Abstract
Background
Beneficial effects of Korean Red Ginseng (KRG) on polycystic ovarian syndrome (PCOS) remains unclear.
Methods
We examined whether pretreatment (daily from 2 hours before PCOS induction) with KRG extract in water (KRGE; 75 and 150 mg/kg/day, p.o.) could exert a favorable effect in a dehydroepiandrosterone (DHEA)-induced PCOS rat model.
Results
Pretreatment with KRGE significantly inhibited the elevation of body and ovary weights, the increase in number and size of ovarian cysts, and the elevation of serum testosterone and estradiol levels induced by DHEA. Pretreatment with KRGE also inhibited macrophage infiltration and enhanced mRNA expression levels of chemokines [interleukin (IL)-8, monocyte chemoattractant protein-1), proinflammatory cytokines (IL-1β, IL-6), and inducible nitric oxide synthase in ovaries induced by DHEA. It also prevented the reduction in mRNA expression of growth factors (epidermal growth factor, transforming growth factor-beta (EGF, TGF-β)) related to inhibition of the nuclear factor kappa-light-chain-enhancer of activated B cell pathway and stimulation of the nuclear factor erythroid–derived 2-related factor 2 pathway. Interestingly, KRGE or representative ginsenosides (Rb1, Rg1, and Rg3(s)) inhibited the activity of inflammatory enzymes cyclooxygenase-2 and iNOS, cytosolic p-IkB, and nuclear p–nuclear factor kappa-light-chain-enhancer of activated B in lipopolysaccharide-induced RAW264.7 cells, whereas they increased nuclear factor erythroid–derived 2-related factor 2 nuclear translocation.
Conclusion
These results provide that KRGE could prevent DHEA-induced PCOS via antiinflammatory and antioxidant activities. Thus, KRGE may be used in preventive and therapeutic strategies for PCOS-like symptoms.
Keywords: Dehydroepiandrosterone, Korean Red Ginseng extract, Polycystic ovarian syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) is the most common cause of female subfertility, affecting approximately 12% of women during and beyond their reproductive years [1], [2]. Its main features include chronic oligo-ovulation or anovulation, impaired fertility, and metabolic syndromes related to type II diabetes mellitus, hyperinsulinemia, hyperandrogenism (clinical or biochemical), hirsutism, acne, increased incidence of endometrial cancer, and polycystic ovaries (histopathological) [1], [3], [4], [5]. Although genetic factors and environmental factors such as diet and stress might be associated to the development of PCOS [6], the pathogenesis of PCOS remains unclear.
Pharmacological agents for treatment of PCOS are very limited. Clomiphene citrate is a standard treatment to produce ovulation in women with PCOS. It is still considered the first line drug [7]. However, clomiphene citrate has some adverse effects, including high failure rate of ovulation (25–30%), significant discrepancy between ovulation and pregnancy rates, antiestrogenic actions on the endometrium and cervical mucous, long half-life, and multifollicular ovulation. In addition, clomiphene citrate is expensive with some adverse effects [7]. Metformin (N,N′-dimethyl-biguanide) is the most extensively used insulin lowering agent in PCOS [8]. According to current recommendations, metformin use should be limited in women with PCOS, who are glucose intolerant [8]. Furthermore, metformin can increase multiple follicular development, the risk of ectopic pregnancy, and congenital malformations, such as neural tube defects [8], [9].
Complementary and alternative medicines such as herbs, phytochemicals, and dietary supplements can mitigate PCOS or decrease the disadvantages of chemicals such as clomiphene citrate and metformin used for PCOS [10]. However, there are minimal evidences that complementary and alternative medicines are safe and efficacious. Thus, new preventive and therapeutic strategies such as complementary and alternative treatment should be evaluated to alleviate PCOS, regulate hormones, and improve quality of life of PCOS women. Panax (P.) ginseng (Korean ginseng) is a popular herbal medicine that has been widely used for more than 2,000 years in Oriental countries. It has long been considered as “the king of herbs” with various pharmacological activities on the immune, circulatory, respiratory, endocrine, gastrointestinal, and central nervous systems [11], [12], [13]. P. ginseng may reduce menopausal symptoms such as sleep disorder, depression, and anxiety. It is often used as a natural estrogen replacement therapy. Furthermore, P. ginseng extract can activate the growth of estrogen receptor (ER) positive (+) cells in vitro. Ginsenoside Rb1 and Rg1 can stimulate ERs with estrogen-like activity [14], [15], [16]. In ovariectomized mice, P. ginseng can recover the estrus cycle and exert significant estrogenic effects as suggested by the reversal of atrophy of the uterus and vagina with upregulated expression of ER α and ER β in reproductive tissues. Meanwhile, P. ginseng can significantly increase serum estradiol and reduced follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in the serum [17]. These reports highlight its possible use for PCOS. Recently, we introduced that Korean Red Ginseng extract in water (KRGE) can reduce the formation of antral follicles and increase the number of corpora lutea in polycystic ovaries associated with normalization of nerve growth factor in an estradiol valerate–treated rat model for PCOS [18]. However, the effect of KRGE on PCOS has not been clearly elaborated yet. Therefore, the objective of this study was to determine the preventive and therapeutic potential of KRGE for PCOS using a dehydroepiandrosterone (DHEA)-mediated rat model and examine the role of its antiinflammatory and antioxidant activities in this regard.
2. Materials and methods
2.1. Animals and ethical statement
Female Sprague Dawley rats (42 days old; Narabiotec Co., Ltd., Seoul, Korea) were kept at a constant temperature of 23 ± 3°C with a 12-hours (hrs) light-dark cycle (lights on from 08:00 to 20:00) and fed food and water ad libitum. The animals were allowed to habituate to the housing facilities for 1 week before the experiments. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Kyung Hee University. In this process, proper randomization of laboratory animals and handling of data were performed in a blind manner in accordance with recent recommendations from an NIH workshop on preclinical models of neurological diseases [6].
2.2. Preparation of KRGE
KRGE (Korea Ginseng Corporation, Daejeon, Korea) was prepared, as previously described [19], from the roots of 6-year-old fresh P. ginseng Meyer. KRGE contained major ginsenosides Rb1 (7.44 mg/g), Rb2 (2.59 mg/g), Rc (3.04 mg/g), Rd (0.91 mg/g), Re (1.86 mg/g), Rf (1.24 mg/g), Rg1 (1.79 mg/g), Rg2s (1.24 mg/g), Rg3s (1.39 mg/g), and Rh1 (1.01 mg/g) and other minor ginsenosides, as determined by high-performance liquid chromatography.
2.3. PCOS induction and KRGE treatment
Female 42-day-old postpubertal rats were divided into an experimental group for pretreatment with KRGE. This group was then divided into five subgroups (n = 9 per group): (1) sham group [saline, subcutaneous (s.c.) + saline, per os (p.o.)], (2) DHEA group [DHEA, 60 mg/kg body weight (BW)/day, s.c. + saline, p.o.], (3) DHEA + KRGE 75 group [DHEA, 60 mg/kg BW/day, s.c. + KRGE 75 mg/kg BW/day, p.o.], (4) DHEA + KRGE 150 group [DHEA, 60 mg/kg BW/day, s.c. + KRGE 150 mg/kg BW/day, p.o.], and (5) KRGE group (saline, s.c. + KRGE 150 mg/kg BW/day, p.o.). The PCOS model was induced by treatment with DHEA as described previously [20] with some modifications [12]. To examine the preventive effects of KRGE in PCOS, KRGE was given orally daily for 20 consecutive days starting 2 hrs before the first DHEA treatment. Rats in the sham or KRGE group were treated with saline or KRGE without DHEA treatment.
