Abstract
BACKGROUND:
Recent data suggest that patients with atopic dermatitis (AD) have increased systemic immune activation and cardiovascular risk. However, unlike psoriasis, evaluation of active vascular inflammation using state-of-the-art imaging is lacking in AD.
OBJECTIVE:
To assess aortic and carotid vascular inflammation using 18F-fluorodeoxyglucose-positron emission tomography/magnetic resonance imaging (18F-FDG-PET/MRI) imaging in moderate-to-severe AD versus healthy individuals.
METHODS:
A total of 27 patients with moderate-to-severe AD and 12 healthy controls were imaged using 18F-FDG-PET/MRI. Target-to-background ratio (TBR) values were calculated in multiple segments of the aorta and carotid vessels.
RESULTS:
Patients with AD had elevated aortic max TBR (fold change [FCH] = 1.45, P = .057) versus healthy controls and significantly elevated mean TBR (FCH = 1.20; P < .05) in the right carotid (RC) arteries versus controls. When examining greatest focal inflammation (most diseased segment [MDS] TBR), patients with AD had higher aortic inflammation (FCH = 1.28; P = .052). AD clinical severity significantly correlated with C-reactive protein (ρ = 0.60, P < .01) and with RC mean TBR levels (ρ = 0.60, P = .04). Stratifying patients into moderate-to-severe and very severe AD showed greater RC mean TBR in patients with very severe AD versus controls (FCH = 1.31; P = .02) and versus patients with moderate/severe AD (FCH = 1.23, P = .05). Aortic inflammation was also significantly greater in patients with very severe AD versus controls (max TBR: FCH = 1.6, P = .04; MDS TBR: FCH = 1.73, P = .03).
CONCLUSIONS:
This preliminary study is the first that establishes greater vascular (aorta and carotid) inflammation in moderate-to-severe AD versus healthy controls. Furthermore, very severe AD showed higher inflammation than both moderate/severe patients and healthy controls. Future studies with larger patient cohorts and evaluation before and after treatment are needed to determine the extent to which vascular inflammation in AD is modifiable.
Keywords: Atopic dermatitis, 18F-FDG-PET/MRI, Cardiovascular inflammation, Systemic inflammation, Aorta, Carotid arteries, Inflammatory skin disease
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, affecting more than 30 million people in the United States.1 Although it often starts in early childhood, most patients have persistent symptoms in adulthood.2,3 AD often also presents with a wide range of allergic and nonallergic comorbidities, including asthma, allergic rhinoconjunctivitis, food allergies, recurrent infections, and other associations.4
Recent data suggest that patients with AD also have increased cardiovascular risk, with higher rates of cardiovascular disease in some populations,5–10 independent of genetic risk,9 similar to psoriasis, another chronic inflammatory skin disease.11 Consistent with this, 48.1% of patients with severe AD without a history of cardiovascular disease showed the presence of coronary plaques, as assessed by coronary computed tomography (CT) angiography, significantly higher than healthy controls who showed a rate of 21.2%.12
A study investigating active vascular inflammation using state-of-the art imaging techniques, however, is lacking. Arterial calcification, shown to be increased in AD, 12 represents a late, burnt-out stage of atherosclerosis that cannot be reversed with medication. 18F-Fluorodeoxyglucose-positron emission tomography/magnetic resonance imaging (18F-FDG-PET/MRI) imaging can directly assess vascular inflammation as a surrogate for active atherosclerotic disease activity13 and has been used extensively to evaluate atherosclerosis in vivo.14 This approach has been applied to psoriasis (in large studies with more than 150 patients) using 18F-FDG-PET/CT, in which it has been shown that patients with psoriasis have increased aortic vascular inflammation, driven by noncalcified plaque burden,15 which improved after psoriasis-targeted treatments.16 However, thus far, 18F-FDG-PET imaging studies have not been performed in patients with AD with significant disease burden. In addition, an emerging technology for detecting vascular inflammation, 18F-FDG-PET/MRI, which has lower radiation exposure, has been successfully used in autoimmune diseases,17,18 but has not yet been applied to inflammatory skin conditions. Therefore, the purpose of this study was to evaluate active vascular inflammation using 18F-FDG-PET/MRI in moderate-to-severe AD versus healthy individuals. Using this technique in a preliminary case-control study of 27 patients with AD and 12 controls, we found that patients with AD had increased vascular inflammation versus healthy controls.
