Abstract
Background
The skin acts as a barrier to protect organisms against harmful exogenous agents. Compound K (CK) is an active metabolite of ginsenoside Rb1, Rb2 and Rc, and researchers have focused on its skin protective efficacy. In this study, we hypothesized that increased expression of the serine protease inhibitor Kazal type-5 (SPINK5) may improve skin barrier function.
Methods
We screened several ginsenosides to increase SPINK5 gene promoter activity using a transactivation assay and found that CK can increase SPINK5 expression. To investigate the protective effect of CK on the skin barrier, RT-PCR and Western blotting were performed to investigate the expression levels of SPINK5, kallikrein 5 (KLK5), KLK7 and PAR2 in UVB-irradiated HaCaT cells. Measurement of transepidermal water loss (TEWL) and histological changes associated with the skin barrier were performed in a UVB-irradiated mouse model and a 1-chloro-2,4-dinitrobenzene (DNCB)-induced atopic dermatitis–like model.
Results
CK treatment increased the expression of SPINK5 and decreased the expression of its downstream genes, such as KLKs and PAR2. In the UVB-irradiated mouse model and the DNCB-induced atopic dermatitis model, CK restored increased TEWL and decreased hydration and epidermal hyperplasia. In addition, CK normalized the reduced SPINK5 expression caused by UVB or DNCB, thereby restoring the expression of the proteins involved in desquamation to a level similar to normal.
Conclusions
Our data showed that CK contributes to improving skin-barrier function in UVB-irradiated and DNCB-induced atopic dermatitis–like models through SPINK5. These results suggest that therapeutic attempts with CK might be useful in treating barrier-disrupted diseases.
Keywords: Atopic dermatitis, Compound K, Skin barrier, SPINK5, UV
1. Introduction
The skin acts as a barrier to protect organisms against exogenous agents, e.g., mechanical, chemical, thermal, radioactive, immunological and microbiological barrier [1]. The skin acts as a mechanical barrier and prevents water, proteins and electrolytes from being lost from the body. The permeability barrier function is located mainly in the stratum corneum (SC) of the epidermis. Maintaining certain layers of SC is significant to maintain the mechanical skin-barrier function. Horny cells are strongly combined with each other via the corneodesmosomes, which consist of desmocollin-1, desmoglein-1, corneodesmosin and plakoglobin in the SC and stratum granulosum (SG) layers [2,3]. These tight contacts decrease toward the surface and disappear from the surface, finally resulting in the desquamation of the corneocytes. This decrease is due to breakdown of the corneodesmosomes caused by the tissue kallikreins (KLKs), a protein family with serine protease activities [4]. The serine protease inhibitor Kazal type-5 (SPINK5) regulates their activities, which is consistent with the proliferation, differentiation and desquamation rate of epidermal keratinocytes [[5], [6], [7]]. This turnover schedule is very stable and is maintained by the serine protease-SPINK5 balance regardless of age [8,9].
SPINK5 deficiency causes Netherton syndrome (NS), which is characterized by impaired skin-barrier function, epidermal hyperplasia, hair anomalies, chronic inflammation of the skin and atopic dermatitis symptoms [[10], [11], [12], [13]]. Decreased SPINK5 activity in Netherton syndrome leads to enhanced activity of serine protease, which impairs the cohesion of the SC, leading to thinning of the SC. Dermal growth is delayed, dehydration occurs and fetal death occurs [12,14]. Expression of KLK in the epidermis appears from the SS layer to the SC layer, and SPINK5 is expressed on the surface of the SG adjacent to the SC [6,12]. That is, KLK and SPINK5 are expressed simultaneously in the SC and can interact with each other. Regulators of local SPINK5 expression have not yet been identified. Presumably, limited expression of SPINK5 in the epidermis is expected to play an important role in controlling desquamation.
Ultraviolet (UV) radiation disrupts the epidermal barrier and induces desquamation of the SC. After UVB irradiation, the decrease in SPINK5 expression and the increase in KLK5 and KLK7 expression may contribute to the exfoliation of the SC [15]. Recent studies have proven that SPINK5 mutation is associated with atopic dermatitis (AD) across some ethnicities and causes defects in skin-barrier function [11,[16], [17], [18]].
Ginseng has been used to maintain good skin condition or improve the condition of it, e.g., as a skin application agent for wrinkle treatment, atopic dermatitis, wounds and other inflammatory skin symptoms. Among several ginsenosides from ginseng, compound K (CK), 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol, is an active metabolite of ginsenoside Rb1, Rb2 and Rc that is biotransformed by the human gut microbiome. Recently, attention to CK on skin protection efficacy has increased; it has been presented that CK decreases MMP-1 expression in tumor necrosis factor-α-stimulated or UVB-irradiated primary human dermal fibroblasts [19]. CK was shown to have antiatopic dermatitis activity in NC/Nga mice administered with dust mite extracts [20]. Although the potential of CK in maintaining good skin condition has been discovered, the precise mechanisms and signaling pathways to its effectiveness have not yet been fully revealed.
To date, research on SPINK5 has focused on mutations in genes, changes in expression and changes in clinical symptoms [[10], [11], [12], [13], [14], [15], [16], [17], [18]]. There has never been a study on natural products or compounds that can control SPINK5. So, we hypothesized that increasing SPINK5 expression may improve skin-barrier function. We screened several ginsenosides to increase SPINK5 gene promoter activity using a transactivation assay and found that CK can increase SPINK5 expression. In this study, we evaluated whether CK contributes to improving skin-barrier function in UVB-irradiated and a 1-chloro-2,4-dinitrobenzene (DNCB)-induced atopic dermatitis–like models and its potential as a therapeutic agent for defective diseases in skin barrier.
2. Materials and methods
2.1. Chemicals and cell cultures
Compound K (purity > 99%) was donated by Professor Si-Kwan Kim (Konkuk University, Chungju, Korea). HaCaT cells (N.E. Fusenig, Deutsches Krebsforschungszentrum, Heidelberg, Germany), which are spontaneously immortalized human keratinocytes, were cultured in Dulbecco's modified essential medium (DMEM; HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) and 100 units/mL penicillin and 100 μg/mL streptomycin (HyClone) at 37 °C in a humidified atmosphere containing 5% CO2. CV-1 cells (KCLB No. 10070) were bought from the Korea Cell Line Bank and grown with DMEM without sodium pyruvate supplemented with 10% FBS and 100 units/mL penicillin and 100 μg/mL streptomycin (HyClone) at 37 °C in a humidified atmosphere containing 5% CO2.
