Abstract
Background
Recently, beneficial roles of ginsenoside F2 (GF2), a minor constituent of Panax ginseng, have been demonstrated in diverse inflammatory diseases. However, its roles in alcoholic liver inflammation and injury have not been clearly understood. Here, we investigated the underlying mechanism by which GF2 ameliorated alcoholic liver injury.
Methods
To induce alcoholic liver injury, C57BL/6J wild type (WT) or interleukin (IL)-10 knockout (KO) mice were orally administered with ethanol (3 g/kg) or ethanol-containing GF2 (50 mg/kg) for 2 wk. Liver injury and infiltration of macrophages and neutrophils were evaluated by serum biochemistry and immunohistochemistry, respectively. The changes of hepatic immune cells were assessed by flow cytometry and polymerase chain reaction analysis. In vitro differentiation of naïve T cells was performed.
Results
GF2 treatment significantly attenuated alcoholic liver injury, in which infiltrations of inflammatory macrophages and neutrophils were decreased. Moreover, the frequencies of Foxp3+ regulatory T cells (Tregs) increased but IL-17–producing T (Th17) cells decreased in GF2-treated mice compared to controls. Furthermore, the mRNA expression of IL-10 and Foxp3 was significantly increased, whereas IL-17 mRNA expression was suppressed in GF2-treated mice. However, these beneficial roles of GF2 were not observed in GF2-treated IL-10 KO mice, suggesting a critical role of IL-10. Similarly, GF2 treatment suppressed differentiation of naïve T cells into Th17 cells by inhibiting RORγt expression and stimulating Foxp3 expression.
Conclusion
The present study suggests that GF2 treatment attenuates alcoholic liver injury by increasing IL-10 expression and Tregs and decreasing IL-17 expression and Th17 cells.
Keywords: Interleukin-10, Interleukin-17, Macrophage, Neutrophil, Panax ginseng
1. Introduction
Ginsenosides are major constituents of Panax ginseng [1] and have multiple pharmacological functions on various diseases including liver disease [[2], [3], [4]]. Among ginsenosides, ginsenoside F2 (GF2) is a minor ginsenoside that exists in very small quantities in Panax ginseng [5]. Regardless of its scarcity, pharmacological efficacies of GF2 on cancer, obesity, hair growth, and skin inflammation have been reported [[6], [7], [8], [9]]. In irritable dermal inflammation, GF2 reduced the frequencies of interleukin (IL)-17 producing cells and neutrophils in the inflamed skin [8]. However, the beneficial effects of GF2 on alcoholic liver disease (ALD) have not been investigated yet.
Long-term alcohol consumption induces various injuries of multiple organs such as brain, heart, kidney, gastrointestinal tract, and liver [10]. Absorbed alcohol is mainly metabolized in hepatocytes by enzymes including alcohol dehydrogenase and cytochrome P450 2E1 (CYP2E1), in which generated oxidative stress, lipid peroxidation, and DNA damage mediate hepatocyte injury [11]. Injured hepatocytes trigger inflammatory processes by releasing chemokines and cytokines, by which different types of immune cells are recruited and activated in ALD. Infiltrated immune cells further exacerbate liver injury by secreting pro-inflammatory mediators [12,13]. Therefore, the control of immune-related responses is a favorable therapeutic strategy for alcoholic liver injury [14].
In ALD, various innate and adaptive immune cells such as Kupffer cells, monocyte-derived macrophages, neutrophils, natural killer (NK) cells, and lymphocytes contribute to the pathogenesis of ALD [[15], [16], [17]]. Among T lymphocytes, T-helper 17 cells (Th17 cells) promote hepatic inflammation through secreting IL-17A, IL-17F, IL-21, IL-22, and tumor necrosis factor (TNF)-α and recruiting leukocytes, mainly neutrophils [18,19]. In contrast, regulatory T cells (Tregs) attenuate inflammation by secreting anti-inflammatory mediators such as IL-10 and transforming growth factor (TGF)-β [20]. Lines of evidence have reported that Tregs are a potential therapeutic target of autoimmune liver disease and they are able to reduce lipotoxicity in hepatocytes of alcoholic liver injury [21,22]. The differentiation of Tregs and Th17 cells depends on the types of stimulating cytokines and transcriptional factors. TGF-β stimulation differentiates naïve CD4+ T cells into anti-inflammatory Tregs with stimulation of IL-21 or IL-23, whereas stimulation of TGF-β with IL-6, IL-12, or IL-1β induces Th17 cell differentiation [20,23,24]. Specifically, Foxp3 is a master regulator in the development and function of Tregs, whereas RORγt is a major transcription factor for Th17 cell differentiation [23]. Because Foxp3 inhibits RORγt transcriptional activity, Foxp3-expressing Th17 cells present different function from conventional Th17 cells [24]. Thus, the balance between Foxp3 and RORγt is critical in regulating functions of Tregs and Th17 cells. Accordingly, the controlling hepatic frequency of Tregs and Th17 cells by regulating Foxp3 expression is a promising therapeutic strategy for ALD.
In the present study, we tested whether GF2 treatment might regulate the frequency of hepatic Tregs and Th17 cells in chronic-binge ethanol-induced liver injury, and we sought to investigate whether GF2 treatment has anti-inflammatory effects in an IL-10–dependent manner.
2. Materials and methods
2.1. Preparation of GF2
GF2 (>95% purity) was prepared as previously described [5] and purified using Recycling Preparative high-performance liquid chromatography (Japan Analytical Industry Co., Ltd., Japan) with JAIGEL-ODS-AP column (10 μm, 500 × 20 mm i.d., Japan Analytical Industry Co., Ltd.). Purified GF2 was suspended in 40% ethanol (Merck, Darmstadt, Germany) for in vivo experiment, and dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA) for in vitro experiment.
