Abstract
Fibroblasts produce collagens and other proteins that form the bulk of the extracellular matrix (ECM) in connective tissues. Emerging data point to functional heterogeneity of fibroblasts. However, the lack of sub-type specific markers hinders our understanding of the different roles of fibroblasts in ECM biology, wound healing, diseases and aging. We have investigated the utility of the cell surface protein CD26 (dipeptidyl peptidase-4) to identify functionally distinct fibroblast subpopulations in human skin. Using flow cytometry and immunohistology, we find that CD26, in combination with the cell surface glycoprotein CD90, identifies a distinct subpopulation of cells which express relatively high levels of type I collagen (COL1A1), a hallmark of fibroblasts. Importantly, the population of CD26+ fibroblasts is selectively increased following wounding of human skin. These cells account for the majority of COL1A1 expression during the ECM remodeling phase of healing. The proportion of CD26+ fibroblasts in the skin of young and aged individuals is similar, indicating that loss of collagen production during aging does not involve selective reduction of CD26+ fibroblasts. In culture, the majority of freshly isolated CD26− fibroblasts gain expression of CD26+. Taken together, these data provide a foundation for targeting CD26+ fibroblasts to modulate wound healing in human skin.
INTRODUCTION
Fibroblasts are a diverse population of cells that produce the stromal extracellular matrix (ECM), which comprises the bulk of connective tissues in multiple organs. Identification of fibroblasts within tissues typically relies on a combination of their morphology, tissue location and expression of proteins that are related to the production of the ECM. Emerging evidence demonstrates fibroblast heterogeneity between different and within the same tissues. This heterogeneity extends to their origins and roles in a variety of physiological processes (Driskell et al., 2013, Driskell and Watt, 2015, Xie et al., 2018).
Several cell surface proteins have been used to identify fibroblasts in different tissues. However, these markers, when used alone or in combination, do not reliably identify fibroblast subpopulations in all tissues. For example, the cell-surface GPI-linked protein CD90 (Thy-1) has been used to identify a functionally distinct population of human fibroblasts in tissues such as the lung and liver (Dudas et al., 2007, Fries et al., 1994). However, reports show that most dermal fibroblasts express CD90, which limits its effectiveness at differentiating between dermal fibroblast subpopulations (Philippeos et al., 2018, Saalbach et al., 1997, Saalbach et al., 1998, Sorrell and Caplan, 2009). In support of this conclusion, Phillippeos et al. identified at least four dermal fibroblast subpopulations, which all expressed CD90+, through a combination of spatial and single-cell transcriptional profiling (Philippeos et al., 2018). In addition, CD90 also displays expression in cell types other than fibroblasts, such as activated hematopoietic cells, neuronal cells and activated endothelial cells (Kisselbach et al., 2009, Wetzel et al., 2004).
Fibroblasts of hematopoietic origin (fibrocytes) can be identified in the circulation by co-expression of collagen and the hematopoietic marker CD45 (Pilling et al., 2009). However, CD45 is rapidly down-regulated when fibrocytes transit from the circulation into tissue, hindering identification of fibrocytes in tissues. Fibrocytes and other fibroblast populations may also express CD34. However, while this marker can identify fibroblasts, it is also associated with hematopoietic and mesenchymal stem cells, reducing its ability to definitively identify fibroblast subpopulations (Diaz-Flores et al., 2014, Sidney et al., 2014). Definitive elucidation of fibroblast identity is required to examine functional heterogeneity of fibroblast subpopulations, which remains elusive due to the lack of reliable surface markers.