2.2. Quantitative RT-PCR
Total RNA was extracted from UVB-irradiated HaCaT cells using RLT buffer (Qiagen, Hilden, Germany). One μg of the extracted RNA was reverse-transcribed using a RevertAid first strand cDNA synthesis kit (Thermo Fisher Scientific, Bremen, Germany) according to the maker's instructions. Real-time PCR was performed using a 7500 Real-time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR1 Green (Power SYBR Green PCR Master Mix, Applied Biosystems). The primer sequences were designed using Primer Express software (Applied Biosystems). The primer sets were as follows: SPINK5, forward 5′- AGC CCC CAG TCT GTA TCC TT -3′ and reverse 5′- CTC CCT TTG CAG AAC TCA GG -3′ glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′- ACC ACA GTC CAT GCC ATC AC-3′ and reverse 5′- TCC ACC ACC CTG TTG CTG TA-3'. The PCR settings were as follows: the initial denaturation for 15 s at 95°C was followed by amplification of 35 cycles at 95°C for 3 s and at 60°C for 31 s and subsequent melting curve analysis was performed by increase of the temperature from 60°C to 95°C. Relative gene expression levels were calculated for GAPDH.
2.3. Immunoblotting analysis
Protein levels of SPINK5, KLK5 and PAR2 were measured by Western blot analysis. Briefly, cells and tissues were lysed in appropriate amounts of RIPA buffer (Bioprince, Chuncheon, Korea) and Phosphatase Inhibitor Cocktail 2 and 3 (Sigma-Aldrich, St. Louis, MO, USA). The lysate was centrifuged at 13,000 rpm for 15 min at 4°C, and the supernatant was collected for further studies. Denatured protein lysates were separated on a sodium dodecyl sulfate polyacrylamide gel by electrophoresis and transferred to polyvinylidene fluoride membranes (Merck, Darmstadt, Germany).
The blots were treated with primary antibodies against SPINK5 (1/1000; Abcam, Cambridge, MA, USA), KLK5 (1/1000; Abcam), protease activated receptor 2 (PAR2, 1/1000; Abcam) and GAPDH (1/3000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by administration with horseradish-peroxidase-conjugated secondary antibodies (1/3000; Santa Cruz) and visualized using an ECL kit (Thermo Fisher Scientific). The band densities were calculated by Image J software and expressed as a ratio to GAPDH.
2.4. Luciferase reporter gene assay
The PCR-amplified products for the SPINK5 promoter (−924 to +72) were inserted into the pGL3 Basic plasmid (Promega, Madison, WI, USA), yielding SPINK5pro-Luc. CV-1 cells were spread into appropriate well plates and cultured for 24 h before transfection. A SPINK5pro-Luc reporter plasmid and a control plasmid pRL-SV-40 were cotransfected using the TransFast™ reagent (Promega), and cultured for an additional 24 h. And then, cells were treated with the indicated concentrations of compound K for other 24 h. Luciferase activities of cell lysates were checked with the Dual-Luciferase® Reporter Assay System according to the maker's guidelines (Promega). The relative luciferase activity was calculated for the corresponding Renilla luciferase activity.
2.5. UVB irradiation
A Sankyo Denki G15T8E (Sankyo Denki, Japan) fluorescent UVB lamp with an emission spectrum between 280 and 360 nm at peak 312 nm was used as the UV source. HaCaT cells were cultured in DMEM for 24 h and pretreated with the indicated concentrations of CK for 1 h before UV irradiation. Then, the cells were washed with phosphate-buffered saline (PBS), exposed to UVB light in small amount of PBS, discarded PBS and retreated with the indicated concentrations of CK. And cells were cultured in cell culture medium for appropriate time periods in a humidified atmosphere containing 5% CO2 at 37°C.
2.6. Animals
Six-week-old female SKH-1 (hairless) mice were obtained from the animal facility of Orientbio (Sungnam, Korea). The mice were maintained in a temperature-controlled (temperature, 23°C ± 2°C; relative humidity, 55% ± 5%) facility with a 12-hr light/dark cycle. Animals were kept free to eat and drink standard animal chow and water in single cages. All laboratory procedures were approved by the Institutional Animal Care and Use Committee of the KIST (Certification No. KIST-2016-011, March 4, 2016).
2.7. UVB-irradiated animal experiments
UVB irradiation was started at 100 mJ/cm2, which corresponds to one minimal erythema dose (MED). Then, the dose was increased by one MED per two weeks until four MED, which was maintained until the end of the experiments. Mice in the UVB + CK group were dermally given 0.3% CK, and the remaining groups received vehicle (propylene glycol: EtOH = 7:3).
2.8. DNCB-induced animal experiments
For sensitization, 200 μl of 1% DNCB was administered to the mice’s back skin once a day for one week. Then, 200 μl of 0.1% DNCB was administered three times a week for two weeks with or without 0.3% CK, twice a day for two weeks.
2.9. Measurement of transepidermal water loss (TEWL) and skin hydration
A SKIN-O-MAT (Cosmomed, Ruhr, Germany) and an Aquaflux AF103 (Biox Systems, London, UK) were used to measure mice skin conditions. TEWL and skin hydration were checked at the beginning and end of the experiment under standard conditions (23 °C ± 2 °C, 55% ± 5% RH).
2.10. Measurement of total serum IgE and IL-4 levels
Blood samples were collected from abdominal aortas and centrifuged at 10,000 rpm for 15 min at 4 °C for serum collection. Total IgE and IL-4 levels were examined using enzyme-linked immunosorbent assay kits (eBioscience, San Diego, CA, USA), according to the supplier's guidelines.
2.11. Histological examination
The dorsal skin (1.5 × 0.5 cm) was detached and fixed in 3.7% formaldehyde (Sigma-Aldrich). Fixed tissues were embedded in paraffin for 24 h and serially cut to 4 μm thickness for histological examination. Tissue sections were stained with hematoxylin and eosin (H&E) and investigated for their general microscopic shape. Images were captured using ProgRes® CapturePro application software (JENOPTIK laser, Jena, Germany) and viewed at 200 X magnification.
2.12. Immunohistochemistry
Four-μm-thick paraffin sections were deparaffinized and immunohistochemically stained for KLK5 (Abcam) and DSC1 (Abcam) with Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488, Abcam) secondary antibody. Immunohistochemical staining was conducted according to common protocols.