2.2. Animal
Male C57BL/6J wild type (WT) and IL-10 knockout (KO) mice (8-week-old and 24–26 g) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and bred in specific-pathogen-free animal facility. All animals were maintained according to the guidelines of the Care and Use of Laboratory Animals published by the National Institutes of Health. The protocol for animal experiment was approved by Institutional Animal Care and Use Committee of the Korea Advanced Institute of Science and Technology (KA2013-36).
2.3. GF2 treatment and alcoholic liver injury model
To obtain the optimal dose and safety of GF2 treatment, 8-week-old mice (24–26 g) were orally administered different dose of GF2 (0, 25, or 50 mg/kg, every day) for 2 wk. To induce chronic-binge ethanol-induced liver injury, mice (5 mice/group) were orally administered ethanol (3 g/kg) alone (EtOH-fed group) or administered ethanol (3 g/kg) containing GF2 (50 mg/kg) (EtOH + GF2 group) once per day (5 PM) for 2 wk. As for controls, mice were treated with isocaloric sugar water instead of ethanol (pair-fed group). After 2 wk, mice were euthanized under anesthesia (Ketamine, Yuhan Co., Korea). The whole blood was harvested by retro-orbital blood collection. Collected liver specimens (5 mice/group) were used for histological analysis and isolation of liver mononuclear cells (MNCs).
2.4. Serum biochemistry
After collection, the whole blood was allowed to clot by leaving it undisturbed at room temperature for 15 min. Serum was harvested from supernatant after centrifugation at 1,000g for 10 min. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total cholesterol, triglyceride, or albumin were measured using VetTest Chemistry Analyzer (IDEXX Laboratory Inc., Westbrook, ME, USA) following the manufacturer's protocol.
2.5. Histopathologic observation
We analyzed all tissue samples from in vivo experiments. Four μm-thick tissue sections from paraffin-embedded blocks were stained with hematoxylin and eosin (H&E). For immunohistochemistry, tissue sections were incubated in 0.01M citrate buffer (pH 6.0) at 90°C for 60 min, followed by blocking with normal rabbit serum (1:75, Abcam, Cambridge, UK) for 20 min. Subsequently, tissue sections were incubated with anti-F4/80 antibody (1:100, Abcam) and anti-Gr1 antibody (1:200, Abcam) for 2 h. Tissue sections were then incubated with biotinylated anti-rabbit IgE and avidin–biotin horse radish peroxidase complex (Vector Laboratories, Burlingame, CA, USA) for 2 h and stained with DAB (Invitrogen, Eugene, OR, USA) for 20 min. Stained tissues were visually inspected using a model Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a CCD camera and computer-assisted image analysis with DP2-BSW (Olympus, Tokyo, Japan). The numbers of inflammatory foci, F4/80+ cells, and Gr1+ cells were counted in two liver sections of each mouse or 10 hepatocytes.
2.6. Isolation of liver MNCs
Liver was mashed and filtered through 70 μm nylon mesh and suspended in phosphate-buffered saline (PBS). Debris and hepatocytes were removed by centrifugation at 35g for 5 min and other cells in supernatant were suspended in 40% Percoll (GE Healthcare Life Sciences, Piscataway, NJ, USA). The cell suspension was centrifuged at 120g for 30 min. After centrifugation, pelleted cells were suspended in 1 × RBC lysis buffer (BioLegend, San Diego, CA, USA) for 5 min, then diluted in PBS. The cell suspension was centrifuged at 65g for 15 min. Finally, liver MNCs were counted and subjected to flow cytometry and quantitative real-time polymerase chain reaction (qRT-PCR).
2.7. Flow cytometry
Liver MNCs were washed with PBS and suspended in PBS containing 0.5% bovine serum albumin and 0.05% sodium azide. The cells were stained with LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit (Invitrogen) following the manufacturer's protocol. After washed, the cells were stained with fluorescence-conjugated surface antibodies for 30 min at 4°C. For intracellular cytokine staining, cells were fixed and permeabilized with Fixation/Permeabilization Solution Kit (BD Bioscience, San Jose, CA, USA). Permeabilized cells were stained with fluorescence-conjugated surface antibodies for 30 min at 4°C. Stained cells were analyzed using LSR II flow cytometer (BD Bioscience). The data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA). Antibodies for flow cytometric analysis included: FITC CD3e (145-2C11), APC CD8a (53-6.7), APC-Cy7 CD11b (M1/70), PE Gr1 (RB6-8C5), PE IL-17A (TC11-18H10), FITC CD4 (RPA-T4), APC IL-10 (JES5-16E3), and PE NK1.1 (PK136) (all from BD Bioscience); eFluor® 450 CD45 (30-F11), FITC F4/80 (BM8), APC Foxp3 (FJK-16s), and PE CD25 (PC61.5) (all from eBioscience, San Diego).
2.8. RNA isolation, cDNA synthesis, and qRT-PCR analysis
Total RNA in liver MNCs were extracted by TRIzol (Invitrogen). cDNA was synthesized from extracted RNA by amfiRivert cDNA synthesis master mix (GenDEPOT, Barker, TX, USA) following the manufacturer's protocol. qRT-PCR was performed with SYBR Green real-time PCR master mix (Toyobo, Osaka, Japan) using the CFX96 system (Bio-Rad, Hercules, CA, USA). The conditions were as follows: initial denaturation at 95°C for 1 min, 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 15 s, and elongation at 72°C for 45 s. The mRNA level of each gene was compared with mRNA level of Actb and analyzed using ΔΔCt values. The primers used for qRT-PCR were listed at Table 1.
