Abstract
Background:
We assessed risks and outcomes of immune checkpoint inhibitor (ICI) rechallenge in patients with resolved grade 3–4 ICI hepatitis, as current guidelines recommend permanent ICI discontinuation in these patients.
Methods:
We performed a retrospective cohort study from 2010–2019 of melanoma patients treated with ≥1 ICIs and who recovered from grade 3–4 ICI hepatitis. The primary outcome was hepatitis recurrence and the secondary outcome was development of any immune-related adverse event (irAE) requiring discontinuation of ICI rechallenge. Best overall response and time to all-cause death were compared between the patients who did or did not undergo ICI rechallenge.
Results:
Of the 102 melanoma patients who developed high-grade ICI hepatitis, 31 underwent ICI rechallenge. While 15/31 (48%) developed an irAE of any grade, only six (19%) required ICI discontinuation due to irAE severity (4/29 [14%] rechallenged with anti-PD-1/PD-L1 and 2/2 [100%] rechallenged with ipilimumab). Recurrent hepatitis accounted for 4/6 of these cases. Rechallenged patients who did not require ICI discontinuation were significantly less likely to receive ipilimumab rather than anti-PD-1/PD-L1 monotherapy (0% vs 33%, RR 0.1, 95% CI 0.1–0.3, p=0.032) and significantly less likely to be rechallenged with their original ICI (8% vs 50%, RR 0.2, 95% CI 0.1–0.7, p=0.038). There was no difference in best overall response or time to death between rechallenged and non-rechallenged patients.
Conclusion:
ICI therapy can be resumed in melanoma patients who have recovered from grade 3–4 ICI hepatitis with a modest risk of serious irAE. It remains unclear whether ICI retreatment improves clinical outcomes.
Keywords: immune checkpoint inhibitor, hepatitis, immunotherapy, melanoma
Precis:
Following resolution of high-grade immune-mediated hepatitis, most patients who are rechallenged with immune checkpoint inhibitors can remain on treatment without developing serious immune-related adverse events. It remains unclear whether immune checkpoint inhibitor retreatment improves clinical outcomes.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) represent an important advance in cancer treatment. These monoclonal antibodies target several key proteins involved in inactivation of T cells, thereby preventing immune evasion by tumor cells. With the rapid expansion of ICI use, adverse reactions based on immune activation are becoming a frequent problem in clinical practice. While immune-related adverse events (irAEs) can involve almost any organ system, the most common sites include the gastrointestinal tract, liver, endocrine glands, and skin.1 Elevated liver enzymes occur in approximately 10% of patients treated with single-agent ICI therapy (more commonly with CTLA-4 inhibitors than PD-1 inhibitors), with grade 3–4 ICI hepatitis (defined as aminotransferase elevations >5 times the upper limit of normal) occurring in 1–2%.2–5 There is ample evidence that patients with metastatic melanoma have improved survival when treated with combined CTLA-4 and PD-1 inhibition versus single agent anti-CTLA-4 therapy, 6–8 and combination therapy also has been shown to be beneficial in renal cell carcinoma and hepatocellular carcinoma.9,10 However, the efficacy of dual checkpoint blockade come at the expense of increased toxicity, with rates of grade 3 or higher hepatitis approaching 15%.1–3,6,11
While current guidelines from the American Society of Clinical Oncology and the European Society for Medical Oncology recommend permanent discontinuation of ICI therapy after a grade 3 or higher ICI hepatitis,2,3 there are limited data regarding the safety and efficacy of resuming ICI therapy in patients who have recovered from high-grade ICI hepatitis. The three largest studies of ICI rechallenge following an irAE contain few patients who were retreated following ICI hepatitis (and even fewer with grade 3–4 hepatitis) and to our knowledge, studies focusing exclusively on ICI rechallenge following ICI hepatitis are lacking.12–14 We therefore sought to examine the outcomes following resumption of ICI therapy in melanoma patients with resolved high-grade ICI hepatitis.
METHODS
Patients and study design
This multicenter, retrospective cohort study includes all melanoma patients treated at the Dana-Farber/Brigham and Women’s Cancer Center and the Mass General Cancer Center between 2010 and 2019 who received at least one dose of ICI therapy and who developed grade 3 or 4 ICI hepatitis. All irAEs were graded using the Common Terminology Criteria for Adverse Events version 5.0. Patients on clinical trials or receiving additional antineoplastic therapy in addition to ICI treatment were not excluded from the study. Grade 3 ICI hepatitis was defined as an ALT of greater than 200 U/L (5 times the upper limit of normal) in patients receiving ICI treatment in the absence of alternative causes of liver injury, including viral hepatitis, ischemia, or other temporally-related medications known to cause drug-induced liver injury. Grade 4 ICI hepatitis was defined similarly, with the exception that a peak ALT of greater than 800 U/L (20 times the upper limit of normal) was required.
Adult patients with a diagnosis of melanoma who received ICI treatment and had an ALT above 200 U/L were identified through the Partners Research Data Registry, a centralized clinical data warehouse that gathers clinical information from the multiple hospital systems within Partners HealthCare. Patients meeting the criteria for grade 3 or 4 ICI hepatitis were identified through physician adjudication by individual chart review. Institutional review board approval was obtained from the Partners Human Research Committee and the Dana Farber Office for Human Research Studies.
