Abstract
Obesity, neurodegenerative diseases, and injury can all lead to cognitive deficits, which can be improved clinically with the implementation of cognitive rehabilitation. Due to a lack of effective cognitive rehabilitation tools in mice, we re-designed a cognitive task utilized to detect problem-solving deficits, to develop a cognitive rehabilitation paradigm for mice. In this study, we developed a modified version of the Puzzle Box task by exposing B6 mice to a variety of obstacles and assessing the escape latencies. We then combined obstacles in order to create a “complex obstacle” for the problem-solving task. We determined that our task was reproducible in different cohorts of mice. Furthermore, with repetition the mice display an improvement in the performance, evident by a shorter escape latency and the ability to maintain this improvement in performance, indicative of long-term memory. Given that this approach is new, we validated whether this task could successfully detect deficits in a mouse model of cognitive impairment, the high-fat diet mouse. We demonstrate that high-fat diet mice have longer escape latencies when exposed to the complex obstacle compared to standard diet control mice. Taken together, these data suggest that the Puzzle Box is a valid task for cognitive rehabilitation in mice.
Keywords: Behavioral task, cognitive impairment, light-dark box, memory, problem-solving, Puzzle Box
1. Introduction
Cognitive rehabilitation is a therapeutic process designed to improve cognitive function in areas related to attention, memory, comprehension, problem-solving and awareness (Barman, Chatterjee, and Bhide, 2016; Cicerone, Dahlberg, Kalmar, Langenbahn, Malec, Bergquist, Felicetti, Giacino, Harley, Harrington, Herzog, Kneipp, Laatsch, and Morse, 2000). Cognitive rehabilitation is divided into two components: restorative and compensatory. (Barman et al., 2016; Cicerone et al., 2000). The restorative approach reinforces, strengthens, and reestablishes previously learned patterns. On the other hand, the compensatory approach enables adaptation to the deficit. For example, stroke survivors experiencing a neurological disorder characterized by the inability to explore or respond to external stimuli contralateral to the lesion, known as spatial neglect, undergo cognitive rehabilitation to enhance visual perception to compensate for the spatial deficits (Barrett and Muzaffar, 2014). Cognitive rehabilitation improves cognitive function in older adults (>65 years and older) with cognitive impairment (Willis, Tennstedt, Marsiske, Ball, Elias, Koepke, Morris, Rebok, Unverzagt, Stoddard, Wright, and Group, 2006). Cognitive rehabilitation also improves cognition in disorders such as schizophrenia (Fiszdon, Whelahan, Bryson, Wexler, and Bell, 2005; Twamley, Vella, Burton, Becker, Bell, and Jeste, 2012; Wexler and Bell, 2005; Wykes, Huddy, Cellard, McGurk, and Czobor, 2011), head injuries (Cicerone, Dahlberg, Malec, Langenbahn, Felicetti, Kneipp, Ellmo, Kalmar, Giacino, Harley, Laatsch, Morse, and Catanese, 2005; Cicerone, Langenbahn, Braden, Malec, Kalmar, Fraas, Felicetti, Laatsch, Harley, Bergquist, Azulay, Cantor, and Ashman, 2011), stroke (Lincoln, Gladman, Berman, Noad, and Challen, 2000; Rohling, Faust, Beverly, and Demakis, 2009), and substance abuse (Vocci, 2008). Furthermore, preliminary results suggests that cognitive rehabilitation also improves cognitive deficits associated with Alzheimer’s disease and Parkinson’s disease (Choi and Twamley, 2013; Sinforiani, Banchieri, Zucchella, Pacchetti, and Sandrini, 2004).
Combining cognitive rehabilitation with a motor task may be useful for elderly to prevent falls, in stroke survivors, and in individuals with traumatic brain injury (Pellecchia, 2005). Tasks that require more cognitive effort are predicted to be more effective for motor learning (Hochstenbach, Mulder, van Limbeek, Donders, and Schoonderwaldt, 1998). Many of the current cognitive tasks for mice are excellent at assessing cognitive function but may not be suitable for cognitive rehabilitation in mice with motor impairments. For example, common cognitive tasks such as the Morris Water Maze and radial arm maze, which assess learning and memory deficits, require normal motor function. Hence, there is a need to develop a cognitive task that can be achieved even with limited motor function.