METHODS
Patients
Twenty-seven untreated adult patients with AD were enrolled in this case-control study. Patients provided institutional review board—approved written consent, and this study was conducted according to the Declaration of Helsinki. Inclusion criteria included AD diagnosis for at least 3 years, with body surface area (BSA) at least 10% and Eczema Area Scoring Index (EASI) greater than 16. Aside from emollients, no topical or systemic medications were used for 2 and 4 weeks, respectively, before inclusion. Exclusion criteria included weight >136 kg due to space limitation in the PET/MR scanner, history of cardiovascular disease, heart failure, uncontrolled arterial hypertension, diabetes, prediabetes, any contraindication to 18F-FDG-PET/MRI, pregnancy, and uncontrolled asthma. Smoking history, weight, systolic and diastolic blood pressure, lipid profiles, C-reactive protein (CRP), electrolytes, and hepatic function tests were collected when available. Twelve healthy adult patients with no known cardiovascular disease were included for comparison. Eligible patients were sequentially enrolled from clinic visits among those who met the inclusion criteria and consented to participate in the study.
Imaging studies
All imaging was performed on a hybrid simultaneous PET/MR system (Biograph mMR; Siemens Healthcare, Erlangen, Germany); 260 MBq of 18F-FDG was injected 90 minutes before image acquisition. Patients were required to have fasted for at least 6 hours and have serum blood glucose levels <200 mg/dL before 18F-FDG administration. PET data were acquired in 2 bed positions, first positioned over the neck to image the carotid arteries for 40 minutes (90–130 minutes after injection), and secondly over the chest to image the thoracic aorta for a further 30 minutes (130–160 minutes after injection). Simultaneously acquired anatomical MRI included, for the neck: 3D time-of-flight MR angiography, and 3D and 2D turbo spin echo, black-blood vessel wall imaging in the carotid arteries, and in the chest: 2D multislice, breath-held bright-blood imaging of the thoracic aorta. PET image reconstruction employed an iterative algorithm with a 344 × 344 × 127 matrix and a 2-mm full-width-at-half-maximum Gaussian post-reconstruction filter. MR-based attenuation correction used the standard approach provided by the scanner in the neck and a specialized respiration-robust approach in the chest.19 Image analysis of PET/MRI data was performed using OsiriX MD (Pixmeo, Geneva, Switzerland). For the carotid arteries, PET images were fused with time-of-flight MR-angiography images in the axial plane. On each axial slice in a volume spanning 1 cm above and below the carotid bifurcation, regions of interest (ROIs) were drawn around the carotid arteries. The left and right carotid arteries were analyzed separately. The mean and maximum standardized uptake values (SUV) of each vessel were measured for each slice. Background was measured in an ROI in the right and left jugular veins. Mean target-to-background ratio (TBR) and maximum (max) TBR were calculated by dividing the mean SUV and max SUV, respectively, by the mean SUV in the background ROI for each slice. The most diseased segment (MDS) TBR was defined as the greatest average of the max TBR for 3 adjacent