Table 1.
Primers for qRT-PCR
| Genes | Forward (5′–3′) | Reverse (5′–3′) | PCR product (base pairs) |
|---|---|---|---|
| Tnf | AAGCCTGTAGCCCACGTCGTA | AAGGTACAACCCATCGGCTGG | 140 |
| Il6 | TCCATCCAGTTGCCTTCTTG | TTCCACGATTTCCCAGAGAAC | 166 |
| Il17a | GCTCCAGAAGGCCCTCAGA | CTTTCCCTCCGCATTGACA | 139 |
| Il10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG | 105 |
| Foxp3 | CCCAGGAAAGACAGCAACCTTTT | TTCTCACAACCAGGCCACTTG | 88 |
| Tgfb1 | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC | 182 |
| Ifng | TAGCCAAGACTGTGATTGCGG | AGACATCTCCTCCCATCAGCAG | 158 |
| Rorc | TGCAAGACTCATCGACAAGG | AGGGGATTCAACATCAGTGC | 176 |
qRT-PCR = quantitative real-time polymerase chain reaction.
2.9. Th17 differentiation
Mouse splenic naïve CD4+ T cells were isolated by naïve CD4+ T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's protocol. Naïve CD4+ T cells were differentiated into Th17 cells by CellXVivo Mouse Th17 Cell Differentiation Kit (R&D systems, Minneapolis, MN, USA) following manufacturer's protocol. Briefly, isolated naïve CD4+ T cells were suspended at 1 × 106 cells/mL in mouse Th17 differentiation media in CD3 antibody-coated 96 well plate for 5 d.
2.10. Statistical analysis
Data are presented as the mean ± SEM. Differences between groups were assessed by means of Student's t test or analysis of variance for comparison of multiple groups. A p value < 0.01 or 0.05 was considered to be statistically significant.
3. Results
3.1. Administration of GF2 does not induce hepatic injury and inflammatory response in healthy mice
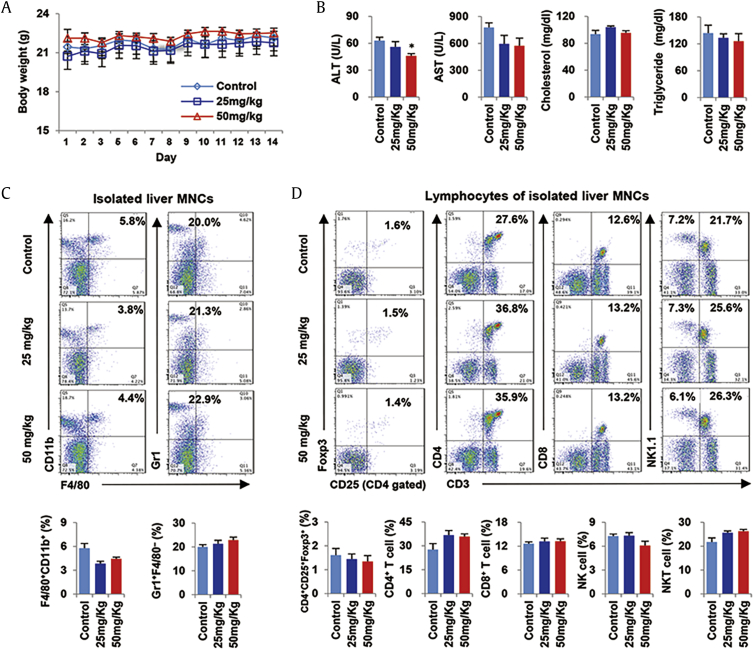
To find optimal dose and avoid unexpected toxicity of GF2, healthy mice were administered different doses of GF2 (0–50 mg/kg) for 2 wk. During the period, there was no difference in changes of body weights (Fig. 1A). At sacrifice, the levels of AST, cholesterol, and triglyceride were different among mice of groups, but ALT levels were significantly decreased in 50 mg/kg GF2-treated mice compared to other mice (Fig. 1B). In flow cytometry analysis, frequencies of F4/80+CD11b+ cells (macrophages) were tend to decrease in GF2-treated mice compared to control mice, whereas those of Gr1+ F4/80‒ cells (neutrophils) were not changed by GF2 treatment (Fig. 1C). Similarly, there were no differences of hepatic frequencies in Tregs (CD4+CD25+Foxp3+), CD4+ T cells (CD4+CD3+), CD8+ T cells (CD8+CD3+), NK cells (NK1.1+CD3‒), and NKT cells (NK1.1+CD3+) (Fig. 1D). Based on the above data, 2-week treatments of GF2 did not induce liver injury and inflammation in healthy mice, and in our study, we decided to use a dose of 50 mg/kg GF2 because of mild protective effect in liver.
Fig. 1.
GF2 does not induce detrimental effects on liver of healthy mice. Wild type (WT) healthy mice were orally administered different doses (0, 25, and 50 mg/kg) of GF2 for 2 wk. (A) Changes of body weights were measured. (B) Serum levels of ALT, AST, cholesterol, and triglyceride were assessed. (C) Frequencies of infiltrated macrophages (F4/80+CD11b+) and neutrophils (Gr1+ F4/80‒) were compared among groups. (D) Frequencies of hepatic lymphocytes such as Tregs (CD4+CD25+Foxp3+), CD4+ T cells, CD8+ T cells, NK cells, and NKT cells were analyzed. Data are expressed as the mean ± SEM. ∗p < 0.05 compared to the corresponding controls. ALT = alanine aminotransferase; AST = aspartate aminotransferase; MNCs = mononuclear cells.
