Summary
Metabolism reprogramming is critical for both cancer progression and effective immune responses in the tumor microenvironment. Amino acid metabolism in different cells and their cross-talk shape tumor immunity and therapy efficacy in patients with cancer. In this review, we focus on multiple amino acids and their transporters, solute carriers (SLCs) members. We discuss their involvement in the regulation of the immune responses in the tumor microenvironment, and assess their associations with cancer immunotherapy, chemotherapy, and radiation therapy; as well as review their potential as targets for cancer therapy. We stress the necessity to understand individual amino acids and their transporters in different cell subsets, the molecular intersection between amino acid metabolism, and effective T cell immunity and its relevance in cancer therapies.
Keywords: Amino acid, solute carriers, CD8+ T cell, cancer, immunotherapy, metabolism, checkpoint blockade
eTOC Blurb
Metabolism reprogramming affects both cancer progression and effective immune responses. Wang and Zou review the transportation and metabolism of multiple amino acids in different cell types in the tumor microenvironment and its relevance in cancer immunity and therapy in patients with cancer.
Introduction
Amino acids are the basic building blocks of protein. However, they are critical for biosynthesis of nucleotides, antioxidant (glutathione), glucosamine, and polyamines - and serve as metabolites that enter into energy production process, such as tricarboxylic-acid cycle (Lukey et al., 2017). The 20 proteinogenic amino acids are traditionally classified as essential or non-essential for animals and humans. Some amino acids may be conditionally essential - depending on cell type, cellular metabolic states, and the microenvironment (Table 1) (Tabe et al., 2019).
Table 1.
Human amino acids
| Essential | Non-essential | Conditionally essential |
|---|---|---|
| Isoleucine (Ile) | Alanine (Ala) | Arginine (Arg) |
| Leucine (Leu) | Asparagine (Asn) | Cysteine (Cys) |
| Lysine (Lys) | Aspartate (Asp) | Glutamine (Gln) |
| Methionine (Met) | Glutamate (Glu) | Glycine (Gly) |
| Phenylalanine (Phe) | Serine (Ser) | Proline (Pro) |
| Threonine (Thr) | Tyrosine (Tyr) | |
| Tryptophan (Trp) | ||
| Valine (Val) | ||
| Histidine (His) |
Amino acid transportation across plasma membrane is mediated by various amino acid transporter systems that are belong to solute carrier (SLC) superfamily. SLC superfamily is comprised of a large variety of transporters of over 400 annotated members, which have a diverse array of substrates. To date, more than 60 SLC members have been identified as amino acids transporters (Kandasamy et al., 2018). Based on their substrate specificity, these amino acid transporters can be classified into neutral, basic, and acidic classes. Each class can be further categorized as sodium-dependent or -independent (Table 2). The transportation of amino acid by its transporter is not one-to-one matched; one amino acid can be transported via several different transporters and one transporter may have multiple substrates. In addition, one cell may express multiple functionally redundant SLCs. Accordingly, depletion of an amino acid may not be biologically equal to one relevant transporter deficiency. Furthermore, the same transporter may have different abundance across distinct cell types, such as tumor cell and immune cell. Moreover, tumor cells and immune cells may preferentially utilize different SLCs to transport the same amino acid. These characteristics raise a significant challenge in biologic and translational research in SLCs.
Table 2.
Amino acid transport systems and their mediators and substrates
| System | SLC | cDNA | Substrates |
|---|---|---|---|
| Neutral amino acid transporters | |||
| Na+ dependent | |||
| A | SLC38A1 | SNAT1 | Gly, Ala, Ser, Cys, Gln, Asn, His, Met, Thr, Pro, Tyr, Val |
| SLC38A2 | SNAT2 | Gly, Pro, Ala, Ser, Cys, Gln, Asn, His, Met | |
| SLC38A4 | SNAT4 | Gly, Ala, Ser, Cys, Gln, Asn, Met | |
| SLC38A8 | FVH2 | Gln, Ala, Arg, His, Asp | |
| SLC38A10 | PP1744 | Gln, Ala, Glu, Asp, Ser | |
| Gly | SLC6A5 | GLYT2 | Gly |
| SLC6A9 | GLYT1 | Gly | |
| SLC6A18 | Xtrp2 | Gly, Ala | |
| B0 | SLC6A15 | SBAT1 | Pro, Met, BCAAs |
| SLC6A17 | NTT4 | Pro, Gly, Leu, Ala, Glu | |
| SLC6A19 | B0AT1 | neutral AA | |
| ASC | SLC1A4 | ASCT1 | Ala, Ser, Cys, Thr |
| SLC1A5 | ASCT2 | Ala, Ser, Cys, Thr, Gln, Asn | |
| N | SLC38A3 | SNAT3 | Gln, Asn, His |
| SLC38A5 | SNAT5 | Gln, Asn, His, Ser, Gly | |
| β | SLC6A6 | TauT | Tau, P-Ala |
| y+L | SLC7A6/SLC3A2 | y+LAT-2/4F2hc | Lys, Arg, Orn, Hys, Met, Leu |
| SLC7A7/SLC3A2 | y+LAT-1/4F2hc | Lys, Arg, Orn, Hys, Met, Leu, Ala, Cys | |
| Na+ independent | |||
| L | SLC7A5/SLC3A2 | LAT1/4F2hc | Leu, Hys, Met, Ile, Val, Phe,Tyr, Trp, Kyn |
| SLC7A8/SLC3A2 | LAT2/4F2hc | neutral AA | |
| SLC43A1 | LAT3 | Leu, Ile, Met, Phe, Val | |
| SLC43A2 | LAT4 | Leu, Ile, Met, Phe, Val | |
| asc | SLC7A10/SLC3A2 | Asc-1/4F2hc | Gly, Ala, Ser, Cys, Thr |
| T | SLC16A10 | TAT1 | Trp, Tyr, Phe |
| b0,+ | SLC7A9/SLC3A1 | BAT1/rBAT | Cys, dibasic and neutral amino acid |
| Basic amino acid transporters | |||
| Na+ dependent | |||
| B0,+ | SLC6A14 | ATB0,+ | Neutral and basic amino acid |
| Na+ independent | |||
| y+ | SLC7A1 | CAT1 | Lys, Arg, Orn |
| SLC7A2 | CAT2 | Lys, Arg, Orn | |
| SLC7A3 | CAT3 | Lys, Arg, Orn | |
| SLC7A4 | CAT4 | Basic amino acid | |
| y+L | Same as above | ||
| b0,+ | Same as above | ||
| Acidic amino acid transporters | |||
| Na+ dependent | |||
| X−AG | SLC1A1 | EAAT3 | Glu, Asp, Cys |
| SLC1A2 | EAAT2 | Glu, Asp | |
| SLC1A3 | EAAT1 | Glu, Asp | |
| SLC1A6 | EAAT4 | Glu, Asp | |
| SLC1A7 | EAAT5 | Glu, Asp | |
| Na+ independent | |||
| x−C | SLC7A11/SLC3A2 | xCT/4F2hc | Cys-Cys |
| Other transporters | |||
| Na+ dependent | |||
| IMINO | SLC6A20 | SIT1 | Pro, hydroxyproline |
| Na+ independent | |||
| Iminoglycine | SLC36A1 | PAT1 | Gly, Pro, Ala |
| SLC36A2 | PAT2 | Gly, Pro, Ala | |
| SLC36A4 | PAT4 | Pro, Trp, Ala | |
Undoubtedly, amino acids are important nutrients for both tumor and immune cells. T cells are the soldiers of the immune system and directly fight again cancer cells. T cells heavily rely on amino acid transportation and metabolism for their activation, differentiation, and function (Nakaya et al., 2014; Siska and Rathmell, 2015). Tumor cells utilize amino acids not only for their own proliferation and invasion (Lukey et al., 2017), but also enables tumor immune evasion (Lemos et al., 2019; O’Sullivan and Pearce, 2015). Here, we first review how amino acids and their transporters regulate T cell activation and effector functions. Then, we discuss the involvement of amino acid metabolism in the immune suppressive networks in the tumor microenvironment (Curiel et al., 2003; Zou, 2005; Zou and Chen, 2008). For instance, tumor cells can generate immune inhibitory metabolites via amino acid catabolism and create an immunosuppressive tumor microenvironment. Furthermore, tumor cells may outcompete immune cells for amino acid supply, impairing immune cell function (Bian et al, 2020; Geiger et al., 2016; Roy et al., 2020). Analogously, recent studies have generated compelling evidence that cancer immunotherapy can alter amino acid metabolism in both immune and tumor cells, and in turn affect therapy efficacy (Palaskas et al., 2019; Siska and Rathmell, 2015; Wang et al., 2019). Therefore, we further explore how cancer therapy reshapes amino acid metabolism in the tumor microenvironment. Finally, we assess the potential strategies for cancer therapy by targeting amino acid metabolism.
Amino acids and T cell activation and effector function
Naïve T cells are quiescent and require relatively small amounts of nutrients to maintain basic energetic and biosynthetic demands. Naïve T cells become activated, rapidly proliferate, and differentiated into effector T cells when the T cell receptor (TCR) encounters a specific MHC-peptide complex accompanied by a costimulatory signal from CD28. This transition is tightly coupled with increased metabolic requirements, such as glucose and amino acids (Horig et al., 1993; O’Sullivan and Pearce, 2015; Siska and Rathmell, 2015). Mechanistically, the activation of the pathways downstream of TCR and transcription factors, including mammalian target of rapamycin (mTOR), cMyc and hypoxia inducible factor (HIF1α), results in an upregulation of key enzymes in glycolysis, amino acid transporters, and amino acid metabolism (Carr et al., 2010; Levring et al., 2012). Amino acids serve as both an energy source and substrate for protein and nucleic acid biosynthesis in the course of T cell activation, differentiation, and function. However, efficient transportation of exogenous amino acids is the prerequisite for their utilization. A variety of amino acids and their transporters are necessary for T cell activation, differentiation, and effector function (Figure 1).