2.4. BW and vaginal smears
BW was measured before treatment with DHEA, KRGE, or saline daily during the experimental period. The stage of estrous cycle was also measured by microscopic observation of the predominant cell type in vaginal smears obtained daily beginning on Day 0 in KRGE-pretreated rats and on Day 25 (48 days after birth) in KRGE-pretreated rats as previously described [21], [22].
2.5. Blood serum preparation and examination of hormone levels
Serum was prepared as described previously [22]. Serum testosterone and estradiol levels were examined using enzyme-linked immunosorbent serologic assay (ELISA). Ultrasensitive ELISA Kits for rat testosterone (Mercodia, Uppsala, Sweden), rat estradiol (Mercodia), rat LH (Enzo Life Sciences, Farmingdale, NY), and rat FSH (Cusabio biotech, Houston, TX) were used according to the manufacturer's instructions.
2.6. Histological examination
Twenty days after DHEA treatment, ovaries were sampled, and paraffin sections (5 μm in thickness) were prepared. After performing hematoxylin-eosin staining, the number of cysts was counted as previously described [22].
2.7. Detection of apoptosis with the terminal deoxynucleotidyl transferase dUTP nick end labeling assay
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed using an ApopTag Peroxidase in situ Apoptosis Detection Kit (S7100) (Millipore, Burlington, MA) according to the manufacturer's instructions and as previously described [23].
2.8. Immunohistological analysis
The paraffin sections were performed by immunohistochemistry as previously described [22]. Briefly, the sections were incubated with rabbit antiionized calcium binding adapter molecule (Iba)-1 (1:1,500; Wako Pure Chemical, Osaka, Japan), followed with biotinylated mouse/rabbit IgG antibody (1:250; Vector Laboratories, Burlingame, CA) and with avidin-biotinylated horseradish peroxidase (1:250; Vector Laboratories).
2.9. Immunoblot analysis
Twenty days after the DHEA treatment, the ovaries were immediately sampled from rats (n = 3 per group) under anesthesia and deep-frozen. Immunoblot analysis was performed as previously described [22]. The following primary antibodies were used for Immunoblot analysis: Rabbit anti-Bad (1:500, Cell Signaling Technology, Beverly, MA), mouse anticleaved caspase-9 (1:1000, Cell Signaling Technology), rabbit anticleaved caspase-3 (1:500; Cell Signaling Technology), rabbit anti-p-IκBα, rabbit anti-p–NF-κB p65 (1:1,500; Cell Signaling Technology), rabbit antinuclear factor erythroid 2-related factor 2 (Nrf2; 1:1500, Santa Cruz Biotechnology, Santa Cruz, CA), mouse antiheme oxygenase-1 (HO-1; 1:1,500; Enzo Life Sciences, Farmingdale, NY), mouse anti-NQO1 (1:1,500; Cell Signaling Technology), rabbit antihistone H3 (1:5,000; Cell Signaling Technology), rabbit anticyclooxygenase-2 (COX-2) (1:1,000, Santa Cruz Biotechnology), rabbit anti-iNOS (1:1,000, Santa Cruz Biotechnology), mouse anti-ER alpha (ERα) (1:1,000, Santa Cruz Biotechnology), and mouse anti-ERß (1:500, Santa Cruz Biotechnology) were used as primary antibodies. Immunoblot images were quantified using Image J analysis software (JAVA image processing program, NIH, Bethesda, MD).
2.10. Real time-polymerase chain reaction analysis
Twenty days after the DHEA treatment, the ovaries were immediately sampled from rats (n = 6 per group) under anesthesia and deep-frozen. Real-time polymerase chain reaction (RT-PCR) was performed using a SYBR Green PCR Master Mix (Applied Biosystems, Franklin Lakes, NJ) as previously described [19]. Fold-induction was calculated using the 2−ΔΔCT method as previously described [24]. Expression levels of each gene were normalized using that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following primer was used for real time RT-PCR: Aromatase forward: 5′-CTC CTC CTG ATT CGG AAT TGT-3’; reverse: 5′-TCT GCC ATG GGA AAT GAG AG-3’.
2.11. RAW264.7 cell culture
RAW264.7 cells (1 × 106 cells/mL) were seeded into a 96-well plate and treated with ginsenosides (1, 10, and 100 μg/mL of KRGE, Rb1, Rg1, and Rg3(S)) in complete Dulbecco's modified Eagle's medium for 7 hours. These cells were then stimulated with lipopolysaccharide (Sigma-Aldrich, St. Louis, MO; 1 μg/mL) for another 6 hour or with estradiol (known as E2; Sigma-Aldrich; 20 nM) for 6 hour. Supernatants were collected and used for Western blot analysis.
2.12. Statistical analysis
All data are presented as means ± standard error of mean. The statistical analyses were performed using the SPSS, 21.0 package (SPSS Inc, Chicago, IL) for Windows. Two-sample comparisons were carried out using the Student t test, and multiple comparisons were made using a two-way analysis of variance with Tukey's post hoc test. A statistical difference was identified at the 5% level unless otherwise indicated.
3. Results
3.1. Effects of KRGE on BW and estrous cycle of DHEA-induced PCOS rats
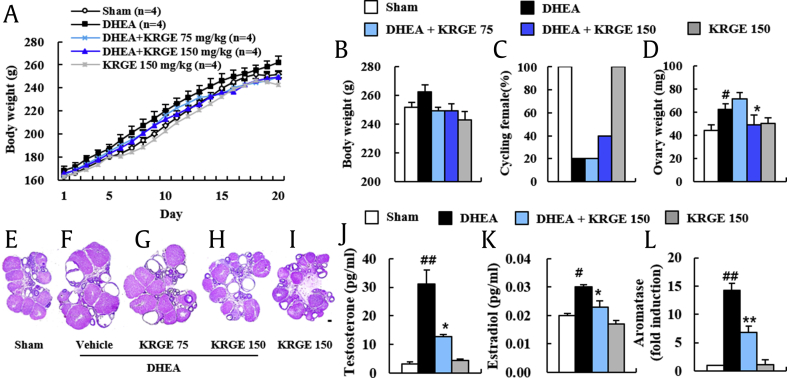
Metabolic syndrome is one of the main features of PCOS [1], [3], [4]. Thus, we first examined whether DHEA or KRGE could induce significant alteration in BW of rats. The BW was gradually increased daily after DHEA-treatment compared with age-matched sham rats. However, the increase was generally reduced in DHEA + KRGE (75 and 150) groups (Fig. 1A). For example, at the end of the experiment, the BW was 262.4 ± 5.1 g in the DHEA group, 249.3 ± 2.5 g in the DHEA + KRGE 75 group, and 249.3 ± 4.8 g in the DHEA + KRGE 150 group (Fig. 1B). Treatment with KRGE (150 mg/kg BW/day) alone did not affect BW compared with that of the sham (Fig. 1A and B).
Fig. 1.
Effect of KRGE on BW gain and estrous cycle in DHEA-induced PCOS rats. (A and B) BW was measured daily during the experimental period. Growth curves for the period of treatment for sham, DHEA, DHEA + KRGE (75 and 150), and KRGE (150) groups (A) and BW at Day 20 after DHEA treatment (B). (C) Estrous cycle was determined by vaginal smear test daily following DHEA treatment. (D) Ovarian weight was measured at Day 20 after DHEA treatment. (E-I) Ovaries were performed by H&E staining at Day 20 after DHEA treatment. Sham (E), DHEA (F), DHEA + KRGE 75 (G), DHEA + KRGE 150 (H), and KRGE 150 (I) groups. (J and K) Serum levels of testosterone (J) and estradiol (K) were measured by ELISA. (L) mRNA expression levels of aromatase in ovaries were measured by real time PCR. Values represent the mean ± SEM. #p < 0.05 and ##p < 0.01 versus Sham group; ∗p < 0.05 and ∗∗p < 0.01 versus DHEA group. Scale bar = 100 μm. FSH, follicle-stimulating hormone; LH, luteinizing hormone; KRGE, Korean Red Ginseng extract; DHEA, dehydroepiandrosterone; BW, body weight; H&E, hematoxylin-eosin; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; PCOS, polycystic ovarian syndrome; SEM, standard error of mean.