3.2. GF2 treatment ameliorates chronic-binge ethanol-induced liver injury and infiltration of macrophages and neutrophils
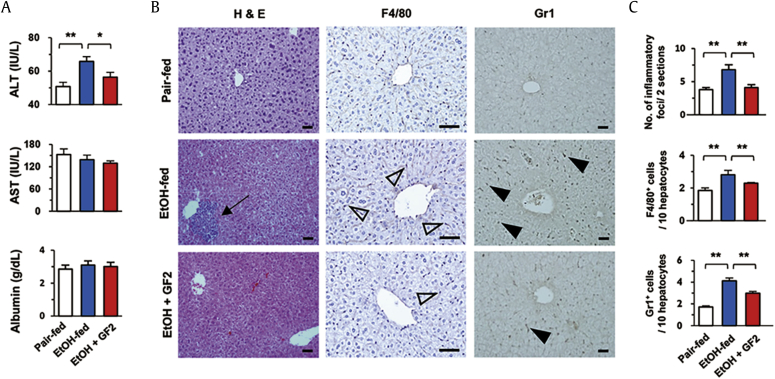
To investigate the effects of GF2 on alcoholic liver injury, WT mice were administered binge ethanol drinking for 2 wk. At sacrifice, EtOH-fed mice showed increased levels of ALT compared to those of pair-fed control mice, whereas GF2 treatment significantly reduced serum ALT levels in ethanol-fed mice (Fig. 2A). However, there were no changes in serum levels of AST and albumin among groups (Fig. 2A). In parallel, H&E staining revealed that increased numbers of inflammatory foci by binge ethanol consumption were reversely decreased in GF2-treated mice compared to non-treated mice (Fig. 2B). Similarly, immunohistochemistry demonstrated that increased infiltration of F4/80+ macrophages and Gr1+ neutrophils were significantly decreased in GF2-treated mice (Fig. 2B, C). These data suggest that GF2 treatment might prevent binge ethanol consumption-mediated liver injury by decreasing inflammatory responses.
Fig. 2.
GF2 attenuates liver injury and immune cell infiltration in chronic-binge ethanol consumption. For 2 wk, WT mice were orally administered binge ethanol (3 g/kg) only (EtOH-fed) or binge ethanol containing 50 mg/kg GF2 (EtOH + GF2) once per day. Pair-fed group mice were treated with isocaloric sugar water. Each group contains five mice. (A) Serum levels of ALT, AST, and albumin were measured. (B) Sections of liver tissues were stained with H&E and antibodies of anti-F4/80 and anti-Gr1. Arrow, closed arrowhead, and open arrowhead indicate inflammatory focus, Gr1+ cells, and F4/80+ cells, respectively. Bar = 50μm. (C) The numbers of inflammatory foci, F4/80+ cells, and Gr1+ cells were counted in two liver sections of each mouse or 10 hepatocytes. Data are expressed as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 compared to the corresponding controls. The results represent three independent experiments. ALT = alanine aminotransferase; AST = aspartate aminotransferase; GF2 = ginsenoside F2; H&E = hematoxylin and eosin.
3.3. GF2 treatment increases hepatic frequency of Tregs in alcoholic liver injury
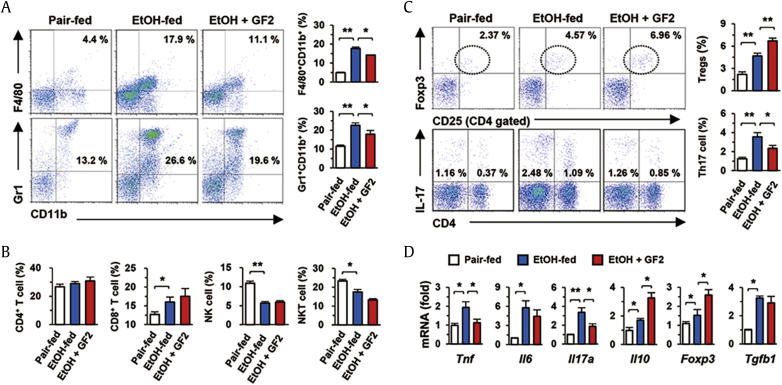
Based on the above findings, we further investigated hepatic inflammatory responses by flow cytometry and qRT-PCR analyses. In liver MNCs, increased frequencies of F4/80+CD11b+ cells (infiltrating macrophages) and Gr1+CD11b+ cells (neutrophils) by binge ethanol drinking were significantly reduced by GF2 treatment (Fig. 3A). Although chronic-binge ethanol consumption increased CD8+ T cells and decreased both populations of NK and NKT cells in the liver, their frequencies were not changed by GF2 treatment (Fig. 3B). Interestingly, both frequencies of Tregs (CD4+CD25+Foxp3+) and Th17 cells (CD4+IL-17+) were significantly increased after chronic-binge ethanol consumption compared to those of pair-fed mice, whereas GF2 treatment significantly increased frequencies of Tregs but decreased those of Th17 cells in EtOH-fed mice (Fig. 3C). In accordance with these findings, qRT-PCR analysis revealed that GF2 treatment suppressed mRNA expression of Tnf and Il17, whereas mRNA expression of Il10 and Foxp3 was significantly increased in liver MNCs of GF2-treated mice compared to those of EtOH-fed mice (Fig. 3D). However, Tgfb1 mRNA expression was not changed by GF2 treatment (Fig. 3D). Based on above findings, we could hypothesize that increased IL-10 expression of Tregs by GF2 might protect alcoholic liver injury.
Fig. 3.