Figure 1. Roles of amino acids and their transporters in T cell function.
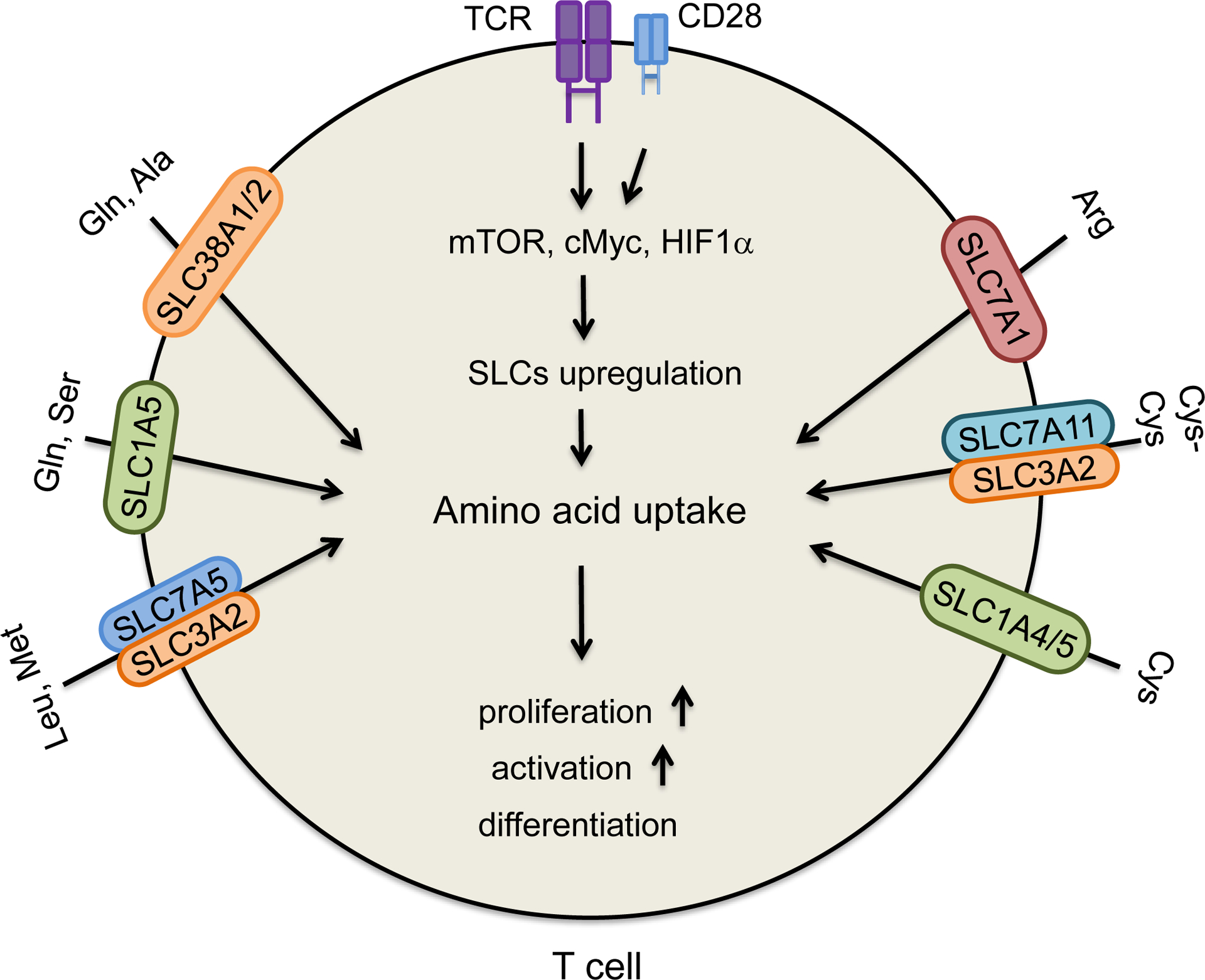
TCR engagement and co-stimulation affect multiple pathways and upregulate amino acid transporters, SLCs in T cells, thereby increasing amino acid uptake. This supports T cell activation, proliferation, and differentiation. Some amino acids and their transporters are required for T cell activation, differentiation, and function: glutamine (Gln), alanine (Ala), serine (Ser), leucine (Leu), methionine (Met), arginine (Arg), cysteine (Cys) and cystine (Cys-Cys). Examples are depicted in the figure.
Alanine.
Alanine, a non-essential amino acid, is transported via SLC38A1 in CD4+ T cells. TCR engagement stimulates SLC38A1 expression (Ron-Harel et al., 2019). Alanine deprivation delays naïve and memory T cell activation, but has no effect on T cell effector function. Extracellular alanine is used for protein synthesis, rather than catabolism in activated T cells (Ron-Harel et al., 2019; Sugden and Cohen, 2015). Thus, it seems that alanine is only necessary for T cell activation.
Arginine.
Arginine is a non-essential amino acid. Its endogenous synthesis can be initiated by the amino acid citrulline. However, some pathological conditions (such as sepsis, wound healing, and cancer) seem to cause high arginine demand, requiring dietary supplementation (Kishton et al., 2016; Luiking et al., 2004). Extracellular arginine is transported across membrane through the y+ system of cationic amino acid transporters, including SLC7A1, SLC7A2, and SLC7A3 (Table 2). SLC7A1 is primarily responsible for arginine uptake in human T cells. Knockdown SLC7A1 expression impairs arginine uptake and T cell proliferation (Werner et al., 2016). Arginine starvation induces T cell cycle arrest via general control nonderepressible 2 (GCN2) activation (Rodriguez et al., 2007), causes loss of the T-cell receptor zeta chain (CD3zeta), and reduces T cell proliferation and cytokine production (Rodriguez et al., 2002; Werner et al., 2016). Arginine supplementation promotes the generation of central memory-like T cells with high survival capacity, consequently enhancing CD8+ T cell-mediated anti-tumor activity in a mouse model (Geiger et al., 2016). Thus, arginine is essential for T cell proliferation, activation, and effector function (Kishton et al., 2016; Rodriguez et al., 2007).
Cysteine and cystine.
Cysteine (Cys) is not an essential amino acid, but is necessary for synthesis of protein, glutathione, and coenzyme A. In extracellular space, cysteine usually exists in its oxidized form - cystine (Cys-Cys) (Srivastava et al., 2010). Different transporters are responsible for cysteine and cystine uptake. Their expression levels are upregulated during T cell activation (Levring et al., 2012). Cysteine is transported by the system ASC (alanine, serine, and cysteine-preferring) transporters, including SLC1A4 and SLC1A5 (Kandasamy et al., 2018). It is important to note that SLC1A5 is also a major transporter for glutamine (Table 2). Cystine is transported through the system xc− transporter, consisting of SLC3A2 and SLC7A11 (xCT) subunits (Robert et al., 2015). Once cystine is transported into cells, it will be immediately reduced into cysteine. For in vitro cultured human T cells, exogenous cysteine and cystine are unnecessary for early activation, but are essential for T cell expansion. Antigen presenting cells (APCs), including dendritic cells (DCs), can export cysteine to support T cell proliferation, whereas myeloid-derived suppressor cells (MDSCs) can sequester extracellular cysteine and cystine to block full T cell activation (Srivastava et al., 2010). However, studies using transporter deficient mouse models have shown different results. In vivo SLC1A5 deficiency has no obvious effect on naive CD4+ T cell proliferation, but impairs Th1 and Th17 cell polarization (Nakaya et al., 2014), although the phenotype may be contributed by the depletion of both cysteine and glutamine. Similarly, although SLC7A11 deficient mouse T cells fail to proliferate in vitro, they can become fully activated in vivo. Furthermore, SLC7A11 deficiency has no effect on anti-tumor T cell response (Arensman et al., 2019). Thus, the roles of cysteine and cystine and their transporters in T cells warrant further investigation.
Glutamine.
Glutamine is not an essential amino acid. As stated above, SLC1A5 is one of the transporters that mediate glutamine transportation. In addition, many other transporters can mediate glutamine uptake: including SLC6A14, SLC6A19 (B0AT1), SLC38A1 (SNAT1), SLC38A2 (SNAT2), SLC38A4 (SNAT4), SLC38A3 (SNAT3), and SLC38A5 (SNAT5) (Carr et al., 2010; Scalise et al., 2020). Among them, SLC1A5 and SLC38A1 have been shown to be upregulated during T cell activation (Sinclair et al., 2013). In line with this, glutamine uptake is enhanced during T cell activation (Horig et al., 1993). Glutamine deprivation blocks murine T cell proliferation and cytokine production in the in vitro culture system (Carr et al., 2010). Extracellular glutamine availability could also regulate T cell differentiation. In both human and murine CD4+ T cells, glutamine limitation suppresses Th1 differentiation and promotes Foxp3+ regulatory T (Treg) cell differentiation(Klysz et al., 2015; Metzler et al., 2016). In SLC1A5 deficient T cells, glutamine uptake and mTORC1 activation upon TCR engagement are largely impaired, and the differentiations of Th1 and Th17 are also blocked (Nakaya et al., 2014).
Leucine.