The Puzzle Box was previously designed as a problem-solving task (Galsworthy, Paya-Cano, Liu, Monleon, Gregoryan, Fernandes, Schalkwyk, and Plomin, 2005). The Puzzle Box has demonstrated consistency and effective for detecting cognitive deficits (Ben Abdallah, Fuss, Trusel, Galsworthy, Bobsin, Colacicco, Deacon, Riva, Kellendonk, Sprengel, Lipp, and Gass, 2011; Galsworthy et al., 2005; Van der Jeugd, Vermaercke, Halliday, Staufenbiel, and Gotz, 2016). The purpose of this study was to modify the Puzzle Box to develop a reliable and valid cognitive rehabilitative task for animals. The development of a cognitive rehabilitation task in mice can be useful to study the benefits of cognitive rehabilitation in preclinical models of stroke, Alzheimer’s and Parkinson’s disease. A good cognitive rehabilitation task must reveal an enhancement in performance with repetition (Barman et al., 2016). Furthermore, an effective cognitive rehabilitation task will lead to retention of performance from day to day (Cicerone et al., 2000). Thus, in this study we characterize the development of a cognitive rehabilitation task for mice by modifying the Puzzle Box paradigm. We also validated the ability of this modified Puzzle Box paradigm to detect cognitive impairment in a mouse model of cognitive impairment, the high-fat diet (HFD) mouse.
2. Materials and Methods
2.1. Puzzle Box design
The Puzzle Box is a two-compartment arena consisting of an open field (12 × 10 inches) and a covered/enclosed compartment (dark box) connected via a 1.5-inch escape door (Fig. 1A). The arena was constructed using three 18 × 10 inches, three 10 × 10 inches, and one 6 × 10 inches acrylic sheets. The dark box compartment was painted black (Rust- Oleum Professional). The acrylic sheets were glued together with 2-part epoxy (Loctite plastic epoxy) and all spaces and cracks were sealed with sealant (Lexel). Five different obstacles were placed in the open door zone (Fig. 1A; red box, 4.25 × 4.25 inches) used to obstruct the escape door (Fig. 1B): 1) nestlet, which is used by the mice as bedding in their home cages, was placed behind the escape door (Fig. 1C); 2) enviro-dri, which was placed in front of the escape door requiring the mouse to burrow through or remove the enviro-dri (Fig. 1D); 3) LEGO bridge, which was placed in front of the escape door requiring the mouse to either go over or under to enter the dark box (Fig. 1E); 4) LEGO stairs, which required the mouse to walk up and then down the stairs to enter the dark box (Fig. 1F); and 5) the tunnel is a T shaped tunnel giving the mouse three ways to enter the dark box (Fig. 1G).
Figure 1. Puzzle box design, escape latency for individual obstacles and reproducibility of the Puzzle Box.
(A) The top view of the puzzle box arena. The puzzle box consists of an open field (*) connected by an (B) open door (black arrow) to a dark box (◊). The open door zone (red box) is obstructed with either (C) the nestlet, (D) enviro-dri, (E) LEGO bridge and (F) LEGO stairs and a (G) tunnel. (H) Escape latency in seconds (sec) with open door, nestlet, enviro-dri, LEGO Bridge (LEGO Brdg), LEGO stairs, and tunnel. (I) Reproducibility of the escape latency in sec for the open door, nestlet, and enviro-dri for 3 different cohorts of mice. White, grey and black bars represent cohort 1, 2, and 3, respectively. (J) Correlation of escape latencies (sec) and age (months) with open door (open circles and solid black line), nestlet (black squares and dashed line) and enviro- dri (grey triangles and black dotted line) in cohort 1, 2, and 3. Data represents mean ± SEM; n=9 per group; *P≤ 0.05.
2.2. Animals
Male and female C57BL6 mice (Jackson Laboratory, Bar Harbor, Maine) 6–24 months of age fed a regular chow diet from Teklad were used for these studies. This age range was used to establish baseline behavior in normal mice at ages typically used in preclinical models with cognitive impairment. Mice were housed in a pathogen-free environment. All protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee, and are in compliance with the university guidelines, state and federal regulations, and the standard of the “Guide for the Care and Use of Laboratory Animals”. The animal welfare assurance number on file with the NIH Office of Laboratory Animal Welfare (OLAW) is A3428–01. The Medical University of South Carolina is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC, Intl.).