GF2 attenuates alcoholic inflammatory responses by increasing Tregs and decreasing IL-17 producing Th17 cells. Isolated liver mononuclear cells (MNCs) from three groups (pair-fed, EtOH-fed, and EtOH + GF2) of WT mice were subjected to flow cytometry or quantitative real-time polymerase chain reaction (qRT-PCR) analysis. (A) Frequencies of infiltrated macrophages (F4/80+CD11b+) and neutrophils (Gr1+CD11b+) were assessed and compared among groups. (B) Frequencies of hepatic lymphocytes such as CD4+ T cells, CD8+ T cells, NK cells, and NKT cells were analyzed. (C) Frequencies of Tregs (CD4+CD25+Foxp3+) and CD4+IL-17+ cells were compared. (D) mRNA expression of diverse inflammation-related genes was evaluated in isolated liver MNCs. Data are expressed as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 compared to the corresponding controls. The results represent three independent experiments. GF2 = ginsenoside F2; IL = interleukin; NK = natural killer; NKT = natural killer T; Tregs = regulatory T cells.
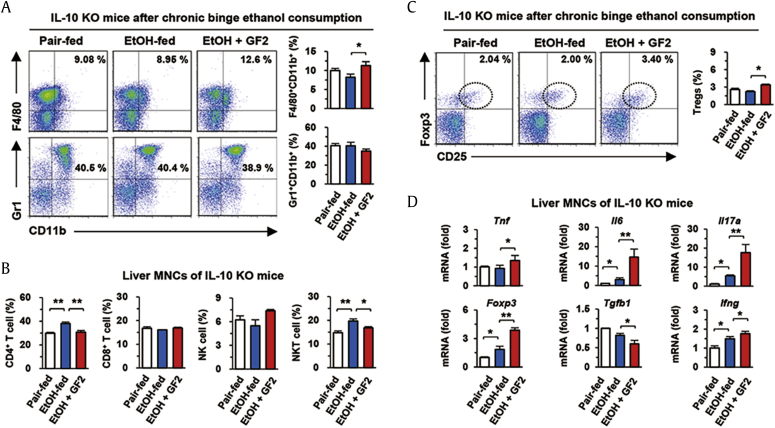
3.4. Protective effect of GF2 is abrogated in IL-10 KO mice
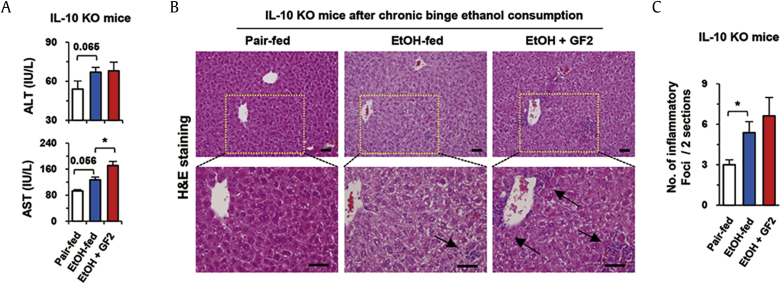
To confirm our hypothesis, we tested whether GF2 treatment might have protective role in IL-10 KO mice. Alcoholic liver injury was induced in IL-10 KO mice with or without treatment of GF2 for 2 wk. Interestingly, GF2 treatment did not attenuate alcoholic liver injury of IL-10 KO mice, in which serum levels of ALT and AST were not decreased by GF2 treatment compared to EtOH-fed mice (Fig. 4A). In H&E staining, inflammatory foci were observed in the liver sections of EtOH-fed IL-10 KO mice compared to those of pair-fed IL-10 KO mice (Fig. 4B). Expectedly, GF2 treatment did not reduce the extent of inflammation (Fig. 4B, C).
Fig. 4.
Alcoholic liver injury and inflammation are not diminished by GF2 in IL-10 KO mice. For 2 wk, IL-10 KO [32] mice were orally administered binge ethanol (3 g/kg) only (EtOH-fed) or binge ethanol containing 50 mg/kg GF2 (EtOH + GF2) once per day. Pair-fed group mice were treated with isocaloric sugar water. Each group contains five mice. (A) Serum levels of ALT and AST were measured. (B) Sections of liver tissues were stained with H&E. Arrows indicate inflammatory foci. Bar = 50μm. (C) The numbers of inflammatory foci were counted in two representative liver sections of each mouse. Data are expressed as the mean ± SEM. ∗p < 0.05 compared to the corresponding controls. The results represent three independent experiments. ALT = alanine aminotransferase; AST = aspartate aminotransferase; GF2 = ginsenoside F2; H&E = hematoxylin and eosin; IL = interleukin; KO = knockout.
In flow cytometric analysis, the frequency of F4/80+CD11b+ macrophages were slightly increased but there was no difference in that of Gr1+CD11b+ neutrophils in GF2-treated IL-10 KO mice compared to those of EtOH-fed IL-10 KO mice (Fig. 5A). In contrast to WT mice (Fig. 3B), chronic-binge ethanol consumption significantly increased the frequencies of CD4+ T and NKT cells in IL-10 KO mice compared to pair-fed mice, but the frequencies of CD4+ T and NKT cells were decreased by GF2 treatment in IL-10 KO mice (Fig. 5B). Interestingly, although GF2 did not attenuate inflammation and alcoholic liver injury in IL-10 KO mice, the frequency of Tregs was still increased by GF2 treatment (Fig. 5C). However, GF2-treated IL-10 KO mice showed much lower frequency of Tregs (~3%) than those (~6%) of GF2-treated WT mice (Fig. 3, Fig. 5C). In qRT-PCR analysis of liver MNCs, mRNA expression of Tnf, Il6, Il17a, and Ifng was slightly increased in EtOH-fed IL-10 KO mice compared to that of pair-fed IL-10 KO mice (Fig. 5D). However, its expression was more significantly increased in GF2-treated IL-10 KO mice than EtOH-fed IL-10 KO mice although Foxp3 mRNA expression was increased in GF2-treated IL-10 KO mice (Fig. 5D). The mRNA expression of Tgfb1 was decreased by GF2 treatment (Fig. 5D). These results suggest that although GF2 treatment increased hepatic Tregs along with increased Foxp3 expression, it did not prevent alcoholic liver injury without IL-10.