2.13. Statistical analyses
The data are expressed as the mean ± standard deviation (SD). Differences in the mean values of the two groups were analyzed using one-way analysis of variance (ANOVA) and a Turkey's multiple comparisons post hoc analysis. The p-values < 0.05 were considered statistically significant.
3. Results
3.1. Effects of compound K on the expression of SPINK5 and downstream genes
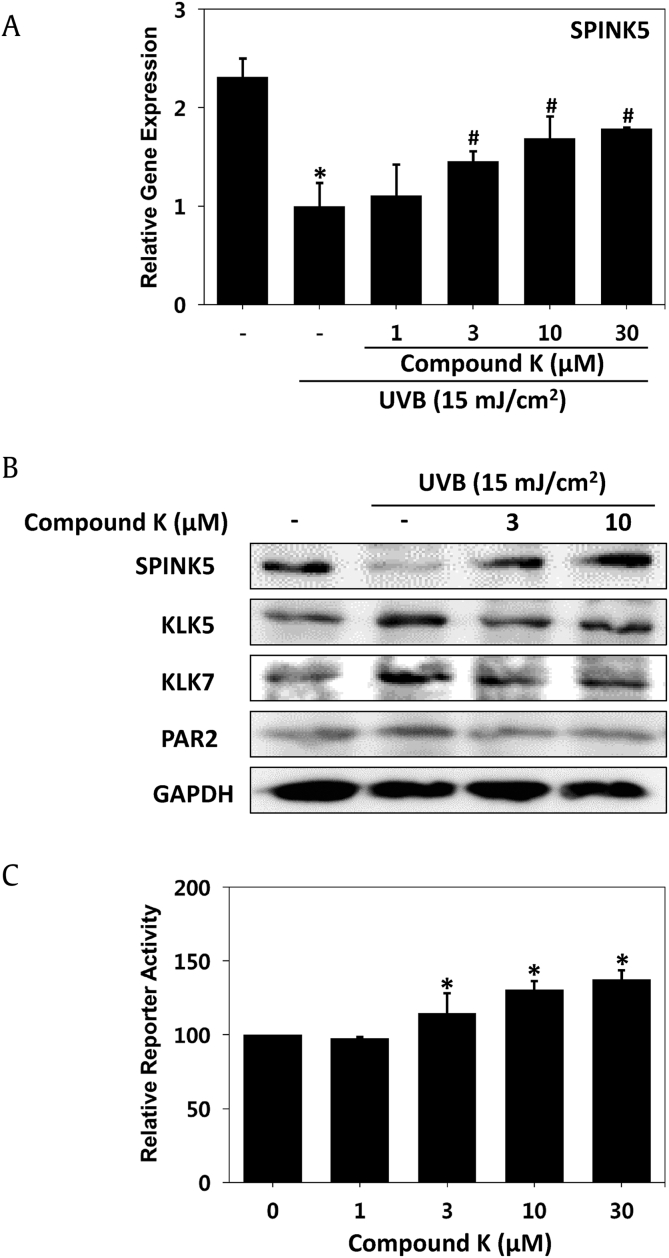
SPINK5 mRNA expression was decreased by UVB irradiation, but CK treatment restored the expression level of SPINK5 in a dose-dependent manner in HaCaT cells (Fig. 1A). The transcriptional activity of SPINK5 was examined by dual-luciferase assays. As a result, the transactivation of SPINK5 was increased by CK in a dose-dependent manner (Fig. 1C). Deficiency of SPINK5 in keratinocytes resulted in hyperactivity of KLK5 and KLK7, and then, hyperactive KLK5 increased PAR2. The protein level of SPINK5 was decreased by UVB, but the expression of SPINK was rescued by treatment with CK in HaCaT cells. KLK5, KLK7 and PAR2, downstream of SPINK5, showed an increase in protein expression after UVB irradiation and a decrease after CK treatment (Fig. 1B). Imbalance of protease and inhibitor and enhanced protease activities in epidermal keratinocytes after UVB irradiation will contribute to a decrease in keratinocyte adhesion and increased exfoliation of the SC [2]. CK treatment seems to solve these phenomena. Taken together, our results demonstrated that UVB irradiation decreases the expression of SPINK in transactivation, mRNA and protein levels, but CK treatment could reverse the UVB effect on SPINK5 and its downstream events.
Fig. 1.
Regulatory effect on SPINK5 transcription and SPINK5 downstream gene expression by CK. (A) Relative gene expression of SPINK5 was determined by Q-PCR and normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) Changed protein levels of SPINK5 and KLK5 were assayed by Western blot analysis of HaCaT cells. UVB was administered at 15 mJ/cm2. (C) CV-1 cells were cotransfected with a vector containing a SPINK5 promoter and a vector containing a reference universal promoter. Each bar represents the mean ± SD of three independent experiments. *p < 0.05 vs. control (CON); #p < 0.05 vs. the UVB-irradiated group.