Leucine is a large neutral amino acid. Leucine transportation is mediated mainly by system L transporter containing SLC7A5 (LAT1) and SLC3A2 subunits. The two subunits are both upregulated upon TCR engagement during T cell activation (Hayashi et al., 2013). SLC7A5 deficient mouse T cells show a defective response to antigen stimulation and do not undergo clonal expansion or effector differentiation, which may be related to rapid inactivation of mTORC1(Sinclair et al., 2013). Inhibition of SLC7A5 impairs human T cell activation and effector function (Hayashi et al., 2013).
Methionine (Figure 2).
Figure 2. Effects of methionine metabolism on T cell function and tumor immunity.
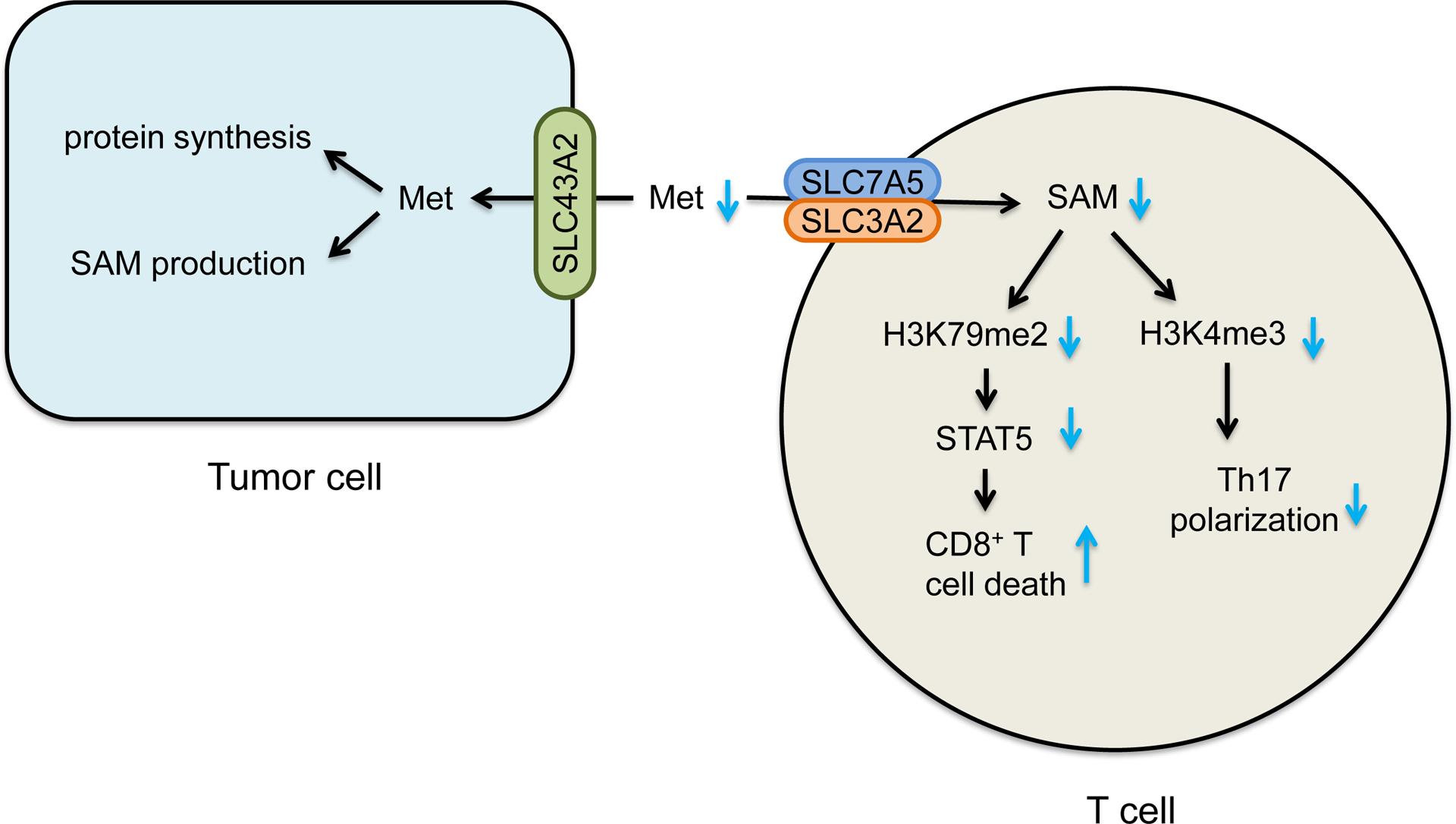
Methionine metabolism generates a universal methyl donor S-adenosyl-methionine (SAM). Tumor cells express high levels of SLC43A2 and consume methionine in the tumor microenvironment. This results in insufficient methionine and SAM for T cells - thus causing loss of CD8+ T cell H3K79me2 and STAT5 expression and function, and impairing H3K4me3 and Th17 polarization.
Methionine is an essential sulfur-containing amino acid. Methionine is required for protein synthesis and the production of S-adenosyl-methionine (SAM), which is a universal methyl donor for DNA, RNA, and protein methyltransferases (Sanderson et al., 2019). Several SLCs (including SLC1A5, SLC7A5, SLC7A6, SLC38A2, and SLC43A2) can mediate methionine transportation. Functional test shows that SLC7A5 may be critical for methionine uptake in mouse CD4+ T cells. SLC7A5 deficient CD4+ T cells manifest a decrease in methionine influx and an impaired activation and differentiation (Sinclair et al., 2019). SLC7A5 also imports leucine, which is necessary for optimal T cell activation. Thus, the phenotype of SLC7A5 deficient T cells may be attributed to insufficient levels of both methionine and leucine. Interestingly, both human and mouse tumor infiltrating effector CD8+ T cells express low levels of SLC43A2, resulting in low levels of methionine and SAM in T cells, impaired expression of H3K79me2 and STAT5, and reduced T cell survival and function (Bian et al, 2020). Accordingly, methionine uptake increases upon T cell activation and helps generate SAM, sustaining histone methylation and RNA methylation (Bian et al, 2020; Sinclair et al., 2019). Methionine starvation can reduce H3K79me2 in CD8+ T cells (Bian et al, 2020) and histone H3K4 methylation (H3K4me3) in Th17 cells (Roy et al., 2020). Thus, methionine is critical for T cell survival and function.
Serine.
Serine is a non-essential amino acid that can be de novo synthesized from glucose. Interestingly, exogenous serine is necessary for Ewing sarcoma (ES) and liposarcoma cell proliferation (Cisse et al., 2020; Issaq et al., 2020). Similarly, in activated T cells the majority of intracellular serine comes from extracellular environment (Ma et al., 2017). Serine uptake is required for optimal T cell proliferation, but is not necessary for effector function. Dietary serine restriction impairs T cell responses to L. monocytogenes infection (Ma et al., 2017). The major transporters for serine uptake in T cells have not yet been identified. Nonetheless, serine and glutamine share several system A and ASC transporters, including SLC1A5 (Table 2). It is speculated that SLC1A5 may be involved in serine uptake in T cells.
Part of fundamental T cell immunobiology is understanding T cell amino acid requirements in physiological and pathological conditions. In the in vitro T cell culture system, single amino acids can be easily supplemented or depleted to investigate its involvement in T cell activation and differentiation. Meanwhile, this approach is not feasible for the in vivo model. Hence, conditional knockout mouse model of a specific SLC is a useful approach to evaluate the importance of certain amino acids in T cell function. However, the functional redundancy of different SLCs may yield inaccurate information on the role of specific amino acids in T cells. Inclusion of multiple complementary and confirmatory experiments will make a compelling case in our understanding of specific amino acids and SLCs in cancer immunity and immunotherapy.
Amino acid metabolism contributes to cancer immune evasion
It is evident that amino acid metabolism is able to regulate anti-tumor immune response. Tumor cells utilize a variety of amino acids to support their proliferation and metastasis. During this process, downstream metabolites generated in tumor cells are released into the tumor microenvironment. Some metabolites exhibit immune regulatory properties, and can directly target and impair APC- and T cell functions. Moreover, tumor cells can compete for amino acids with T cells via high expression of selective transporters, thereby causing the shortage of extracellular amino acids and impairing T cell proliferation, survival, and effector function. Here, we examine arginine, tryptophan, and methionine as examples to discuss their involvement in the regulation of tumor immunity.
Arginine (Arg) catabolism (Figure 3).
Figure 3. Role of arginine and Try-Kyn metabolisms in tumor immune evasion.