2.3. Development of problem-solving task
The problem-solving task was performed in the Puzzle Box as described above. A video camera (Besteker HD) was mounted above the open field and Panlab Harvard Apparatus’s SMART video tracking software (Barcelona, Spain) is used to record the movements of the mouse and the escape latency from the open field to the dark box. The mice were allowed to explore the empty Puzzle Box until they escaped to the dark box or for 300 seconds. Following three trials with the open door, the escape latency with the nestlet, enviro-dri, LEGO bridge, LEGO stairs, and tunnel trials were recorded. Three different complex obstacles were designed by combing 2–3 of the aforementioned obstacles: complex (1) LEGO stairs and nestlet; complex (2) LEGO bridge, enviro-dri, and nestlet; and complex (3) the tunnel, enviro-dri and nestlet. The mice were subjected to three trials for each obstacle. The arena, LEGO and tunnel obstacles were thoroughly cleaned with ethanol between each trial and mouse. Furthermore, fresh enviro-dri and nestlet were used for each trial and mouse.
2.4. Validation in a mouse model of cognitive impairment
To generate a mouse model of cognitive impairment, B6 mice purchased from Jackson Laboratory (Bar Harbor, Maine) were placed on either the standard diet (STD) consisting of 10% kcal from fat (Research Diets Inc.; #D12450B, New Brunswick, NJ) or a HFD with 54% kcal from fat (Research Diets Inc.; D05090701) ad libitum at 4 weeks of age per our previously published protocol (Sims-Robinson, Bakeman, Bruno, Jackson, Glasser, Murphy, and Feldman, 2016; Watson, Stone, Williams, Williams, and Sims-Robinson, 2020a). After 28 weeks of diet, both groups were subjected to the Puzzle Box and performed 3 trials each of open door, nestlet, enviro-dri and tunnel. In addition, the mice were also subjected to 3 trials of the complex obstacle 3. Recording and tracking of the mice was done according to the protocol above.
2.5. Statistical Analysis
Data analyses were performed using Prism v6 (GraphPad Software, Inc.). All sample sizes represent the number of animals. All data were analyzed using either a one-way ANOVA with Tukey’s Multiple Comparison Test (Evidence of learning for problem-solving the complex obstacle and long-term memory retention for problem-solving the complex obstacle) or a mixed-effects repeated measures analysis with Bonferroni’s multiple comparisons test (Validation of the Puzzle Box: Problem-solving deficits in high-fat diet mice). All analyses significance was determined using an alpha-level of 0.05.
3. Results
3.1. Latency of individual obstacles
The escape latency of the third trial was evaluated, to establish the latency for each individual obstacle (Fig. 1H; n=9). The shortest escape latency occurs with the open door (18.9 ±12.8 seconds) and the tunnel (21.8±13.8 seconds). The escape latency is approximately 4–5 times longer with the nestlet (87.3±33.5 seconds), enviro-dri (73.1±45.1 seconds), LEGO bridge (69.5±24.9 seconds) compared with the open door. The longest escape latency occurs with LEGO stairs (133.3±41.5 seconds).
3.2. Reproducibility of the Puzzle Box task
To investigate the reproducibility of the problem-solving task, the escape latency for 3 different cohorts of mice following the third trial were evaluated (Fig. 1I; n=9). No significant differences were found in the escape latencies between cohorts 1 (18.9±12.8 seconds), 2 (60.0±18.2 seconds), and 3 (22.2±9.3 seconds) with the open door. Similarly, there were no significant differences in the escape latencies for cohorts 1 (87.3±33.5 seconds), 2 (149.8±31.6 seconds) and 3 (155.2±26.6 seconds) with the nestlet. The escape latency for the enviro-dri was also similar among cohorts 1 (93.1±45 seconds), 2 (42.5±14.2 seconds), and 3 (64.9±18.2 seconds).