Outcomes and covariates
The primary outcome was recurrence of at least a grade 2 ICI hepatitis (defined as an ALT of greater than 100 U/L, or 2.5 times the upper limit of normal). The secondary outcome was rechallenge discontinuation due to immune-related toxicity. Additional outcomes comparing the rechallenged and non-rechallenged groups were best overall response (BOR), disease control, and time to all-cause death. Response was determined based on documentation in oncology clinical notes.
Baseline demographic data, clinical parameters, and laboratory values were collected at the time of initiation of ICI therapy. Laboratory data were additionally collected at the time of diagnosis of the incident grade 3 or higher ICI hepatitis and at the time of peak ALT elevation. Comorbidities included a history of smoking (defined as greater than or equal to a 10 pack-year smoking history, and further subdivided into active or former smoking), alcohol use (defined as greater than an average of 2 drinks/day for males and 1 drink/day for females), and underlying liver disease (based on available diagnosis codes, imaging, and/or histology). The date of commencement of systemic corticosteroid therapy at a dose of at least 1 mg/kg prednisone equivalents was recorded for all patients who received corticosteroid treatment. If 1 mg/kg corticosteroid therapy was started prior to the time of diagnosis of grade 3 hepatitis (e.g., for lower-grade hepatitis or for other irAEs), the start date was considered to be the date of initial diagnosis of grade 3 hepatitis. For patients who underwent retreatment with ICI therapy, lower toxicity therapy was defined as administration of an ICI known to be less likely to cause an irAE (e.g., combination therapy to monotherapy or an anti-CTLA-4 to an anti-PD-1/PD-L1). ECOG performance status was recorded at the time of rechallenge for patients who were retreated and at the time of ALT normalization (defined as <50 U/L) for patients who were not retreated. For retreated patients, the rationale for pursuing rechallenge was determined from oncology notes if available. For survival analysis, data on all-cause mortality, total length of follow-up, time to peak ALT level, and time to return of ALT to below the upper limit of normal were collected.
Statistical analysis
Baseline patient characteristics are reported as means and standard deviations for continuous normal data, medians and interquartile ranges for continuous non-normal data, and frequencies and percentages for categorical data. Univariable analyses were performed using two-sided Student’s t-test/ Wilcoxon rank sum test for continuous variables and Chi-squared test/ Fisher’s exact test for categorical variables. Kaplan-Meier analysis with log-rank testing was utilized for time-to-event data and the start date for all time-to-event analyses was the first day that grade 3–4 ICI hepatitis was diagnosed. Cox regression was employed to control for potential confounders in the survival analysis. Patients were censored at the time of last follow-up or death. Statistical significance was defined as p≤0.05. All statistical analyses were performed using SAS version 9.4 (Cary, NC, USA).
RESULTS
Cohort Selection
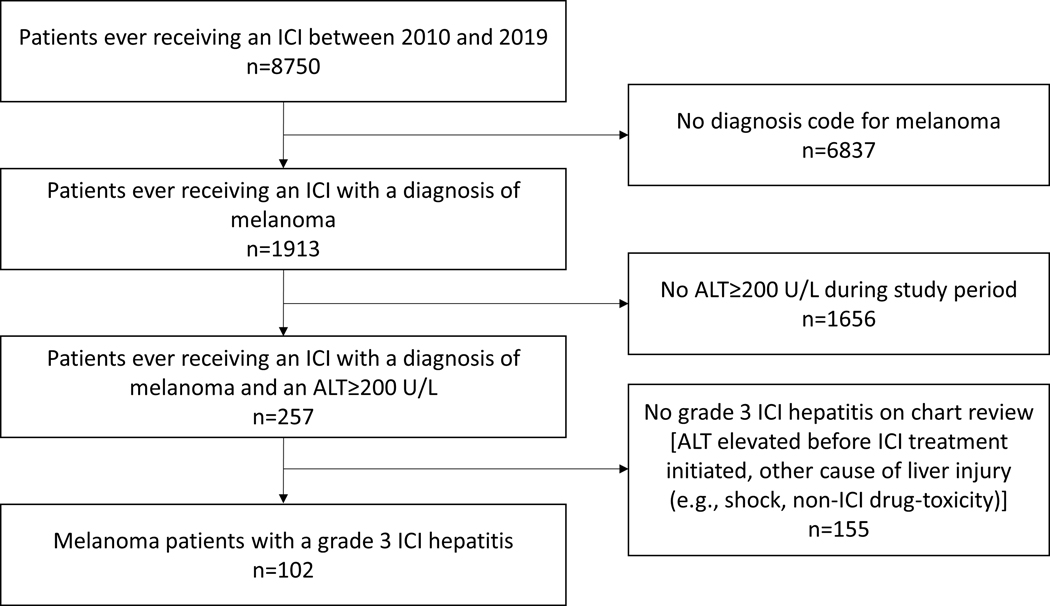
We identified 8750 patients (6613 from Dana-Farber/Brigham and Women’s Cancer Center and 2137 from the Mass General Cancer Center) who received at least one dose of an ICI between 2010–2019, of which 1913 had a diagnosis code of melanoma (Figure 1). Of the 257 melanoma patients who had an ALT value of ≥200 U/L, 102 were established to have grade 3 or higher ICI hepatitis by physician-adjudication. Following resolution of ICI hepatitis, 31 (30.4%) patients subsequently were retreated with an ICI, with a median length of total follow-up of 815 (IQR 357–1293) days.