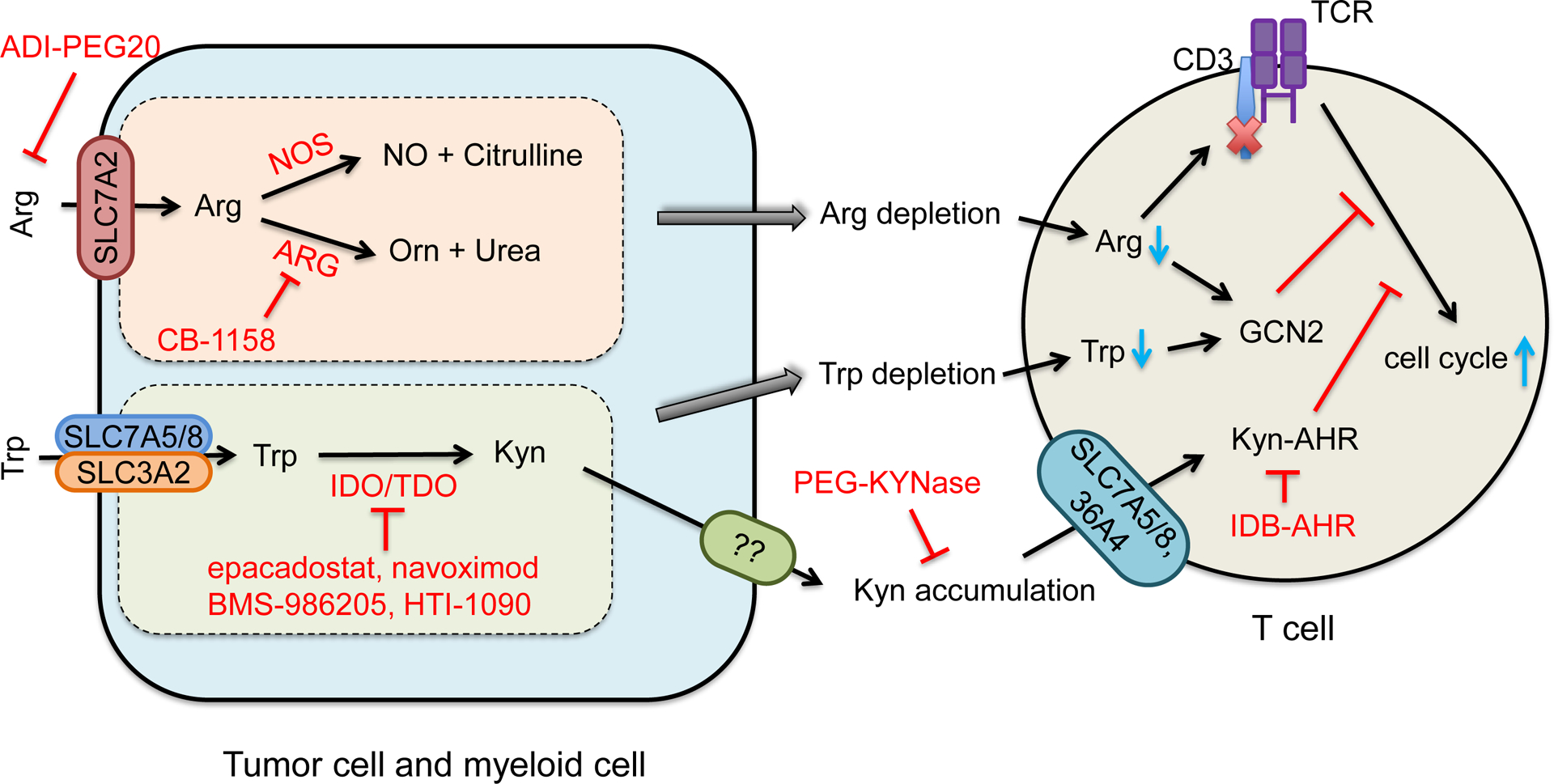
Arginine (Arg) is transported by SLC7A2. Intracellular Arg metabolism results in extracellular Arg depletion and NO production. Arg deficiency impairs and NO inhibits T cell function.
Trp is transported by SLC3A2 and SLC7A5 or SLC7A8. Trp is catalyzed by indoleamine-2,3-dioxygenase (IDO) or tryptophan-2,3-dioxygenase (TDO) to become kynurenine (Kyn). Trp-Kyn metabolism results in extracellular Trp depletion and Kyn accumulation, subsequently causing T cell inhibition.
Arginine deiminase (ADI-PEG20) degrades arginine. PEG-KYNase degrades Kyn. Small inhibitors that target key enzymes in Arg and Trp-Kyn metabolic pathways are listed in red.
Apart from protein synthesis, intracellular arginine is used for the production of many substances - including nitric oxide (NO), proline, ornithine (Orn), creatine, agmatine, and polyamines (Szefel et al., 2019). The initial step of arginine catabolism is mediated by two enzymes: nitric oxide synthase (NOS) and arginase (ARG). Three isozymes of NOS - NOS1, NOS2 (also known as inducible NOS; iNOS) and NOS3 (Rodriguez et al., 2017) - catalyze and convert arginine into NO and citrulline. Two isoforms of arginase, Arg1 and Arg2, convert Arg into Orn and urea. Orn is further metabolized to proline by Orn aminotransferase (OAT) or to polyamines (putrescine, spermidine and spermine) by Orn decarboxylase (ODC) (Lemos et al., 2019; Rodriguez et al., 2017). Hence, Arg metabolism depends on the expression levels and activities of NOS and ARG enzymes. Human tumor and myeloid cells express high level of iNOS - causing high levels of NO, which promoted tumor growth and resistance to therapy (Kryczek et al., 2006; Rodriguez et al., 2017). Furthermore, tumor associated myeloid cells - including macrophages (TAMs) (Lepique et al., 2009), MDSCs (Gabrilovich and Nagaraj, 2009), and DCs (Norian et al., 2009) - express high levels of SLC7A2 and Arg1, which transport extracellular Arg and consume Arg, respectively, leading to insufficient arginine to T cells and impaired T cell-mediated antitumor immunity in vivo (Geiger et al., 2016). Furthermore, Arg catabolism generates NO, which inhibits anti-tumor T cell response. NO-derived peroxynitrite can cause nitration of tyrosine residues to block protein tyrosine phosphorylation, resulting in decreased T cell proliferation and activation (Brito et al., 1999). Moreover, MDSCs can inhibit NK cell cytokine production (Stiff et al., 2018) and mediate T cell activation and recruitment (Gehad et al., 2012) through NO-related pathways. Thus, arginine catabolism contributes to cancer immune evasion by restricting T cell access to arginine and impairing immune cell function via NO.
Tryptophan (Trp) metabolism (Figure 3).
Tryptophan is an essential amino acid. Extracellular Trp is transported into cells by the neutral amino acid transporter system L, which is composed of a heavy chain SLC3A2 and one of two light chains - SLC7A5 or SLC7A8 (Table 2) (Kandasamy et al., 2018). A small fraction of intracellular Trp is used for protein synthesis and the production of tryptamine and serotonin. More than 95% of free Trp is claimed by the kynurenine (Kyn) pathway for degradation (Cervenka et al., 2017). Trp is catalyzed by indoleamine-2,3-dioxygenase 1 (IDO1), IDO2, or tryptophan-2,3-dioxygenase (TDO) to become Kyn. Then, Kyn is hydroxylated to 3- hydroxykynurenine (3-HK), which is converted to 3- hydroxyanthranilic acid (3-HAA). 3-HAA is rapidly converted to quinolinic acid (QA), then finally converted to nicotinamide adenine dinucleotide (NAD). NAD is a key coenzyme in energy metabolism and redox reactions (Cervenka et al., 2017). As IDO1 is highly expressed in tumor cells, stromal cells, DCs, and macrophages in the tumor microenvironment, Trp catabolism can result in Trp depletion and accumulation of Trp associated metabolites, which mediate tumor immune evasion. For example, Kyn and 3-HAA can be up-taken by T cells and impair T cell function. Furthermore, aryl hydrocarbon receptor (AHR) is a direct target of Kyn (Opitz et al., 2011). AHR promotes Treg differentiation (Mezrich et al., 2010), decreases DC function, suppresses effector T cell function (Nguyen et al., 2010), and is negatively associated with cancer patient survival (Cheong and Sun, 2018). AHR also drives macrophage CD39 expression, thereby inhibiting T cell activation (Takenaka et al., 2019). Kyn can be directly transferred into CD8+ T cells via SLC7A5 (Sinclair et al., 2018), SLC7A8, and SLC36A4 (Liu et al., 2018) to activate AHR and upregulate PD-1 expression in CD8+ T cells (Liu et al., 2018). Blocking this Kyn-AHR pathway enhances antitumor efficacy of adoptive T cell transfer (Liu et al., 2018). Thus, Trp depletion and Trp-Kyn-AHR related metabolites contribute to tumor immune evasion.
Methionine metabolism (Figure 2).
Intracellular methionine is converted to SAM, the universal donor for epigenetic methylation (Mentch et al., 2015). Effector T cells are programmed to become functionally exhausted in the tumor microenvironment. Exhausted effector T cells exhibit distinct histone modification profiles and limit the efficacy of immunotherapy (Pauken et al., 2016). Abnormal epigenetic patterns correlate with effector T cell malfunction in tumors. A recent study demonstrated that tumor cells disrupt methionine metabolism in CD8+ T cells, subsequently lowering intracellular methionine and SAM levels and resulting in loss of H3K79me2. Consequently, loss of H3K79me2 leads to low STAT5 expression and impairs T cell immunity. Mechanistically, tumor cells avidly consume and outcompete T cells for methionine via high expression of SLC43A2, a methionine transporter (Bian et al, 2020). Thus, potent cancer methionine consumption is an unappreciated immune evasion mechanism.
Cancer therapy reprograms amino acid metabolism in the tumor microenvironment
The shortage of specific amino acids and the immunosuppressive nature of some amino acid metabolites can impair activation and function of immune cells, particularly effector T cells in the tumor microenvironment. On the other hand, emerging studies demonstrate that efficacy of immunotherapy, chemotherapy, and radiation therapy correlates with T cell function in animal models and patients with cancer (Weichselbaum et al., 2017; Zou et al., 2016). Thus, cancer therapy may alter amino acid metabolism, thereby impacting T cell phenotype in the tumor microenvironment and shaping therapeutic response.
Immunotherapy and amino acids (Figure 4).
Figure 4. Immune-directed amino acid metabolism reprogramming in cancer therapy.