slices, as previously described.20 For the aorta, PET images were fused with axial 2D breath-held bright-blood images with an example image shown in Figure 1. Similar to the carotids, ROIs were drawn around the vessel wall of the ascending aorta from 2 slices above the aortic root to include the slice caudal to the inferior curve of the aortic arch, and SUVs were recorded. ROIs were then drawn in the superior vena cava and mean SUV recorded to calculate mean TBR and max TBR for each slice of the aortic bed.
FIGURE 1.
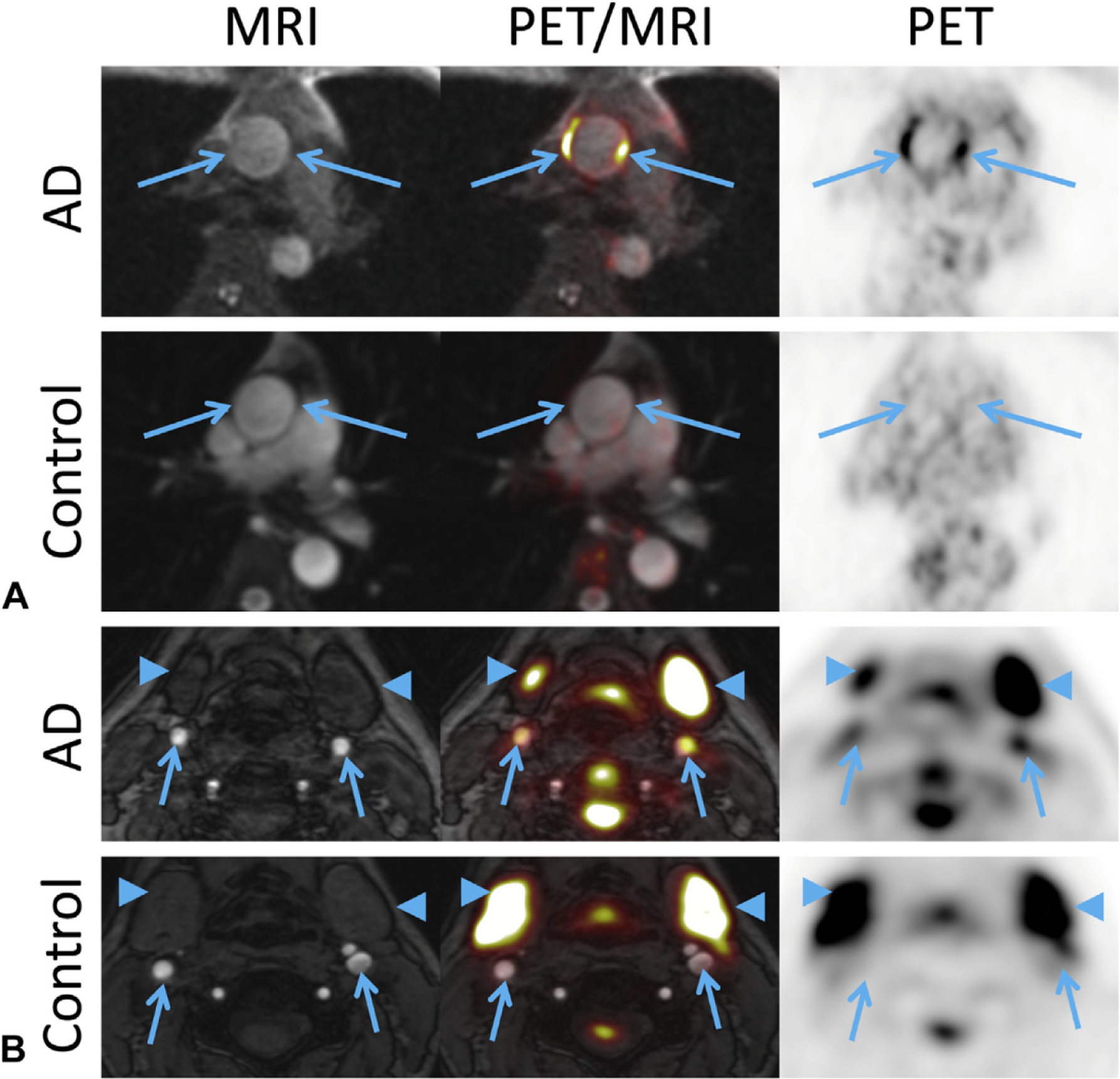
Representative MRI and PET images from patients with atopic dermatitis (AD) and normal control subjects. A, In the aorta, FDG-PET uptake is elevated in the ascending aorta vessel wall compared with controls (arrows). B, In the carotid arteries, elevated FDG-PET uptake is seen localized to the vessel wall in AD compared with controls (arrows). Intense physiological FDG-PET uptake is seen in the salivary glands (arrowhead). FDG, Fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography.
Statistical analysis
Analyses were performed using R language (R-project.org) and packages available through the Bioconductor Project (www.bioconductor.org). Differences between demographic groups were assessed using 2-side Student’s t-test for continuous variables and the χ2 test for categorical variables. TBR measures were normalized by log2 transformation to reduce outlier effects, and 2-sided Student’s t-test was applied to determine significant differences between groups. Differences between groups are reported as fold change (FCH) between the geometric means of the 2 groups. Correlations were calculated using the Spearman correlation coefficient on log2(TBR).
RESULTS
A total of 27 patients with AD and 12 healthy controls were included in this study. Demographics are included in Table I. No significant differences were found between groups in terms of age, sex, smoking, weight, high-density lipoprotein cholesterol, systolic blood pressure, and diastolic blood pressure. The AD group had a significantly higher proportion of white patients (P = .002) and total cholesterol (P = .011).
TABLE I.
Demographics
| Characteristic | AD (N = 27) | Control (N = 12) | P value |
|---|---|---|---|
| Age, mean (SD) | 39.2 (14.3) | 38.1 (11.0) | .461 |
| Sex, n (%) | .089 | ||
| Male | 19 (70.1) | 5 (41.7) | |
| Female | 8 (29.9) | 7 (68.3) | |
| Race, n (%) | .002 | ||
| White | 22 (75.9) | 3 (25.0) | |
| Asian | 3 (10.3) | 2 (16.7) | |
| Black | 2 (6.9) | 4 (33.3) | |
| Other | 0 (0.0) | 3 (25.0) | |
| Smoker, n (%) | .211 | ||
| Yes | 3 (11.1) | 0 (0.0) | |
| No | 24 (88.9) | 12 (100.0) | |
| Weight, mean (SD) | 84.6 (19.2) | 76.9 (16.5) | .212 |
| Total cholesterol, mean (SD) | 200.4 (44.5) | 169.1 (21.8) | .011 |
| HDL cholesterol, mean (SD) | 51.8 (15.7) | 60.1 (17.8) | .194 |
| CRP, mean (SD) | 10.3 (15.0) | N/A | N/A |
| Systolic BP, mean (SD) | 125.2 (13.7) | 124.3 (10.0) | .837 |
| Diastolic BP, mean (SD) | 79.4 (6.8) | 80.1 (7.6) | .802 |
| EASI score, mean (SD) | 38.5 (13.8) | N/A | N/A |
| BSA, mean (SD) | 66.7 (25.3) | N/A | N/A |
AD, Atopic dermatitis; BP, blood pressure; BSA, body surface area; CRP, C-reactive protein; EASI, Eczema Area and Severity Index; HDL, high-density lipoprotein; SD, standard deviation.