Figure 1 -.
Flowchart of patient identification and exclusion
Comparison of ICI-rechallenged versus non-rechallenged patients
Table I provides baseline characteristics and details regarding the initial ICI hepatitis episode in the overall cohort, stratified by whether patients did or did not undergo rechallenge. As compared to the 71 patients who were not rechallenged with an ICI following initial immune related hepatitis, patients who were rechallenged were significantly younger (mean age 52.2 ± 13.3 vs 58.8 ± 16.5, p=0.050) and more likely to have been treated with combination CTLA-4/PD-1 therapy (71.0% vs 45.1%, p=0.002). The patients who were rechallenged with an ICI also had lower overall severity of hepatitis, as evidenced by the proportion of patients not needing steroid treatment (16.1% vs 0%, p=0.002), the median maximum steroid dose (1.0 mg/kg [0.8–2.0] vs 2.0 mg/kg [1.0–2.0], p=0.023), the proportion of patients developing steroid-refractory hepatitis (15.4% vs 42.9%, p=0.016), and the proportion of patients undergoing Gastroenterology/Hepatology consultation (45.2% vs 81.7%, p<0.001). On the other hand, laboratory values at baseline, at the time of diagnosis of grade 3 hepatitis, and at the time of peak ALT were broadly similar between those patients who were and were not rechallenged. There also was no difference in ECOG performance status between the two groups. Liver biopsy was performed at similar proportions in both groups (51.6% vs 54.9%, p=0.757); 93% of histology was documented by pathologists as compatible with ICI hepatitis in their written reports with findings ranging from nonspecific lobular inflammation to histiocytic infiltration.15–17 Of the remaining four biopsies, three were performed later in the hepatitis course and showed mild nonspecific changes consistent with resolving liver injury and one was a sample with extensive malignant infiltration which made identification of a possible cause of liver injury impossible. Notably, the latter patient experienced normalization of liver enzymes following withdrawal of the causative ICI which ruled out that patient’s liver metastases as a possible cause of their elevated liver tests.
Table 1 -.
Characteristics, laboratory values, and outcomes of rechallenged and non-rechallenged patients
| No rechallenge (n=71) | Rechallenge (n=31) | p-value | |
|---|---|---|---|
| Age | 58.8 +/− 16.5 | 52.2 +/− 13.3 | 0.050 |
| Males | 42 (59.2%) | 15 (48.4%) | 0.314 |
| Ethnicity | |||
| White | 67 (94.4%) | 30 (96.8%) | |
| Black | 0 (0.0%) | 0 (0.0%) | |
| Hispanic | 2 (2.8%) | 0 (0.0%) | |
| Other/unknown | 2 (2.8%) | 1 (3.2%) | |
| Body Mass Index | 27.3 +/− 5.6 | 26.7 +/− 6.1 | 0.619 |
| Baseline liver disease | 18 (25.4%) | 5 (16.1%) | 0.440 |
| Smoking | 0.489 | ||
| Nonsmoker | 57 (80.3%) | 26 (83.4%) | |
| Current smoker | 7 (9.9%) | 1 (3.2%) | |
| Former smoker | 7 (9.9%) | 4 (12.9%) | |
| Alcohol use | 11 (15.7%) | 2 (6.5%) | 0.334 |
| Metastatic disease | 55 (77.5%) | 27 (87.1%) | 0.291 |
| Liver metastases | 25 (35.2%) | 9 (29.0%) | 0.543 |
| ECOG performance status | |||
| 0 | 33 (50.8%) | 21 (67.7%) | |
| 1 | 23 (35.4%) | 9 (29.0%) | |
| 2 | 7 (10.8%) | 1 (3.2%) | |
| 3 | 2 (3.1%) | 0 (0%) | |
| ≥2 | 9 (13.9%) | 1 (3.2%) | 0.160 |
| ICI at time of initial hepatitis | |||
| Ipilimumab + nivolumab | 32 (45.1%) | 22 (71.0%) | |
| Ipilimumab | 24 (33.8%) | 4 (12.9%) | |
| Nivolumab | 9 (12.7%) | 2 (6.5%) | |
| Pembrolizumab | 5 (7.0%) | 2 (6.5%) | |
| Atezolizumab | 1 (1.4%) | 1 (3.2%) | |
| Combination ICI use at time of hepatitis | 32 (45.1%) | 22 (71.0%) | 0.016 |
| Development of grade 4 ICI hepatitis | 22 (31.0%) | 3 (9.7%) | 0.025 |
| Other non-hepatitis irAEs | 47 (66.2%) | 10 (32.3%) | 0.002 |
| Treatment of hepatitis | |||
| Initial hepatitis resolved without steroids | 0 (0.0%) | 5 (16.1%) | 0.002 |
| Maximum steroid dose, mg/kg | 2.0 (1.0–2.0) | 1.0 (0.8–2.0) | 0.023 |
| Days to initiation of steroids (at least 1 mg/kg) | 0 (0–2) | 0 (0–1) | 0.206 |
| Steroid-refractory hepatitis | 30 (42.9%) | 4 (15.4%) | 0.016 |
| Mycophenolate mofetil treatment | 28 (39.4%) | 4 (12.9%) | 0.010 |
| Azathioprine treatment | 2 (2.8%) | 0 (0.0%) | 1 |
| Gastroenterology consultation | 58 (81.7%) | 14 (45.2%) | <0.001 |
| Liver biopsy | 39 (54.9%) | 16 (51.6%) | 0.757 |
| Compatible with ICI hepatitis per pathology note | 36 (92.3%) | 15 (93.8%) | |