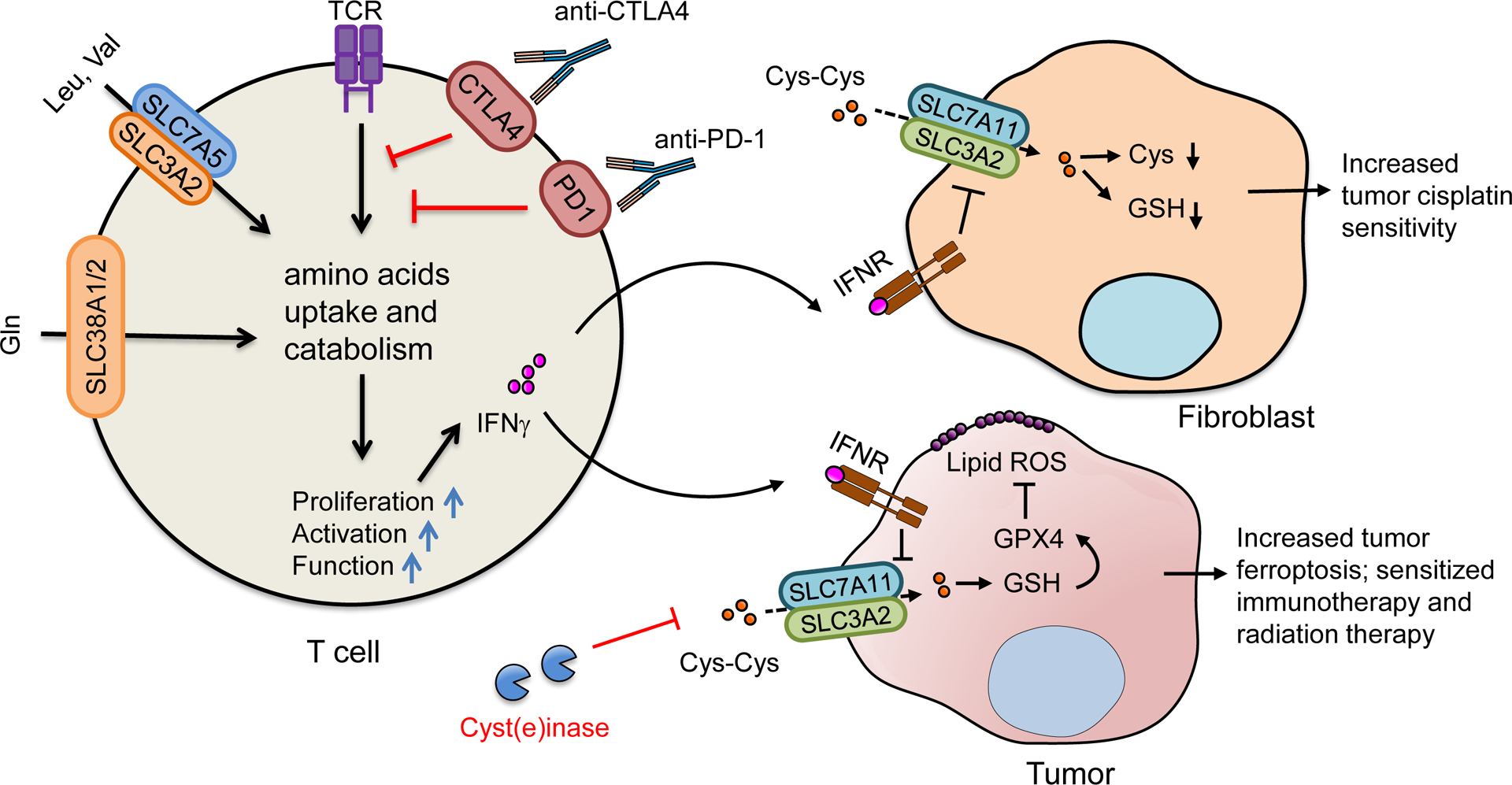
Checkpoint receptors, including CTLA-4 and PD-1, negatively regulate T cell activation in part by limiting the uptake and catabolism of amino acids. Checkpoint blockade may reprogram T cell amino acid metabolism and improve T cell function. Immunotherapy-induced IFNγ downregulates the expression of cystine (Cys-Cys) transporter, SLC7A11, in cancer associated fibroblasts - resulting in a decrease in cysteine (Cys) and glutathione (GSH) for tumor cells. Decreased intracellular glutathione in tumor cells augments intracellular cisplatin accumulation and sensitizes platin-based chemotherapy. IFNγ also suppresses cystine uptake by downregulating SLC7A11 and SLC3A2 in cancer cells, resulting in an increase in tumor lipid peroxidation and ferroptosis, and synergizing immunotherapy, chemotherapy, and radiation therapy.
Immunotherapy leads to enhanced T cell tumor infiltration and effector function (Zou et al., 2016). Checkpoint receptors, including CTLA-4 and PD-1, negatively regulate T cell activation in part by limiting the metabolic fitness and uptake of glucose and amino acids (Lim et al., 2017). PD-1 signaling causes a decrease in glucose transporter 1 (Glut1) expression, glucose uptake, and glycolysis (Parry et al., 2005; Patsoukis et al., 2015). Furthermore, PD-1 signaling also results in decreased induction of SLC38A1 and SLC38A2, restrains glutamine transportation, and reduces the catabolism of branched-chain amino acids, including valine and leucine(Siska and Rathmell, 2015). Moreover, PD-1 promotes lipolysis and β-oxidation of fatty acids in T cells by upregulation of carnitine palmitoyltransferase 1A (CPT1A), the rate-limiting enzyme of mitochondrial fatty acid oxidation (FAO). CTLA4 engagement can also inhibit the expression of Glut1, SLC38A1, and SLC38A2 (Patsoukis et al., 2015). Thus, it is unsurprising that checkpoint blockade can directly reprogram T cell metabolism and increase their metabolic fitness and function.
In addition to T cells, checkpoint therapy can reprogram amino acid metabolism in tumor cells via IFNγ. Immunotherapy activated effector T cells release IFNγ. IFNγ suppresses cystine uptake by downregulating SLC7A11 and SLC3A2 in cancer cells, resulting in an increase in tumor lipid peroxidation, tumor ferroptosis, and tumor regression. In line with this, treatment with anti-PD-L1 augments lipid peroxidation and ferroptosis in cancer cells in tumor bearing mice (Wang et al., 2019). Using cyst(e)inase, an engineered enzyme that degrades both cystine and cysteine, in combination with PD-L1 blockade synergistically enhances T cell-mediated anti-tumor immunity and induces potent tumor cell ferroptosis. Moreover, the number of infiltrating CD8+ cells negatively correlates with the expression of SLC7A11 and SLC3A2 in human melanoma tissues (Wang et al., 2019). Thus, immunotherapy-activated T cells can reprogram amino acid metabolism in tumor cells and eventually contribute to T cell-mediated anti-tumor immunity.
Chemotherapy and amino acids (Figure 4).
Several cancer chemotherapy agents can regulate metabolic profile in tumor cells, including amino acid metabolism (Maria et al., 2017). Cisplatin, tamoxifen, and doxorubicin affect tyrosine and alanine in breast cancer cell lines, which may contribute to drug resistance (Maria et al., 2017). Interestingly, platinum-based chemotherapy efficacy is regulated by effector T cells via cysteine and cysteine metabolism. Effector CD8+ T cell-derived IFNγ downregulates the expression of cystine transporter SLC7A11 and upregulates the expression of gamma-glutamyltransferases (GGTs) in ovarian cancer associated fibroblasts, resulting in a decrease in glutathione release - thereby reducing the source of glutathione for tumor cells. Intracellular glutathione reacts with cisplatin to form a Pt(GS)2 conjugate. This Pt(GS)2 conjugate can be eliminated across the membrane through the ATP-dependent glutathione S-conjugate export pump (Ishikawa and Ali-Osman, 1993). Hence, high levels of intracellular glutathione in tumor cells lead to reduced cisplatin nuclear accumulation and enhanced tumor cell resistance to cisplatin-based chemotherapy (Wang et al., 2016). Thus, cysteine metabolism is involved in the interplay between cisplatin and IFNγ, and regulates chemotherapy efficacy.
Radiation therapy and amino acids.
Radiation therapy results in tumor cell death via inducing mitotic catastrophe, autophagy, apoptosis, and necrosis (Rodriguez-Ruiz et al., 2020). Radiotherapy could also induce tumor cell ferroptosis. Radiation activates ataxia-telangiectasia mutated gene (ATM), in collaboration with IFNγ signaling, synergistically suppresses SLC7A11, resulting in reduced tumor cell cystine uptake, enhancing tumor lipid oxidation and ferroptosis, and improving tumor control (Lang et al., 2019). Notably, radiation may regulate tumor ferroptosis through upregulation of acyl-CoA synthetase long-chain family member 4 (ACSL4) (Lei et al., 2020) or inducing direct oxidation of cytoplasmic lipids (Ye et al., 2020). Given that immunotherapy activates T cells and enhances T cell IFNγ production, tumor ferroptosis may be a novel intersection between two cancer treatment modalities. The data suggest that amino acid and lipid metabolisms contribute to radiation therapy efficacy.
Targeting amino acid metabolism to treat cancer
Cancer cells depend on amino acids for replication, energy, and redox homeostasis. This creates a metabolic vulnerability for cancer therapy. Targeting amino acids, their enzymes, and transporters may be a potential cancer therapy approach.
Targeting amino acids and their enzymes
Arginine (Figure 3).
