Abstract
Background
Mechanical thrombectomy is the standard of care, in selected patients, for acute ischemic stroke with large vessel occlusion but its use in patients with stroke secondary to infective endocarditis is controversial. We report three cases of acute ischemic stroke treated by mechanical thrombectomy and we propose an extensive review of the literature to evaluate the clinical safety and efficacy of thrombectomy in patients with stroke secondary to infective endocarditis.
Methods
A comprehensive literature search was performed following a pre-specified protocol of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Case reports, cases series, cross-sectional studies, case control studies, randomized controlled trials or nonrandomized controlled trials were considered that included endocarditis-related acute ischemic stroke patients who underwent mechanical thrombectomy.
Results
The database search yielded 431 relevant records published until January 2020. Nineteen articles fulfilled the eligibility criteria that described thirty patients. After the thrombectomy, 13.3% of the patients experienced intracranial haemorrhage. After the procedure, the median National Institutes of Health Stroke Scale score dropped from 15 (IQR 7) to 2.5 (IQR 5.75). At 90 days, mortality was 23.3% while 46.7% of the patients were functionally independent (mRS ≤ 2).
Discussion
Based on our review, the use of mechanical thrombectomy in patients with large vessel occlusion due to endocarditis-associated stroke might improve patient outcome but it should be considered on a case by case base as the safety has not been well established yet. Further research on risk stratification is needed to drive clinician during the decision-making process.
Keywords: Infective endocarditis, Stroke, Mechanical thrombectomy
Introduction
Acute ischemic stroke is the most common neurological complication of infective endocarditis, manifesting clinically in 20–40% of the patients [1, 2]. Conversely, complications may be completely silent as asymptomatic ischemia can occur in another 30–40% of patients [3]. Patients with stroke secondary to infective endocarditis have a severe prognosis leaving only less than one-third of patients alive with functional independence [4].
Treatment of patients with acute stroke secondary to infective endocarditis is suboptimal as thrombolytic therapy is contraindicated due to high risk of haemorrhagic transformation of the infarct [5]. Mechanical thrombectomy is the standard of care, in selected patients, for acute ischemic stroke with large vessel occlusion. However, its efficacy and safety in patients with stroke secondary to infective endocarditis have limited evidence in the literature. Then, there is an urgent need of improved treatment for patients with stroke secondary to infective endocarditis.
We report here a case series of patients with stroke secondary to infective endocarditis treated with mechanical thrombectomy and a review of the current available literature on this issue.
Methods
Search strategy and study selection
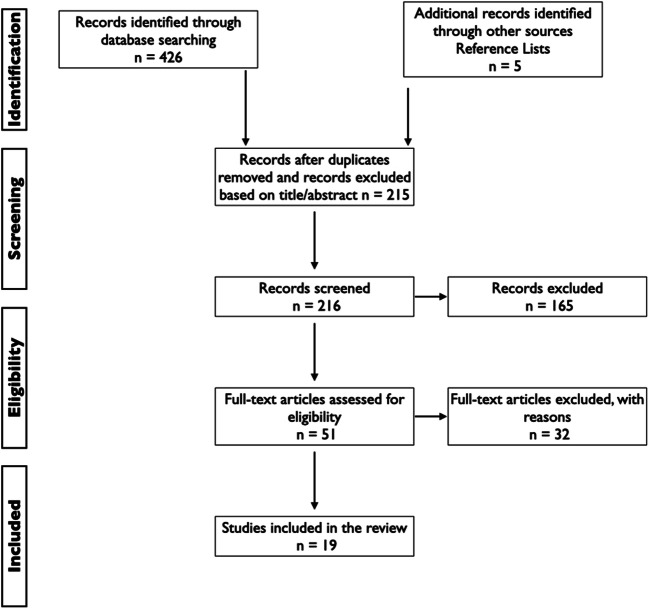
We performed a systematic review following PRISMA (Fig. 1) guidance [6]. Systematic search for the reports published until January 2020 was conducted in PubMed, Cochrane, SciELO, B-on, Google scholar and clinical trial registries for relevant articles. Reference lists from all included articles and abstracts were also assessed for any additional relevant studies not identified through the initial search. For those meeting the eligibility criteria, full-text articles were obtained. Pre-defined eligibility criteria were applied. For the search strategy, we combined the terms ‘stroke and endocarditis’ with ‘thrombectomy’. We did also the same search, but without the word ‘stroke’.
Fig. 1.
PRISMA flowchart
Eligibility criteria
Only reports published in English were considered. We included randomized or nonrandomized controlled trials, case control studies, cross-sectional studies, case series and case reports that reported the treatment of patients (> 18 years old) with acute stroke secondary to infective endocarditis with mechanical thrombectomy. Title and abstracts of all retrieved articles were assessed for inclusion.