Identification of fibroblast subpopulations is of particular interest to the studies of cutaneous aging and wound healing, and is an active area of research. During aging, collagen production by dermal fibroblasts declines (Quan et al., 2010). This decline contributes to skin thinning, fragility and impairment of wound healing in the elderly. However, there is ambiguity as to whether this age-related reduction of collagen production is caused by alterations in the relative abundance of fibroblast subpopulations or other factors. Marsh et al. show that loss of neighboring fibroblasts in mouse skin does not trigger fibroblast migration or proliferation. Instead, fibroblast membranes extend to fill the space, which provides one explanation for how skin homeostasis could lead to loss of collagen production (Marsh et al., 2018). Salzer et al. propose that acquisition of adipogenic traits by dermal fibroblasts in mouse skin during aging could explain loss of ECM production (Millar, 2018, Salzer et al., 2018).
Reports indicate that fibroblast subpopulations may participate in distinct aspects of wound healing, which if lost during aging, could explain a gradual loss of collagen. For instance, fibroblasts in the lower dermis of mice may contribute higher levels of ECM during initial wound healing, while fibroblasts in the upper dermis may support re-epithelialization (Driskell et al., 2013). A better understanding of fibroblast subpopulations involved in the deposition of ECM during wound healing has the potential to improve recalcitrant wound healing, reduce scar formation, and improve our understanding of age-related changes in human skin.
Interestingly, the cell surface protein CD26 has recently been recognized to identify a fibroblast population in mice that produces the bulk of new collagen during development, healing of incisional wounds, and scarring (Rinkevich et al., 2015). CD26 possesses dipeptidyl-peptidase A activity and is expressed in a variety of immune cell types and endothelial cells in different tissues (Abbott et al., 1994, Klemann et al., 2016, Matheeussen et al., 2011). In a recent study investigating fibroblast heterogeneity, two major populations characterized by SFRP/CD26 and FMO1/LSP1 were found in human skin (Tabib et al., 2018). Both of these populations expressed CD34 and the SFRP/CD26 subpopulation was found to be present at a higher frequency in the dermis. However, it remains unclear whether CD26 can identify a functionally important fibroblast subpopulation in human skin that is disproportionally involved in aging or wound healing. To address these issues, we have investigated the ability of CD26 to identify dermal fibroblast subpopulations that contribute to collagen production during wound healing and aging in human skin.
RESULTS
CD26-positive fibroblasts are present in human dermis
We initially investigated the presence of CD26+ resident fibroblast subpopulations in human dermis by flow cytometry. Full thickness skin samples were digested with collagenase and cells were labeled with fluorescently tagged antibodies to CD26. We also labeled the cells with antibodies to cell surface marker proteins CD90, CD34 and CD45. CD90 and CD34 are expressed on fibroblasts as well as other cell types. In contrast, CD45 is expressed in bone-marrow-derived cells. We analyzed the cells by four-color flow cytometry for these cell surface markers.
We identified four populations of cells by staining for CD26 and CD90 (Figure 1A, Supplemental Figure 1 for contour plot). The largest population of cells (~60% of total cells) expressed high levels of CD26 and low levels of CD90 (CD26+/CD90− single positive). The next most abundant cell population (~20% of total cells) lacked expression of both CD26 and CD90 (CD26−/CD90− double negative). Approximately 10% of the total cells expressed CD90. The CD90+ cell population was nearly equally divided between CD26− cells (CD26−/CD90+ single positive) and CD26+ cells (CD26+/CD90+ double positive) .(Figure 1B). Almost all (98%) of the CD26+/CD90+ double positive cells were also positive for CD34 (data not shown), a protein expressed on mesenchymal stem and tissue progenitor cells. Since we were interested in resident dermal cells, we checked whether these populations expressed CD45. However, CD45 expression was negligible in CD26+ and CD90+ cells (data not shown).
Figure 1. Identification of CD26+ cell populations in human dermis.