Certain types of tumors, such as melanoma and hepatocellular carcinoma (HCC), may depend on extracellular arginine for survival due to their lack of de novo synthesis of arginine. This phenomenon is defined as arginine auxotrophy (Ensor et al., 2002). PEGylated arginine deiminase (ADI-PEG20) (Figure 3) breaks down arginine into citrulline and eliminates arginine in the tumor microenvironment, resulting in arginine auxotrophic tumor cell death (Ensor et al., 2002; Miraki-Moud et al., 2015) via mitochondrial damage and nuclear leakage (Changou et al., 2014). In combination with PD-L1 blockade, ADI-PEG20 treatment inhibits tumor growth in animal models, along with increased T cell activation and reduced Treg tumor accumulation (Brin et al., 2017). In a phase II randomized clinical trial, ADI-PEG20 improves progression-free survival (PFS) in patients with arginine auxotrophic mesothelioma (Szlosarek et al., 2017). However, in a Phase III study in patients with HCC, ADI-PEG 20 (as a second line therapy) fails to demonstrate an overall survival benefit (Abou-Alfa et al., 2018). Nonetheless, the combination of ADI-PEG20 with pembrolizumab is ongoing in the clinical trial (NCT03254732).
Opposite to arginine depletion, given that the shortage of Arg impairs T cell proliferation and function in the tumor microenvironment, arginine supplementation may recover T cell function and improve T cell mediated anti-tumor immunity. Indeed, supplementation of arginine during the in vitro T cell expansion induced global metabolic changes - including reduced glucose consumption and increased oxidative phosphorylation, which results in increased T cell persistence and anti-tumor activity in mouse B16 melanoma model (Geiger et al., 2016). Moreover, arginine administration synergizes the effect of anti-PD-L1 in a murine osteosarcoma model (He et al., 2017). CB-1158, a potent small-molecule inhibitor of Arg1, relieves MDSC-mediated suppression of T cells in vitro, increases CD8+ T cell numbers and function in the tumor microenvironment, and inhibits tumor growth in multiple tumor models (Steggerda et al., 2017). Treatment with CB-1158 synergistically enhances anti-tumor effect of checkpoint blockade in murine models (Steggerda et al., 2017). This approach is being tested in a clinical study in patients with advanced or metastatic solid tumors (NCT02903914). Therefore, Arg depletion (e.g. ADI-PEG20) and supplementation may be clinically beneficial for arginine auxotrophic tumors and non-auxotrophic tumors, respectively.
Asparagine.
L-asparaginase is the only FDA-approved anticancer agent that directly targets amino acid metabolism to treat pediatric and adult patients with acute lymphoblastic leukemia (ALL) (Couturier et al., 2015; Touzart et al., 2019). L-asparaginase is a bacteria-derived enzyme that catalyzes the degradation of asparagine into ammonia and aspartate (Keating et al., 1993; Tabe et al., 2019). Although asparagine is a non-essential amino acid, leukemia cells are highly dependent on exogenous asparagine due to asparagine synthetase (ASNS) deficiency - the only enzyme capable of de novo asparagine synthesis (Su et al., 2008). L-asparaginase treatment causes the depletion of extracellular asparagine, leading to leukemia cell death (Berenbaum et al., 1970). In addition to ALL, extranodal NK/T-cell lymphoma is sensitive to L-asparaginase (Jaccard et al., 2011). Similarly, heterologous enzymes or engineered enzymes targeting cysteine or arginine are being investigated in animal models and clinical trials (Cramer et al., 2017; Szlosarek et al., 2017). Notably, these enzymes are originally isolated from prokaryotes. They may provoke an undesired immune response in vivo in humans (Peterson et al., 1971).
Cysteine and cystine.
Cyst(e)inase is an engineered human cyst(e)inase enzyme that can mediate the degradation of the extracellular cysteine and cystine (Cramer et al., 2017). Treatment with cyst(e)inase causes the depletion of intracellular glutathione and the accumulation of ROS, resulting in cell cycle arrest and death in multiple cancer cell lines. In vivo cyst(e)inase administration reduces tumor growth in both prostate and breast cancer xenografts and causes the depletion of serum cysteine and cystine pool (Cramer et al., 2017). Cell death caused by cystine depletion was later identified as ferroptosis (Dixon et al., 2012). Indeed, cyst(e)inase can induce ferroptosis in cultured human and mouse tumor cells; its cytotoxicity could be further increased by IFNγ derived from activated CD8+ T cells (Wang et al., 2019). Interestingly, cyst(e)inase as single agent mediates antitumor activity and enhances antitumor T cell response in mouse ID8 ovarian cancer model. Combinatorial therapy of cyst(e)inase and PD-L1 blockade synergistically enhances anti-tumor immunity and suppress tumor growth (Wang et al., 2019). In addition, cyst(e)inase inhibits tumor progression in mouse pancreatic ductal adenocarcinoma (PDAC) (Badgley et al., 2020). Therefore, cystine uptake might be a metabolic vulnerability for some tumor cells.
Methionine.
Methionine is essential for tumor cell growth in the in vitro culture (Cavuoto and Fenech, 2012). Dietary methionine restriction inhibits tumor growth and metastasis, and increases tumor sensitivity to chemotherapy and radiation therapy in immune deficient murine models (Gao et al., 2019). In line with this, multiple human and mouse tumor cells highly express SLC43A2, a methionine transporter. Notably, tumor cells outcompete T cells for methionine via SLC43A2 and diminishes T cell-mediated anti-tumor immunity, while CD8+ T cells are extremely sensitive to methionine deprivation (Bian et al, 2020). Interestingly, methionine supplementation, rather than restriction, restores T cell immunity in immune competent murine models bearing different tumors (Bian et al, 2020). Furthermore, methionine supplementation recovers STAT5 and H3K79me2 expression and function in CD8+ T cells in patients with colon cancer (Bian et al, 2020). Thus, similar to targeting arginine, it is essential to selectively (or predominantly) target methionine in tumor cells, and to avoid methionine starvation in T cells in order to gain potential clinical benefit for cancer therapy (Bian et al, 2020).
Tryptophan (Figure 3).
IDO is positioned at the rate-limiting step in Trp catabolism. Several IDO1 inhibitors (including epacadostat, navoximod, and BMS-986205) have distinct action of modes, either competing with tryptophan for the catalytic site of IDO1 or irreversibly binding with IDO1 with high affinity (Cully, 2018). Dual IDO1 and TDO inhibitors, such as HTI-1090, are also in development. These inhibitors reverse the immunosuppressive phenotype mediated by Trp catabolism in preclinical tumor models, and have entered clinical evaluations in combination with immunotherapy. Among them, epacadostat was tested in a phase III clinical trial ECHO-301 in combination with pembrolizumab in unresectable or metastatic melanoma. However, this combination failed to meet its primary end point and resulted in the early termination of ECHO-301 trial (Muller et al., 2019). Many trials involving epacadostat or BMS-986205 in combination with checkpoint blockade have been terminated or discontinued. This clinical trial failure represents a setback in the development of IDO inhibitors; the reason for failure remains mechanistically and clinically elusive. It may be essential to functionally determine if IDO1-mediated immunosuppression is a major immune evasion mechanism in the tested patients. Nonetheless, several clinical trials are ongoing to test epacadostat in combination with other types of therapy - including cancer vaccination, radiotherapy, and chemotherapy. These trials may hopefully shed new light on IDO1 inhibitor biology in humans.
IDO1 locates upstream of the Trp-Kyn-AHR pathway. Inhibitors targeting AHR or enzyme degrading Kyn can inhibit tumor progression through reinforcing the antitumor immune response (Joseph et al., 2018; Triplett et al., 2018). IDB-AHRi, an AHR antagonist, can block the nuclear translocation of AHR and increase the expression of IFNγ and TNFα in human peripheral blood mononuclear cells. In a mouse model bearing CT26 colon cancer, IDB-AHRi inhibits tumor growth, increases CD8+ T cell tumor infiltration, and reduces Treg cells and tumor-associated macrophages (Joseph et al., 2018). PEG-KYNase, a recombinant enzyme, degrades Kyn into immunologically inert metabolites. PEG-KYNase has therapeutic effects when administered alone or in combination with checkpoint blockades in multiple mouse tumor models (Triplett et al., 2018). This antitumor activity is associated with increased tumor infiltrating effector CD8+ T cells (Triplett et al., 2018). Moreover, an engineered Kyn consuming bacterial strain decreases tumor Kyn level in vivo and inhibits tumor growth in combination with anti-PD1 antibody (West et al., 2018). These preclinical studies may be translated into clinical trials in the near future.
Targeting amino acid transporters
SLC3A2.
SLC3A2 in combination with one of the other SLCs (including SLC7A5, SLC7A6, SLC7A7, SLC7A8, SLC7A10, and SLC7A11) constitutes a heterodimeric amino acid transporter(Cantor and Ginsberg, 2012). Notably, SLC3A2 may not be considered a direct amino acid transporter, and it can interact with integrins to regulate cell adhesion. SLC3A2 has been shown to regulate tumor survival, proliferation, migration and radiotherapy sensitivity (Bajaj et al., 2016; Cantor and Ginsberg, 2012; Fenczik et al., 1997). High expression of SLC3A2 is associated with poor prognosis in many types of tumor (Cantor and Ginsberg, 2012). Treatment with IGN523, a humanized anti-SLC3A2 monoclonal antibody (mAb), has shown anti-tumor efficacy in leukemia-derived and non-small cell lung cancer xenograft models (Hayes et al., 2015). IGN523 mediates tumor cell death via NK-cell mediated antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), and can also inhibit tumor cell amino acid uptake - such as phenylalanine (Phe) (Hayes et al., 2015). However, it is unknown whether IGN523-inhibited amino acid uptake contributes to its in vivo antitumor activity. Nonetheless, a phase I clinical trial of IGN523 is ongoing in patients with acute myeloid leukemia (NCT02040506).
SLC7A5.