Because regular estrous cycles are a key index of healthy ovarian function in women, we measured the stage of estrous cycle by a vaginal smear test (Fig. 1C). All rats of the sham and KRGE groups displayed regular cycles. The ratio of rats with regular cycle was 20% in the DHEA group. This ratio was recovered to 40% in the DHEA + KRGE group (150 mg/kg) (Fig. 1C). These findings suggest that pretreatment with KRGE exerts a favorable effect for the maintenance of a regular estrous cycle.
3.2. Effects of KRGE on ovarian weight and morphology in DHEA-induced PCOS rats
Ovarian weight of rats can be increased or decreased because of PCOS [3]. Thus, we tested ovarian weight from each group after the end of the experiment. Ovarian weights in the DHEA group were increased by 40.5% (62.5 ± 4.6 g) relative to that of the sham group (44.5 ± 5.0 g). However, the increase was significantly inhibited [by 21.0% (49.4 ± 8.0 g)] in the DHEA + KRGE (150 mg/kg) group compared with that of the DHEA group (Fig. 1D), consistent with alteration in size of ovaries (Fig. 1E–I). KRGE itself did not significantly affect the alteration of ovarian weight or appearance compared with that of the sham group. The formation of multiovarian cysts reflects pivotal pathological features in the ovary of PCOS patient [3]. Thus, we also examined whether KRGE blocked the formation of multiovarian cysts by DHEA (Fig. 1E–I). Ovaries from the sham and KRGE groups displayed normal appearance and normal histological structure consisting of a fibromuscular stroma and many blood vessels with a cortex containing large numbers of follicles and large corpora lutea in different stages of growth and regression (Fig. 1E). However, ovaries from the DHEA group displayed multiple cystic follicles (Fig. 1F), in line with previously described results [25], [26], [27]. The number of follicular cysts in the ovaries was 6.2 ± 0.7 in the DHEA group. However, it was 3.4 ± 0.7 in the DHEA + KRGE 75 group and 3.4 ± 0.7 in the DHEA + KRGE 150 group (Fig. 1F–H). These results provide that pretreatment with KRGE can successfully disrupt the development of follicular cysts induced by DHEA.
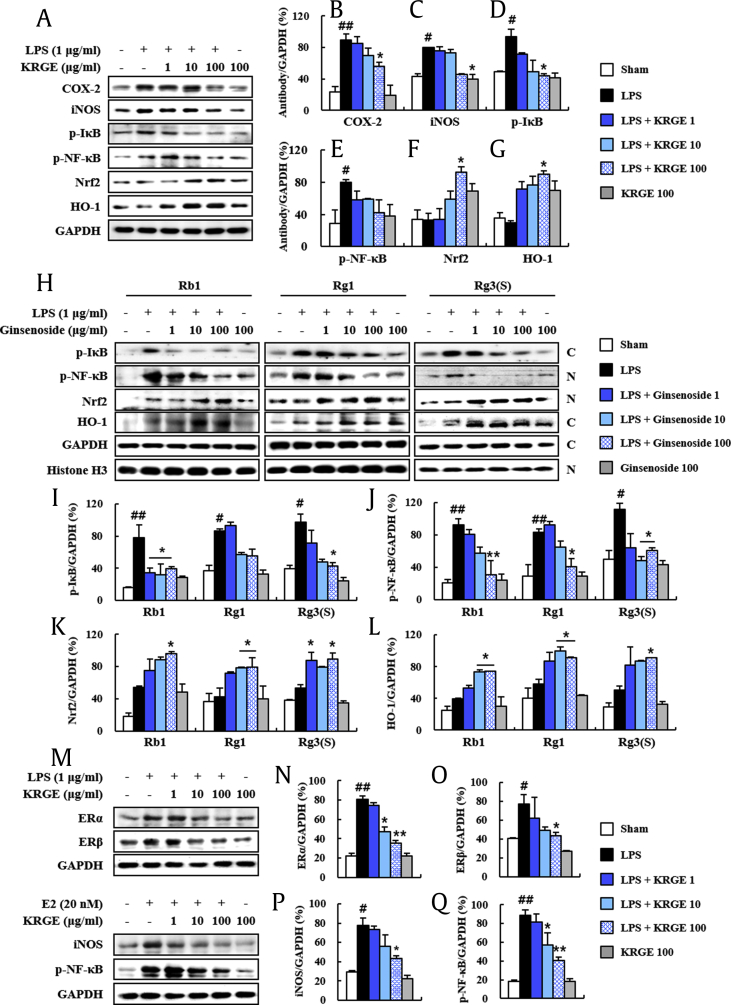
Fig. 5.
Antiinflammatory and antioxidant effects of KRGE in the lipopolysaccharide-induced RAW264.7 cells. (A-G) Levels of activation of inflammatory and antioxidant mediators in lipopolysaccharide-induced RAW264.7 cells were measured by immunoblot analysis (A) and quantified (B-G). COX-2 (B), iNOS (C), p-IkB (D), p–NF-kB (E), Nrf2 (F), and HO-1 (G). (H-L) Levels of activation of inflammatory and antioxidant mediators in lipopolysaccharide-induced RAW264.7 cells following treatment with ginsenosides (Rb1, Rg1, and Rg3(S)) were measured by immunoblot analysis (H) and quantified (I-L). Cytosolic p-IkB (I), nuclear p–NF-kB (J), nuclear Nrf2 (K), and cytosolic HO-1 (L). (M-P) Protein expression of ERα, ERβ, iNOS, and p–NF-kB in the lipopolysaccharide or E2-stimulated RAW264.7 cells following treatment with KRGE were measured by immunoblot analysis (M) and quantified (N–P). ERα (N), ERβ (O), iNOS (P), and p–NF-kB (Q). The C and N in the photo H display cytosolic and nuclear extracts, respectively. Values represent the mean ± SEM. #p < 0.05 and ##p < 0.01 versus vehicle-treated group; *p < 0.05 and **p < 0.05 versus lipopolysaccharide-treated or E2-treated group. KRGE, Korean Red Ginseng extract; SEM, standard error of mean; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cell; Nrf2, nuclear factor erythroid–derived 2-related factor; HO-1, antiheme oxygenase-1; COX-2, cyclooxygenase-2; ER, estrogen receptor.
3.3. Effects of KRGE on ovarian steroidogenic function following DHEA treatment
The formation of ovarian cysts and the presence of oligoovulation or anovulation and hyperandrogenism are critical features of PCOS patients [1], [3], [4]. Thus, we investigated serum levels of testosterone, LH, FSH, and estradiol as markers for steroidogenic function in DHEA-induced PCOS rats (Fig. 1J and Supplementary data 1). Testosterone level was significantly enhanced in the DHEA group (by 848.5%) compared with that in the sham group, whereas its level was inhibited by 59.2% in the DHEA + KRGE group (Fig. 1J). However, LH or FSH level was not significantly affected by DHEA or KRGE (Supplementary data 1). Continuously, we examined serum levels of estradiol as additional marker for steroidogenic function in DHEA-induced PCOS rats (Fig. 1K). Estradiol level was significantly increased by 50.0% in the DHEA group compared with that in the sham group, while its level was reduced by 23.3% in the DHEA + KRGE group (Fig. 1K). In addition, mRNA expression level of aromatase, also called estrogen synthase, was significantly increased by 142.0% in the ovaries of the DHEA group compared with that in the sham group, while its level was reduced by 52.1% in the DHEA + KRGE group (Fig. 1L). These results provide evidence that positive regulation of KRGE on steroid hormones may reduce the induction of PCOS by DHEA.