Data collection process
We extracted the following information from the reports: age, gender, site of the infective endocarditis, presence or not of atrial fibrillation, treatment with oral anticoagulant or not, blood culture pathogen, baseline National Institutes of Health Stroke Scale (NIHSS) scores, treatment with intravenous thrombolysis and site of the large vessel occlusion. We also extracted as neuroradiological outcome the presence of intracranial haemorrhage after treatment and the thrombolysis in cerebral infarction (TICI) [7] scale score defined as grade 0, no perfusion; grade 1, penetration with minimal perfusion; grade 2A, only partial filling of the entire vascular territory visualised; grade 2B, complete filling of all the expected vascular territories visualised but the filling is slower than normal; and grade 3, complete perfusion. We documented the endovascular revascularization technique used, the NIHSS score after treatment and the modified Rankin Scale (mRS) at follow-up. Whenever any data is not provided, it was documented as not reported (NR).
Risk of bias in individual studies
The quality of the articles was examined according to the Quality Assessment Tool for Case Series Studies of National Heart, Lung, and Blood Institute and to the Case Reporting Guidelines of Care (2013).
Data synthesis
We summarised the data obtained in Table 1. In Table 2, we provided the % of patients with mRS score 0–1 and 0–2 at 90 days. We have also calculated the median (IQR) of the NIHSS at 24 h after the mechanical thrombectomy and the median (IQR) change in NIHSS score from baseline to 24 h after the procedure.
Table 1.
Characteristics of patients with stroke and infective endocarditis treated with mechanical thrombectomy
| Authors | Age/sex | Site of the IE | AF | Treatment of OAC | Blood culture pathogen | Onset NIHSS score | Treatment with IV thrombolysis | LVO site | TICI | Endovascular revascularization technique | ICH | NIHSS outcome | mRS at follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D’Anna | 67/M | Mitral valve | No | No | Gemella morbillorum | 17 | No | M1 | 3 | Aspiration | No | 0–24 h after | 0 at 90 days |
| D’Anna | 30/F | Aortic valve | No | No | Neisseria gonorrhoeae | 22 | No | Distal ICA | 2a | Aspiration and stent retriever | Yes | 22–24 h after | 3 at 90 days |
| D’Anna | 65/F | Mitral valve | No | No | Staphylococcus aureus | 18 | NR | M2 | 0 | Aspiration | No | 21–24 h after | 4 at 90 days |
| Sloane et al. | 59/F | Mitral valve | No | No | Negative | 21 | No | M1 | 2B | Stent retriever | No | 6 after the procedure | 1 at 180 days |
| Distefano et al. | 75/M | Aortic valve | No | No | Enterococcus faecalis | 16 | Yes | M1 | NR (recanalised) | Aspiration | Yes | NR | 6 few days later |
| Sgreccia et al. | 31/M | Aortic valve | No | No | Candida parapsilosis | 18 | Yes | ICA bifurcation | 3 | Stent retriever | No | 0–48 h later | 0 at 90 days |
| Ambrosioni et al. | 79/M | Prosthetic (mechanical) | NR | Yes | Staphylococcus aureus | 9 | NR | M1 and ICA | 0 | Stent retriever | No | 35–24 h after | 6 at 7 days |
| Ambrosioni et al. | 69/ F | Prosthetic (mechanical) | NR | Yes | Streptococcus oralis | 10 | NR | Basilar | 3 | Stent retriever | No | 2–24 h after | 0 at 7 and 90 days |
| Ambrosioni et al. | 56/F | Native | NR | No | Negative culture | 19 | NR | M1 | 3 | Stent retriever | No | 2–24 h after | 0 at 7 and 90 days |
| Ambrosioni et al. | 72/M | Native | NR | No | Streptococcus dysgalactiae | 35 | NR | Basilar | 3 | Stent retriever | No | 35–24 h after | 6 at 7 days |