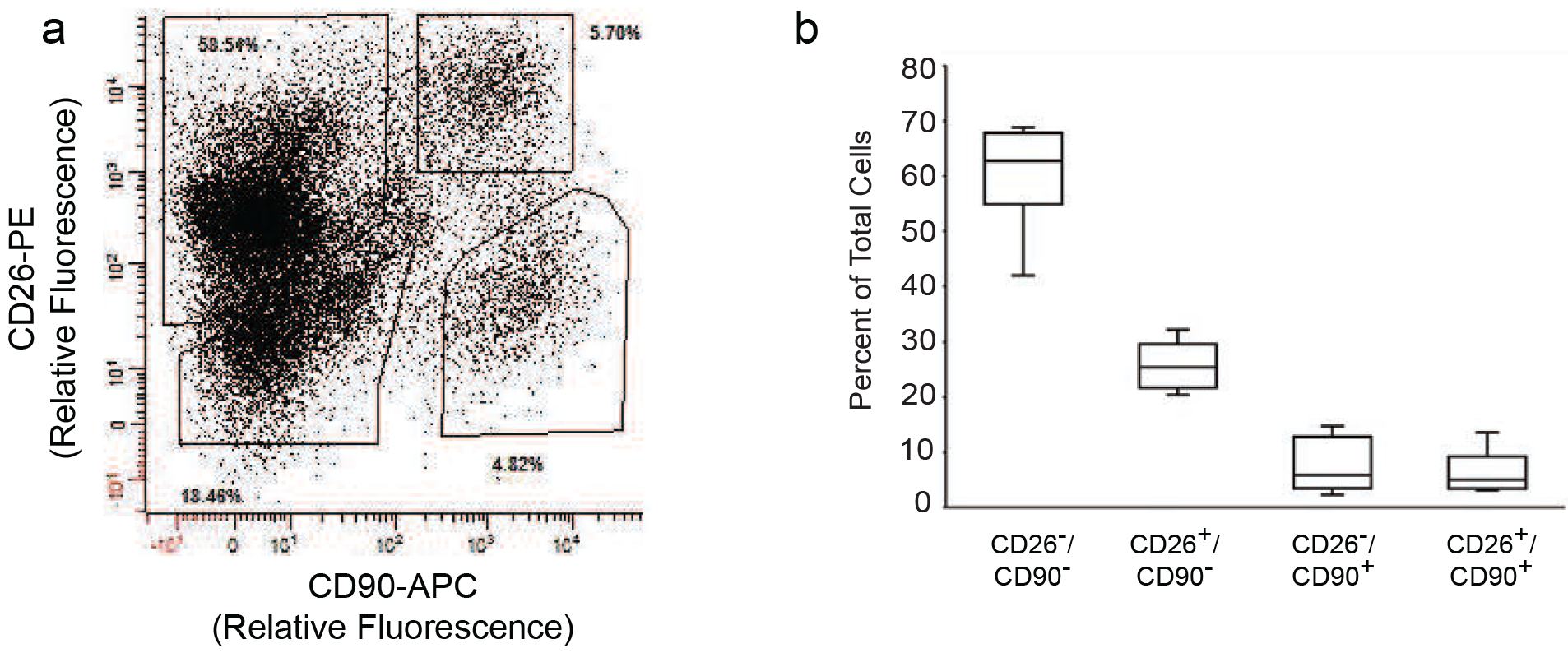
Dermal rich skin cell suspensions were prepared by collagenase digestion of adult human buttocks skin samples. Cells were labeled with fluorophore-conjugated antibodies to CD26, CD90, CD34 and CD45, followed by four-color flow cytometry analysis. A) Representative flow cytometry analysis showing distribution of cell populations based on expression of CD26 and CD90. The solid polygons depict gates that were used for sorting. Percentages of total cells within each gate are indicated. B) Quantitation of CD26 and CD90 cell populations in adult human dermis. Results are means+SEM, n=5 subjects.