3.2. Effects of CK on skin-barrier function in the UVB-irradiated barrier disrupted model
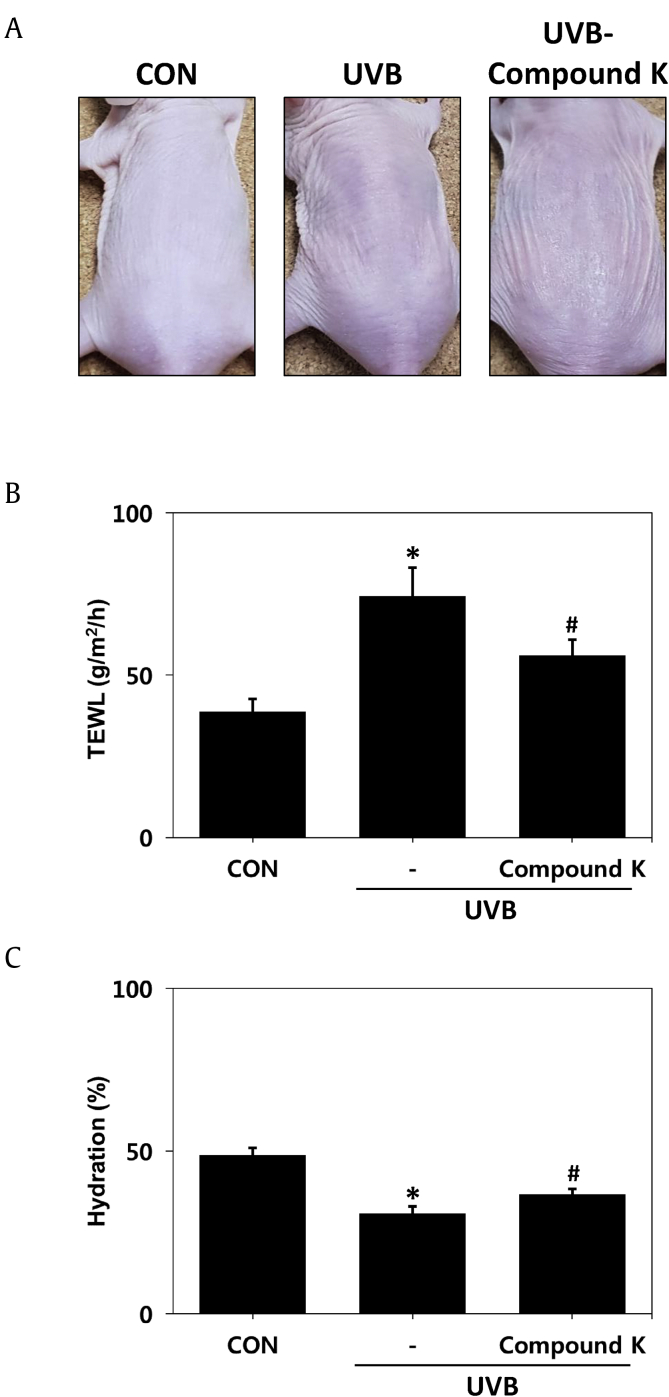
To verify that CK can improve the skin-barrier function, a mouse model with a damaged barrier due to UVB irradiation on the back was established. Groups irradiated with UVB for eight weeks showed typical skin-barrier damage phenomena, such as hyperkeratosis, parakeratosis, sunburn and dry skin. In contrast, the above symptoms were reduced in the group treated with CK at the same time as UVB irradiation (Fig. 2A). One of the most useful methods of assessing skin-barrier function is the quantitative measurement of TEWL [1]. Therefore, we measured TEWL and skin hydration to evaluate barrier function. The TEWL of the control group without any treatment was 30 g/m2/h, increased to 85 g/m2/h in the UVB irradiation group and decreased to 57 g/m2/h in the UVB and CK cotreated group (Fig. 2B). Skin hydration was approximately 50% in normal skin, 31% in the UV irradiation group and 37% in the CK treatment group (Fig. 2C). Taken together, these results indicated that UVB destroys the skin barrier, but CK apparently improves skin-barrier function.
Fig. 2.
Improvement in epidermal barrier permeability function by CK in the UVB-irradiated model. (A) Clinical features of dryness and skin-barrier impairment. (B) The level of transepidermal water loss (TEWL). (C) Estimation of skin hydration. The results are expressed as the mean ± SD (n = 7). *p < 0.05 vs. CON; #p < 0.05 vs. the UVB-irradiated group. CON: control group, UVB: UVB-irradiated group, UVB-CK: UVB-irradiated group and 0.3% compound K-treated group.
3.3. Effects of CK on epidermal thickness and barrier-function-related proteins in the UVB-irradiated animal model
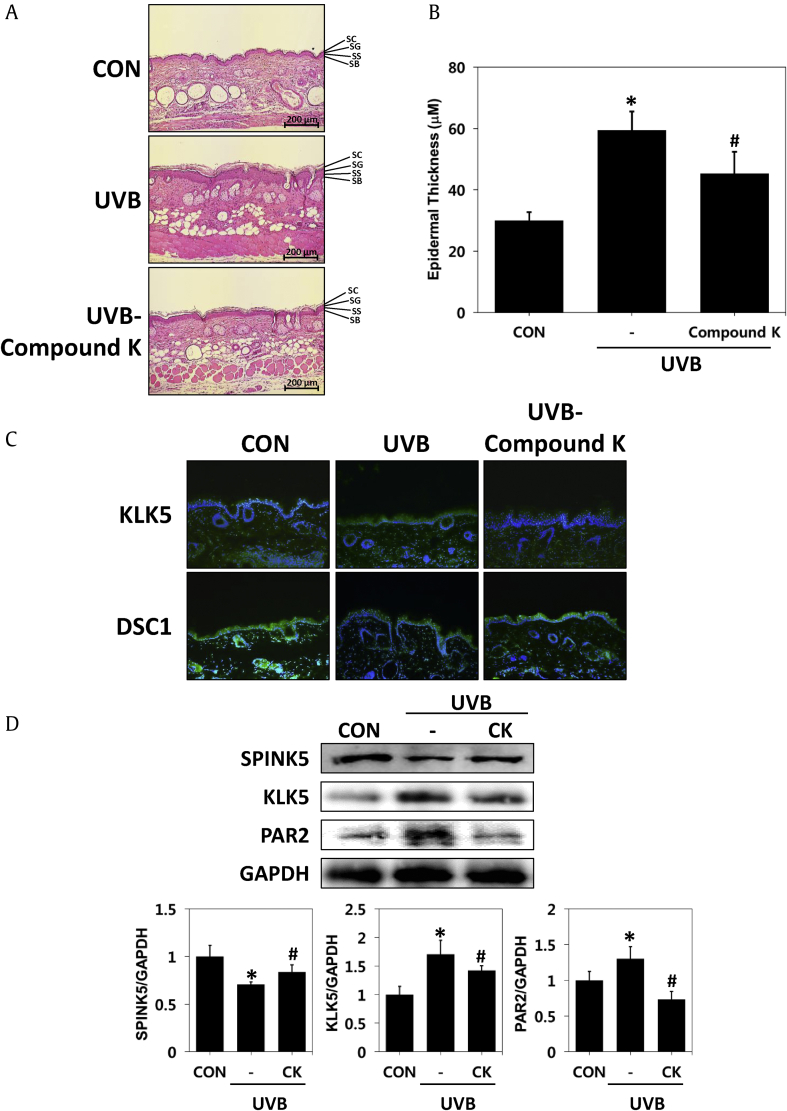
To determine whether CK can restore the damaged skin barrier, we examined the thickness of the epidermis, histological appearance and proteins related to skin-barrier function using dorsal tissue samples obtained from the mice. As a result of H&E staining, epidermal hyperkeratosis, which was prominent in the UVB irradiation group, was improved in the CK-treated group (Fig. 3A). Additionally, we confirmed that the same result was obtained in the measurement results of the skin thickness. UVB irradiation for 56 days significantly increased the thickness of the epidermis by two-fold compared to the control, and CK inhibited the increase in skin thickness by UVB irradiation to 73.5% (Fig. 3B).