| Resolving liver injury | 0 (0%) | 3 (6.2%) | |
| Nondiagnostic* | 1 (7.7%) | 0 (0%) | |
| Total length of follow-up (days) | 525 (202–1047) | 815 (357–1293) | 0.041 |
| Baseline labs | |||
| AST (U/L) | 19 (17–24) | 21 (17–24) | 0.289 |
| ALT (U/L) | 18 (13–26) | 20 (15–31) | 0.352 |
| Alkaline phosphatase (U/L) | 75 (63–90) | 69 (54–90) | 0.373 |
| Total bilirubin (mg/dL) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.944 |
| Albumin (g/dL) | 4.3 +/− 0.5 | 4.2 +/− 0.4 | 0.549 |
| Creatinine (mg/dL) | 0.9 +/− 0.2 | 0.9 +/− 0.2 | 0.681 |
| Platelet count (x109/L) | 235 (205–260) | 244 (217–275) | 0.192 |
| INR | 1.0 (1.0–1.1) | 1.0 (1.0–1.0) | 0.690 |
| LDH (U/L) | 168 (150–200) | 188 (160–288) | 0.028 |
| PMN count (x103/uL) | 4.3 (3.4–5.8) | 4.2 (3.3–5.0) | 0.643 |
| Lymphocyte count (x103/uL) | 1.4 (1.0–2.1) | 1.5 (1.2–1.8) | 0.867 |
| Labs at time of grade 3 ICI hepatitis diagnosis | |||
| AST (U/L) | 255 (145–412) | 262 (163–402) | 0.448 |
| ALT (U/L) | 326 (230–589) | 421 (270–479) | 0.543 |
| Alkaline phosphatase (U/L) | 179 (109–256) | 137 (79–255) | 0.389 |
| Total bilirubin (mg/dL) | 0.8 (0.5–1.1) | 0.5 (0.4–0.7) | 0.028 |
| Albumin (g/dL) | 3.8 +/− 0.6 | 3.7 +/− 0.5 | 0.737 |
| Creatinine (mg/dL) | 0.9 +/− 0.2 | 0.9 +/− 0.2 | 0.792 |
| Platelet count (x109/L) | 214 (165–248) | 204 (162–238) | 0.779 |
| INR | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 0.743 |
| LDH (U/L) | 381 (274–476) | 368 (295–609) | 0.447 |
| PMN count (x103/uL) | 4.4 (3.0–6.7) | 3.1 (1.9–7.1) | 0.004 |
| Lymphocyte count (x103/uL) | 1.3 (0.7–2.0) | 1.2 (0.7–1.5) | 0.299 |
| Labs at time of peak ALT | |||
| AST (U/L) | 295 (170–514) | 260 (156–399) | 0.377 |
| ALT (U/L) | 496 (312–978) | 460 (289–580) | 0.144 |
| Alkaline phosphatase (U/L) | 179 (100–356) | 156 (80–259) | 0.304 |
| Total bilirubin (mg/dL) | 0.9 (0.5–1.5) | 0.5 (0.3–0.7) | <0.001 |
| Albumin (g/dL) | 3.7 +/− 0.5 | 3.7 +/− 0.5 | 0.832 |
| Creatinine (mg/dL) | 0.8 +/− 0.2 | 0.8 +/− 0.2 | 0.803 |
| Platelet count (x109/L) | 211 (162–262) | 213 (178–259) | 0.735 |
| INR | 1.1 (0.9–1.1) | 1.1 (1.0–1.1) | 0.525 |
| LDH (U/L) | 353 (232–453) | 381 (271–609) | 0.529 |
| PMN count (x103/uL) | 6.0 (4.2–8.4) | 3.0 (2.0–5.5) | 0.808 |
| Lymphocyte count (x103/uL) | 1.2 (0.7–2.1) | 1.2 (0.9–1.7) | 0.231 |
| Best overall response | |||
| Complete response | 14 (19.7%) | 8 (25.8%) | |
| Partial response | 14 (19.7%) | 12 (38.7%) | |
| Stable disease | 17 (23.9%) | 3 (9.7%) | |
| Progressive disease | 17 (23.9%) | 8 (23.8%) | |
| Complete or partial response | 28 (45.2%) | 20 (64.5%) | 0.078 |
| Disease control rate | 45 (72.6%) | 23 (74.2%) | 0.869 |
| Death | 33 (46.5%) | 12 (38.7%) | 0.467 |
Data are mean +/− SD, median (IQR), or n (%)
Extensive liver metastases precluded identification of a possible etiology
The primary rationale for ICI rechallenge for those patients in whom it was documented included disease progression while off treatment (38.7%), failure of interval non-ICI treatment (16%; defined as patients who received treatment other than ICIs following the resolution of their ICI hepatitis and developed progressive disease), and not requiring steroid treatment for the initial ICI hepatitis episode (9.7%). Notably, the patients who did not receive steroids all had resolution of their liver injury after withdrawal of the causative ICI and all five patients underwent liver biopsy with four out the five biopsies noted to be consistent with ICI hepatitis on the pathology report; the fifth had diffuse infiltration of the liver with metastatic melanoma. Although the mean time to peak ALT from the time of first grade 3 ALT elevation was significantly shorter among the rechallenged patients (2.6 vs 10.0 days, log-rank p=0.003; Supplemental Figure S1a), this effect lost significance on Cox regression analysis after controlling for use of combination therapy and development of steroid-refractory ICI hepatitis (HR 1.5, 95% CI 0.9–2.6, p=0.11). The mean time to normalization of ALT from time of first grade 3 ALT elevation also was significantly shorter in the rechallenged patients (39.2 vs 68.9 days, log-rank p=0.001; Supplemental Figure Sb), and this effect remained significant even after adjusting for combination therapy and development of steroid-refractory ICI hepatitis (HR 1.9, 95% CI 1.1–3.3, p=0.014). These data support that patients who were rechallenged had less severe hepatotoxicity overall.