We next quantified the hallmark of fibroblasts, type I collagen (COL1A1) gene expression, in the four populations of dermal cells (CD26−/CD90− double negative, CD26+/CD90− single positive, CD26−/CD90+ single positive and CD26+/CD90+ double positive). The cell populations were isolated by FACS and COL1A1 gene expression was determined by real-time PCR (Figure 2A). Both CD90+ cell populations (CD26−/CD90+ and CD26+/CD90+) had relatively high COL1A1 gene expression, while both CD90− cell populations (CD26−/CD90− and CD26+/CD90−) had little to non-detectable COL1A1 expression. The levels of COL1A1 gene expression were similar in both CD90+ cell populations (i.e. CD26− and CD26+). These data support the conclusion that CD90 is a valid fibroblast marker in human skin. Furthermore, the data reveal the presence of approximately two equal size subpopulations of CD90+ fibroblasts in human dermis. These two subpopulations are distinguished by expression of CD26.
Figure 2. CD26 identifies a population of collagen-expressing fibroblasts in adult human dermis.
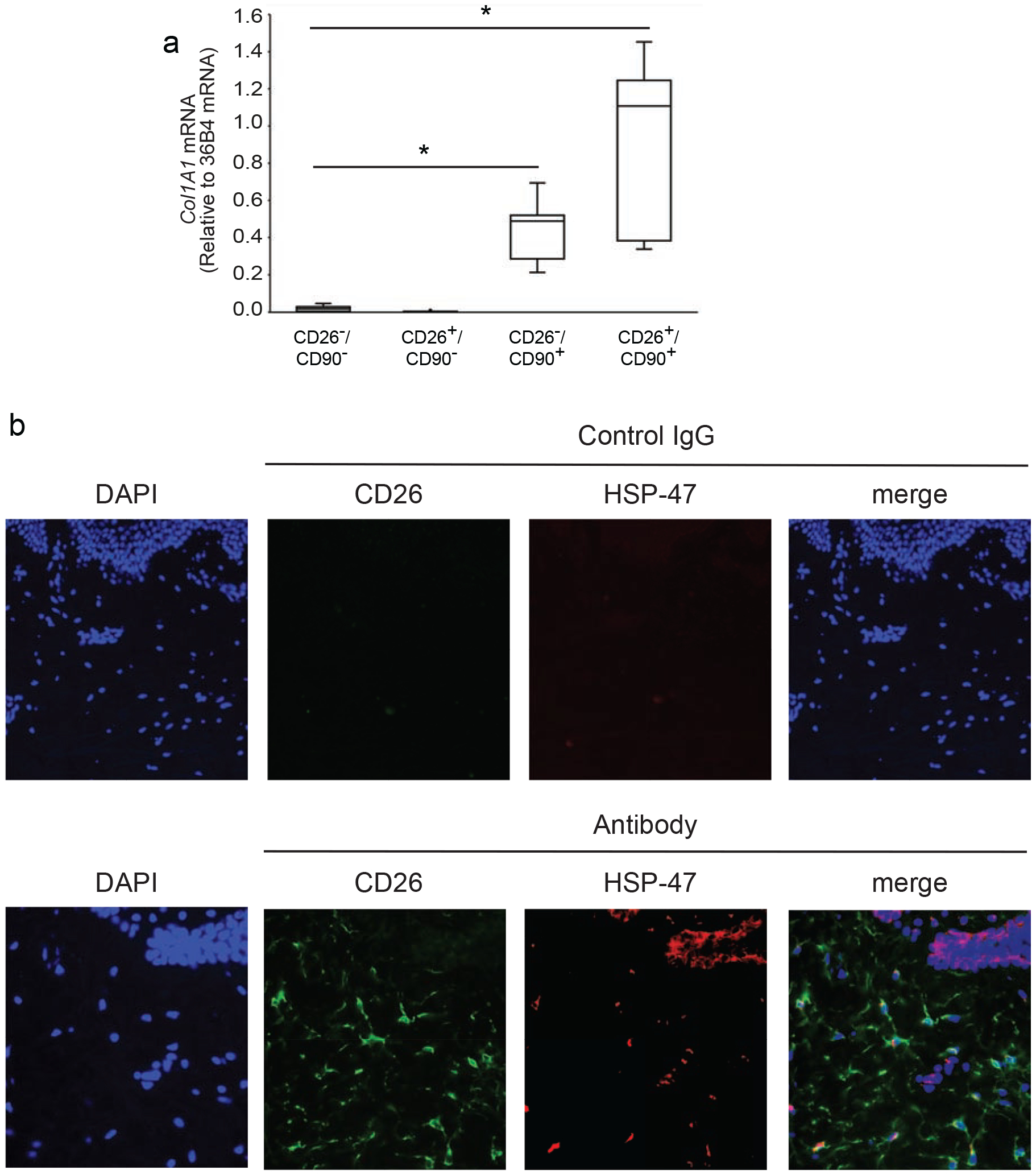
A) Dermal cells were prepared from buttocks skin by collagenase digestion and sorted by FACS, as described in the Figure 1 legend. Total RNA was prepared from each of the four indicated cell populations and type I collagen (COL1A1) gene expression was determined by real-time PCR. Data are normalized to internal control housekeeping gene 36B4. Results are means+SEM, n=5 biological, n=2 technical replicates: *p<0.05 vs CD26−/CD90− or CD26+/CD90+. B) Localization of CD26+ fibroblasts in the dermis of adult human skin. Paraformaldehyde-fixed human skin sections were double-immunostained for CD26 (green) and HSP-47 (red). HSP-47 is a chaperone for collagen and serves as a marker for collagen-producing cells. Nuclei were stained blue with DAPI. CD26+/HSP-47+ double-positive cells (closed arrows) and CD26+ single positive (open triangles) cells are observed in the dermis. In addition, HSP-47+ staining is observed in basal keratinocytes (open arrows), which express basement membrane collagen. Representative images from five experiments (40x objective). Scale bars are 50 microns.
