investment in an energetically costly behaviour. However, whether this reduction is enough to offset the costs of breeding is uncertain, particularly for females, who often produce multiple litters per year [3] and for whom a single litter weighs on average 27.9% of a female's post-parturition mass (n = 47 captive litters; see electronic supplementary material).
Our data aligns well with recent studies on captive groups [10–12] and suggests that division of labour in mole-rat groups may be less pronounced than in primitively eusocial insects and resembles that of other cooperatively breeding vertebrates, where breeder contributions can vary widely. In the primitively eusocial paper wasp (Polistes sp.), queens rarely contribute to foraging [23], whereas in cooperatively breeding vertebrates, the breeders often show more substantial contributions to cooperative activities [24–26]. However, cooperation can take many forms and the degree to which the contributions of breeders and helpers diverge in their cooperative effort varies widely across different taxa and is likely to depend on the nature of the behaviour and the relative costs and benefits of carrying it out. For example, in cichlid fishes, breeders do less brood care compared with subordinate helpers [24], whereas in callitrichid primates, breeders and helpers both engage in energetically demanding infant-carrying behaviour, but the contribution of helpers is typically lower than that of breeders [25]. Some of the most detailed information on contrasts in cooperative behaviour in mammals comes from Kalahari meerkats, where reproduction is monopolized by a breeding pair and individuals engage in an unusually diverse array of cooperative behaviours, including pup-feeding, babysitting, digging and guarding [26]. Across these behaviours, dominant individuals of both sexes rarely babysit [27], but feed pups and dig at similar rates to subordinates [27], and dominant males are the most frequent sentinels in the group [28].
Our results indicate that there are pronounced contrasts in contribution to a potential public good between breeders and non-breeders in wild social mole-rats. However, we did not find differences in foraging activity across non-breeders of different sizes as suggested by some studies in captivity [5,8,11,12]. As breeders are generally the oldest individuals, we could not strictly separate the effect of reproductive status from age, and to overcome this confound experimental manipulations of breeding status in the wild would be needed. Our data also do not allow us to directly identify behaviours and we suggest that future studies of division of labour in wild mole-rats should make use of bio-logging techniques that facilitate the identification of behavioural modes [29] to study division of labour across non-breeding mole-rats, and thereby answer the question of whether non-breeders specialize permanently or temporarily on specific tasks, and whether variation in collective foraging affects development and future fitness prospects.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dave Gaynor, Tim Vink, Philippe Vullioud and all managers, students and volunteers for their contribution to the data collection and to organizing the field site, and Hanna Bensch and Shay Rotics for comments on a previous draft of the manuscript. We thank the Kalahari Research Trust for access to the research facilities, Marta Manser for her contribution to maintaining the Kalahari Research Centre, and Nigel Bennett for generous support and invaluable advice during this project. We are grateful to the Mammals Research Institute at the University of Pretoria for their continuous support and to the Northern Cape Department of Environment and Nature Conservation for permission to conduct research in the Northern Cape.
Ethics
The protocol used in this study was approved by the animal ethics committee of the University of Pretoria, EC032-13.
Data accessibility
The data and code are uploaded to Dryad (https://doi.org/10.5061/dryad.8931zcrnm [30]).
Authors' contributions
M.Z. and T.C.-B. conceived the study; K.F. and J.T. collected the data; Y.F. analysed the data; M.Z., J.T. and Y.F. wrote the paper; all authors commented on the manuscript and approved the final version of the manuscript. All authors agree to be held accountable for the content of the manuscript.
Competing interests
We have no competing interests.
Funding
This study was supported by Vetenskapsrådet (2017-05296), Crafoordska Stiftelsen (2018-2259) and H2020 European Research Council (294494 and 742808).
References
- 1.Bourke A. 2011. Principles of social evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Sherman PW, Jarvis JUM, Alexander RD. 1991. The biology of the naked mole-rat. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Bennett NC, Faulkes CG. 2000. African mole-rats: ecology and eusociality. Oxford, UK: Cambridge University Press. [Google Scholar]
- 4.O'Riain M, Jarvis J, Alexander R, Buffenstein R, Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 197. ( 10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis JU, O'Riain MJ, Bennett NC, Sherman PW. 1994. Mammalian eusociality: a family affair. Trends Ecol. Evol. 9, 47–51. ( 10.1016/0169-5347(94)90267-4) [DOI] [PubMed] [Google Scholar]
- 6.Scantlebury M, Speakman J, Oosthuizen M, Roper T, Bennett N. 2006. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature 440, 795–797. ( 10.1038/nature04578) [DOI] [PubMed] [Google Scholar]