Regarding tumor response, there were eight patients in the non-rechallenged group who received adjuvant ICI therapy and had no evidence of disease at initiation of treatment for whom BOR was not able to be determined. In the remaining 94 patients, there was no significant difference in the disease control rate between those who were rechallenged and those who were not (71.4% vs 72.6%, RR 0.9, 95% CI 0.4–1.8, p=0.780). However, there was a trend towards rechallenged patients being more likely to achieve a complete or partial response (20 [64.5%] vs 28 [44.4%], RR 1.5, 95% CI 1.0–2.1, p=0.055). Of note, one out of the 94 patients included in this analysis died before restaging; for the purposes of assessing disease response, this patient was considered to have progressive disease.
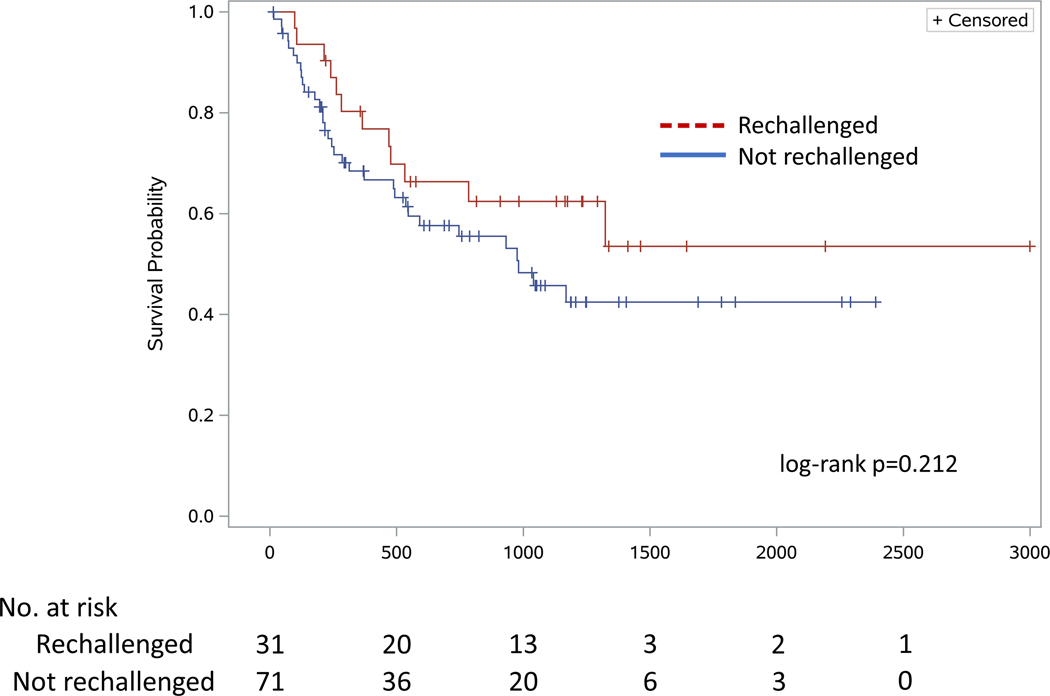
A total of 45 patients died during the study period. There was no significant difference in time to death between patients who were rechallenged and those who were not (961 vs 752 days respectively, log-rank p=0.212; Figure 2), even after adjusting for age, combination CTLA-4/PD-1 therapy, and presence of stage 4 melanoma using Cox regression (HR 0.88, 95% CI 0.44–1.79, p=0.729). Notably, initial treatment with combination ICI therapy was associated with a significantly longer time to all-cause death (HR 0.46, 95% CI 0.24–0.90, p=0.023) as compared with all other ICI treatment regimens, despite ICI discontinuation in patients who developed high-grade ICI hepatitis.
Figure 2 -.
Time to all-cause death
Incidence of immune-related adverse events following ICI rechallenge
Of the 31 patients who resumed ICI therapy, 28 (90.3%) were treated with PD-1 inhibitor monotherapy (48.4% nivolumab, 41.9% pembrolizumab), two received ipilimumab (6.5%) and one received atezolizumab (3.2%). All 23 patients (74.2%) who initially received combination ipilimumab/nivolumab were rechallenged with anti-PD-1 monotherapy (Table II). Six patients (19.4%) were retreated with the same class of ICI (two anti-CTLA4, three anti-PD-1, and one anti-PD-L1). The median duration of ICI treatment following rechallenge was 223 (IQR 63–615) days. Six of 31 (19.4%) patients who resumed ICI therapy developed an irAE that necessitated medication discontinuation at a median time of 91 (IQR 21–448) days from rechallenge initiation, which was longer than their median time to initial grade 3 ICI hepatitis (41 [IQR 30–80] days). Of these, four patients (12.9%) developed recurrent ICI hepatitis (two grade 2, one each grade 3 and grade 4), one (1.6%) developed grade 2 pneumonitis, and one (1.6%) developed grade 3 hypophysitis (Table III). Notably, 3 of these 6 patients (50%) were retreated with the same single agent (including both patients who received ipilimumab). Out of the six patients who did not undergo rechallenge with a lower risk ICI regimen, 3 experienced an irAE requiring discontinuation. An additional nine patients (29.0%) developed other irAEs (i.e., dermatitis, colitis, nephritis, arthritis, hypophysitis) that were not severe enough to require treatment with corticosteroids or ICI discontinuation.