SLC7A5 binds to SLC3A2 to form system L transporter and transports several substrates, including Leu, Hys, Met, Ile, Val, Phe, Tyr, Trp, and Kyn (Yahyaoui and Perez-Frias, 2019). Anti-SLC7A5 mAb mediates antitumor activity against human colon cancer in xenograft model. This antibody could inhibit branched chain amino acids (Leu, Ile, and Val) (Ueda et al., 2019) and Kyn (Sinclair et al., 2018) uptake and manifest ADCC activity (Ueda et al., 2019). Kyn mediates T cell dysfunction by activating AHR signal (Sinclair et al., 2018). Therefore, anti-SLC7A5 may block Kyn uptake by T cells and enhance antitumor T cell immunity.
SLC7A11.
SLC7A11 is involved in cystine transportation. Differing from other amino acids that correspond to multiple transporters, cystine can only be transported through SLC7A11 (Arensman et al., 2019; Cramer et al., 2017). Hence, SLC7A11 may be a promising target for cystine addictive cancer therapy. Similar to cyst(e)inase, a blocking antibody against SLC7A11 may inhibit cystine uptake, increase intracellular ROS and lipid peroxidation, induce tumor ferroptosis, and synergize the therapeutic efficacy of checkpoint blockade (Wang et al., 2019). However, the identification of SLC7A11 structure is needed to define an epitope that can be utilized to block its transporter activity.
SLC43A2.
SLC43A2 can transport methionine. Cancer cells express high levels of SLC43A2 to outcompete T cells for methionine, resulting in impaired T cell function and tumor immunity. Inhibition of tumoral methionine uptake by knocking down SLC43A2 or using system L inhibitor BCH slows down tumor growth, improves anti-PD-L1 therapy, and enhances antitumor T cell response. Therefore, specifically targeting tumor SLC43A2 may serve as an immunotherapeutic approach for cancer (Bian et al, 2020).
Concluding remarks
Compelling evidence has shown that amino acids, their transporters, and metabolites participate in the regulation of immune responses in the tumor microenvironment. Reprogramming amino acid metabolism is relevant in tumor immunity and therapy. However, there are substantial knowledge gaps in our understanding of individual amino acids and their SLCs on different immune cell phenotype and function. For example, our current amino acid and SLC knowledge is largely built upon the in vitro immune cell and tumor cell culture systems. Furthermore, approximately 60 SLC members can mediate amino acid transportation (Kandasamy et al., 2018). Currently, only some SLCs have been examined in immune cells using knockout mouse models. Moreover, different SLCs are heterogeneously expressed, differentially regulated, and functionally redundant and/or potentially interdependent. The phenotype in specific SLC genetic deficient model may be attributed to multiple amino acid uptakes and metabolism. Furthermore, the studies on amino acids, their transporters, and metabolism in the human immune system are rare. Nevertheless, current knowledge suggests that metabolic redundancies can be exploited for selective targeting of cancer. Future research is needed to fill the aforementioned biology gaps and pave the way in utilizing amino acid metabolism targeting for cancer immunotherapy.
Acknowledgments
This work was supported in part by the research grants from the U.S. NIH/NCI R01 grants (W.Z) (CA217648, CA123088, CA099985, CA193136 and CA152470) and the NIH through the University of Michigan Rogel Cancer Center Grant (P30 CA046592) (W.Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Abou-Alfa GK, Qin S, Ryoo BY, Lu SN, Yen CJ, Feng YH, Lim HY, Izzo F, Colombo M, Sarker D, et al. (2018). Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 29, 1402–1408. [DOI] [PubMed] [Google Scholar]
- Arensman MD, Yang XS, Leahy DM, Toral-Barza L, Mileski M, Rosfjord EC, Wang F, Deng S, Myers JS, Abraham RT, et al. (2019). Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America 116, 9533–9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, et al. (2020). Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj J, Konuma T, Lytle NK, Kwon HY, Ablack JN, Cantor JM, Rizzieri D, Chuah C, Oehler VG, Broome EH, et al. (2016). CD98-Mediated Adhesive Signaling Enables the Establishment and Propagation of Acute Myelogenous Leukemia. Cancer cell 30, 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MC, Ginsburg H, and Gilbert DM (1970). Effects of L-asparaginase on lymphocyte-target cell reactions in vitro. Nature 227, 1147–1148. [DOI] [PubMed] [Google Scholar]
- Bian et al. , Y.W.L.e.a.Z. W (2020). Cancer SLC43A2 reprograms methionine metabolism and histone methylation 1 in T cells. Nature in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brin E, Wu K, Lu HT, He Y, Dai Z, and He W (2017). PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget 8, 58948–58963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, and Cayota AM (1999). Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol 162, 3356–3366. [PubMed] [Google Scholar]
- Cantor JM, and Ginsberg MH (2012). CD98 at the crossroads of adaptive immunity and cancer. Journal of cell science 125, 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, and Frauwirth KA (2010). Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185, 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavuoto P, and Fenech MF (2012). A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer treatment reviews 38, 726–736. [DOI] [PubMed] [Google Scholar]
- Cervenka I, Agudelo LZ, and Ruas JL (2017). Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357. [DOI] [PubMed] [Google Scholar]
- Changou CA, Chen YR, Xing L, Yen Y, Chuang FY, Cheng RH, Bold RJ, Ann DK, and Kung HJ (2014). Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proceedings of the National Academy of Sciences of the United States of America 111, 14147–14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JE, and Sun L (2018). Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends in pharmacological sciences 39, 307–325. [DOI] [PubMed] [Google Scholar]
- Cisse MY, Pyrdziak S, Firmin N, Gayte L, Heuillet M, Bellvert F, Fuentes M, Delpech H, Riscal R, Arena G, et al. (2020). Targeting MDM2-dependent serine metabolism as a therapeutic strategy for liposarcoma. Sci Transl Med 12. [DOI] [PubMed] [Google Scholar]
- Couturier MA, Huguet F, Chevallier P, Suarez F, Thomas X, Escoffre-Barbe M, Cacheux V, Pignon JM, Bonmati C, Sanhes L, et al. (2015). Cerebral venous thrombosis in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma during induction chemotherapy with l-asparaginase: The GRAALL experience. Am J Hematol 90, 986–991. [DOI] [PubMed] [Google Scholar]
- Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al. (2017). Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nature medicine 23, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully M (2018). Metabolic disorders: IDO inhibitors could change tack to treat metabolic disorders. Nat Rev Drug Discov 17, 544. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. (2003). Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nature medicine 9, 562–567. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensor CM, Holtsberg FW, Bomalaski JS, and Clark MA (2002). Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer research 62, 5443–5450. [PubMed] [Google Scholar]
- Fenczik CA, Sethi T, Ramos JW, Hughes PE, and Ginsberg MH (1997). Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390, 81–85. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, and Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, Richie JP Jr., Ciccarella A, Calcagnotto A, Mikhael PG, et al. (2019). Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehad AE, Lichtman MK, Schmults CD, Teague JE, Calarese AW, Jiang Y, Watanabe R, and Clark RA (2012). Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. The Journal of investigative dermatology 132, 2642–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. (2016). L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 167, 829–842 e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Jutabha P, Endou H, Sagara H, and Anzai N (2013). LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol 191, 4080–4085. [DOI] [PubMed] [Google Scholar]
- Hayes GM, Chinn L, Cantor JM, Cairns B, Levashova Z, Tran H, Velilla T, Duey D, Lippincott J, Zachwieja J, et al. (2015). Antitumor activity of an anti-CD98 antibody. International journal of cancer. Journal international du cancer 137, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Lin H, Yuan L, and Li B (2017). Combination therapy with L-arginine and alpha-PD-L1 antibody boosts immune response against osteosarcoma in immunocompetent mice. Cancer biology & therapy 18, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horig H, Spagnoli GC, Filgueira L, Babst R, Gallati H, Harder F, Juretic A, and Heberer M (1993). Exogenous glutamine requirement is confined to late events of T cell activation. Journal of cellular biochemistry 53, 343–351. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, and Ali-Osman F (1993). Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem 268, 20116–20125. [PubMed] [Google Scholar]
- Issaq SH, Mendoza A, Kidner R, Rosales TI, Duveau DY, Heske CM, Rohde JM, Boxer MB, Thomas CJ, DeBerardinis RJ, et al. (2020). EWS-FLI1-regulated Serine Synthesis and Exogenous Serine are Necessary for Ewing Sarcoma Cellular Proliferation and Tumor Growth. Molecular cancer therapeutics 19, 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, et al. (2011). Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117, 1834–1839. [DOI] [PubMed] [Google Scholar]
- Joseph J, Gonzalez-Lopez M, Galang C, Garcia C, Lemar H, Lu J, Vaidya K, Fischer M, Frey C, Alam M, et al. (2018). Abstract 4719: Small-molecule antagonists of the Aryl Hydrocarbon Receptor (AhR) promote activation of human PBMCs in vitro and demonstrate significant impact on tumor growth and immune modulation in vivo. Cancer research 78, 4719–4719. [Google Scholar]
- Kandasamy P, Gyimesi G, Kanai Y, and Hediger MA (2018). Amino acid transporters revisited: New views in health and disease. Trends in biochemical sciences 43, 752–789. [DOI] [PubMed] [Google Scholar]
- Keating MJ, Holmes R, Lerner S, and Ho DH (1993). L-asparaginase and PEG asparaginase--past, present, and future. Leuk Lymphoma 10 Suppl, 153–157. [DOI] [PubMed] [Google Scholar]
- Kishton RJ, Sukumar M, and Restifo NP (2016). Arginine Arms T Cells to Thrive and Survive. Cell Metab 24, 647–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, Mongellaz C, Floess S, Fritz V, Matias MI, et al. (2015). Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Science signaling 8, ra97. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. (2006). B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. The Journal of experimental medicine 203, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al. (2019). Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer discovery 9, 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, et al. (2020). The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell research 30, 146–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos H, Huang L, Prendergast GC, and Mellor AL (2019). Immune control by amino acid catabolism during tumorigenesis and therapy. Nature reviews. Cancer 19, 162–175. [DOI] [PubMed] [Google Scholar]