Fig. 5.
GF2 aggravates alcoholic inflammatory response in IL-10 KO mice. Isolated liver MNCs from three groups (pair-fed, EtOH-fed, and EtOH + GF2) of IL-10 KO mice were subjected to flow cytometry or qRT-PCR analysis. (A) Frequencies of infiltrated macrophages (F4/80+CD11b+) and neutrophils (Gr1+CD11b+) were compared among groups. (B) Frequencies of hepatic lymphocytes such as CD4+ T cells, CD8+ T cells, NK cells, and NKT cells were assessed. (C) Frequencies of regulatory T cells (CD4+CD25+Foxp3+) were compared. (D) mRNA expression of diverse inflammation-related genes was evaluated in isolated liver MNCs of IL-10 KO mice. Data are expressed as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 compared to the corresponding controls. The results represent three independent experiments. GF2 = ginsenoside F2; IL = interleukin; KO = knockout; MNCs = mononuclear cells; NK = natural killer; NKT = natural killer T.
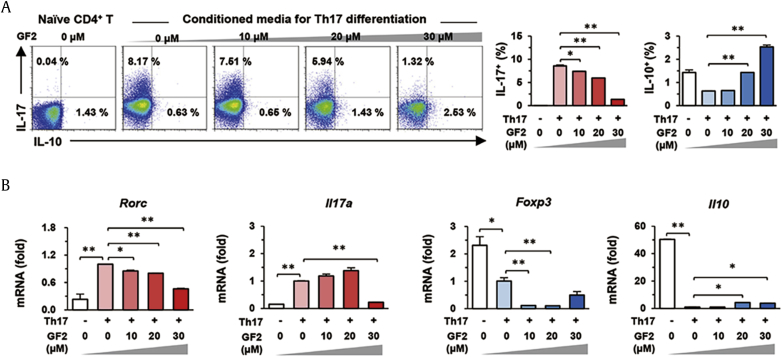
3.5. GF2 is involved in the differentiation of naïve T cells into Th17 cells
Based on the above data, we could speculate that GF2 might regulate the expression of IL-10 and IL-17 in Tregs and Th17 cells, respectively. To prove our hypothesis, we first tested whether GF2 treatment could inhibit Th17 differentiation from naïve T cells in vitro system. In Th17 differentiation condition, 8.17 % of naïve CD4+ T cells were successfully differentiated into IL-17-producing Th17 cells compared to negative controls (0.04 %; Fig. 6A). In differentiated Th17 cells, the mRNA expression of Rorc and Il17a was significantly increased, but that of Foxp3 and Il10 was remarkably suppressed compared to those of naïve T cells (Fig. 6B). However, GF2 treatment significantly inhibited Th17 differentiation in a dose-dependent manner, whereas it gradually increased IL-10 production of CD4+ T cells (Fig. 6A). Concordantly, qRT-PCR analysis revealed that GF2 treatment decreased increased mRNA expression of Rorc and Il17a, whereas it significantly increased Il10 mRNA expression compared to those of Th17 cells (Fig. 6B). Although 10 μM GF2 treatment decreased Foxp3 mRNA expression in Th17 cells, Foxp3 mRNA expression was gradually increased by high doses (20 and 30 μM) of GF2 compared to10 μM GF2 treatment (Fig. 6B). These results suggest that GF2 might suppress not only IL-17 producing Th17 differentiation but also stimulate IL-10 expression in differentiating CD4+ T cells.
Fig. 6.
GF2 inhibits Th17 differentiation of naïve CD4+ T cells. Splenic naïve CD4+ T cells were isolated and then they were differentiated using Th17 differentiation media for 5 d in the presence of diverse doses of GF2 (0–30 μM). Naïve CD4+ T cells are used for negative controls. (A) Differentiated CD4+ T cells were analyzed and the frequencies of IL-17 or IL-10 producing cells were assessed by flow cytometry. (B) Collected cells were subjected to qRT-PCR analysis. Data are expressed as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01 compared to the corresponding controls. The results represent three independent experiments. GF2 = ginsenoside F2; IL = interleukin.
4. Discussion
The present study clearly demonstrated that GF2 treatment attenuated chronic-binge ethanol-induced liver injury in an IL-10 dependent manner. Moreover, the frequency of hepatic Tregs was increased, whereas Th17 cell frequency was decreased in the liver of GF2-treated mice. Furthermore, direct treatment of GF2 suppressed Th17 differentiation of naïve CD4+ T cells by inhibiting IL-17 but enhancing IL-10 expression in vitro. Therefore, GF2 treatment has a protective role against alcoholic liver injury and inflammation.