3.3. Correlation of escape latency with age
To investigate the correlation between escape latency and age, simple linear regression analysis was performed (Fig. 1J). There was no correlation between escape latency and age with the open door (F= 0.04; P= 0.836) and enviro-dri (F= 3.94; P= 0.058) obstacles. On the other hand, a significant correlation between escape latency and age was observed with the nestlet (F= 5.03; P= 0.034) obstacle.
3.4. Development of the cognitive rehabilitation task
3.4.1. Evidence of learning with the problem-solving task
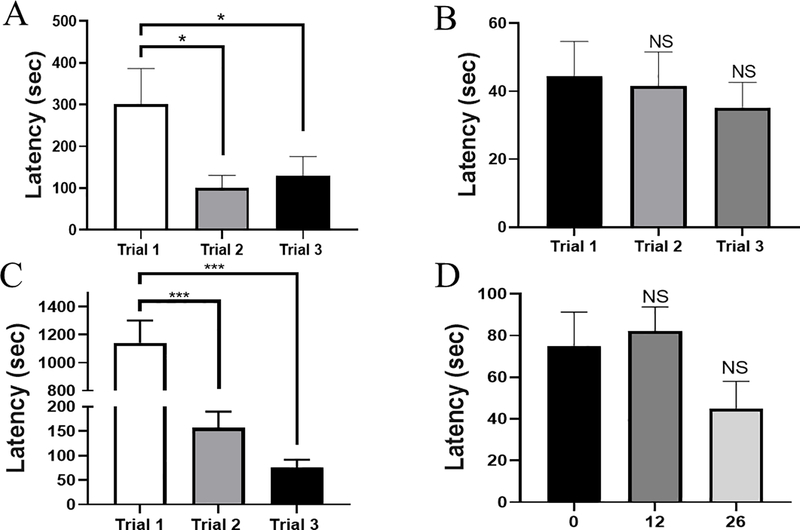
To develop a problem-solving task, the escape latency for 3 trials in a subset of mice (cohort 2; n=9), were assessed using multiple obstacles combined to create 3 different complex obstacles. For complex 1, the escape latency decreased by 70% from trial 1 (301.0 ± 85.61 seconds) to trial 2 (100.5 ± 30.03 seconds) and decreased 60% from trial 1 to trial 3 (130.0 ± 45.5 seconds) (Fig. 2A). For complex 2 (Fig. 2B), there were no significant differences among trial 1 (44.4 ± 10.3 seconds), trial 2 (41.6 ± 10.0 seconds), and trial 3 (35.2 ± 7.4 seconds). Finally, complex 3 consisting of the tunnel, enviro-dri and nestlet resulted in the most significant improvement in subsequent trials (Fig. 2C), which led us to select complex 3 for subsequent analysis. There was a 90% decrease from trial 1 (1142.1±159.6 seconds) to trial 2 (156.5± 33.2 seconds) and from trial 2 to trial 3 (75.1± 16.2 seconds).
Figure 2. Evidence of learning for problem-solving the complex obstacle and long-term memory retention for problem-solving the complex obstacle.
Escape latency in seconds (sec) during 3 consecutive trials with (A) complex 1 obstacle, which consists of LEGO stairs and nestlet; (B) complex 2 obstacle, which consists of the LEGO bridge, enviro- dri and nestlet; (C) complex 3 obstacle, which consists of the tunnel, enviro- dri, and the nestlet; Repeated measures one-way ANOVA and Tukey’s post-hoc used for multiple comparisons. (D) Escape latency in sec at 0, 12, and 26 days with complex 3 obstacle; Ordinary one-way ANOVA and Tukey’s post-hoc used for multiple comparisons. Data represents mean ± SEM; n=9; * P ≤ 0.05; *** P≤ 0.001; not significant (NS).
3.4.2. Evidence of retention with the problem-solving task
To assess retention for performance using complex 3, trials were performed using cohort 2 (n=9), 12 and 26 days later. The data reveals no significant differences at day 0 (75.1± 16.2 seconds), 12 (82.2± 11.6 seconds), and 26 (44.9± 13.2 seconds) (Fig. 2D).