| Ambrosioni et al. | 79/F | Prosthetic (biological) | NR | Yes | Negative culture | 5 | NR | M1 | 2B | Stent retriever | No | 2–24 h after | 2 at 7 and 90 days |
| Ambrosioni et al. | 85/M | Prosthetic (biological) | NR | No | Staphylococcus epidermidis | 8 | NR | M1 | 3 | Stent retriever | No | 0–24 h after | 0 at 7 days and 6 at 90 days |
| Bolognese et al. | 42/M | Aortic valve | No | No | Streptococcus viridans | 3 | No | M2 | 2B | Aspiration | No | 0 at 4 weeks | NR |
| Elodie et al. | 70/ F | Aortic valve | Yes | Yes | Negative culture | 10 | No | M1 | NR (recanalised) | Stent retriever | No | 1 immediately after | 1 at 90 days |
| Nishino et al. | 72/M | Mitral valve | Yes | Yes | Streptococcus salivarius | NR | No | M2 | NR (recanalised) | Stent retriever | No | NR | 6 at 9 days |
| Scharf et al. | NR/NR | Mechanical mitral valve and native aortic valve | No | Yes | Streptococcus | 12 | No | M1 | 3 | Stent retriever and aspiration | No | 1 | 0 at discharge |
| Sveinsson et al. | 33/M | Prosthetic mitral valve | No | Yes | Serratia marcescens | 14 | No | M1 | NR (recanalised) | NR | No | 1 at discharge | 1 at discharge |
| Sveinsson et al. | 67/M | Prosthetic mitral valve | Yes | Yes | Enterococcus faecalis | 13 | No | M1 | NR (recanalised) | NR | No | 3 at discharge | 1 after few months |
| Sveinsson et al. | 39/F | Mitral valve | No | No | NR | 15 | No | M2 | NR (recanalised) | NR | No | 4 at discharge | 2 after 3 months |
| Ladner et al. | 40/NR | Aortic valve | No | No | Enterococcus faecalis | 3 | No | M1 | 3 | Aspiration | No | 0 at 13 days | 0 at 13 days |
| Kim et al. | 40/F | Mitral valve | No | No | Streptococcus mitis | 15 | No | M2 | NR (recanalised) | Aspiration | No | 3 at 2 days | 2 at 3 months |
| Toeg et al. | 73/M | Bioprosthetic aortic valve (replaced 8 weeks prior) | No | No | Gram-positive cocci | 20 | No | M1, A1 and distal ICA | NR (recanalised) | NR | No | 2 immediately after; 0 after 8 months | NR |
| Akkoyunlu et al. | 23/F | Mitral valve | No | No | Gram-positive coccobacillus | NR | No | M1 | NR (recanalised) | NR | No | NR | NR |
| Kang et al. | 39/F | Mitral valve | No | No | Streptococcus gordonii | 16 | No | M1 | 2b | Stent retriever | No | 3 at 4 weeks | NR |
| Dababneh et al. | 67/F | Bovine mitral valve (replaced 6 months prior) | Yes | Yes | Gram-negative vancomycin-resistant rods | NR | No | Between segments M1 and M2 | 2 or 3 | Stent retriever | No | NR | 6 at 7 days |
| Kan et al. | 78/F | Aortic valve | No | No | No | 16 | No | M2 | 3 | Stent retriever | No | 12–24 h later | NR |
| Liang et al. | 70/F | Mitral valve | Yes | Yes | Group B Streptococcus agalactiae | 24 | No | M2 | NR (recanalised) | Stent retriever | No | NR, reported no residual neurology | NR |
| Walker et al. | NR/NR | NR | NR | NR | Coagulase-negative Staphylococcus | 14 | Yes | NR | NR | Stent retriever | Yes | NR | 6 |
| Walker et al. | NR/NR | NR | NR | NR | Enterococcus faecalis | 14 | Yes | NR | NR | Stent retriever | Yes | NR | 6 |
| Bain et al. | 24/F | NR | No | Yes | Gram-positive bacilli | 18 | No | M1 | NR (recanalised) | Stent retriever | No | 7–24 h after; 2 after 2 months | NR |
M male; F female; IE infective endocarditis; IV intravenous; AF atrial fibrillation; OAC oral anticoagulant; NIHSS National Institutes of Health Stroke Scale; ICA internal carotid artery; LVO large vessel occlusion; TICI thrombolysis in cerebral infarction; ICH intracranial haemorrhage; mRS modified Rankin Scale; NR not reported
Table 2.