- Lepique AP, Daghastanli KR, Cuccovia IM, and Villa LL (2009). HPV16 tumor associated macrophages suppress antitumor T cell responses. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 4391–4400. [DOI] [PubMed] [Google Scholar]
- Levring TB, Hansen AK, Nielsen BL, Kongsbak M, von Essen MR, Woetmann A, Odum N, Bonefeld CM, and Geisler C (2012). Activated human CD4+ T cells express transporters for both cysteine and cystine. Sci Rep 2, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Phillips JB, Madeira da Silva L, Zhou M, Fodstad O, Owen LB, and Tan M (2017). Interplay between Immune Checkpoint Proteins and Cellular Metabolism. Cancer research 77, 1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, Fiskesund R, Xie J, Liu J, Yin X, et al. (2018). Tumor-Repopulating Cells Induce PD-1 Expression in CD8(+) T Cells by Transferring Kynurenine and AhR Activation. Cancer cell 33, 480–494 e487. [DOI] [PubMed] [Google Scholar]
- Luiking YC, Poeze M, Dejong CH, Ramsay G, and Deutz NE (2004). Sepsis: an arginine deficiency state? Crit Care Med 32, 2135–2145. [DOI] [PubMed] [Google Scholar]
- Lukey MJ, Katt WP, and Cerione RA (2017). Targeting amino acid metabolism for cancer therapy. Drug discovery today 22, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, Mainolfi N, Suri V, Guak H, Balmer ML, et al. (2017). Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab 25, 345–357. [DOI] [PubMed] [Google Scholar]
- Maria RM, Altei WF, Selistre-de-Araujo HS, and Colnago LA (2017). Effects of Doxorubicin, Cisplatin, and Tamoxifen on the Metabolic Profile of Human Breast Cancer MCF-7 Cells As Determined by (1)H High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance. Biochemistry 56, 2219–2224. [DOI] [PubMed] [Google Scholar]
- Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, Gomez Padilla P, Ables G, Bamman MM, Thalacker-Mercer AE, et al. (2015). Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism. Cell Metab 22, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B, Gfeller P, and Guinet E (2016). Restricting Glutamine or Glutamine-Dependent Purine and Pyrimidine Syntheses Promotes Human T Cells with High FOXP3 Expression and Regulatory Properties. J Immunol 196, 3618–3630. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, and Bradfield CA (2010). An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185, 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraki-Moud F, Ghazaly E, Ariza-McNaughton L, Hodby KA, Clear A, Anjos-Afonso F, Liapis K, Grantham M, Sohrabi F, Cavenagh J, et al. (2015). Arginine deprivation using pegylated arginine deiminase has activity against primary acute myeloid leukemia cells in vivo. Blood 125, 4060–4068. [DOI] [PubMed] [Google Scholar]
- Muller AJ, Manfredi MG, Zakharia Y, and Prendergast GC (2019). Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol 41, 41–48. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, and Sun SC (2014). Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, and Kishimoto T (2010). Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America 107, 19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, and Allen PM (2009). Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer research 69, 3086–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, and Pearce EL (2015). Targeting T cell metabolism for therapy. Trends Immunol 36, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203. [DOI] [PubMed] [Google Scholar]
- Palaskas NJ, Garcia JD, Shirazi R, Shin DS, Puig-Saus C, Braas D, Ribas A, and Graeber TG (2019). Global alteration of T-lymphocyte metabolism by PD-L1 checkpoint involves a block of de novo nucleoside phosphate synthesis. Cell Discov 5, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, and Riley JL (2005). CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology 25, 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al. (2015). PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6, 6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. (2016). Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RG, Handschumacher RE, and Mitchell MS (1971). Immunological responses to L-asparaginase. The Journal of clinical investigation 50, 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert SM, Buckingham SC, Campbell SL, Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid MA, Eschbacher JM, et al. (2015). SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med 7, 289ra286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, and Galluzzi L (2020). Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol 21, 120–134. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC, and Al-Khami AA (2017). Arginine Metabolism in Myeloid Cells Shapes Innate and Adaptive Immunity. Frontiers in immunology 8, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, and Ochoa AC (2007). L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, and Ochoa AC (2002). Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem 277, 21123–21129. [DOI] [PubMed] [Google Scholar]
- Ron-Harel N, Ghergurovich JM, Notarangelo G, LaFleur MW, Tsubosaka Y, Sharpe AH, Rabinowitz JD, and Haigis MC (2019). T Cell Activation Depends on Extracellular Alanine. Cell Rep 28, 3011–3021 e3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DG, Chen J, Mamane V, Ma EH, Muhire BM, Sheldon RD, Shorstova T, Koning R, Johnson RM, Esaulova E, et al. (2020). Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metab 31, 250–266 e259. [DOI] [PubMed] [Google Scholar]
- Sanderson SM, Gao X, Dai Z, and Locasale JW (2019). Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nature reviews. Cancer 19, 625–637. [DOI] [PubMed] [Google Scholar]
- Scalise M, Pochini L, Galluccio M, Console L, and Indiveri C (2020). Glutamine transporters as pharmacological targets: From function to drug design. Asian J Pharm Sci 15, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Howden AJ, Brenes A, Spinelli L, Hukelmann JL, Macintyre AN, Liu X, Thomson S, Taylor PM, Rathmell JC, et al. (2019). Antigen receptor control of methionine metabolism in T cells. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Neyens D, Ramsay G, Taylor PM, and Cantrell DA (2018). Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat Commun 9, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, and Cantrell DA (2013). Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol 14, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siska PJ, and Rathmell JC (2015). T cell metabolic fitness in antitumor immunity. Trends Immunol 36, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, and Ostrand-Rosenberg S (2010). Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer research 70, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier G, et al. (2017). Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. Journal for immunotherapy of cancer 5, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S, Abood D, et al. (2018). Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 1891–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N, Pan YX, Zhou M, Harvey RC, Hunger SP, and Kilberg MS (2008). Correlation between asparaginase sensitivity and asparagine synthetase protein content, but not mRNA, in acute lymphoblastic leukemia cell lines. Pediatric blood & cancer 50, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden S, and Cohen EA (2015). Attacking the Supply Lines: HIV-1 Restricts Alanine Uptake to Prevent T Cell Activation. Cell host & microbe 18, 514–517. [DOI] [PubMed] [Google Scholar]
- Szefel J, Danielak A, and Kruszewski WJ (2019). Metabolic pathways of L-arginine and therapeutic consequences in tumors. Advances in medical sciences 64, 104–110. [DOI] [PubMed] [Google Scholar]
- Szlosarek PW, Steele JP, Nolan L, Gilligan D, Taylor P, Spicer J, Lind M, Mitra S, Shamash J, Phillips MM, et al. (2017). Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol 3, 58–66. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Lorenzi PL, and Konopleva M (2019). Amino acid metabolism in hematologic malignancies and the era of targeted therapy. Blood 134, 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, Gutierrez-Vazquez C, Kenison J, Tjon EC, Barroso A, et al. (2019). Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nature neuroscience 22, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzart A, Lengline E, Latiri M, Belhocine M, Smith C, Thomas X, Spicuglia S, Puthier D, Pflumio F, Leguay T, et al. (2019). Epigenetic Silencing Affects l-Asparaginase Sensitivity and Predicts Outcome in T-ALL. Clinical cancer research : an official journal of the American Association for Cancer Research 25, 2483–2493. [DOI] [PubMed] [Google Scholar]
- Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, Qerqez A, Dekker JD, Tanno Y, Lu WC, et al. (2018). Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nature biotechnology 36, 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Hayashi H, Miyamoto T, Abe S, Hirai K, Matsukura K, Yagi H, Hara Y, Yoshida K, Okazaki S, et al. (2019). Anti-tumor effects of mAb against L-type amino acid transporter 1 (LAT1) bound to human and monkey LAT1 with dual avidity modes. Cancer science 110, 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. (2019). CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L, Lu F, Wei S, Maj T, Peng D, et al. (2016). Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 165, 1092–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum RR, Liang H, Deng L, and Fu YX (2017). Radiotherapy and immunotherapy: a beneficial liaison? Nature reviews. Clinical oncology 14, 365–379. [DOI] [PubMed] [Google Scholar]
- Werner A, Amann E, Schnitzius V, Habermeier A, Luckner-Minden C, Leuchtner N, Rupp J, Closs EI, and Munder M (2016). Induced arginine transport via cationic amino acid transporter-1 is necessary for human T-cell proliferation. Eur J Immunol 46, 92–103. [DOI] [PubMed] [Google Scholar]
- West KA, Fisher A, Leventhal D, Sokolovska A, Li N, Plescia C, Castillo M, Isabella V, Kolodziej S, Miller P, et al. (2018). Abstract 2920: Metabolic modulation of the tumor microenvironment using Synthetic Biotic™ Medicines. Cancer research 78, 2920–2920. [Google Scholar]
- Yahyaoui R, and Perez-Frias J (2019). Amino Acid Transport Defects in Human Inherited Metabolic Disorders. International journal of molecular sciences 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang Y, et al. (2020). Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS chemical biology 15, 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W (2005). Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature reviews. Cancer 5, 263–274. [DOI] [PubMed] [Google Scholar]
- Zou W, and Chen L (2008). Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8, 467–477. [DOI] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, and Chen L (2016). PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 8, 328rv324. [DOI] [PMC free article] [PubMed] [Google Scholar]