Fig. 3.
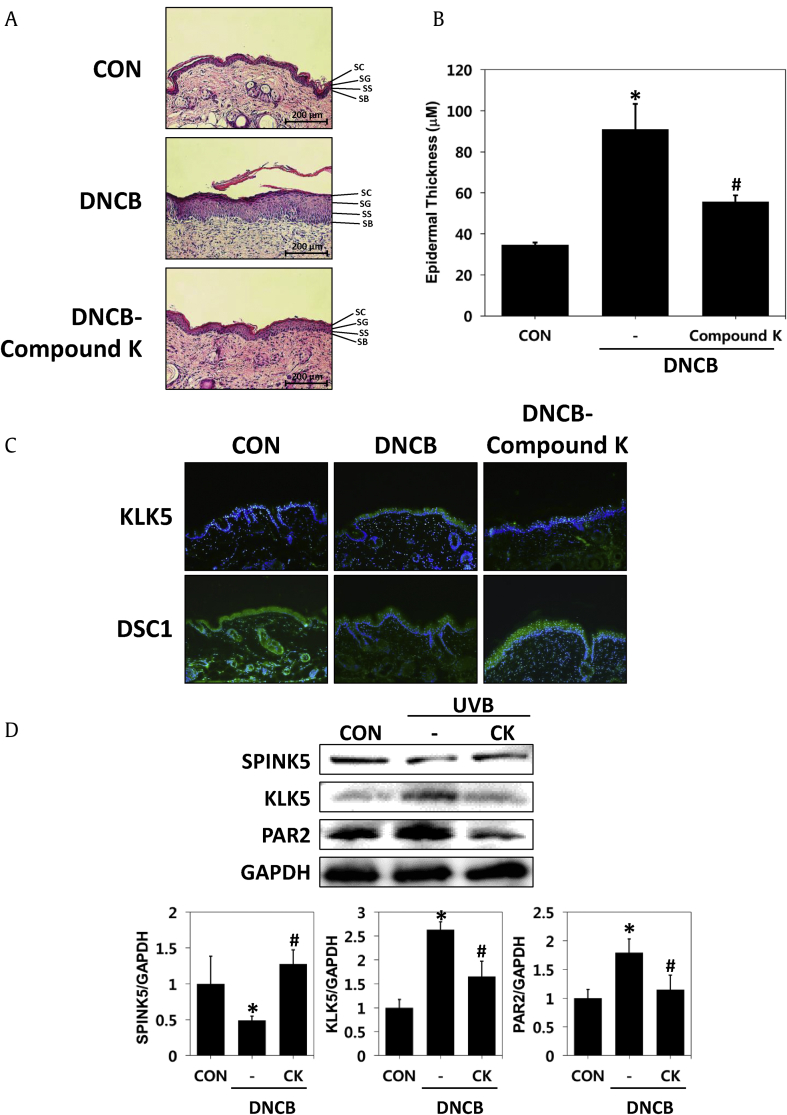
Histopathological analysis of CK in hairless mice with UVB-irradiated skin barrier dysfunction. Tissues were excised, fixed in 10% formaldehyde, embedded in paraffin and sectioned. (A) The sections were stained with hematoxylin and eosin (H&E) (magnification, x 100). (B) Epidermal thickness. (C) Immunohistochemistry of KLK5 and DSC1 expression (magnification × 100). (D) Changed protein levels of SPINK5, KLK5 and PAR2 were assayed by a Western blot analysis. The results are expressed as the mean ± SD (n = 7). *p < 0.05 vs. CON; #p < 0.05 vs. the UVB-irradiated group. CON: control group, UVB: UVB-irradiated group, UVB-CK: UVB-irradiated and 0.3% Compound K-treated group, KLK5: kallikrein 5, DSC1: desmocollin 1, SPINK5: serine protease inhibitor Kazal type-5, PAR2: protease activated receptor 2.
Several studies have shown that both KLK5 (kallikrein 5) and DSC1 (desmocollin 1) are located in the SC of the epidermis, and KLK5 contributes to desquamation by collapsing desmosomal cadherins such as Desmocollin 1. The location and level of proteins expressed in the epidermis related to desquamation of the skin, such as KLK5 and DSC1, were identified. Immunohistochemical staining of the tissues revealed that the expression of KLK5 was increased in the epidermis of the UVB-irradiated group compared to the untreated group. However, expression of KLK5 was decreased in the epidermis of the group treated with CK. In addition, desmocollin1 was decreased in the epidermal keratinocytes of the UVB-irradiated group but increased in the CK treatment group. These results suggested that the CK treatment inhibited the degradation of DSC1 by inhibiting KLK5 expression induced by UVB in the stratum corneum (Fig. 3C). Proteins were extracted from dorsal tissues of mouse models and used to measure the expression levels of SPINK5, KLK5 and PAR2 by Western blotting. UVB irradiation decreased SPINK5 and increased KLK5 and PAR2. However, CK administration reversed the phenomena caused by UVB (Fig. 3D). These results suggested that CK reduces epidermal hyperplasia and normalizes the expression of reduced SPINK5 caused by UVB, thereby restoring the expression of the protein involved in abnormal desquamation to a level similar to that of normal skin.