3.5. Validation of problem-solving task using HFD mice
To validate that the complex 3 obstacle detects cognitive deficits, the escape latencies were assessed in STD (n=4) and HFD mice (n=11), a model of cognitive impairment, after three trials. A mixed-effects repeated measures analysis revealed a significant effect of obstacle on escape latency (F= 17.8; P= <0.0001). Analysis also revealed interaction effect between diet and obstacle on escape latency (F= 5.4; P= 0.0010). There were no significant differences in the escape latencies between STD versus HFD mice, for the open door (20.9± 6.5 versus 9.1± 1.4), nestlet (64.7± 25.4 versus 56.2± 21.8), enviro-dri (31.0± 8.0 versus 20.4± 3.3), and tunnel (19.7± 2.8 versus 12.6± 1.6). The escape latency in the HFD (201.6± 21.7) mice was increased by 115% compared with the STD (93.7± 23.6) mice when completing complex 3. There was a significant interaction with obstacles (individual and complex 3; F= 15.3); however, there was no significant interaction with diet (STD and HFD; F= 0.5; Fig. 3A). To evaluate the potential impact of HFD on mobility, total distance for mice was assessed in STD and HFD mice. There were no significant differences in total distance between STD versus HFD mice for open door (191.5± 117.0 versus 30.9± 4.1), nestlet (207.7 ± 95.4 versus 169.6 ± 53.9), enviro- dri (49.0± 14.4 versus 52.8± 11.9), tunnel (34.9± 10.4 versus 30.5± 5.6) and complex 3 (99.2± 52.6 versus 218.2± 36.7; Fig. 3B). To evaluate potential differences in exploratory behavior, we assessed the time in the open door zone, which corresponds to time exploring/ interacting with the obstacles. There were no significant differences between STD versus HFD mice the time spent interacting with, open door (6.2±1.3 versus 4.8± 1.3), nestlet (10.2± 4.2 versus 17.5± 4.6), enviro- dri (15.9± 3.8 versus 13.1± 2.2), tunnel (12.6± 3.3 versus 7.1± 1.6) and complex 3 (65.8± 45.6 versus 34.4± 6.8; Fig. 3C).
Figure 3. Validation of the Puzzle Box: Problem-solving deficits in high-fat diet mice.
The (A) escape latency (seconds; sec), (B) total distance (centimeters; cm), (C) time (sec) interacting within the open door zone indicative of exploratory behavior in both standard diet (STD; white bars) and high-fat diet (HFD; black bars) mice with individual obstacles and the complex 1 obstacle. Data represents mean ± SEM; n=4 (STD) and n=11 (HFD); *P≤ 0.05. A mixed-effects repeated measures analysis with Bonferroni’s multiple comparisons test used for analysis.
4. Discussion
Cognitive rehabilitation has been effective for improving attention, memory, and executive function in patients with traumatic brain injury, stroke (Arciniegas, Held, and Wagner, 2002; Barman et al., 2016; Cicerone et al., 2000; Fiszdon et al., 2005). Currently, there are no established cognitive rehabilitation paradigms for preclinical models. We provide for the first-time evidence for the use of the Puzzle Box as a cognitive rehabilitation task. Similar to the initial Puzzle Box design described by Ben Abdallah et al (Ben Abdallah et al., 2011), our arena was made of Plexiglas with an open field and a dark box connected by an escape door. In our study, we used objects to obstruct the door that closely resembled objects used in previous studies (Ben Abdallah et al., 2011; O’Connor, Burton, Leamey, and Sawatari, 2014; Van der Jeugd et al., 2016). On average the escape latency in our study is comparable to those observed in other Puzzle Box studies (Ben Abdallah et al., 2011; O’Connor et al., 2014). Furthermore, similar escape latencies among three different cohorts of mice for the open door, nestlet, and enviro-dri suggests that the escape latencies are consistent among three cohorts of mice.
In order for the Puzzle Box to be a viable cognitive rehabilitation task, there must be evidence of improvement with repetition, indicative of learning (O’Connor et al., 2014) and the ability to retain this improvement long-term. We demonstrated that the escape latency decreases between consecutive trials. This suggests that the animals learn, evident by the enhancement in performance with repetition when completing the problem-solving task. Furthermore, the mice retained this enhancement of performance 12 and 26 days after the initial trial. Overall, this data suggests that this modified version of the Puzzle Box may be a useful paradigm for cognitive rehabilitation in mice.