3.4. Effects of KRGE on ovarian apoptosis following DHEA treatment
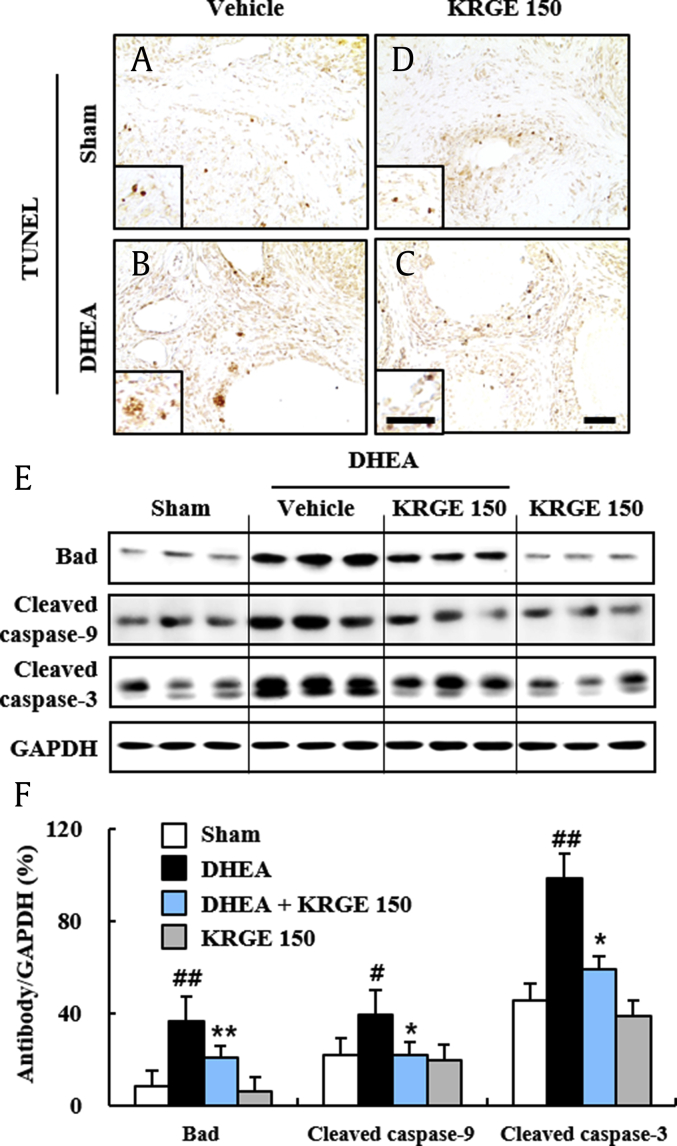
Apoptosis is known to be increased in ovaries from DHEA-induced PCOS rats [28]. Thus, we examined whether KRGE could reduce ovarian apoptosis at 20 days after DHEA treatment using TUNEL staining (Fig. 2). The number of TUNEL (+) cells was increased around cystic follicles in ovaries from the DHEA group, whereas it was reduced in the DHEA + KRGE group (Fig. 2A–D). In agreement with this result, protein expression levels of Bad (a proapoptotic factor), cleaved caspase-9 (an initiator), and cleaved caspase-3 (an executioner) were increased in ovaries (453.1%, 179.4%, and 216.9%, respectively) after DHEA treatment, whereas their expression levels were inhibited by KRGE pretreatment (43.9%, 34.1%, and 60.6%, respectively) (Fig. 2E and F). These results provide evidence that pretreatment with KRGE may inhibit ovarian apoptosis following DHEA treatment.
Fig. 2.
Effect of KRGE on ovarian apoptosis in DHEA-induced PCOS rats. (A-D) The level of apoptosis was measured using ovarian sections by TUNEL staining at day 20 after DHEA treatment. Sham (A), KRGE 150 (D), DHEA (B), and DHEA + KRGE 150 (C) groups. (E-F) Protein expression level of Bad, cleaved caspase-9, cleaved caspase-3 was measured in ovaries by immunoblot analysis at Day 20 after DHEA treatment (E) and quantified (F). Values represent the mean ± SEM. Scale bar = 100 μm. #p < 0.05 and ##p < 0.01 versus Sham group; ∗p < 0.05 and ∗∗p < 0.01 versus DHEA group. KRGE, Korean Red Ginseng extract; DHEA, dehydroepiandrosterone; PCOS, polycystic ovarian syndrome; SEM, standard error of mean; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
3.5. Effects of KRGE on macrophage infiltration and expression of inflammatory mediators
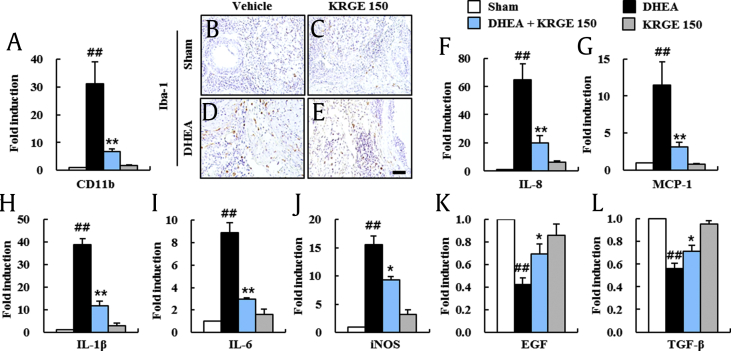
PCOS in humans is related to chronic inflammation [29]. The main pathological events shown in ovaries of PCOS are macrophage infiltration and macrophage-derived products [30]. Therefore, we measured the effects of KRGE on macrophage infiltration in ovarian tissues of PCOS (Fig. 3A–E). The mRNA expression of CD11b was elevated significantly by 31.2-fold in ovaries from the DHEA group compared with that of the sham group. However, the elevation in the mRNA expression of CD11b was reduced by 78.2% in ovaries from the DHEA + KRGE group (Fig. 3A). We also measured the distribution of macrophages in ovarian tissues by immunohistochemical assay using Iba-1 antibody as a marker of macrophages. In agreement with the alteration in mRNA expression of CD11b, the number of Iba-1 (+) macrophages was elevated in the theca cell layer of follicles and stromal tissue of the DHEA group (Fig. 3D). However, the number was reduced in the DHEA + KRGE group (Fig. 3E). Sequentially, we investigated the regulatory effect of KRGE on mRNA expression of chemokines (interleukin [IL]-8 and MCP-1), proinflammatory cytokines (IL-1β and IL-6), iNOS, and growth factors (EGF and TGF-β) in ovaries of each group at 20 days after DHEA treatment and/or KRGE treatment (Fig. 3F–L). The mRNA levels of IL-8, MCP-1, IL-1β, IL-6, and iNOS were elevated significantly by 64.6-, 11.5-, 38.7-, 8.9-, and 15.5-fold, respectively, in ovaries from the DHEA group compared with those in the sham group. However, the elevation in mRNA expressions was inhibited significantly by 69.5%, 73.0%, 70.0%, 67.4%, and 40.0%, respectively, in the DHEA + KRGE group (Fig. 3F–J). On the other hand, mRNA expression levels of EGF and TGF-β were reduced significantly by 60.0% and 40.0%, respectively, in the DHEA group compared with those in the sham group, but their levels were significantly elevated by 0.7-fold and 0.7-fold, respectively, in the KRGE group (Fig. 3K and L). KRGE itself did not exert a significant effect on the expression of these mediators (Fig. 3). These findings suggested that KRGE can inhibit macrophage infiltration and inflammatory response in the ovarian tissue of PCOS model.