3.4. Effects of CK on epidermal-barrier function in a DNCB-induced atopic dermatitis–like animal model
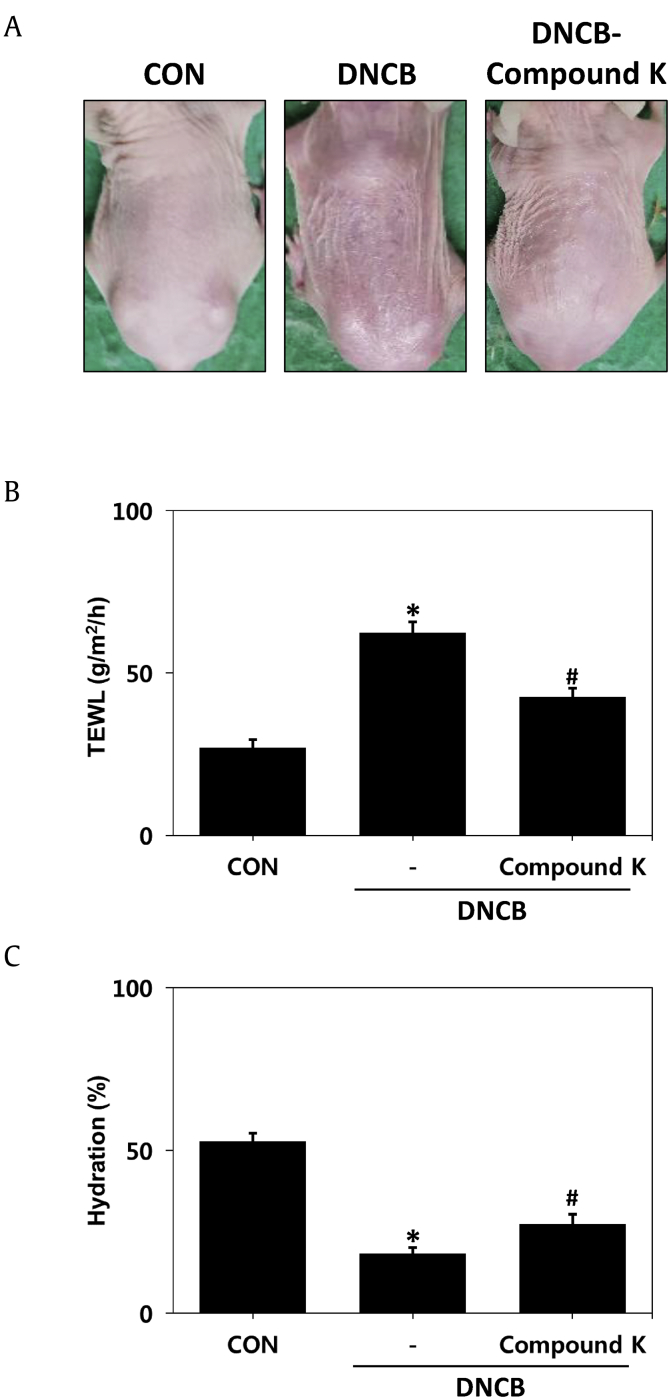
To demonstrate that CK can improve the function of the broken skin barrier, another mouse model of atopic dermatitis with impaired barrier function induced by DNCB was introduced. DNCB-treated mice showed skin-barrier abnormality, such as hyperkeratosis and dryness, as well as dermatitis symptoms. In contrast, in the group treated with DNCB and CK simultaneously, the above symptoms were reduced (Fig. 4A). To evaluate skin-barrier function, we measured TEWL and skin hydration. The TEWL of the control group without any treatment was 28 g/m2/h, increased to 65 g/m2/h in the DNCB-treated group and decreased to 42 g/m2/h in the DNCB- and CK-treated groups (Fig. 4B). Skin hydration was approximately 52% in normal skin, decreased to 20% in the DNCB-treated group and increased to 28% in the DNCB- and CK-treated groups (Fig. 4C). Taken together, these results indicated that the skin-barrier function, which is caused by atopic dermatitis, is restored when treated with CK.
Fig. 4.
Improvement of epidermal barrier and permeability function by CK in a DNCB-induced atopic dermatitis-like model. (A) Clinical features of atopic dermatitis-like skin lesions. (B) The level of transepidermal water loss (TEWL). (C) Estimation of skin hydration. The results are expressed as the mean ± SD (n = 7). *p < 0.05 vs. CON; #p < 0.05 vs. the DNCB-induced group. CON: control group, DNCB: DNCB-induced group, DNCB-CK: DNCB-induced and 0.3% CK-treated group.
3.5. Effects of CK on skin thickness and barrier function–related proteins in the DNCB-induced barrier disrupted animal model
To examine whether CK could ameliorate damaged skin barriers caused by atopic dermatitis, we measured epidermal thickness, histological appearance and proteins that were associated with skin-barrier function, as in the UVB-irradiated mouse model. First, samples obtained from dorsal tissue of mice were stained with H&E to investigate histological changes. As shown by H&E staining, epidermal hyperkeratosis was alleviated in the CK-treated group compared to the DNCB-induced group (Fig. 5A). Application with DNCB for 14 days resulted in a three-fold increase in epidermal thickness compared to the control, while CK suppressed DNCB-induced skin thickening by 50.5% (Fig. 5B).
Fig. 5.
Histopathological analysis of compound K in hairless mice with DNCB-induced atopic dermatitis-like model. Tissues were excised, fixed in 10% formaldehyde, embedded in paraffin and sectioned. (A) The sections were stained with hematoxylin and eosin (H&E) (magnification, x 100). (B) Epidermal thickness. (C) Immunohistochemistry of KLK5 and DSC1 expression (magnification × 100). (D) Changed protein levels of SPINK5, KLK5 and PAR2 were assayed by Western blot analysis. The results are expressed as the mean ± SD (n = 7). *p < 0.05 vs. CON; #p < 0.05 vs. the DNCB-induced group. CON: control group, DNCB: DNCB-induced group, DNCB-CK: DNCB-induced and 0.3% CK-treated group, KLK5: kallikrein 5, DSC1: desmocollin 1, SPINK5: serine protease inhibitor Kazal type-5, PAR2: protease activated receptor 2.
The localization and levels of KLK5 and DSC1 in the skin tissues of mice treated with CK were measured. KLK5 expression in the immunohistochemical staining of tissues was found to be increased compared to the untreated group in the epidermis of the DNCB-induced group. However, KLK5 expression decreased in the epidermis of the group treated with DNCB and CK. In addition, desmocolin 1, which was degraded by KLK5, was decreased in epidermal keratinocytes of the DNCB-inducible group. However, this molecule increased in the DNCB and CK treatment groups (Fig. 5C). Next, proteins expressed in the epidermis related to skin desquamation were identified. Proteins were extracted from the dorsal tissues of the normal, DNCB-, or DNCB- and CK-treated mouse groups, and the levels of SPINK5, KLK5 and PAR2 were evaluated using Western blotting. DNCB treatment reduced SPINK5 and enhanced KLK5 and PAR2. However, the expression of the SPINK5 protein was increased, and KLK5 and PAR2 were decreased by the CK treatment (Fig. 5D). These results suggest that CK restores the expression of various proteins associated with atopic hyperplasia and hyperkeratosis caused by atopic dermatitis to normal levels.