Elevated arterial inflammation found in patients with moderate-to-severe AD versus healthy controls
Using 18F-FDG-PET/MRI, we calculated the TBR (a measure of vascular inflammation) in the aorta, left carotid, and right carotid of patients with moderate-to-severe AD and healthy controls. Furthermore, multiple TBR measures were used to evaluate overall levels of inflammation (mean TBR), maximal levels of inflammation observed over the entire vessel (max TBR), and peak levels of inflammation found at any point along the most diseased part of the vessel (MDS TBR; see the Methods section for details). Overall, there was a trend for increased TBR in AD compared with controls. In some comparisons of TBR measures in some vessels, the results showed statistically significant differences. We found that patients with AD had significantly elevated mean TBR versus controls in the right carotid artery (log2FCH = 0.17, FCH = 1.12, P < .05; Figure 2). Comparing max TBR between groups, inflammation was elevated in the aortas of patients with AD versus controls, approaching statistical significance (log2FCH = 0.32, FCH = 1.24, P = .057). Finally, when examining the greatest focal inflammation using the MDS TBR measure, higher aortic inflammation was found in AD (log2FCH = 0.36, FCH = 1.28; P = .052). FCHs and P values for all comparisons are listed in Table II.
FIGURE 2.

Mean target-to-background ratio (TBR) in healthy controls and patients with atopic dermatitis (AD). Mean TBR in the carotid arteries and aortas of healthy controls and patients with AD. *P < .05.
TABLE II.
TBR comparisons
| Left carotid | Right carotid | Aorta | |||||||
|---|---|---|---|---|---|---|---|---|---|
| log2(FCH) | FCH* | P value | log2(FCH) | FCH* | P value | log2(FCH) | FCH* | P value | |
| AD (n = 27) vs Control (n = 12) | |||||||||
| Mean TBR | −0.01 | −1.01 | .873 | 0.17 | 1.12 | .047† | 0.24 | 1.18 | .120 |
| Max TBR | −0.09 | −1.07 | .552 | 0.12 | 1.09 | .251 | 0.32 | 1.24 | .057‡ |
| MDS TBR | −0.14 | −1.10 | .467 | 0.12 | 1.09 | .371 | 0.36 | 1.28 | .052‡ |
| Very severe AD (n = 7) vs Control (n = 12) | |||||||||
| Mean TBR | 0.05 | 1.03 | .933 | 0.39 | 1.31 | .016† | 0.54 | 1.45 | .114 |
| Max TBR | 0.00 | 1.00 | 1.000 | 0.40 | 1.32 | .074‡ | 0.68 | 1.60 | .044† |
| MDS TBR | −0.06 | −1.04 | .964 | 0.47 | 1.39 | .106 | 0.79 | 1.73 | .031† |
| Very severe AD (n = 7) vs Moderate/severe AD (n = 20) | |||||||||
| Mean TBR | 0.09 | 1.06 | .782 | 0.30 | 1.23 | .05‡ | 0.40 | 1.32 | .235 |
| Max TBR | 0.12 | 1.09 | .766 | 0.38 | 1.30 | .067‡ | 0.49 | 1.40 | .139 |
| MDS TBR | 0.11 | 1.08 | .855 | 0.47 | 1.39 | .072‡ | 0.59 | 1.50 | .097‡ |
AD, Atopic dermatitis; FCH, fold change; TBR, target-to-background ratio.
Positive FCH represents greater value in the first group, and negative FCH represents lower value in the first group.
P < .05.
P < .1.
Further evaluating the relationship of clinical disease severity of patients with AD with inflammatory measures, we noted that AD severity measures (EASI and BSA) significantly correlated with CRP (ρ = 0.60, P < .01 for both), confirming previous findings.21 Congruent with our findings of elevated inflammation in the right carotid arteries of patients with AD, we found a significant correlation of right carotid mean TBR levels with EASI (ρ = 0.60, P = .04). Max TBR and MDS TBR measures also correlated with clinical severity assessed by EASI; however, these correlations were not significant (ρ = 0.30, P = .13 and ρ = 0.32, P = .11, respectively).