Fig. 3.
Effect of KRGE on macrophage infiltration and expression levels of inflammatory mediators in ovaries of DHEA-induced PCOS rats. (A) The mRNA expression of CD11b in ovaries was measured by real-time PCR at day 20 after DHEA treatment. (B-E) The distribution of Iba-1 (+) macrophage was measured using ovarian sections by immunohistochemistry staining at day 20 after DHEA treatment. Sham (B), KRGE 150 (C), DHEA (D), and DHEA + KRGE 150 (E) groups. (F-L) The mRNA expression levels of chemokines (IL-8, MCP-1), proinflammatory cytokines (IL-1β, IL-6), iNOS, and growth factors (EGF, TGF-β) in ovaries were measured by real-time PCR analysis at Day 20 after DHEA treatment. Values represent the mean ± SEM. Scale bar = 100 μm. ##p < 0.01 versus Sham group; *p < 0.05 and **p < 0.01 versus DHEA group. KRGE, Korean Red Ginseng extract; DHEA, dehydroepiandrosterone; PCOS, polycystic ovarian syndrome; SEM, standard error of mean; IL, interleukin; PCR, polymerase chain reaction.
3.6. Antiinflammatory and antioxidant effects of KRGE in the ovarian tissue of DHEA-induced PCOS model
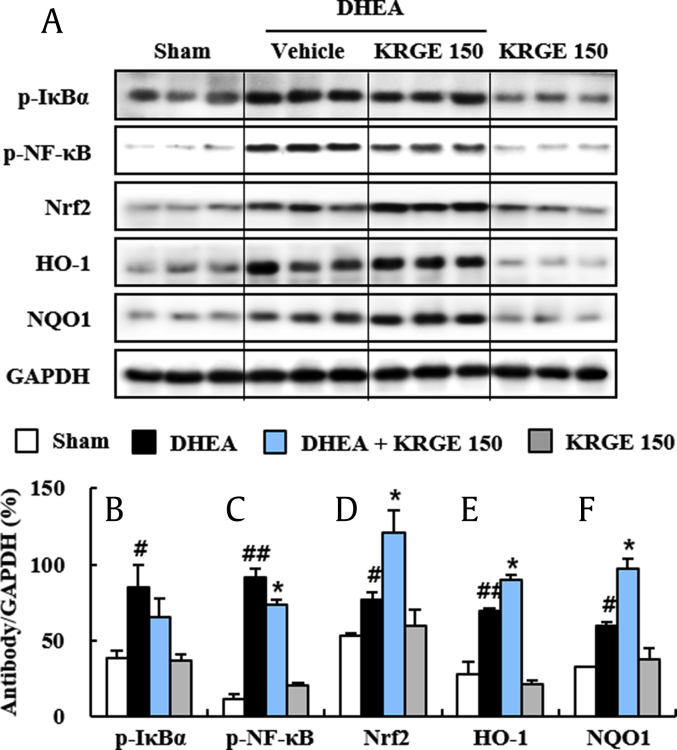
KRGE and ginsenosides (Rb1, Rd, Rg1, etc.) have an antiinflammatory action through prevention of the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) pathway, a representative inflammation mechanism in various diseases [11], [31], [32]. However, the effect of KRGE remains unclear. Thus, we examined whether KRGE could inhibit the upregulation of the NF-κB pathway in ovaries following DHEA treatment. Phosphorylation levels of IκBα and NF-κB were significantly increased by 119.5% and 713.4%, respectively, in ovaries at 20 days after the DHEA treatment compared with that of the sham group, whereas their phosphorylation levels were significantly decreased by 22.9% and 19.7%, respectively, by pretreatment with KRGE (150 mg/kg/day). KRGE treatment alone did not induce significant alteration of the NF-κB pathway (Fig. 4A–C). These results indicate that KRGE exerts antiinflammatory activity in ovaries after DHEA treatment via inhibiting the NF-κB pathway.
DHEA can increase oxidative stress in a human cell line during differentiation [33]. KRGE and ginsenosides have antioxidative effects through Nrf2 transcriptional stimulation in various diseases [23], [34]. However, the antioxidant effects of KRGE on DHEA-induced PCOS in the rat model are unclear. In the present study, Nrf2 protein expression was markedly increased in ovaries of the DHEA group (43.6%) compared with that of the sham group. Its expression was further increased by pretreatment with KRGE (150 mg/kg, 57.5%) compared with that of the DHEA group (Fig. 4A and D). Protein expression levels of phase II enzymes HO-1 and NQO-1 from immunoblot analysis were markedly upregulated by 153.7% and 84.7%, respectively, in ovaries in the DHEA group. Such upregulations in protein expression were further enhanced by 28.6% and 63.3%, respectively, in the DHEA + KRGE group (Fig. 4A, E, and F). Taken together, these findings indicate that KRGE exerts antioxidant activity through upregulation of the Nrf2 pathway in ovaries of the DHEA-induced PCOS rat model.
Fig. 4.
Effect of KRGE on NF-κB and Nrf2 pathways in ovaries of DHEA-induced PCOS rats. (A-F) Levels of activation of NF-κB and Nrf2 pathways in ovaries were measured by immunoblot analysis at Day 20 after DHEA treatment (A) and quantified (B–F). p-IκBα (B), p–NF-κB (C), Nrf2 (D), HO-1 (E), and NQO-1 (F). Values represent the mean ± SEM. #p < 0.05 and ##p < 0.01 versus Sham group; *p < 0.05 versus DHEA group. KRGE, Korean Red Ginseng extract; DHEA, dehydroepiandrosterone; PCOS, polycystic ovarian syndrome; SEM, standard error of mean; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cell; Nrf2, nuclear factor erythroid–derived 2-related factor; HO-1, antiheme oxygenase-1.
3.7. Antiinflammatory and antioxidant effects of KRGE in lipopolysaccharide-induced RAW264.7 cells
We demonstrated antiinflammatory and antioxidant activities of KRGE in DHEA-induced PCOS in rats (Fig. 1, Fig. 2, Fig. 3, Fig. 4). To further understand both activities of KRGE, we performed in vitro studies using lipopolysaccharide-induced RAW264.7 cells. KRGE inhibited the elevation of protein expression of inflammatory enzymes COX-2 and iNOS (Fig. 5A–C), the p-IkB (Fig. 5A and D), and p–NF-kB (Fig. 5A and E) after lipopolysaccharide stimulation. However, KRGE upregulated Nrf2 and HO-1 expression after lipopolysaccharide stimulation (Fig. 5A, F, and G). Sequentially, to further investigate the mechanism of KRGE, we examined effects of representative components (ginsenosides) in the same in vitro study. Interestingly, ginsenoside Rb1, Rg1, and Rg3(s) prevented the elevation in protein expression of cytosolic p-IkB and nuclear p–NF–kB induced by lipopolysaccharide stimulation (Fig. 5H–J), whereas they upregulated Nrf2 nuclear translocation after lipopolysaccharide stimulation (Fig. 5H, K, and L). These results suggest that KRGE might alleviate DHEA-induced polycystic ovarian syndrome in rats via its antiinflammatory and antioxidant activities.
Additionally, to examine whether antiinflammatory effects of KRGE might be related to estrogen expression, we investigated the effect of KRGE on protein expression of ERα and ERβ in the lipopolysaccharide-induced RAW264.7 cell model. Protein expression levels of ERα and ERβ were increased by lipopolysaccharide stimulation, whereas their levels were reduced by KRGE (Fig. 5M–O). Protein expression levels of iNOS and p–NF-kB were increased by E2 stimulation, whereas their levels were reduced by KRGE (Fig. 5M, P, and Q). These results suggest that the antiinflammatory effects of KRGE might be associated with direct or indirect effect of KRGE on estrogen expression.