Finally, in order to validate that this modified version of the Puzzle Box is capable of detecting cognitive deficits, we performed the task in a mouse model of cognitive impairment, the HFD mouse model. We previously demonstrated that cognitive impairment is evident in HFD mice by 28 weeks of age using both novel object recognition test and Morris Water Maze (Sims-Robinson et al., 2016; Watson, Stone, Williams, Williams, and Sims-Robinson, 2020b). While there appears to be no differences in the escape latencies between STD and HFD mice with the open door, HFD mice demonstrate a significant increase in escape latency with the complex obstacle. This data suggests that HFD mice have deficits in problem-solving. Previous tasks such as the novel object recognition task and the Morris water maze detect deficits in recognition and working memory, respectively. On the other hand, the Puzzle Box task detects problem-solving deficits (Galsworthy, Paya-Cano, Monleon, and Plomin, 2002). The advantage of using the Puzzle Box is that it can be potentially used for rehabilitation. Interestingly, a previous study revealed that consumption of “good fats”, omega-3-polyunsaturated fatty acids-enriched, formula can improve problem-solving abilities in 9 month old babies (Drover, Hoffman, Castaneda, Morale, and Birch, 2009). Our previous studies demonstrates that HFD mice spend similar amounts of time exploring during object recognition tasks (Sims-Robinson et al., 2016; Watson et al., 2020b). Our current data reveals that the distance traveled by the STD and HFD mice are comparable, suggesting that diet does not impact mobility. Furthermore, STD and HFD mice spend similar amounts of time interacting with obstacles and in the open field, which indicates that diet does not affect the motivation to explore. Thus, in addition to memory deficits previously reported (Sims-Robinson et al., 2016; Watson et al., 2020b), our data suggests that HFD also contributes to impairments with problem-solving.
In summary, we have demonstrated that the Puzzle Box can be modified for cognitive rehabilitation training. Our data reveals that the Puzzle Box task is reproducible among various cohorts of mice. Furthermore, we demonstrate that mice display an enhancement in performance evident by the decrease in escape latency with repetition. The enhancement in performance is retained for several days suggesting that the tasks augments long-term memory. We also observed problem-solving deficits in a mouse model of cognitive impairment, the HFD mouse. We conclude that the Puzzle Box is a useful task for assessing cognitive impairment and can be used as a cognitive rehabilitation task in mice.
Supplementary Material
Highlights.
The Puzzle Box task detects problem solving deficits in a mouse model of cognitive impairment.
The Puzzle Box may be a useful paradigm for cognitive rehabilitation in mice.
High-fat diet contributes to problem solving deficits.
Acknowledgements
The authors wish to acknowledge Dr. Serena Kinley-Cooper Sims and Luke Watson for editorial assistance and Madison Patrick, Tyler Stone, and Janet Boggs for technical assistance.
Funding. This work was supported by the National Institute of Health (NINDS 1R01NS099595-01A1; NIGMS P20 GM109040; NHLBI R25 HL092611 to A.W.) and the Alzheimer’s Association (AARGD-16-440893).