- 7.Dammann P, Burda H. 2006. Sexual activity and reproduction delay ageing in a mammal. Curr. Biol. 16, R117–R118. ( 10.1016/j.cub.2006.02.012) [DOI] [PubMed] [Google Scholar]
- 8.Lacey EA, Sherman PW. 1991. Social organization of naked mole-rat colonies: evidence for divisions of labor. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD), pp. 275–336. Princeton, NJ: Princeton Universtiy Press. [Google Scholar]
- 9.Houslay TM, Vullioud P, Zöttl M, Clutton-Brock TH. 2020. Benefits of cooperation in captive Damaraland mole-rats. Behav. Ecol. 31, 711–718. ( 10.1093/beheco/araa015) [DOI] [Google Scholar]
- 10.Mooney SJ, Filice DC, Douglas NR, Holmes MM. 2015. Task specialization and task switching in eusocial mammals. Anim. Behav. 109, 227–233. ( 10.1016/j.anbehav.2015.08.019) [DOI] [Google Scholar]
- 11.Zöttl M, Vullioud P, Mendonça R, Ticó MT, Gaynor D, Mitchell A, Clutton-Brock T. 2016. Differences in cooperative behavior among Damaraland mole rats are consequences of an age-related polyethism. Proc. Natl Acad. Sci. USA 113, 10 382–10 387. ( 10.1073/pnas.1607885113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorley J, Mendonça R, Vullioud P, Torrents-Ticó M, Zöttl M, Gaynor D, Clutton-Brock T. 2018. No task specialization among helpers in Damaraland mole-rats. Anim. Behav. 143, 9–24. ( 10.1016/j.anbehav.2018.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovegrove B. 1988. Colony size and structure, activity patterns and foraging behaviour of a colony of the social mole-rat Cryptomys damarensis (Bathyergidae). J. Zool. 216, 391–402. ( 10.1111/j.1469-7998.1988.tb02437.x) [DOI] [Google Scholar]
- 14.Lövy M, Šklíba J, Šumbera R. 2013. Spatial and temporal activity patterns of the free-living giant mole-rat (Fukomys mechowii), the largest social bathyergid. PLoS ONE 8, e55357 ( 10.1371/journal.pone.0055357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Šklíba J, Lövy M, Hrouzková E, Kott O, Okrouhlík J, Šumbera R. 2014. Social and environmental influences on daily activity pattern in free-living subterranean rodents: the case of a eusocial bathyergid. J. Biol. Rhythms 29, 203–214. ( 10.1177/0748730414526358) [DOI] [PubMed] [Google Scholar]
- 16.Šklíba J, Lövy M, Burda H, Šumbera R. 2016. Variability of space-use patterns in a free living eusocial rodent, Ansell's mole-rat indicates age-based rather than caste polyethism. Scient. Rep. 6, 37497 ( 10.1038/srep37497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seney ML, Kelly DA, Goldman BD, Šumbera R, Forger NG. 2009. Social structure predicts genital morphology in African mole-rats. PLoS ONE 4, e7477 ( 10.1371/journal.pone.0007477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrents-Ticó M, Bennett NC, Jarvis JU, Zöttl M. 2018. Sex differences in timing and context of dispersal in Damaraland mole-rats (Fukomys damarensis). J. Zool. 306, 252–257. ( 10.1111/jzo.12602) [DOI] [Google Scholar]
- 19.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 20.R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 21.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 22.Lovegrove B. 1989. The cost of burrowing by the social mole rats (Bathyergidae) Cryptomys damarensis and Heterocephalus glaber: the role of soil moisture. Physiol. Zool. 62, 449–469. ( 10.1086/physzool.62.2.30156179) [DOI] [Google Scholar]
- 23.Dew HE. 1983. Division of labor and queen influence in laboratory colonies of Polistes metricus (Hymenoptera; Vespidae). Z. Tierpsychol. 61, 127–140. ( 10.1111/j.1439-0310.1983.tb01333.x) [DOI] [Google Scholar]
- 24.Zöttl M, Fischer S, Taborsky M. 2013. Partial brood care compensation by female breeders in response to experimental manipulation of alloparental care. Anim. Behav. 85, 1471–1478. ( 10.1016/j.anbehav.2013.03.045) [DOI] [Google Scholar]
- 25.Santos CV, French JA, Otta E. 1997. Infant carrying behavior in callitrichid primates: Callithrix and Leontopithecus. Int. J. Primatol. 18, 889–907. ( 10.1023/A:1026340028851) [DOI] [Google Scholar]
- 26.Clutton-Brock TH, Manser M. 2016. Meerkats: cooperative breeding in the Kalahari. In Cooperative breeding in vertebrates (eds Koenig WD, Dickinson JL), pp. 294–317. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Clutton-Brock TH, Russell AF, Sharpe LL. 2004. Behavioural tactics of breeders in cooperative meerkats. Anim. Behav. 68, 1029–1040. ( 10.1016/j.anbehav.2003.10.024) [DOI] [Google Scholar]
- 28.Clutton-Brock TH, O'Riain MJ, Brotherton PNM, Gaynor D, Kansky R, Griffin AS, Manser M. 1999. Selfish sentinels in cooperative mammals. Science 284, 1640–1644. ( 10.1126/science.284.5420.1640) [DOI] [PubMed] [Google Scholar]
- 29.Hammond TT, Springthorpe D, Walsh RE, Berg-Kirkpatrick T. 2016. Using accelerometers to remotely and automatically characterize behavior in small animals. J. Exp. Biol. 219, 1618–1624. ( 10.1242/jeb.136135) [DOI] [PubMed] [Google Scholar]
- 30.Francioli Y, Thorley J, Finn K, Clutton-Brock T, Zöttl M. 2020. Data From: Breeders are less active foragers than non-breeders in wild Damaraland mole-rats Dryad Digital Repository. ( 10.5061/dryad.8931zcrnm) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Francioli Y, Thorley J, Finn K, Clutton-Brock T, Zöttl M. 2020. Data From: Breeders are less active foragers than non-breeders in wild Damaraland mole-rats Dryad Digital Repository. ( 10.5061/dryad.8931zcrnm) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and code are uploaded to Dryad (https://doi.org/10.5061/dryad.8931zcrnm [30]).