4. Discussion
Because PCOS is associated to metabolic disturbances, chronic inflammation, and oxidative stress, it can affect quality-of-life and long-term health of affected individuals [35], [36]. Thus far, no therapeutic intervention has been successful in preventing or treating PCOS. KRG is one of the most important medicinal plants for patients with age-related disorders in traditional Oriental medicine [11], [12], [13]. But, the effect and underlying mechanism of KRGE on the female reproductive disorders has not been analyzed systematically. Here, we demonstrated beneficial effects of KRGE in the DHEA-induced PCOS rat model via its antiinflammatory and antioxidant activities. Thus, KRGE has preventive and therapeutic potential for PCOS.
The DHEA-induced PCOS rat model mimics the main features of PCOS in women, although its detailed biology remains unknown [25], [26], [37], [38]. Our DHEA-induced PCOS rat model exhibited increased weights of animals and ovaries (Fig. 1), corresponding to previous reports [12], [22]. Here, pretreatment with KRGE prevented the increase in BW and ovarian weight of rats with PCOS, corresponding to improved PCOS in rats (Fig. 1). These possibilities were partially explained by the finding that exercise and Oriental medicine Kyung-Ok-Ko can reduce these factors associated with improved PCOS [39], [40]. Our results suggest that KRGE may prevent increased weights of the body and ovaries induced by DHEA treatment.
Because DHEA can induce PCOS in animal models by inducing hyperandrogenization [20], the level of serum testosterone is very important following DHEA treatment. In the present study, the level of serum testosterone was increased markedly after DHEA treatment compared with that of the sham treatment. However, the increase was significantly blocked by pretreatment with KRGE (Fig. 1J). The level of serum estradiol increase is related to formation of follicular cysts, estrous cycle arrest, altered ovarian steroidogenesis, and anovulation as a result of hyperandrogenism in PCOS patients [41] and in DHEA-induced/testosterone-induced animal models [26]. According to recent reports, metformin and Oriental medicine Kyung-Ok-Ko can restore ovulation disorder in PCOS [22], [42] and improve ovary-related parameters in DHEA-induced hyperandrogenized animal models [37]. In agreement with these reports, pretreatment with KRGE also inhibited the enhancement in serum estradiol levels and aromatase activity in ovaries from the DHEA group, mitigated the pathology of multi cystic ovaries, and recovered estrous cycle arrest in the DHEA group of rats (Fig. 1K) in the present study. These findings provide evidence that KRGE has a favorable effect in the prevention and treatment of PCOS.
DHEA promotes the apoptosis of ovarian granulosa or theca cells via phosphoinositide-3-kinase–protein kinase B/Akt, SIRT1 (member of the sirtuin family), adenosine monophosphate-activated protein kinase, or Wnt signal pathway [43], [44]. In the present study, KRGE reduced the number of TUNEL positive cells and inhibited protein expressions of Bad (a proapoptotic factor), cleaved caspase-9 (an initiator), and cleaved caspase-3 (an executioner) in the ovary with PCOS. These results suggest that KRGE exert antiapoptotic activity in the ovary with PCOS by inhibiting Bax/capase-9/capase-3 pathway. Its antiapoptotic activity has been explained by abundant previous reports. Ginseng extract and its compounds have antiapoptotic activity in MPP + -induced apoptosis of PC12 cells by stimulating ERs with consequent activation of extracellular-signal-regulated kinase 1/2, Akt, and inhibition of c-Jun N-terminal kinase, p38 MAPK [45]. They also have antiapoptotic activity in neuroblastoma cells via ER β-mediated phosphatidylinositol-3 kinase/Akt signaling [46].
PCOS is related to a chronic inflammation processes [29]. Its main mechanism is macrophage contribution [30]. Ovarian macrophages can secrete proinflammatory cytokines/chemokines and trophic factors in normal or abnormal processes of the ovary. These mediators released from macrophages can regulate tissue remodeling and apoptosis implicated in folliculogenesis, ovulation and corpus luteum/albicans formation [30]. Here, KRGE blocked infiltration of Iba-1 (+) cells (macrophages) in ovarian stroma of DHEA-induced PCOS rats, corresponding with increased mRNA expression of Iba-1 in ovaries (Fig. 3B–E). Because macrophage infiltration has a critical activity in PCOS patients [30], numerous studies have reported alteration in expression of inflammatory mediators in serum, follicular fluid, or ovarian tissue of PCOS patients. Follicular fluid and serum levels of IL-6 and TNF-α are increased in nonobese/nondiabetic PCOS patients [47]. Serum levels of MCP-1 and macrophage inflammatory protein-1 alpha are increased in PCOS patients [48]. Activities of iNOS and COX-2 are increased in ovaries of PCOS patients [38], [49]. Activities of iNOS and COX-2 are inhibited by metformin treatment [38]. Interestingly, here, pretreatment with KRGE significantly inhibited the stimulation of the NF-κB signal pathway and the increase in mRNA levels of IL-1β, IL-6, IL-8, MCP-1, and iNOS in ovaries from PCOS rats, whereas KRGE recovered reduction in mRNA expression of EGF and TGF-β in ovaries from PCOS rats (Fig. 3F–L). In agreement with these results from in vivo study, KRGE and ginsenosides (Rb1, Rg1, and Rg3(S)) also inhibited the elevation in protein expression of inflammatory enzymes COX-2 and iNOS, cytosolic p-IkB, and nuclear p–NF–kB in lipopolysaccharide-induced RAW264.7 cell model (Fig. 5A–E). Conclusively, KRGE has an antiinflammatory activity in ovaries of the DHEA-induced PCOS model. Therefore, targeting the inflammatory process such as activation of NF-κB signaling and/or production of inflammatory mediators using KRGE and its components might be a therapeutic alternative to current treatment.
Oxidative stress is characterized by an imbalance between production and scavenging of reactive oxygen species [50]. Oxidative stress is implicated in PCOS pathogenesis because woman with PCOS display more serious oxidative stress compared with healthy woman [51]. Because transcription factor Nrf2 is responsible for strong antioxidant activity, which protects various cells from oxidative stress, targeting Nrf2 might exert a pivotal role in protection against various inflammatory disorders [52]. Given that KRGE could stimulate Nrf2 and its products (HO-1 and NQO-1) in ovaries from DHEA-induced PCOS rats (Fig. 4) and that KRGE and ginsenosides (Rb1, Rg1, and Rg3(s)) upregulated Nrf2 nuclear translocation in lipopolysaccharide-induced RAW264.7 cell model (Fig. 5), it is conceivable that triggering the Nrf2 pathway by KRGE could inhibit the NF-κB pathways, thus contributing to KRGE's antiinflammatory effect to protect ovaries against DHEA-induced hyperandrogenization. These findings might be directly/indirectly explained by the antioxidant effect saponin metabolite Rh3 of and ginsenoside Rg1 in cultured cells through Nrf2 stimulation [53], [54] and its antiinflammatory activity (decreased expression of cytokines and chemokines) by suppressing the NF-κB pathways through Nrf2 stimulation [34], [55], [56]. Although our results suggest that KRGE might suppress the activation of the NF-κB pathways via Nrf2 mechanism, reciprocal interaction between Nrf2 and NF-κB activities remains to be clarified. Nevertheless, our results suggest that KRGE can directly or indirectly exert a potent preventive and therapeutic effect in the DHEA-induced PCOS rat model by its antiinflammatory and antioxidative effects possibly through activating the Nrf2 pathway and suppressing the NF-κB pathways.