As total cholesterol was elevated in the AD versus control group, we also performed a subset analysis of patients with AD and control subjects with matched total cholesterol (AD: mean 171.3, standard deviation [SD] 22.7, n = 13; controls: mean 169.1, SD 21.8; P = .65, n = 12). We found trends toward elevated max TBR (log2FCH = 0.40, FCH = 1.32; P = .18) and MDS TBR (log2FCH = 0.43, FCH = 1.1.34; P = .21) in the aortas of the AD versus healthy subjects. We also found a trend toward elevated mean TBR in the right carotid arteries for the AD group (log2FCH = 0.15, FCH = 1.1.11; P = .37). Furthermore, total cholesterol levels among patients with AD did not correlate with either aortic TBR or right carotid TBR (measured by mean TBR, max TBR, or MDS TBR).
More severe AD is associated with greater vascular inflammation
To further assess whether clinical severity of AD is associated with higher levels of systemic inflammation, we stratified patients into “moderate/severe AD” (EASI ≤ 50, n = 20) and “very severe AD” (EASI > 50, n = 7), based on previously reported criteria.22 No differences in weight or total cholesterol were noted between these groups. Patients with very severe AD had significantly greater mean TBR than healthy controls in their right carotids (log2FCH = 0.39, FCH = 1.31, P = .02; Figure 3). Moreover, patients with very severe AD also had significantly elevated mean TBR versus patients with moderate/severe AD in their right carotids (log2FCH = 0.30, FCH = 1.23, P = .05, Figure 3). When evaluating max TBR, elevated inflammation in the right carotid of patients with very severe AD versus healthy controls and patients with very severe AD versus patients with moderate/severe AD was also found, trending to significance (log2FCH = 0.40, FCH = 1.32, P = .076 and log2FCH = 0.38, FCH = 1.30, P = .067, respectively). In the aorta, cardiovascular inflammation was also found to be significantly elevated in patients with very severe AD versus healthy controls when measured by max TBR (log2FCH = 0.68, FCH = 1.60, P = .04) and by MDS TBR (log2FCH = 0.79, FCH = 1.73, P = .03). FCHs and P values for all comparisons are listed in Table II.
FIGURE 3.

Mean target-to-background ratio (TBR) in health controls, patients with moderate and severe atopic dermatitis (AD), and patients with very severe AD. Mean TBR in the carotid arteries and aortas of healthy controls, patients with moderate and severe AD, and patients with very severe AD. *P < .05.
DISCUSSION
Inflammatory diseases such as rheumatoid arthritis,23 and more recently, psoriasis,12,16,24 have been associated with systemic inflammation, as well as with increased risk of cardiovascular disease. These diseases were also associated with increased vascular inflammation as assessed by different imaging modalities, including 18F-FDG-PET/CT16,18,25,26 and CT angiography.12,15 Furthermore, these imaging techniques were further applied to show that vascular inflammation can be reversed with new anti-inflammatory treatments.16
In AD, there are emerging epidemiological data of increased cardiovascular comorbidities, including hypertension and heart disease in certain populations.5–10 These epidemiological data are also supported by increases in circulation of moderate-to-severe AD patients of inflammatory proteins, including cardiovascular/atherosclerosis markers, compared to controls, and even to moderate-to-severe psoriasis patients. The increases in systemic immune and cardiovascular markers in patients with moderate-to-severe AD have been recently suggested to potentially originate and correlate with levels in lesional skin.27 Consistent with this, coronary calcification has been shown in patients with severe AD without established cardiac disease. Nevertheless, imaging with 18F-FDG-PET/MRI to identify increased cardiovascular inflammation, which is a surrogate for active atherosclerotic disease,13 has not been used in AD. In view of rapid development of therapeutics in AD, its application to AD can potentially serve as a valuable tool to assess reversibility of vascular inflammation with various treatments.
It is currently thought that chronic inflammation may accelerate atherosclerosis, potentially due to repetitive vascular injury.28 In psoriasis, cutaneous and systemic inflammation is centered on elevated levels of IL-17, IL-22, and TNF-α, and these cytokines are currently believed to contribute to the increased cardiovascular risk in psoriasis.24,29 In AD skin, there is significant upregulation of IL-13 and IL-22 cytokines.30,31 Increased systemic immune activation has also been observed in blood of patients with moderate-to-severe AD,27,32,33 with increases in both skin homing and systemic T-cell subsets when compared with healthy controls.34 IL-13 has also been shown to be elevated in blood from patients with AD.33,35 Of note, AD is associated with even higher activation of skin homing T-cell subsets when compared with psoriasis.36 These observations point to an increased burden of not only skin, but also systemic inflammation in patients with moderate-to-severe AD.