Efficacy outcome
| mRS score at 90 days | IE patients treated with MT |
|---|---|
| mRS score 0–1 at 90 days | 36.7% (11/30)* |
| mRS score 0–2 at 90 days | 46.7% (14/30)* |
| NIHSS at 24 h | |
| Median score | 2.5 (IQR 5.75) |
| Change in NIHSS score from baseline to 24 h | |
| Median change | − 14 (IQR 10) |
MT mechanical thrombectomy; IE infective endocarditis; NIHSS National Institutes of Health Stroke Scale; mRS modified Rankin Scale; IQR interquartile range
*mRS score at 90 days is not available for seven patients
Case series
Case 1
A 30-year-old female patient was brought to our emergency department 4 h after she developed right-sided hemiparesis, aphasia, right homonymous hemianopia, right central facial paralysis and reduced sensation in the right side of the body while she was at the gym (NIHSS 22). A month earlier, she presented to her local general practitioner (GP) with symptoms including sore throat, lethargy and general malaise. Her GP found no specific abnormalities on thorough general examination. CT of the brain showed already established changes in the left middle cerebral artery territory with an Alberta stroke program early CT score (ASPECTS) of 6. CT angiography of the brain showed a thrombus in the terminal segment of the left internal carotid artery (ICA). Thrombolysis was not performed because of the low ASPECT score on the CT brain. Mechanical thrombectomy was performed using a combination of aspiration and stent retriever, and partial recanalization was obtained (TICI 2a). A day later, the CT of the brain showed haemorrhagic transformation with an intraparenchymal haematoma centred in the left lentiform nucleus and her NIHSS was unchanged. At the same time, she developed rising fever and elevated CRP (145 mg/L). On day 4 of her admission, transthoracic echocardiogram showed vegetation on the aortic valve with associated severe aortic regurgitation. Left ventricular function was unimpaired. Blood cultures were positive for Gram-negative diplococcus, identified as Neisseria gonorrhoeae. She was therefore treated with ceftriaxone and azithromycin. After 3 months, her mRS was 3.
Case 2
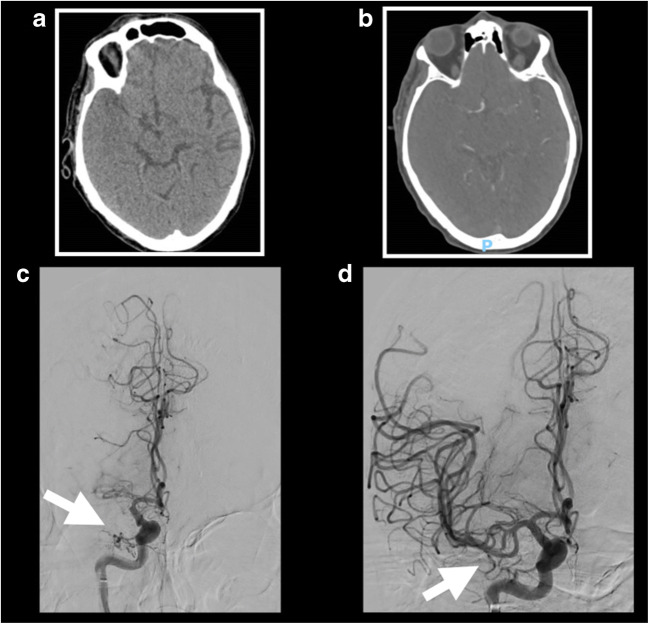
A 67-year-old male patient was transferred from a local hospital to our emergency department 3 h after sudden onset of left sided weakness, sensory disturbance and dysarthria. His NIHSS was 17. The patient was initially admitted for infective endocarditis of his native mitral valve and he was supposed to valve replacement surgery soon. Blood culture was positive for Gemella morbillorum. Computer tomography (CT) with CT angiography showed an occlusion of the M1 segment of the right middle cerebral artery. ASPECT score was 8 (Fig. 2a and b). Because of the evidence of endocarditis, intravenous thrombolysis was not considered an option. However, based on the large vessel occlusion, mechanical thrombectomy was performed under local anaesthetic with sedation. Endovascular thrombectomy was then performed with successful aspiration of the clot (TICI 3) (Fig. 2c and d). No immediate complications were recorded after the procedure. After 24 h, his NIHSS dropped to 13. Mitral valve replacement surgery was successfully performed 1 month later. At neurological follow-up after 3 months, the patient showed no neurological deficits (NIHSS 0) and mRS score of 0.
Fig. 2.
Case 2. a Axial CT head showing hyperdensity of the M1 segment of the right middle cerebral artery consistent with acute thrombus, and focal hypodensity of the right temporal lobe with loss of grey-white matter differentiation. b CT angiography demonstrating abrupt cutoff at the proximal M1 segment of the right middle cerebral artery. c Angiography confirmed occlusion of the right middle cerebral artery. d Post-angiographic images demonstrating the restoration of the flow in the right middle cerebral artery following successful thrombectomy
Case 3
A 65-year-old female patient with staphylococcal native mitral valve infective endocarditis had a witnessed onset of right-sided hemiparesis and aphasia (NIHSS 18). The patient was initially admitted in a local hospital for infective endocarditis and treated with teicoplanin and gentamicin. The patient was brought to our emergency department 3 h and 45 min after she developed her stroke symptoms. Her past medical history included also liver cirrhosis, type 2 diabetes, hypertension and breast cancer. CT angiography showed an occlusion of both M2 divisional branches. Intravenous thrombolysis was contraindicated because of the evidence of endocarditis. Mechanical thrombectomy was considered due to the large vessel occlusion. Despite several attempts at aspiration, the clot remained in situ with the final angiography demonstrating a subtotal occlusion of both the M2 divisional branches (TICI 1). After 24 h, her NIHSS was 21 and the CT showed no haemorrhagic transformation. At neurological follow-up after 3 months, her mRS score was 4.