A line of evidence has reported that diverse ginsenosides including Rg1 have beneficial effects on ALD by upregulating hepatic levels of glutathione and superoxide dismutase, or downregulating the expression of CYP2E1 and NF-κB-mediated inflammatory signaling pathways [[25], [26], [27]]. In our study, we demonstrated novel anti-inflammatory mechanisms of GF2 in ALD. First, GF2 treatment increased mRNA expression of Foxp3, a master regulator of Tregs, in both liver MNCs of EtOH-fed WT and IL-10 KO mice (Fig. 3, Fig. 5D), subsequently leading to increased hepatic Tregs. Second, in vitro GF2 treatment enhanced Foxp3 mRNA expression, which resulted in the suppression of Th17 differentiation. These findings suggested that GF2 treatment contributed to hepatic population of Tregs through upregulation of Foxp3 transcriptional factor. In addition, our findings are in consistent with other studies that activation of Tregs inhibits inflammation of liver diseases [21,28].
In Th17 differentiation, it is well known that transcription factor Foxp3 inhibits RORγt expression [23]. In contrast, TGF-β and other factors suppress Foxp3 expression of naïve CD4+ T cells to achieve Th17 differentiation through RORγt [23]. In our study, we also confirmed that Foxp3 expression was decreased but RORγt expression was increased in differentiated Th17 cells compared to those of naïve CD4+ T cells (Fig. 6B). However, high dose of GF2 treatment significantly suppressed the expression of RORγt and IL-17A (Rorc and Il17a), whereas the production and expression of IL-10 in differentiating naïve CD4+ T cells were dose-dependently increased by GF2 treatment in vitro. These data suggested that GF2 treatment might contribute to hepatic population of Th17 cells by suppressing RORγt expression.
It has been well known that anti-inflammatory effects of Tregs are mainly mediated by IL-10 signaling [20]. In the present study, GF2 treatment increased IL-10 expression in vivo and in vitro models that might contribute to the attenuation of ALD by decreasing infiltration of macrophages, neutrophils, and Th17 cells and reducing expression of pro-inflammatory cytokines. However, although Foxp3 expression was increased by GF2, its protective effects were not observed in IL-10 KO mice. Anti-inflammatory function of Tregs is maintained by IL-10/signal transducer and activator of transcription signaling, in which Tregs themselves exert anti-inflammatory function through producing IL-10 [29]. Accordingly, IL-10-deficient condition should attenuate anti-inflammatory function of Tregs and its population in the liver. In contrast, toll-like receptor signaling and proinflammatory cytokines adversely induce proinflammatory cytokine production of Tregs, in which TGF-β expression was decreased but expression of IFN-γ and IL-17 was increased in Tregs [29,30]. Thus, IL-10-mediated anti-inflammation and Treg are critical mechanisms of GF2 in alcoholic liver injury.
Similarly, ginsenoside Rd (GRd) and ginsenoside Rp1 (GRp1) promote differentiation of Tregs [31,32]. GRd and GRp1 increase Foxp3 expression and production of TGF-β, IL-10, and IL-35. In addition to GF2, these ginsenosides belong to protopanaxadiol-type ginsenoside. Protopanaxadiol-type ginsenosides have been considered as regulators of glucocorticoid and estrogen receptor-mediated gene expression [3,33,34], which are also involved in the cytokine production and upregulated Foxp3 expression in Tregs [35,36]. Therefore, GF2-mediated anti-inflammatory function might be related with these receptors. However, further studies are required to elucidate the exact molecular mechanism of GF2 in this matter.
In conclusion, the present study clearly demonstrates that GF2 treatment attenuates alcoholic liver injury by increasing IL-10 expression and modulating the frequencies of hepatic Tregs and Th17 cells. This study might raise our attention to pharmacological significance of GF2 in the treatment of ALD.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MEST) (2018R1A2A1A05077608), Korea Mouse Phenotyping Project (2014M3A9D5A01073556), and the Intelligent Synthetic Biology Center of Global Frontier Project (2011-0031955) funded by the Ministry of Science, ICT and Future Planning.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.03.002.
Contributor Information
Jin-Seok Byun, Email: jsbyun@knu.ac.kr.
Won-Il Jeong, Email: wijeong@kaist.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee S.M., Bae B.-S., Park H.-W., Ahn N.-G., Cho B.-G., Cho Y.-L., Kwak Y.-S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39(4):384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J.-H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42(3):264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung K.W., Wong A.S.-T. Pharmacology of ginsenosides: a literature review. Chinese Med. 2010;5(1):20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huu Tung N., Uto T., Morinaga O., Kim Y.H., Shoyama Y. Pharmacological effects of ginseng on liver functions and diseases: a minireview. Evidence-based complementary and alternative medicine. eCAM. 2012;2012:173297. doi: 10.1155/2012/173297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui C.H., Kim J.K., Kim S.C., Im W.T. Characterization of a ginsenoside-transforming beta-glucosidase from Paenibacillus mucilaginosus and its application for enhanced production of minor ginsenoside F(2) PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siraj F.M., SathishKumar N., Kim Y.J., Kim S.Y., Yang D.C. Ginsenoside F2 possesses anti-obesity activity via binding with PPARgamma and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J Enzyme Inhib Med Chem. 2015;30(1):9–14. doi: 10.3109/14756366.2013.871006. [DOI] [PubMed] [Google Scholar]