CRP, an acute phase reactant, is a widely accepted biomarker for atherosclerotic disease, with prognostic value in predicting cardiovascular events.37 In psoriasis, CRP is significantly elevated and associated with skin disease severity.38 Importantly, CRP serum levels were identified as significantly increased in patients with moderate-to-severe AD when compared with matched healthy controls.21 Furthermore, a robust signature in blood from patients with moderate-to-severe AD (defined through high-throughput proteomic assays) highlighted significant dysregulation of inflammatory and cardiovascular risk markers,35 perhaps originating from the robust skin inflammation.27,33,35,39,40 The increases in skin and serum inflammatory and cardiovascular risk factors, together with hyperactivated circulating T cells and increased coronary artery calcifications, strongly support the systemic nature of AD extending beyond classic atopic/allergic associations.
This preliminary study is the first to establish a trend for greater vascular inflammation in patients with moderate-to-severe AD, as seen in the aortas and right carotid arteries versus healthy controls in this study cohort. Based on the measurements of mean TBR, a conservative estimate of total vessel inflammation, max TBR, and MDS TBR, an estimate of the greatest focal inflammation in the vessel, different levels of statistical significance were observed; however, regardless of the specific TBR measure used, there was a clear trend toward greater inflammation in the AD group versus controls. In addition, clinical severity of patients with AD measured by EASI correlated with both the CRP level and the extent of inflammation within their right carotid arteries. Furthermore, stratifying patients with AD by clinical severity into patients with moderate/severe and very severe AD revealed higher TBRs for very severe AD than both moderate/severe patients and healthy controls. In contrast, patients with moderate/severe AD did not have elevated TBRs versus controls. Taken together, these observations suggest that there may be a relationship between the level of systemic inflammation in patients with AD and their level of vascular inflammation. Of note, no differences in TBRs were identified in the left carotid arteries between patients with AD and healthy controls. The asymmetric distribution of vascular injury might be rooted in the different anatomy between the right and the left carotid arteries (arising from the brachiocephalic trunk versus from the aortic arch, respectively), which influences local hemodynamic forces.41
Differential carotid intima media thickness has also been observed in multiple other studies,42–44 typically with greater thickening of the left carotid vessels. The differential results between the carotids observed in this study will require investigation in future studies larger cohorts.
We must acknowledge several limitations. The sample size was small (particularly for healthy controls); thus it is likely that the study was underpowered to detect differences with higher levels of confidence. In addition, only 4 patients with moderate AD were included, preventing further stratification and analysis separating moderate from severe and very severe patients. Lastly, total cholesterol levels were significantly elevated among patients with AD compared with healthy controls. As cholesterol levels are known to contribute to vascular inflammation, this may represent a confounding factor. When comparing a subset of patients with AD with matched total cholesterol levels with healthy controls, there was a trend to significant elevation of vascular inflammation that supports the validity of our findings; however, this comparison was limited by a small sample size. Future studies with much larger patient cohorts are needed to elucidate the relationship between chronic systemic inflammation in AD, cardiovascular inflammation as a surrogate for atherosclerotic disease, and ultimately, risk for cardiovascular events. Furthermore, evaluation of vessel inflammation before and after treatment, as has been done in psoriasis,16,45 showing decreased inflammation after treatment, will help to determine the extent to which vascular inflammation in AD is modifiable.
What is already known about this topic?
In moderate-to-severe atopic dermatitis (AD), elevated systemic immune activation is increasingly recognized, and epidemiological studies suggest increased cardiovascular risk.
What does this article add to our knowledge?
This study is the first to show increased cardiovascular inflammation using fluorodeoxyglucose-positron emission tomography/magnetic resonance imaging in moderate-to-severe AD.
How does this study impact current management guidelines?
This study suggests that taking into account the potential for cardiovascular inflammation may be warranted in deciding on the need to use systemic treatment.
Acknowledgments
This study was supported by NIH grant HL071021.