Results
The database search yielded 431 relevant records published until January 2020. Nineteen articles fulfilled the eligibility criteria [8–26]. Three articles (16%) were case series while sixteen (84%) were single case report.
Synthesis of results
Table 1 shows the characteristics of patients with stroke and infective endocarditis treated with mechanical thrombectomy. The median age of the patients was 67 years old (IQR 32.75). For three patients, their age was not reported. Eleven patients were men (36.7%) while for four patients (13.3%), the gender was not documented. The median baseline NIHSS resulted to be 15 (IQR 7). The most common site of the large vessel occlusion was the M1 (56.7%). After the thrombectomy, nine patients (30%) had TICI score of 3 while the TICI score of 2b was obtained in four patients (13.3%). After the treatment, four patients (13.3%) experienced intracranial haemorrhage. After the procedure, the median NIHSS dropped to 2.5 (IQR 5.75) (Table 2).
After a follow-up of 90 days, seven patients were dead (mRS = 6) (23.3%) while fourteen (46.7%) were functionally independent (mRS ≤ 2) (Table 2) (Fig. 3). Of note, mRS score at 90 days was not available for seven patients.
Fig. 3.
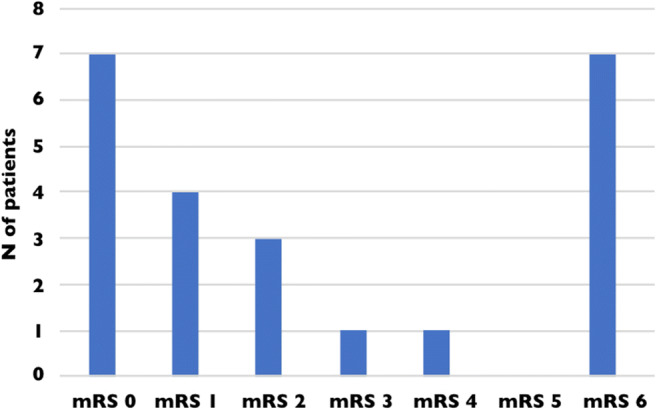
Distribution of the mRS at 90 days. mRS score at 90 days is not available for seven patients
Discussion
Our review described the clinical presentation, management and outcome of patients treated with mechanical thrombectomy for thrombotic stroke due to infective endocarditis. Although mechanical thrombectomy is not recognised as the standard of care for acute stroke secondary to infective endocarditis, this review provides evidence of consistent benefit for endovascular treatment on disability in these patients. Indeed, in our review, 36.7% of the patients with stroke due to infective endocarditis treated with mechanical thrombectomy had a 90-day mRS ≤ 1 while 46.7% of the patients had a mRS ≤ 2 at 90 days. Overall, almost half of the patients with stroke due to infective endocarditis treated with mechanical thrombectomy were functionally independent after 3 months. Many previous studies showed that the occurrence of neurologic complications during infective endocarditis were associated with an increased risk of mortality [1, 2, 4, 27, 28]. More specifically, Thuny et al. [29] demonstrated that the risk of death differed according to the type of cerebrovascular disease. Patients with silent brain infarcts or transient ischemic attack had a better prognosis, whereas large stroke resulted to be a strong predictor of mortality independently of the other prognostic factors. Moreover, the cause of death in these patients was a direct consequence of this neurologic event. One explanation of this finding is the fact that the absence of large brain injuries carried a better prognosis and allowed early surgery to be performed with a low operative risk. This result underlines the critical need of safe reperfusion therapy for acute ischemic stroke due to infective endocarditis for salvaging ischemic brain that is not already infarcted to avoid dramatic cerebral damages.