- 7.Shin H.S., Park S.Y., Hwang E.S., Lee D.G., Song H.G., Mavlonov G.T., Yi T.H. The inductive effect of ginsenoside F2 on hair growth by altering the WNT signal pathway in telogen mouse skin. Eur J Pharmacol. 2014;730:82–89. doi: 10.1016/j.ejphar.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Park S.H., Seo W., Eun H.S., Kim S.Y., Jo E., Kim M.H., Choi W.M., Lee J.H., Shim Y.R., Cui C.H. Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem Biophys Res Commun. 2016;478(4):1713–1719. doi: 10.1016/j.bbrc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Mai T.T., Moon J., Song Y., Viet P.Q., Phuc P.V., Lee J.M., Yi T.H., Cho M., Cho S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Canc Lett. 2012;321(2):144–153. doi: 10.1016/j.canlet.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 10.Shield K.D., Parry C., Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2014;35(2):155–173. [PMC free article] [PubMed] [Google Scholar]
- 11.Louvet A., Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12(4):231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 12.Albano E., Vidali M. Immune mechanisms in alcoholic liver disease. Genes Nutr. 2010;5(2):141–147. doi: 10.1007/s12263-009-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H.J., Gao B., Zakhari S., Nagy L.E. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32(1):343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandrekar P., Ambade A. Immunity and inflammatory signaling in alcoholic liver disease. Hepatol Int. 2014;8(Suppl 2):439–446. doi: 10.1007/s12072-014-9518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y.-S., Kim M.-H., Yi H.-S., Kim S.Y., Kim H.-H., Kim J.H., Yeon J.E., Byun K.S., Byun J.-S., Jeong W.-I. CX3CR1 differentiates F4/80low monocytes into pro-inflammatory F4/80high macrophages in the liver. Sci Rep. 2018;8(1):15076. doi: 10.1038/s41598-018-33440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albano E. Role of adaptive immunity in alcoholic liver disease. Int J Hepatol. 2012;2012:893026. doi: 10.1155/2012/893026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammerich L., Heymann F., Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.H., Shim Y.R., Seo W., Kim M.H., Choi W.M., Kim H.H., Kim Y.E., Yang K., Ryu T., Jeong J.M. Mitochondrial double-stranded RNA in exosome promotes interleukin-17 production through toll-like receptor 3 in alcoholic liver injury. Hepatology. 2019 doi: 10.1002/hep.31041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffery H.C., Braitch M.K., Brown S., Oo Y.H. Clinical potential of regulatory T cell therapy in liver diseases: an overview and current perspectives. Front Immunol. 2016;7:334. doi: 10.3389/fimmu.2016.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Wu T., Wang Y., Wan X., Qi J., Li L., Wang X., Luo X., Ning Q. Regulatory T cells suppress excessive lipid accumulation in alcoholic liver disease. J Lipid Res. 2019;60(5):922–936. doi: 10.1194/jlr.M083568. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Ziegler S.F., Buckner J.H. FOXP3 and the regulation of Treg/Th17 differentiation. Microb Infect. 2009;11(5):594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du R., Zhao H., Yan F., Li H. IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J Leukoc Biol. 2014;96(1):39–48. doi: 10.1189/jlb.1RU0114-010RR. [DOI] [PubMed] [Google Scholar]
- 25.Wang M., Zhang X.-J., Liu F., Hu Y., He C., Li P., Su H., Wan J.-B. Saponins isolated from the leaves of Panax notoginseng protect against alcoholic liver injury via inhibiting ethanol-induced oxidative stress and gut-derived endotoxin-mediated inflammation. J Funct Foods. 2015;19:214–224. [Google Scholar]
- 26.Li J-p, Gao Y., Chu S-f, Zhang Z., Xia C-y, Mou Z., Song X-y, He W-b, Guo X-f, Chen N-h. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol-and CCl 4-induced hepatic fibrosis. Acta Pharmacol Sin. 2014;35(8):1031. doi: 10.1038/aps.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y., Chu S., Li J., Li J., Zhang Z., Xia C., Heng Y., Zhang M., Hu J., Wei G. Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J Ethnopharmacol. 2015;173:231–240. doi: 10.1016/j.jep.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Ni S., Li S., Yang N., Tang X., Zhang S., Hu D., Lu M. Deregulation of regulatory T cells in acute-on-chronic liver failure: a rat model. Mediators Inflamm. 2017;2017:1390458. doi: 10.1155/2017/1390458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhry A., Samstein R.M., Treuting P., Liang Y., Pils M.C., Heinrich J.-M., Jack R.S., Wunderlich F.T., Brüning J.C., Müller W. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandiyan P., Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine. 2015;76(1):13–24. doi: 10.1016/j.cyto.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J., Byeon H., Im K., Min H. Effects of ginsenosides on regulatory T cell differentiation. Food Sci Biotechnol. 2017;27(1):227–232. doi: 10.1007/s10068-017-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae J., Koo J., Kim S., Park T.-Y., Kim M.-Y. Ginsenoside Rp1 exerts anti-inflammatory effects via activation of dendritic cells and regulatory T cells. J Ginseng Res. 2012;36(4):375–382. doi: 10.5142/jgr.2012.36.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T., Liang Y., Zuo P., Yan M., Jing S., Li T., Wang Y., Zhang J., Wei Z. Identification of 20(R, S)-protopanaxadiol and 20(R, S)-protopanaxatriol for potential selective modulation of glucocorticoid receptor. Food Chem Toxicol. 2019:110642. doi: 10.1016/j.fct.2019.110642. [DOI] [PubMed] [Google Scholar]
- 34.Hien T.T., Kim N.D., Pokharel Y.R., Oh S.J., Lee M.Y., Kang K.W. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol Appl Pharmacol. 2010;246(3):171–183. doi: 10.1016/j.taap.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Ugor E., Prenek L., Pap R., Berta G., Ernszt D., Najbauer J., Németh P., Boldizsár F., Berki T. Glucocorticoid hormone treatment enhances the cytokine production of regulatory T cells by upregulation of Foxp3 expression. Immunobiology. 2018;223(4–5):422–431. doi: 10.1016/j.imbio.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Tai P., Wang J., Jin H., Song X., Yan J., Kang Y., Zhao L., An X., Du X., Chen X. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.