Table 2 -.
ICI regimens of patients who underwent rechallenge
| Rechallenge, no discontinuation^ (n=25) | Rechallenge, discontinuation^ (n=6) | p-value | |
|---|---|---|---|
| ICI at time of initial hepatitis | |||
| Ipilimumab + nivolumab | 21 (84%) | 2 (33.3%) | |
| Ipilimumab | 1 (4%) | 3 (50%) | |
| Nivolumab | 2 (8%) | 0 (0%) | |
| Pembrolizumab | 0 (0%) | 1 (16.7%) | |
| Atezolizumab | 1 (4%) | 0 (0%) | |
| Combination ICI use at time of initial hepatitis | 21 (84%) | 2 (33.3%) | 0.024 |
| ICI used for rechallenge | |||
| Ipilimumab | 0 (0%) | 2 (33.3%) | |
| Nivolumab | 13 (52%) | 2 (33.3%) | |
| Pembrolizumab | 11 (44%) | 2 (33.3%) | |
| Atezolizumab | 1 (4%) | 0 (0%) | |
| Non-ICI agents used in conjunction with ICI rechallenge* | 2 (8%) | 0 (0%) | 1 |
| Trametinib | 1 (4%) | 0 (0%) | |
| Binimetinib | 1 (4%) | 0 (0%) | |
| Rechallenge with anti-PD-1/PD-L1 versus anti-CTLA-4 | 0.032 | ||
| Ipilimumab | 0 (0%) | 2 (33.3%) | |
| Nivolumab/pembrolizumab/atezolizumab | 25 (100%) | 4 (66.7%) | |
| ICI regimen (initial to rechallenge) | |||
| Ipilimumab to ipilimumab | 0 (0%) | 2 (33.3%) | |
| Ipilimumab to PD-1 | 1 (4%) | 1 (16.7%) | |
| Ipilimumab + nivolumab to PD-1 | 21 (84%) | 2 (33.3%) | |
| PD-1 to PD-1 | 2 (8%) | 1 (16.7%) | |
| Atezolizumab to atezolizumab | 1 (4%) | 0 (0%) | |
| Rechallenge with lower hepatitis risk regimen | 22 (88.0%) | 3 (50.0%) | 0.069 |
| Rechallenge with original ICI | 2 (8%) | 3 (50%) | 0.038 |
Data are n (%)
Discontinuation indicates ICI retreatment was stopped due to an irAE
Both patients who received a MEK inhibitor in conjunction with their ICI rechallenge were treated with pembrolizumab
Table III -.
Characteristics and outcomes of patients who underwent ICI rechallenge
| Rechallenge, no discontinuation^ (n=25) | Rechallenge, discontinuation^ (n=6) | p-value | |
|---|---|---|---|
| Age | 52.4 (43.7-57.9) | 55.9 (44.5-67.7) | 0.537 |
| Males | 12 (48%) | 3 (50%) | 1 |
| Ethnicity | 1 | ||
| White | 24 (96%) | 6 (100%) | |
| Black | 0 (0%) | 0 (0%) | |
| Hispanic | 0 (0%) | 0 (0%) | |
| Other/unknown | 1 (4%) | 0 (0%) | |
| Body Mass Index | 24.9 (22.8–28.7) | 27.1 (23.5–31.6) | 0.570 |
| Baseline liver disease | 3 (12%) | 2 (33.3%) | 0.241 |
| Smoking | |||
| Nonsmoker | 22 (88%) | 4 (66.7%) | |
| Current smoker | 1 (4%) | 0 (0%) | |
| Former smoker | 2 (8%) | 2 (33.3%) | |
| Alcohol use | 1 (4%) | 1 (16.7%) | 0.355 |
| Metastatic disease | 22 (88%) | 5 (83.3%) | 1 |
| Liver metastases | 6 (24%) | 3 (50%) | 0.320 |
| ICI as first-line cancer therapy | 19 (76%) | 5 (83.3%) | 1 |
| Development of grade 4 ICI hepatitis | 3 (12%) | 0 (0%) | 1 |
| Other non-hepatitis irAEs | 9 (36%) | 1 (16.7%) | 0.634 |
| Liver biopsy for initial hepatitis | 11 (44%) | 5 (83.3%) | 0.172 |
| Treatment of initial hepatitis | |||
| Initial hepatitis resolved without steroids | 2 (8%) | 3 (50%) | 0.038 |
| Maximum steroid dose, mg/kg | 1.5 (1.0–2.0) | 0.4 (0–2.0) | 0.114 |
| Steroid-refractory hepatitis | 3 (13.0%) | 1 (33.3%) | 0.408 |
| Prednisone treatment ongoing at time of rechallenge | 9 (37.5%) | 1 (33.3%) | 1 |
| Time from ICI initiation to development of initial ICI hepatitis (days) | 44 (24–63) | 41 (30–80) | 0.740 |
| Time to normalization of ALT after initial ICI hepatitis episode (days) | 30 (21–55) | 35 (21–40) | 0.823 |
| Rationale for rechallenge | |||
| Disease progression while off treatment | 11 (44%) | 1 (16.7%) | |
| Failed interval non-ICI treatment | 5 (20%) | 0 (0%) | |
| Did not require steroids for initial ICI hepatitis | 1 (4%) | 2 (33.3%) | |
| Anecdotal evidence of safety | 1 (4%) | 1 (16.7%) | |
| None documented | 7 (28%) | 2 (33.3%) | |
| Time to rechallenge from initial ICI hepatitis episode (days) | 84 (47–224) | 51 (15–77) | 0.179 |
| Days of treatment with rechallenge agent | 238 (63–567) | 91 (21–448) | 0.430 |
| Type of irAE leading to discontinuation | |||
| Hepatitis | N/A | 4 (66.7%) | |
| Pneumonitis | N/A | 1 (16.7%) | |