P. ginseng, KRG, or its major active ingredients such as ginsenosides, polysaccharides, and gintonin can enhance physical strength, prevent aging, increase vigor [11], [12], [13], and induce immune, endocrine, cardiovascular, nervous, and cancer-related benefits associated with its antiinflammatory and antioxidant activities [57]. KRGE can reduce the number of antral follicles and increase the number of corpora lutea in polycystic ovaries associated with regulation of NGF expression in an estradiol valerate-induced PCOS rat model [18]. Rg1 can enhance the antiaging capacity of ovary and fertility abilities of premature ovarian failure mice through enhancing antiinflammatory and antioxidant capacities of the ovary [58]. Ginsenosides can activate proliferation of granulosa cells from chicken prehierarchical follicles through PKC (protein kinase C) activation and upregulation of cyclin gene expression [59]. Oral administration of Rh2, a component of red ginseng, has strong inhibitory effects on human ovarian cancer cell growth in nude mice [60]. Taken together, the anti-PCOS effect of KRGE in the present study might be because of a combination action of various components. Although it remains unknown which components in KRGE exert positive effect on PCOS, the present findings have academic value as basic data for further study.
5. Conclusions
We confirmed that KRGE had beneficial effects in a DHEA-induced PCOS rat model. Pretreatment of KRGE significantly inhibited the enhancement of the weight of bodies and ovaries, size and number of follicular cysts, and serum levels of testosterone and estradiol following DHEA induction associated with its antiinflammatory effects (reduced infiltration of macrophages and reduced mRNA expression of inflammatory mediators, enhanced growth factors, and inhibited NF-kB pathway) and antioxidant activity (Nrf2, HO-1, and NQO-1) in the ovary. Taken together, KRGE might synergistically prevent DHEA-induced PCOS via antiinflammatory and antioxidant activities, suggesting its preventive and therapeutic potential for PCOS.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was supported by the 2014 grant from the Korean Society of Ginseng and the National Research Foundation of Korea (NRF) funded by the Ministry of Science, and ICT (NRF-2012R1A1A2008505).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.08.007.
Contributor Information
Ik-Hyun Cho, Email: ihcho@khu.ac.kr.
Chun-Sik Bae, Email: csbae210@chonnam.ac.kr.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Carmina E., Lobo R.A. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–1899. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi M.O., Louwers Y.V., Taylor K.D., Jones M.R., Cui J., Kwon S., Chen Y.D., Guo X., Stolk L., Uitterlinden A.G. Replication of association of a novel insulin receptor gene polymorphism with polycystic ovary syndrome. Fertil Steril. 2011;95:1736–1741. doi: 10.1016/j.fertnstert.2011.01.015. e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodarzi M.O., Dumesic D.A., Chazenbalk G., Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Norman R.J., Homan G., Moran L., Noakes M. Lifestyle choices, diet, and insulin sensitizers in polycystic ovary syndrome. Endocrine. 2006;30:35–43. doi: 10.1385/ENDO:30:1:35. [DOI] [PubMed] [Google Scholar]
- 5.Taylor A.E., McCourt B., Martin K.A., Anderson E.J., Adams J.M., Schoenfeld D., Hall J.E. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 6.Landis S.C., Amara S.G., Asadullah K., Austin C.P., Blumenstein R., Bradley E.W., Crystal R.G., Darnell R.B., Ferrante R.J., Fillit H. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kousta E., White D.M., Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 1997;3:359–365. [Google Scholar]
- 8.Mulligan K., Yang Y., Wininger D.A., Koletar S.L., Parker R.A., Alston-Smith B.L., Schouten J.T., Fielding R.A., Basar M.T., Grinspoon S. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS. 2007;21:47–57. doi: 10.1097/QAD.0b013e328011220e. [DOI] [PubMed] [Google Scholar]
- 9.Kar S., Sanchita S. Clomiphene citrate, metformin or a combination of both as the first line ovulation induction drug for Asian Indian women with polycystic ovarian syndrome: a randomized controlled trial. J Hum Reprod Sci. 2015;8:197–201. doi: 10.4103/0974-1208.170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raja-Khan N., Stener-Victorin E., Wu X., Legro R.S. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2011;301:E1–E10. doi: 10.1152/ajpendo.00667.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho I. Effects of Panax ginseng in neurodegenerative diseases. J Ginseng Res. 2012;36:342–353. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E.J., Jang M., Choi J.H., Park K.S., Cho I.H. An improved dehydroepiandrosterone-induced rat model of polycystic ovary syndrome (PCOS): post-pubertal improves PCOS’s feature. Front Endocrinol (Lausanne) 2018 Dec 4;9:735. doi: 10.3389/fendo.2018.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42:239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding J., Xu Y., Ma X., An J., Yang X., Liu Z., Lin N. Estrogenic effect of the extract of Renshen (Radix Ginseng) on reproductive tissues in immature mice. J Tradit Chin Med. 2015;35:460–467. doi: 10.1016/s0254-6272(15)30125-4. [DOI] [PubMed] [Google Scholar]
- 15.Cho J., Park W., Lee S., Ahn W., Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab. 2004;89:3510–3515. doi: 10.1210/jc.2003-031823. [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Pan Z., Wang Z., Zhu W., Shen Y., Cui R., Lin J., Yu H., Wang Q., Qian J. Ginsenoside Rg1 protection against beta-amyloid peptide-induced neuronal apoptosis via estrogen receptor alpha and glucocorticoid receptor-dependent anti-protein nitration pathway. Neuropharmacology. 2012;63:349–361. doi: 10.1016/j.neuropharm.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Ding J., Ma X.P., Ma Y.H., Liu Z.Q., Lin N. Treatment with Panax ginseng antagonizes the estrogen decline in ovariectomized mice. Int J Mol Sci. 2014;15:7827–7840. doi: 10.3390/ijms15057827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Jung J.H., Park H.T., Kim T., Jeong M.J., Lim S.C., Nah S.Y., Cho I.H., Park S.H., Kang S.S., Moon C.J. Therapeutic effect of Korean Red ginseng extract on infertility caused by polycystic ovaries. J Ginseng Res. 2011;35:250–255. doi: 10.5142/jgr.2011.35.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang M., Lee M.J., Kim C.S., Cho I.H. Korean Red ginseng extract attenuates 3-nitropropionic acid-induced Huntington's-like symptoms. Evid Based Complement Alternat Med. 2013;2013:237207. doi: 10.1155/2013/237207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchetti C.G., Solano M.E., Sander V., Arcos M.L., Gonzalez C., Di Girolamo G., Chiocchio S., Cremaschi G., Motta A.B. Effects of dehydroepiandrosterone on ovarian cystogenesis and immune function. J Reprod Immunol. 2004;64:59–674. doi: 10.1016/j.jri.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Marcondes F.K., Bianchi F.J., Tanno A.P. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 22.Jang M., Lee M.J., Lee J.M., Bae C.S., Kim S.H., Ryu J.H., Cho I.H. Oriental medicine Kyung-Ok-Ko prevents and alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats. PLoS One. 2014:9. doi: 10.1371/journal.pone.0087623. e87623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi J.H., Jang M., Nah S.Y., Oh S., Cho I.H. Multitarget effects of Korean Red Ginseng in animal model of Parkinson's disease: antiapoptosis, antioxidant, antiinflammation, and maintenance of blood-brain barrier integrity. J Ginseng Res. 2018;42:379–388. doi: 10.1016/j.jgr.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Solano M.E., Sander V.A., Ho H., Motta A.B., Arck P.C. Systemic inflammation, cellular influx and up-regulation of ovarian VCAM-1 expression in a mouse model of polycystic ovary syndrome (PCOS) J Reprod Immunol. 2011;92:33–44. doi: 10.1016/j.jri.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Mannerås L., Cajander S., Holmäng A., Seleskovic Z., Lystig T., Lönn M., Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 27.Maharjan R., Nagar P.S., Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med. 2011;1:273–279. doi: 10.4103/0975-9476.74090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bas D., Abramovich D., Hernandez F., Tesone M. Altered expression of Bcl-2 and Bax in follicles within dehydroepiandrosterone-induced polycystic ovaries in rats. Cell Biol Int. 2011;35:423–429. doi: 10.1042/CBI20100542. [DOI] [PubMed] [Google Scholar]