4. Discussion
The skin barrier is one of the most important protective factors in the body. This barrier is sustained by a balance between the firm contact of corneocytes and the desquamation of the SC layer. The skin is turned over at regular intervals during proliferation, differentiation and desquamation of keratinocytes. This turnover is precisely controlled by the balance between desquamation-related serine protease and SPINK5. In skin exposed to UV rays or with atopic dermatitis, the expression of SPINK5 is disturbed, leading to a weakening of the skin barrier. In this study, we focused on the regulation of SPINK5 expression to enhance skin-barrier function. Screening of various components of ginseng, which is known to have protection efficacy on skin, revealed that compound K enhanced the transcriptional activity of SPINK5, and the efficacy of CK in an animal model with reduced SPINK5 expression was verified.
UVB irradiation in the skin has been presented to lead the exfoliation of keratinocytes [21], but enzymes in keratinocytes whose expression is regulated by UVB irradiation has not been clearly elucidated. To elucidate the role of these enzymes in the exfoliation of the SC by UVB, we investigated molecules whose expression is altered by UVB irradiation in human epidermal keratinocytes. When UVB was irradiated with HaCaT cells, SPINK5 gene and protein expression was decreased, while expression of KLK5, KLK7 and PAR2 was increased. Activation and upregulation of PAR2 can be facilitated by other unregulated proteases such as KLK5, KLK7 or other factors secreted from the skin cells, which sustain the induction of multiple signal transduction pathways in SPINK5-deficient skin. These results provide evidence that the activation of a biological cascade that causes skin inflammation in NS is an intrinsic property of SPINK5-deficient keratinocytes, namely a cellular autonomous process. In addition, increased expression of PAR2 induces an increase in TSLP, causing an abnormal immune response [22]. Therefore, SPINK5 increased by CK not only enhances skin-barrier function, but also inhibits the activity of serine protease in the epidermis and inhibits the expression of PAR2, enhancing the skin barrier function. It can also inhibit inflammatory responses. It is reported that SPINK5 gene expression in keratinocytes is regulated by transcription factors targeting the promoter regions −676/–532 and −318/–146 [23]. However, it is not yet known which cis-elements play this role, but it is speculated that CK will also raise SPINK5 expression by regulating transcription factors in putative cis-elements located at these promoter sites. Transient receptor potential V1 (TRPV1) acts as a temperature sensor and is known to be involved in epidermal permeability barrier homeostasis in keratinocytes. It is activated at about 42°C and above and its expression is increased by UVB. Some reports indicate that activators of TRPV1 delay barrier recovery [24,25]. Therefore, it is expected that CK may restore barrier function by regulating TRPV1, one of the causes of skin barrier damage caused by UVB. This time, we did not attempt to study this, but the next study will investigate whether CK affects expression or activation of TRPV1. Based on the above results, we demonstrated the effectiveness of CK with two animal models with a weakened skin barrier.
In the first model, based on in vitro experiments, UVB irradiation on the back of hairless mice weakened the skin barrier. As a result, characteristics such as hyperkeratosis, dry skin and water loss were observed. Histological analysis showed that expression of KLK5 was increased in the epidermis due to the decrease in SPINK5, and the expression of DSC1 was decreased. However, treatment with CK increased SPINK5 expression, which resulted in decreased expression of KLK5 and increased expression of DSC. UVB irradiation weakened the skin barrier, but CK treatment improved the skin.
In the second model, DNCB-induced lesions such as atopic dermatitis were then treated with CK to prove that the lesions improved. Changes in the skin due to DNCB induction have been characterized by excessive eczema with persistent scaling of erythema, skin dryness and bleeding. An increased level of IgE and DNCB-induced hypersensitivity due to the development of immune responses regulated by T cells were also observed [26]. In skin-barrier function, these changes reduce skin-barrier proteins, such as filaggrin, loricrin and involucrin [27]. CK treatment enhanced moisturization by strengthening the skin barrier weakened by DNCB, tightening the skin barrier and increasing the expression of DSC1 in the epidermis. Protein expression of KLK5 and PAR2 was also decreased compared to the DNCB induction group.
Thus, skin-barrier function weakened by UVB or DNCB was improved by the CK-mediated increase in SPINK5 and decrease in the expression of serine proteases such as KLK5 and KLK7. Decreased expression of serine proteases reduced the degradation of corneodesmosomes such as DSC1 and DSG1, which can protect against skin dehydration and entry of external antigens.
In summary, CK is expected to enhance skin-barrier function, not by blocking UVB but by increasing SPINK5, which is reduced by UVB. In addition, CK improved the expression of SPINK5 reduced by DNCB in various lesions of DNCB-induced atopic dermatitis mice. Therefore, CK is expected not only to increase SPINK5 reduced by UVB but also to restore the weakened skin-barrier function by increasing SPINK5 in other skin-barrier dysfunction models, such as atopic dermatitis and Netherton syndrome.
5. Conclusion
Taken together, we identified for the first time that CK can regulate the expression of SPINK5. This means that CK can be used as an excellent substance for improving skin-barrier function weakened by UVB or various skin weakening factors, as well as diseases such as atopic dermatitis. In addition, few studies have been done regarding the relevance of SPINK5 among many factors that cause sunburn, hyperkeratosis, desquamation and erythema caused by UVB. Therefore, our study is also expected to contribute to clarifying the connection between UVB and SPINK5.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, the Ministry of Health & Welfare, Republic of Korea (Grant No. HI14C2687) and by the Korea Institute of Science and Technology, Republic of Korea (Grant Nos. 2Z05610 and 2Z05650).