To date, treatment with intravenous alteplase is not recommended for patients with acute ischemic stroke and symptoms consistent with infective endocarditis because of the increased risk of intracranial haemorrhage [30]. Despite the fact that the fibrinolysis might promote reperfusion through cerebral vessels occluded by septic emboli, histopathological studies suggested that cerebral infarcts caused by septic emboli are particularly prone to haemorrhagic transformation as a result of septic arteritis with erosion of the arterial wall in the recipient vessel, with or without the formation of mycotic aneurysms. The use of mechanical thrombectomy for ischemic stroke with large vessel occlusion due to septic emboli has been documented in few patients in literature so far [28]. In our review, we found that only 13.3% of the patients with endocarditis-related acute stroke treated with thrombectomy suffered an intracranial haemorrhage after the treatment. Bettencourt et al. [28] showed that the use of intravenous alteplase alone or combined with mechanical thrombectomy was associated with a 4-fold increased risk of intracranial haemorrhage compared with mechanical thrombectomy alone. The severe bleeding complications associated with the use of intravenous alteplase may suggest that mechanical thrombectomy alone should be considered in selected patients with infective endocarditis if there is a documented large vessel occlusion.
The patterns of endocarditis-associated stroke observed in previous studies are very heterogenous. Previous studies, using conventional and DWI MRI [31] [32, 33], showed that patients with acute ischemic stroke and infective endocarditis can have a variety of ischemic lesions, including single cortical, territorial, disseminated punctate and disseminated small and large lesions in multiple vascular territories. Infarction of the middle cerebral artery territory resulted to be the most common anatomical lesion, with involvement of the middle cerebral artery tree [31]. In this review, 93.3% of the patients (28/30) treated with mechanical thrombectomy had a large vessel occlusion in the anterior circulation. Previous studies proved the value of thrombectomy in anterior circulation acute ischemic stroke within the first 6 h of symptom onset [34–36]. In MR CLEAN [34], 33% of patients achieved a good clinical outcome being functionally independent with thrombectomy versus 19% with medical therapy; in EXTEND-IA [35], the respective outcomes were 71% versus 40%; in SWIFT PRIME [36], they were 60% versus 35%. Recently, two recent multicentre randomized controlled trials of mechanical thrombectomy of the anterior circulation initiated at a later time windows of up to 16 h and 24 h from symptom onset have shown that endovascular therapy is safe and highly effective in carefully selected patients with advanced imaging in comparison with medical management alone [37, 38]. The results of our review suggest that the use of thrombectomy might be an efficient and safe treatment for patients with acute large vessel occlusion of the anterior circulation associated with endocarditis and might help improve outcome; however, age, time from symptom onset, clinical severity of stroke symptoms, pre-stroke level of functioning and anatomic location of the large vessel occlusion are the most important determinants of candidacy for mechanical thrombectomy in this cohort of patients.
This study has different limitations. In first instance, our search did not find any randomized controlled trials to investigate the efficacy and safety of the mechanical thrombectomy in patients with acute stroke due to infective endocarditis as our review was based only on single case reports or case series. Secondly, there were some data missing and this made difficult to compare the results. Finally, we draw our conclusions in favour of the use of thrombectomy based on a potential publication bias.
In conclusion, based on our review, the use of mechanical thrombectomy in patients with large vessel occlusion due to endocarditis-associated stroke should be considered on a case by case base as the safety has not well established yet. Further research on risk stratification is needed to drive clinician during the decision-making process.
Data availability
The data that support the findings of this study are available from the corresponding author, LD, upon reasonable request.
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
Not applicable
Statement of informed consent
Informed consent was obtained by the patients.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sotero FD, Rosário M, Fonseca AC, Ferro JM (2019) Neurological complications of infective endocarditis. Curr Neurol Neurosci Rep 19. 10.1007/s11910-019-0935-x [DOI] [PubMed]