| Hypophysitis | N/A | 1 (16.7%) | |
| Grade 2 or higher hepatitis recurrence after rechallenge | N/A | 4 (66.7%) | |
| Non dose-limiting toxicities | |||
| Skin (grade 1 ×2, grade 2 ×1) | N/A | 3 (50%) | |
| Colitis (grade 1) | N/A | 1 (16.7%) | |
| Nephritis (grade 2) | N/A | 1 (16.7%) | |
| Arthritis (grade 2) | N/A | 1 (16.7%) | |
| Hypophysitis (grade 1 ×1, grade 2 ×2) | N/A | 3 (50%) | |
| Best overall response prior to rechallenge* | |||
| Complete response | 1 (4%) | 0 (0%) | |
| Partial response | 10 (40%) | 1 (25%) | |
| Stable disease | 5 (20%) | 2 (50%) | |
| Progressive disease | 9 (36%) | 1 (25%) | |
| Disease control rate prior to rechallenge* | 16 (64%) | 3 (75%) | 1 |
| Best overall response, entire study period | |||
| Complete response | 8 (32%) | 0 (0%) | |
| Partial response | 10 (40%) | 2 (33.3%) | |
| Stable disease | 1 (4%) | 2 (33.3%) | |
| Progressive disease | 6 (24%) | 2 (33.3%) | |
| Disease control rate, entire study period | 19 (76%) | 4 (66.7%) | 0.634 |
| Non dose-limiting toxicities |
Data are median (IQR) or n (%)
Discontinuation indicates ICI retreatment was stopped due to an irAE
Excludes two patients who were rechallenged without interval restaging scans following their hepatitis
When comparing patients who required ICI discontinuation to those who did not, there were no significant differences in age, sex, ethnicity, melanoma stage, liver metastases, smoking, alcohol use, underlying liver disease, time from ICI initiation to development of grade 3 hepatitis, or time to rechallenge from initial hepatitis episode (Table III). There also was no difference in the proportion of patients being administered corticosteroids at the time of rechallenge (33.3% vs 37.5%), although all were receiving a dose of 10 mg or less of prednisone equivalents. Patients who were successfully rechallenged were more likely to have previously received combination ipilimumab/nivolumab therapy (84% vs 33%, p=0.024; Table II). While the small number of patients who experienced rechallenge discontinuation due to immune-related toxicity precluded multivariable analysis, on univariable analysis, patients who did not develop an irAE severe enough to require treatment discontinuation were significantly less likely to be rechallenged with ipilimumab versus anti-PD-1/PD-L1 monotherapy (0% vs 33%, RR 0.1, 95% CI 0.1–0.3, p=0.032) and significantly less likely to be rechallenged with the same ICI that cause the original ICI hepatitis (8% vs 50%, RR 0.2, 95% CI 0.1–0.7, p=0.038). There also was a strong trend towards these patients being more likely to receive a lower hepatitis risk regimen (88% vs 50%, p=0.069).
DISCUSSION
While immune checkpoint inhibitors have had a substantial positive impact on outcomes in patients with advanced malignancies, data regarding the safety and utility of ICI rechallenge in patients who previously developed high-grade ICI hepatitis are limited.12–14 In the present multicenter, retrospective cohort study of melanoma patients who previously developed a grade 3 or higher ICI hepatitis, 13% of those who were rechallenged with an ICI developed recurrent (grade 2 or higher) hepatitis. While half of patients who were restarted on an ICI developed any irAE, most instances were mild and only 20% required treatment discontinuation. Approximately 90% of patients were rechallenged with PD-1/PD-L1 monotherapy, with discontinuation of rechallenge due to immune-related toxicity in these patients being less common (13.7%). Both patients who were readministered a CTLA-4 inhibitor, and three of the five patients who were retreated with their original ICI, developed toxicity that required cessation of treatment. There also was no significant difference in best overall response between patients who were and were not rechallenged with an ICI, although there was a trend towards the rechallenged patients having a higher likelihood of achieving a complete or partial response.