References
- 1.Antonov D., Schliemann S., Elsner P. Methods for the assessment of barrier function. Curr Probl Dermatol. 2016;49:61–70. doi: 10.1159/000441546. [DOI] [PubMed] [Google Scholar]
- 2.North A.J., Bardsley W.G., Hyam J., Bornslaeger E.A., Cordingley H.C., Trinnaman B., Hatzfeld M., Green K.J., Magee A.I., Garrod D.R. Molecular map of the desmosomal plaque. J Cell Sci. 1999;112:4325–4336. doi: 10.1242/jcs.112.23.4325. [DOI] [PubMed] [Google Scholar]
- 3.Angst B.D., Marcozzi C., Magee A.I. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 4.Yousef G.M., Diamandis E.P. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- 5.Komatsu N., Takata M., Otsuki N., Toyama T., Ohka R., Takehara K., Saijoh K. Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J Invest Dermatol. 2003;121:542–549. doi: 10.1046/j.1523-1747.2003.12363.x. [DOI] [PubMed] [Google Scholar]
- 6.Komatsu N., Saijoh K., Toyama T., Ohka R., Otsuki N., Hussack G., Takehara K., Diamandis E.P. Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. Br J Dermatol. 2005;153:274–281. doi: 10.1111/j.1365-2133.2005.06754.x. [DOI] [PubMed] [Google Scholar]
- 7.Pierard G.E., Goffin V., Hermanns-Le T., Pierard-Franchimont C. Corneocyte desquamation. Int J Mol Med. 2000;6:217–221. doi: 10.3892/ijmm.6.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Ya-Xian Z., Suetake T., Tagami H. Number of cell layers of the stratum corneum in normal skin - relationship to the anatomical location on the body, age, sex and physical parameters. Arch Dermatol Res. 1999;291:555–559. doi: 10.1007/s004030050453. [DOI] [PubMed] [Google Scholar]
- 9.Harding K. Challenges for skin and wound care in the new century. Adv Skin Wound Care. 2000;13(212):215. [PubMed] [Google Scholar]
- 10.Raghunath M., Tontsidou L., Oji V., Aufenvenne K., Schurmeyer-Horst F., Jayakumar A., Stander H., Smolle J., Clayman G.L., Traupe H. SPINK5 and Netherton syndrome: novel mutations, demonstration of missing LEKTI, and differential expression of transglutaminases. J Invest Dermatol. 2004;123:474–483. doi: 10.1111/j.0022-202X.2004.23220.x. [DOI] [PubMed] [Google Scholar]
- 11.Chavanas S., Bodemer C., Rochat A., Hamel-Teillac D., Ali M., Irvine A.D., Bonafe J.L., Wilkinson J., Taieb A., Barrandon Y. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu N., Takata M., Otsuki N., Ohka R., Amano O., Takehara K., Saijoh K. Elevated stratum corneum hydrolytic activity in Netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5-derived peptides. J Invest Dermatol. 2002;118:436–443. doi: 10.1046/j.0022-202x.2001.01663.x. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu N., Saijoh K., Jayakumar A., Clayman G.L., Tohyama M., Suga Y., Mizuno Y., Tsukamoto K., Taniuchi K., Takehara K. Correlation between SPINK5 gene mutations and clinical manifestations in Netherton syndrome patients. J Invest Dermatol. 2008;128:1148–1159. doi: 10.1038/sj.jid.5701153. [DOI] [PubMed] [Google Scholar]
- 14.Stoll C., Alembik Y., Tchomakov D., Messer J., Heid E., Boehm N., Calvas P., Hovnanian A. Severe hypernatremic dehydration in an infant with Netherton syndrome. Genet Couns. 2001;12:237–243. [PubMed] [Google Scholar]
- 15.Nin M., Katoh N., Kokura S., Handa O., Yoshikawa T., Kishimoto S. Dichotomous effect of ultraviolet B on the expression of corneodesmosomal enzymes in human epidermal keratinocytes. J Dermatol Sci. 2009;54:17–24. doi: 10.1016/j.jdermsci.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Nishio Y., Noguchi E., Shibasaki M., Kamioka M., Ichikawa E., Ichikawa K., Umebayashi Y., Otsuka F., Arinami T. Association between polymorphisms in the SPINK5 gene and atopic dermatitis in the Japanese. Genes Immun. 2003;4:515–517. doi: 10.1038/sj.gene.6363889. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L.P., Di Z., Zhang L., Wang L., Ma L., Lv Y., Hong Y., Wei H., Chen H.D., Gao X.H. Association of SPINK5 gene polymorphisms with atopic dermatitis in Northeast China. J Eur Acad Dermatol Venereol. 2012;26:572–577. doi: 10.1111/j.1468-3083.2011.04120.x. [DOI] [PubMed] [Google Scholar]
- 18.Walley A.J., Chavanas S., Moffatt M.F., Esnouf R.M., Ubhi B., Lawrence R., Wong K., Abecasis G.R., Jones E.Y., Harper J.I. Gene polymorphism in Netherton and common atopic disease. Nat Genet. 2001;29:175–178. doi: 10.1038/ng728. [DOI] [PubMed] [Google Scholar]
- 19.Kim E., Kim D., Yoo S., Hong Y.H., Han S.Y., Jeong S., Jeong D., Kim J.H., Cho J.Y., Park J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J Ginseng Res. 2018;42:218–224. doi: 10.1016/j.jgr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.R., Choi J., Kim J., Kim H., Kang H., Kim E.H., Chang J.H., Kim Y.E., Choi Y.J., Lee K.W. 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365–371. doi: 10.1016/j.jep.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Corcuff P., Leveque J.L. Corneocyte changes after acute UV irradiation and chronic solar exposure. Photodermatol. 1988;5:110–115. [PubMed] [Google Scholar]
- 22.Briot A., Deraison C., Lacroix M., Bonnart C., Robin A., Besson C., Dubus P., Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le N.A., Katsuyama M., Demura M., Tanii H., Katsuyama H., Saijoh K. Regulation of serine protease inhibitor Kazal type-5 (SPINK5) gene expression in the keratinocytes. Environ Health Prev Med. 2014;19:307–313. doi: 10.1007/s12199-014-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denda M., Sokabe T., Fukumi-Tominaga T., Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007;127:654–659. doi: 10.1038/sj.jid.5700590. [DOI] [PubMed] [Google Scholar]
- 25.Huang K.F., Ma K.H., Liu P.S., Chen B.W., Chueh S.H. Ultraviolet B irradiation increases keratin 1 and keratin 10 expressions in HaCaT keratinocytes via TRPV1 activation and ERK phosphorylation. Exp Dermatol. 2017;26:832–835. doi: 10.1111/exd.13292. [DOI] [PubMed] [Google Scholar]
- 26.Inagaki N., Nagai H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment:mouse models for the development of remedies for human allergic dermatitis. J Pharmacol Sci. 2009;110:251–259. doi: 10.1254/jphs.09r01fm. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.W., Wu Q., Jang Y.P., Choung S.Y. Pinus densiflora bark extract ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by regulating Th1/Th2 balance and skin barrier function. Phytother Res. 2018;32:1135–1143. doi: 10.1002/ptr.6061. [DOI] [PubMed] [Google Scholar]