- 2.Morris NA, Matiello M, Lyons JL, Samuels MA. Neurologic complications in infective endocarditis: identification, management, and impact on cardiac surgery. Neurohospitalist. 2014;4:213–222. doi: 10.1177/1941874414537077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snygg-Martin U, Gustafsson L, Rosengren L, Alsiö Å, Ackerholm P, Andersson R, Olaison L. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. 2008;47:23–30. doi: 10.1086/588663. [DOI] [PubMed] [Google Scholar]
- 4.Sonneville R, Mirabel M, Hajage D, Tubach F, Vignon P, Perez P, Lavoué S, Kouatchet A, Pajot O, Mekontso Dessap A, Tonnelier JM, Bollaert PE, Frat JP, Navellou JC, Hyvernat H, Hssain AA, Tabah A, Trouillet JL, Wolff M, ENDOcardite en REAnimation Study Group Neurologic complications and outcomes of infective endocarditis in critically ill patients: the ENDOcardite en REAnimation prospective multicenter study. Crit Care Med. 2011;39:1474–1481. doi: 10.1097/CCM.0b013e3182120b41. [DOI] [PubMed] [Google Scholar]
- 5.Masuda J, Yutani C, Waki R, Ogata J, Kuriyama Y, Yamaguchi T. Histopathological analysis of the mechanisms of intracranial hemorrhage complicating infective endocarditis. Stroke. 1992;23:843–850. doi: 10.1161/01.STR.23.6.843. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol. 2003;34:1923–1924. doi: 10.1161/01.str.0000082720.85129.0a. [DOI] [PubMed] [Google Scholar]
- 8.Sloane KL, Raymond SB, Rabinov JD, Singhal AB. Mechanical thrombectomy in stroke from infective endocarditis: case report and review. J Stroke Cerebrovasc Dis. 2020;29:104501. doi: 10.1016/j.jstrokecerebrovasdis.2019.104501. [DOI] [PubMed] [Google Scholar]
- 9.Distefano M, Calandrelli R, Arena V, Pedicelli A, Della Marca G, Pilato F. A puzzling case of cryptogenic stroke. J Stroke Cerebrovasc Dis. 2019;28:e33–e35. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim J-M, Jeon J-S, Kim Y-W, Kang D-H, Hwang Y-H, Kim Y-S. Forced arterial suction thrombectomy of septic embolic middle cerebral artery occlusion due to infective endocarditis: an illustrative case and review of the literature. Neurointervention. 2014;9:101–105. doi: 10.5469/neuroint.2014.9.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toeg HD, Al-Atassi T, Kalidindi N, Iancu D, Zamani D, Giaccone R, Masters RG. Endovascular treatment for cerebral septic embolic stroke. J Stroke Cerebrovasc Dis. 2014;23:e375–e377. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Akkoyunlu Y, Iraz M, Kocaman G, Ceylan B, Aydin C, Aslan T (2013) Abiotrophia defectiva endocarditis presenting with hemiplegia. Jundishapur J Microbiol 6. 10.5812/jjm.8907
- 13.Kang G, Yang TK, Choi JH, Heo ST. Effectiveness of mechanical embolectomy for septic embolus in the cerebral artery complicated with infective endocarditis. J Korean Med Sci. 2013;28:1244–1247. doi: 10.3346/jkms.2013.28.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dababneh H, Shushrutha Hedna V, Ford J, Taimeh Z, Peters K, Mocco J, Waters MF. Endovascular intervention for acute stroke due to infective endocarditis. Neurosurg Focus. 2012;32:E1. doi: 10.3171/2011.11.FOCUS11263. [DOI] [PubMed] [Google Scholar]
- 15.Kan P, Webb S, Siddiqui AH, Levy EI. First reported use of retrievable stent technology for removal of a large septic embolus in the middle cerebral artery. World Neurosurg. 2012;77:591.e1–591.e5. doi: 10.1016/j.wneu.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 16.Liang Infective endocarditis complicated by acute ischemic stroke from septic embolus: successful solitaire FR thrombectomy. Cardiol Res. 2012;3:277–280. doi: 10.4021/cr235e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker KA, Sampson JB, Skalabrin EJ, Majersik JJ. Clinical characteristics and thrombolytic outcomes of infective endocarditis-associated stroke. Neurohospitalist. 2012;2:87–91. doi: 10.1177/1941874412446199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bain MD, Hussain MS, Gonugunta V, Katzan I, Gupta R. Successful recanalization of a septic embolus with a balloon mounted stent after failed mechanical thrombectomy. J Neuroimaging. 2011;21:170–172. doi: 10.1111/j.1552-6569.2009.00457.x. [DOI] [PubMed] [Google Scholar]
- 19.Sgreccia A, Carità G, Coskun O, Di Maria F, Benamer H, Tisserand M, Scemama A, Rodesch G, Lapergue B, Consoli A (2019) Acute ischemic stroke treated with mechanical thrombectomy and fungal endocarditis: a case report and systematic review of the literature. J Neuroradiol. 10.1016/j.neurad.2019.03.003 [DOI] [PubMed]