Our findings are consistent with three prior studies evaluating the safety of ICI rechallenge, with reported incidences of recurrent irAE ranging from 20–50%;12–14 however, none of these studies focused exclusively on ICI hepatitis. Simonaggio et al.12 examined a cohort of 40 patients with varying cancers who underwent ICI rechallenge, including 5 with ICI hepatitis. The overall rate of recurrent irAE was 55%, with hepatitis recurrence in 3/5 (60%). Santini et al.13 reported on 68 patients with non-small cell lung cancer who developed an irAE necessitating treatment interruption. Of the 38 patients who were readministered an ICI (including 3 patients with ICI hepatitis), 52% experienced an irAE (though the specific outcomes for the patients who initially developed ICI hepatitis were not described. Finally, Pollack et al.14 evaluated 80 patients with melanoma who discontinued treatment after experiencing an irAE from combined CTLA-4 and PD-1 blockade, including 29 with ICI hepatitis (19 with grade 3 or higher). Five (17%) of these patients developed a grade 3 or higher irAE after resuming anti-PD-1 therapy, although these data were not stratified by severity of initial ICI hepatitis.
To our knowledge, our study represents the largest cohort of patients who were retreated with an ICI following development of high-grade ICI hepatitis. Strengths of the study include robust clinical data for each patient (including liver histology in 54%), and physician adjudication of ICI hepatitis. While focusing exclusively on melanoma patients mitigates heterogeneity and confounding, it effectively limits the study population to Caucasians and may thereby constrain generalizability to other ethnicities, as well as to other malignancies. As our study is retrospective, baseline patient characteristics, tumor response, and toxicities may not have been assessed as rigorously as in the context of a clinical trial. In addition, selection bias is implicit in the observation that rechallenged patients were younger, more likely to have received combination CTLA-4/PD-1 treatment, and had less severe hepatotoxicity (i.e., fewer steroid-refractory patients, shorter time to peak ALT, shorter time to ALT normalization). Only a very small number of patients underwent rechallenge with the same ICI regimen. We speculate that this may be due to the understandable desire to use a lower-toxicity risk regimen in those retreated as 74% of the rechallenged patients initially received combination nivolumab/ipilimumab therapy. Despite the small numbers, the patients who were retreated with the same ICI had a significantly higher risk of recurrence of toxicity. Finally, conclusions regarding risk factors for the development of ICI hepatitis following rechallenge are constrained by the limited number of patients who experienced this outcome.
In summary, our data indicate that melanoma patients who experience high-grade ICI hepatitis have a relatively modest risk of recurrence upon ICI rechallenge, particularly when utilizing PD-1/PD-L1 monotherapy as the rechallenge agent. The fact that both patients retreated with ipilimumab developed recurrent ICI hepatitis raises concern that this may be a high-risk treatment approach. However, while the development of ICI hepatitis does not appear to be an absolute contraindication to the resumption of immunotherapy in patients with melanoma, it remains unclear whether reinitiating ICI therapy improves clinical outcomes in these individuals. Further studies are also needed to evaluate whether specific subgroups of patients may benefit from ICI rechallenge.
Supplementary Material
a. Time to peak ALT
b. Time to normalization of ALT
Acknowledgments
Funding: NIH grant 5 T32 DK007533-35
Footnotes
Disclaimers:
F. Stephen Hodi
Stock and other ownership interests: Apricity, Torque
Consulting or advisory role: Merck Sharp & Dohme, Novartis, Genentech/Roche, EMD Serono, Sanofi, Bayer, Aduro Biotech, Pfizer, Verastem, Bristol-Myers Squibb, Takeda, Surface, Compass Therapeutics, Partners Therapeutics, Pionyr, 7Hills Pharma, Torque, Rheos, Amgen, Boston Pharmaceuticals Research funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Novartis (Inst)
Osama E. Rahma
Employment: Outcomes4me
Honoraria: Merck, Clinical Care Options, MI Bioresearch, PRIMA Consulting, Leerink, Alaunusglobal Consulting or advisory role: Celgene, Alcimed, Gfk, Merck, Five Prime Therapeutics, Putnam Associates, Defined Health, PureTech, Leerink, Genentech, Imvax, GSK, Maverick Therapeutics, Bayer, Sobi Research funding: Amgen (Inst), Merck
Shilpa Grover
Employment: UpToDate
References:
- 1.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv264–iv266. [DOI] [PubMed] [Google Scholar]
- 4.Grover S, Rahma OE, Hashemi N, Lim RM. Gastrointestinal and Hepatic Toxicities of Checkpoint Inhibitors: Algorithms for Management. Am Soc Clin Oncol Educ Book. 2018;38:13–19. [DOI] [PubMed] [Google Scholar]
- 5.Sanjeevaiah A, Kerr T, Beg MS. Approach and management of checkpoint inhibitor-related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270–1271. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Rollin L, Larkin J. Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(25):2503–2504. [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 9.Yau T, Kang Y-K, Kim T-Y, et al. Nivolumab (NIVO)+ ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. In: American Society of Clinical Oncology; 2019. [Google Scholar]
- 10.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346–1353. [DOI] [PubMed] [Google Scholar]
- 12.Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett J, Srivastava A, Misdraji J. Fibrin Ring Granulomas in Checkpoint Inhibitor-induced Hepatitis. Am J Surg Pathol. 2017;41(1):134–137. [DOI] [PubMed] [Google Scholar]
- 16.Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31(6):965–973. [DOI] [PubMed] [Google Scholar]
- 17.De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a. Time to peak ALT
b. Time to normalization of ALT