Abbreviations used
- AD
Atopic dermatitis
- BSA
Body surface area
- CRP
C-reactive protein
- CT
Computed tomography
- EASI
Eczema Area Scoring Index
- 18F-FDG
18F-Fluorodeoxyglucose
- FCH
Fold change
- MDS
Most diseased segment
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- ROI
Region of interest
- SD
Standard deviation
- SUV
Standardized uptake value
- TBR
Target-to-background ratio
Footnotes
Conflicts of interest: P. Brunner reports personal fees from LEO Pharma, Pfizer, Sanofi Genzyme, Eli Lilly, Novartis, Celgene, UCB Pharma, Biotest, Boehringer Ingelheim, AbbVie, Amgen, and Arena Pharmaceuticals; and is an investigator for Novartis (grant paid to his institution). J. G. Krueger reports grants and personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, Biogen Idec, Delenex, AbbVie, Sanofi, Baxter, Parexel, Xenoport, and Kineta. M. G. Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Astra-Zeneca, Boehringer Ingelheim, Celgene, Clinuvel, Eli Lilly, Incyte, Janssen Research & Development, LLC, Kadmon Corp., Leo Pharmaceuticals, MedImmune, Novartis, Ortho Dermatologics, Pfizer, Sciderm, UCB, Inc., and ViDac; is a consultant for Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed, Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, NeuroDerm, Pfizer, Promius/Dr. Reddy’s Laboratories, Theravance, and Verrica. Z. A. Fayad reports grants from Daiichi-Sankyo, Amgen, Bristol Myers Squibb, and Siemens Healthineers; and personal fees from Alexion, GlaxoSmithKline, and Trained Therapeutix Discovery. E. Guttman-Yassky is a consultant for AbbVie, Amgen, Allergan, Asana Bioscience, Celgene, Concert, Dermira, DS Biopharma, Escalier, Galderma, Glenmark, Kyowa Kirin, LEO Pharmaceuticals, Lilly, Mitsubishi Tanabe, Novartis, Pfizer, Regeneron, Sanofi, and Union Therapeutics; has board membership in Allergan, Asana Bioscience, Celgene, DBV, Dermavant, Dermira, Escalier, Galderma, Glenmark, Kyowa Kirin, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, and Sanofi; reports research grants from AbbVie, AnaptysBio, AntibioTx, Asana Bioscience, Boehringer Ingelheim, Celgene, DBV, Dermavant, DS Biopharma, Galderma, Glenmark, Innovaderm, Janssen Biotech, Kiniska Pharma, LEO Pharmaceuticals, Lilly, MedImmune, Sienna Biopharmaceuticals, Novan, Novartis, Ralexar, Regeneron, Pfizer, UCB, and Union Therapeutics. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Hanifin JM, Reed ML, Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis 2007;18:82–91. [DOI] [PubMed] [Google Scholar]
- 2.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol 2014;150:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg JI. Persistence of childhood eczema into adulthood. JAMA Dermatol 2014;150:591–2. [DOI] [PubMed] [Google Scholar]
- 4.Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol 2017;137:18–25. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol 2015;135:721–728.e6. [DOI] [PubMed] [Google Scholar]
- 6.Andersen YMF, Egeberg A, Gislason GH, Hansen PR, Skov L, Thyssen JP. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J Allergy Clin Immunol 2016;138:310–312.e3. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy 2015;70:1300–8. [DOI] [PubMed] [Google Scholar]
- 8.Su VY, Chen TJ, Yeh CM, Chou KT, Hung MH, Chu SY, et al. Atopic dermatitis and risk of ischemic stroke: a nationwide population-based study. Ann Med 2014;46:84–9. [DOI] [PubMed] [Google Scholar]
- 9.Standl M, Tesch F, Baurecht H, Rodriguez E, Muller-Nurasyid M, Gieger C, et al. Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol 2017;137:1074–81. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg JI, Becker L, Kwasny M, Menter A, Cordoro KM, Paller AS. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol 2015;151:144–52. [DOI] [PubMed] [Google Scholar]
- 11.Guttman-Yassky E, Krueger JG, Lebwohl MG. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp Dermatol 2018;27:409–17. [DOI] [PubMed] [Google Scholar]
- 12.Hjuler KF, Bottcher M, Vestergaard C, Deleuran M, Raaby L, Botker HE, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med 2015;128:1325–1334.e2. [DOI] [PubMed] [Google Scholar]
- 13.Rudd JH, Myers KS, Bansilal S, Machac J, Woodward M, Fuster V, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging 2009;2: 107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alie N, Eldib M, Fayad ZA, Mani V. Inflammation, atherosclerosis, and coronary artery disease: PET/CT for the evaluation of atherosclerosis and inflammation. Clin Med Insights Cardiol 2014;8:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol 2018;3:949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenendyk JW, Shukla P, Dey AK, Elnabawi YA, Aksentijevich M, Choi H, et al. Association of aortic vascular uptake of (18)FDG by PET/CT and aortic wall thickness by MRI in psoriasis: a prospective observational study. Eur J Nucl Med Mol Imaging 2019;46:2488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011; 378:1547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvera SS, Aidi HE, Rudd JH, Mani V, Yang L, Farkouh M, et al. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis 2009; 207:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robson PM, Dweck MR, Trivieri MG, Abgral R, Karakatsanis NA, Contreras J, et al. Coronary artery PET/MR imaging: feasibility, limitations, and solutions. JACC Cardiovasc Imaging 2017;10:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachi K, Mani V, Kaufman AE, Alie N, Goldstein RZ, Fayad ZA, et al. Imaging plaque inflammation in asymptomatic cocaine addicted individuals with simultaneous positron emission tomography/magnetic resonance imaging. World J Radiol 2019;11:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vekaria AS, Brunner PM, Aleisa AI, Bonomo L, Lebwohl MG, Israel A, et al. Moderate-to-severe atopic dermatitis patients show increases in serum C-reactive protein levels, correlating with skin disease activity. F1000Res 2017;6: 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015;172:1353–7. [DOI] [PubMed] [Google Scholar]
- 23.Crowson CS, Liao KP, Davis JM III, Solomon DH, Matteson EL, Knutson KL, et al. Rheumatoid arthritis and cardiovascular disease. Am Heart J 2013;166: 622–628.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vena GA, Vestita M, Cassano N. Psoriasis and cardiovascular disease. Dermatol Ther 2010;23:144–51. [DOI] [PubMed] [Google Scholar]
- 25.Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis 2013;3:273–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Emami H, Vijayakumar J, Subramanian S, Vucic E, Singh P, MacNabb MH, et al. Arterial 18F-FDG uptake in rheumatoid arthritis correlates with synovial activity. JACC Cardiovasc Imaging 2014;7:959–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavel AB, Zhou L, Diaz A, Ungar B, Dan J, He H, et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol 2020;82:690–9. [DOI] [PubMed] [Google Scholar]
- 28.Steyers CM III, Miller FJ Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014;15:11324–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kupetsky EA, Mathers AR, Ferris LK. Anti-cytokine therapy in the treatment of psoriasis. Cytokine 2013;61:704–12. [DOI] [PubMed] [Google Scholar]
- 30.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol 2019;139:1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashida S, Uchi H, Takeuchi S, Esaki H, Moroi Y, Furue M. Significant correlation of serum IL-22 levels with CCL17 levels in atopic dermatitis. J Dermatol Sci 2011;61:78–9. [DOI] [PubMed] [Google Scholar]
- 33.He H, Li R, Choi S, Zhou L, Pavel A, Estrada YD, et al. Increased cardiovascular and atherosclerosis markers in blood of older atopic dermatitis patients. Ann Allergy Asthma Immunol 2020;124:70–8. [DOI] [PubMed] [Google Scholar]
- 34.Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol 2015;136:104–115.e7. [DOI] [PubMed] [Google Scholar]
- 35.Brunner PM, Suarez-Farinas M, He H, Malik K, Wen HC, Gonzalez J, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017;7:8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czarnowicki T, He HY, Wen HC, Hashim PW, Nia JK, Malik K, et al. Alopecia areata is characterized by expansion of circulating Th2/Tc2/Th22, within the skin-homing and systemic T-cell populations. Allergy 2018;73:713–23. [DOI] [PubMed] [Google Scholar]
- 37.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 2013;62:397–408. [DOI] [PubMed] [Google Scholar]
- 38.Strober B, Teller C, Yamauchi P, Miller JL, Hooper M, Yang YC, et al. Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. Br J Dermatol 2008;159:322–30. [DOI] [PubMed] [Google Scholar]
- 39.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res 2011;39:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selwaness M, van den Bouwhuijsen Q, van Onkelen RS, Hofman A, Franco OH, van der Lugt A, et al. Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right. Stroke 2014;45:3226–30. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Lin CH, Lu CJ, Yip PK, Chen RC. Carotid atherosclerosis, intima media thickness and risk factors—an analysis of 1781 asymptomatic subjects in Taiwan. Atherosclerosis 2002;164:89–94. [DOI] [PubMed] [Google Scholar]
- 43.Onbas O, Kantarci M, Okur A, Bayraktutan U, Edis A, Ceviz N. Carotid intima-media thickness: is it correlated with stroke side? Acta Neurol Scand 2005;111:169–71. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez Hernandez SA, Kroon AA, van Boxtel MP, Mess WH, Lodder J, Jolles J, et al. Is there a side predilection for cerebrovascular disease? Hypertension 2003;42:56–60. [DOI] [PubMed] [Google Scholar]
- 45.Kim BS, Lee WK, Pak K, Han J, Kim GW, Kim HS, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: feasibility study using (18)F-fluorodeoxyglucose PET/CT. J Am Acad Dermatol 2019;80:1322–31. [DOI] [PubMed] [Google Scholar]