Abbreviations
- HFD
high fat diet
- STD
standard diet
- ANOVA
analysis of variance
- LEGO Brdg
LEGO Bridge
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arciniegas DB, Held K, & Wagner P (2002). Cognitive Impairment Following Traumatic Brain Injury. Curr Treat Options Neurol, 4, 43–57. [DOI] [PubMed] [Google Scholar]
- Barman A, Chatterjee A, & Bhide R (2016). Cognitive Impairment and Rehabilitation Strategies After Traumatic Brain Injury. Indian J Psychol Med, 38, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AM, & Muzaffar T (2014). Spatial cognitive rehabilitation and motor recovery after stroke. Curr Opin Neurol, 27, 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, Fuss J, Trusel M, Galsworthy MJ, Bobsin K, Colacicco G, Deacon RM, Riva MA, Kellendonk C, Sprengel R, Lipp HP, & Gass P (2011). The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol, 227, 42–52. [DOI] [PubMed] [Google Scholar]
- Choi J, & Twamley EW (2013). Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychol Rev, 23, 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, Felicetti T, Giacino JT, Harley JP, Harrington DE, Herzog J, Kneipp S, Laatsch L, & Morse PA (2000). Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil, 81, 1596–1615. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, Ellmo W, Kalmar K, Giacino JT, Harley JP, Laatsch L, Morse PA, & Catanese J (2005). Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil, 86, 1681–1692. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, Felicetti T, Laatsch L, Harley JP, Bergquist T, Azulay J, Cantor J, & Ashman T (2011). Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil, 92, 519–530. [DOI] [PubMed] [Google Scholar]
- Drover J, Hoffman DR, Castaneda YS, Morale SE, & Birch EE (2009). Three randomized controlled trials of early long-chain polyunsaturated Fatty Acid supplementation on means-end problem-solving in 9-month-olds. Child Dev, 80, 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszdon JM, Whelahan H, Bryson GJ, Wexler BE, & Bell MD (2005). Cognitive training of verbal memory using a dichotic listening paradigm: impact on symptoms and cognition. Acta Psychiatr Scand, 112, 187–193. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Liu L, Monleon S, Gregoryan G, Fernandes C, Schalkwyk LC, & Plomin R (2005). Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav Genet, 35, 675–692. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleon S, & Plomin R (2002). Evidence for general cognitive ability (g) in heterogeneous stock mice and an analysis of potential confounds. Genes Brain Behav, 1, 88–95. [DOI] [PubMed] [Google Scholar]
- Hochstenbach J, Mulder T, van Limbeek J, Donders R, & Schoonderwaldt H (1998). Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke. J Clin Exp Neuropsychol, 20, 503–517. [DOI] [PubMed] [Google Scholar]
- Lincoln NB, Gladman JR, Berman P, Noad RF, & Challen K (2000). Functional recovery of community stroke patients. Disabil Rehabil, 22, 135–139. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Burton TJ, Leamey CA, & Sawatari A (2014). The use of the puzzle box as a means of assessing the efficacy of environmental enrichment. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellecchia GL (2005). Dual-task training reduces impact of cognitive task on postural sway. J Mot Behav, 37, 239–246. [DOI] [PubMed] [Google Scholar]
- Rohling ML, Faust ME, Beverly B, & Demakis G (2009). Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.’s (2000, 2005) systematic reviews. Neuropsychology, 23, 20–39. [DOI] [PubMed] [Google Scholar]
- Sims-Robinson C, Bakeman A, Bruno E, Jackson S, Glasser R, Murphy GG, & Feldman EL (2016). Dietary Reversal Ameliorates Short- and Long-Term Memory Deficits Induced by High-fat Diet Early in Life. PLoS One, 11, e0163883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinforiani E, Banchieri L, Zucchella C, Pacchetti C, & Sandrini G (2004). Cognitive rehabilitation in Parkinson’s disease. Arch Gerontol Geriatr Suppl, 387–391. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Becker DR, Bell MD, & Jeste DV (2012). The efficacy of supported employment for middle-aged and older people with schizophrenia. Schizophr Res, 135, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Jeugd A, Vermaercke B, Halliday GM, Staufenbiel M, & Gotz J (2016). Impulsivity, decreased social exploration, and executive dysfunction in a mouse model of frontotemporal dementia. Neurobiol Learn Mem, 130, 34–43. [DOI] [PubMed] [Google Scholar]
- Vocci FJ (2008). Cognitive remediation in the treatment of stimulant abuse disorders: a research agenda. Exp Clin Psychopharmacol, 16, 484–497. [DOI] [PubMed] [Google Scholar]
- Watson LS, Stone TD, Williams D, Williams AS, & Sims-Robinson C (2020a). High-Fat Diet Impairs Tactile Discrimination Memory in the Mouse. Behav Brain Res, 382, 112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LS, Stone TD, Williams D, Williams AS, & Sims-Robinson C (2020b). High-Fat diet impairs tactile discrimination memory in the mouse. Behav Brain Res, 112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, & Bell MD (2005). Cognitive remediation and vocational rehabilitation for schizophrenia. Schizophr Bull, 31, 931–941. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E, & Group AS (2006). Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA, 296, 2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P (2011). A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry, 168, 472–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.