- 29.Benson S., Janssen O.E., Hahn S., Tan S., Dietz T., Mann K., Pleger K., Schedlowski M., Arck P.C., Elsenbruch S. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun. 2008;22:177–184. doi: 10.1016/j.bbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Wu R., Van der Hoek K.H., Ryan N.K., Norman R.J., Robker R.L. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–133. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
- 31.He B., Chen P., Yang J., Yun Y., Zhang X., Yang R., Shen Z. Neuroprotective effect of 20(R)-ginsenoside Rg(3) against transient focal cerebral ischemia in rats. Neurosci Lett. 2012;526:106–111. doi: 10.1016/j.neulet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-kappaB activation. Neurochem Int. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Izumo K., Horiuchi M., Komatsu M., Aoyama K., Bandow K., Matsuguchi T., Takeuchi M., Takeuchi T. Dehydroepiandrosterone increased oxidative stress in a human cell line during differentiation. Free Radic Res. 2009;43:922–931. doi: 10.1080/10715760903137093. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y.M., Yoon H., Park H.M., Song B.C., Yeum K.J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J Ginseng Res. 2017;41:113–119. doi: 10.1016/j.jgr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn S., Janssen O.E., Tan S., Pleger K., Mann K., Schedlowski M., Kimmig R., Benson S., Balamitsa E., Elsenbruch S. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. 2005;153:853–860. doi: 10.1530/eje.1.02024. [DOI] [PubMed] [Google Scholar]
- 36.Zuo T., Zhu M., Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev. 2016;2016:8589318. doi: 10.1155/2016/8589318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sander V., Luchetti C.G., Solano M.E., Elia E., Di Girolamo G., Gonzalez C., Motta A.B. Role of the N, N’-dimethylbiguanide metformin in the treatment of female prepuberal BALB/c mice hyperandrogenized with dehydroepiandrosterone. Reproduction. 2006;131:591–602. doi: 10.1530/rep.1.00941. [DOI] [PubMed] [Google Scholar]
- 38.Elia E., Sander V., Luchetti C.G., Solano M.E., Di Girolamo G., Gonzalez C., Motta A.B. The mechanisms involved in the action of metformin in regulating ovarian function in hyperandrogenized mice. Mol Hum Reprod. 2006;12:475–481. doi: 10.1093/molehr/gal057. [DOI] [PubMed] [Google Scholar]
- 39.Manneras L., Jonsdottir I.H., Holmang A., Lonn M., Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology. 2008;149:3559–3568. doi: 10.1210/en.2008-0053. [DOI] [PubMed] [Google Scholar]
- 40.Ross R., Janssen I., Dawson J., Kungl A.M., Kuk J.L., Wong S.L., Nguyen-Duy T.B., Lee S., Kilpatrick K., Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 41.Diamanti-Kandarakis E., Christakou C., Kandarakis H. Polycystic ovarian syndrome: the commonest cause of hyperandrogenemia in women as a risk factor for metabolic syndrome. Minerva Endocrinol. 2007;32:35–47. [PubMed] [Google Scholar]
- 42.Johnson N. Metformin is a reasonable first-line treatment option for non-obese women with infertility related to anovulatory polycystic ovary syndrome--a meta-analysis of randomised trials. Aust N Z J Obstet Gynaecol. 2011;51:125–129. doi: 10.1111/j.1479-828X.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Zheng Q., Sun D., Cui X., Chen S., Bulbul A., Liu S., Yan Q. Dehydroepiandrosterone stimulates inflammation and impairs ovarian functions of polycystic ovary syndrome. J Cell Physiol. 2019;234:7435–7447. doi: 10.1002/jcp.27501. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y.C., Ma J.X. The role of MiR-324-3p in polycystic ovary syndrome (PCOS) via targeting WNT2B. Eur Rev Med Pharmacol Sci. 2018;22:3286–3293. doi: 10.26355/eurrev_201806_15147. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto R., Yu J., Koizumi H., Ouchi Y., Okabe T. Ginsenoside Rb1 prevents MPP(+)-Induced apoptosis in PC12 cells by stimulating estrogen receptors with consequent activation of ERK1/2, Akt and inhibition of SAPK/JNK, p38 MAPK. Evid Based Complement Alternat Med. 2012;2012:693717. doi: 10.1155/2012/693717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen C.T., Luong T.T., Kim G.L., Pyo S., Rhee D.K. Korean Red Ginseng inhibits apoptosis in neuroblastoma cells via estrogen receptor beta-mediated phosphatidylinositol-3 kinase/Akt signaling. J Ginseng Res. 2015;39:69–75. doi: 10.1016/j.jgr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amato G., Conte M., Mazziotti G., Lalli E., Vitolo G., Tucker A.T., Bellastella A., Carella C., Izzo A. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101:1177–1182. doi: 10.1016/s0029-7844(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 48.Glintborg D., Andersen M., Richelsen B., Bruun J.M. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf). 2009;71:652–658. doi: 10.1111/j.1365-2265.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 49.Hatzirodos N., Bayne R.A., Irving-Rodgers H.F., Hummitzsch K., Sabatier L., Lee S., Bonner W., Gibson M.A., Rainey W.E., Carr B.R. Linkage of regulators of TGF-beta activity in the fetal ovary to polycystic ovary syndrome. Faseb J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 51.Murri M., Luque-Ramirez M., Insenser M., Ojeda-Ojeda M., Escobar-Morreale H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 52.Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V.R. Oxidative stress and Parkinson's disease. Front Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du X., Xu H., Jiang H., Xie J. Akt/Nrf2 activated upregulation of heme oxygenase-1 involves in the role of Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells. Neurotox Res. 2013;24:71–79. doi: 10.1007/s12640-012-9362-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee Y.Y., Park J.S., Lee E.J., Lee S.Y., Kim D.H., Kang J.L., Kim H.S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharidestimulated microglia: critical role of 5’-adenosine monophosphate-activated protein kinase signaling pathway. J Agric Food Chem. 2015;63:3472–3480. doi: 10.1021/jf506110y. [DOI] [PubMed] [Google Scholar]
- 55.Chen X.C., Zhu Y.G., Zhu L.A., Huang C., Chen Y., Chen L.M., Fang F., Zhou Y.C., Zhao C.H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- 56.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J Ginseng Res. 2015;39:299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 58.He L., Ling L., Wei T., Wang Y., Xiong Z. Ginsenoside Rg1 improves fertility and reduces ovarian pathological damages in premature ovarian failure model of mice. Exp Biol Med (Maywood). 2017;242:683–691. doi: 10.1177/1535370217693323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan T.Q., Ge C., Mi Y., Jin Y., Zhang C. Ginsenosides promote proliferation of granulosa cells from chicken prehierarchical follicles through PKC activation and up-regulated cyclin gene expression. Cell Biol Int. 2010;34:769–775. doi: 10.1042/CBI20090244. [DOI] [PubMed] [Google Scholar]
- 60.Tode T., Kikuchi Y., Hirata J., Kita T., Imaizumi E., Nagata I. [Inhibitory effects of oral administration of ginsenoside Rh2 on tumor growth in nude mice bearing serous cyst adenocarcinoma of the human ovary] Nihon Sanka Fujinka Gakkai Zasshi. 1993;45:1275–1282. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.