- 20.Ambrosioni J, Urra X, Hernández-Meneses M, Almela M, Falces C, Tellez A, Quintana E, Fuster D, Sandoval E, Vidal B, Tolosana JM, Moreno A, Chamorro A, Miró JM, Hospital Clínic Infective Endocarditis Study Group. J M M, J A, Pericàs JM, A T, M H M, A M, de la Mària CG, Garcia-Gonzalez J, Marco F, M A, Vila J, E Q, E S, Paré JC, C F, Pereda D, Cartañá R, Ninot S, Azqueta M, Sitges M, B V, Pomar JL, Castella M, J M T, Ortiz J, Fita G, Rovira I, D F, Ramírez J, Brunet M, Soy D, Castro P, Llopis J. Mechanical thrombectomy for acute ischemic stroke secondary to infective endocarditis. Clin Infect Dis. 2018;66:1286–1289. doi: 10.1093/cid/cix1000. [DOI] [PubMed] [Google Scholar]
- 21.Bolognese M, von Hessling A, Müller M. Successful thrombectomy in endocarditis-related stroke: case report and review of the literature. Interv Neuroradiol. 2018;24:529–532. doi: 10.1177/1591019918774761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elodie O, Labeyrie PE, Aubry M, Cecile D, Roux S, Ferry T, Nighoghossian N. Whipple’s endocarditis diagnosed by thrombus analysis retrieved by successful mechanical thrombectomy. J Neurol Sci. 2019;400:42–43. doi: 10.1016/j.jns.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Nishino W, Tajima Y, Inoue T, Hayasaka M, Katsu B, Ebihara K, Kawauchi D, Kubota M, Suda S. Severe vasospasm of the middle cerebral artery after mechanical thrombectomy due to infective endocarditis: an autopsy case. J Stroke Cerebrovasc Dis. 2017;26:e186–e188. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Scharf EL, Chakraborty T, Rabinstein A, Miranpuri AS. Endovascular management of cerebral septic embolism: three recent cases and review of the literature. J Neurointerv Surg. 2017;9:463–465. doi: 10.1136/neurintsurg-2016-012792. [DOI] [PubMed] [Google Scholar]
- 25.Sveinsson O, Herrman L, Holmin S. Intra-arterial mechanical thrombectomy: an effective treatment for ischemic stroke caused by endocarditis. Case Rep Neurol. 2016;8:229–233. doi: 10.1159/000452213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladner TR, Davis BJ, He L, Kirshner HS, Froehler MT, Mocco J. Complex decision-making in stroke: preoperative mechanical thrombectomy of septic embolus for emergency cardiac valve surgery. BMJ Case Rep. 2014;2014:1–4. doi: 10.1136/bcr-2014-011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson DJ, Goldstein LB, Wilkinson WE, Corey GR, Cabell CH, Sanders LL, Sexton DJ. Stroke location, characterization, severity, and outcome in mitral vs aortic valve endocarditis. Neurology. 2003;61:1341–1346. doi: 10.1212/01.WNL.0000094359.47929.E4. [DOI] [PubMed] [Google Scholar]
- 28.Bettencourt S, Ferro JM. Acute ischemic stroke treatment in infective endocarditis: systematic review. J Stroke Cerebrovasc Dis. 2020;29:104598. doi: 10.1016/j.jstrokecerebrovasdis.2019.104598. [DOI] [PubMed] [Google Scholar]
- 29.Thuny F, Avierinos JF, Tribouilloy C, Giorgi R, Casalta JP, Milandre L, Brahim A, Nadji G, Riberi A, Collart F, Renard S, Raoult D, Habib G. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study. Eur Heart J. 2007;28:1155–1161. doi: 10.1093/eurheartj/ehm005. [DOI] [PubMed] [Google Scholar]
- 30.Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE, American Heart Association Stroke Council and Council on Epidemiology and Prevention Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke. Stroke. 2016;47:581–641. doi: 10.1161/str.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 31.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.STR.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Lee JY, Kim TH, Kim SC, Choi YH, Pai H, Choi WS. Imaging of the neurological complications of infective endocarditis. Neuroradiology. 1998;40:109–113. doi: 10.1007/s002340050549. [DOI] [PubMed] [Google Scholar]
- 33.Bakshi R, Wright PD, Kinkel PR, Bates VE, Mechtler LL, Kamran S, Pullicino PM, Sirotkin I, Kinkel WR. Cranial magnetic resonance imaging findings in bacterial endocarditis: the neuroimaging spectrum of septic brain embolization demonstrated in twelve patients. J Neuroimaging. 1999;9:78–84. doi: 10.1111/jon19999278. [DOI] [PubMed] [Google Scholar]
- 34.Berkhemer OA, Fransen PSS, Beumer D, van den Berg L, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen M, Staals J, Hofmeijer J, van Oostayen J, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk L, Kappelle LJ, Lo RH, van Dijk E, de Vries J, de Kort PL, van Rooij W, van den Berg J, van Hasselt B, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog H, Gerrits DG, van den Berg-Vos R, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam W, Roos YB, van der Lugt A, van Oostenbrugge R, Majoie CB, Dippel DW, MR CLEAN Investigators A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 35.Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 36.Saver JL, Goyal M, Bonafe A et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 10.1056/NEJMoa1415061 [DOI] [PubMed]
- 37.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, LD, upon reasonable request.