Since reports have studied CD26 as a fibroblast marker in the mouse dermis (Rinkevich et al., 2015), we examined whether collagen expressing CD26 cells are expressed in human skin. Frozen skin sections were immunostained by double fluorescence for CD26 and heat shock protein-47 (HSP-47) (Figure 2B). HSP-47 is a collagen-specific chaperone that serves as a marker for collagen expressing cells (Kuroda and Tajima, 2004). CD26 and HSP-47 double positive cells with fibroblast-like morphology (Rittie, 2005) were found throughout the dermis, substantiating our finding that CD26-positive fibroblasts are present in human skin.
CD26-positive fibroblasts increase in number and produce elevated levels of collagen during wound healing
Production of new collagen by fibroblasts is a critical aspect of the later phases of the wound healing response. Therefore, we explored fibroblast subpopulations in wounded human skin. Wounds were created on buttocks skin by a fully ablative CO2 laser, which completely removes the epidermis and superficial dermis, as shown previously (Fisher and Rittié, 2018). The resulting partial thickness wound induces a well-characterized wound healing response (Orringer et al., 2004). We obtained skin samples from subject-matched non-wounded and wounded skin three weeks post wounding, which corresponds to the period of maximum COL1A1 production (Orringer et al., 2004). Dermal cells were labeled with antibodies to CD26, CD90, CD34 and CD45, followed by four-color flow cytometry analysis, as described above. Interestingly, wounding caused significant increases in both CD26+/CD90− and CD26+/CD90+ cell populations.
The proportion of CD26+/CD90+ double-positive fibroblasts significantly increased 2.2-fold compared to non-wounded skin (Figures 3A and 3B, Supplemental Figure 1 for contour plot), while the expression of CD34 did not change (data not shown). In contrast, the relative size of the CD26−/CD90+ single positive fibroblast population did not significantly change. These data indicate that the CD26+ fibroblast population in human skin is specifically enriched during wound healing.
Figure 3. Wounding induces significantly greater collagen gene expression in CD26+ than CD26− fibroblasts in adult human skin.
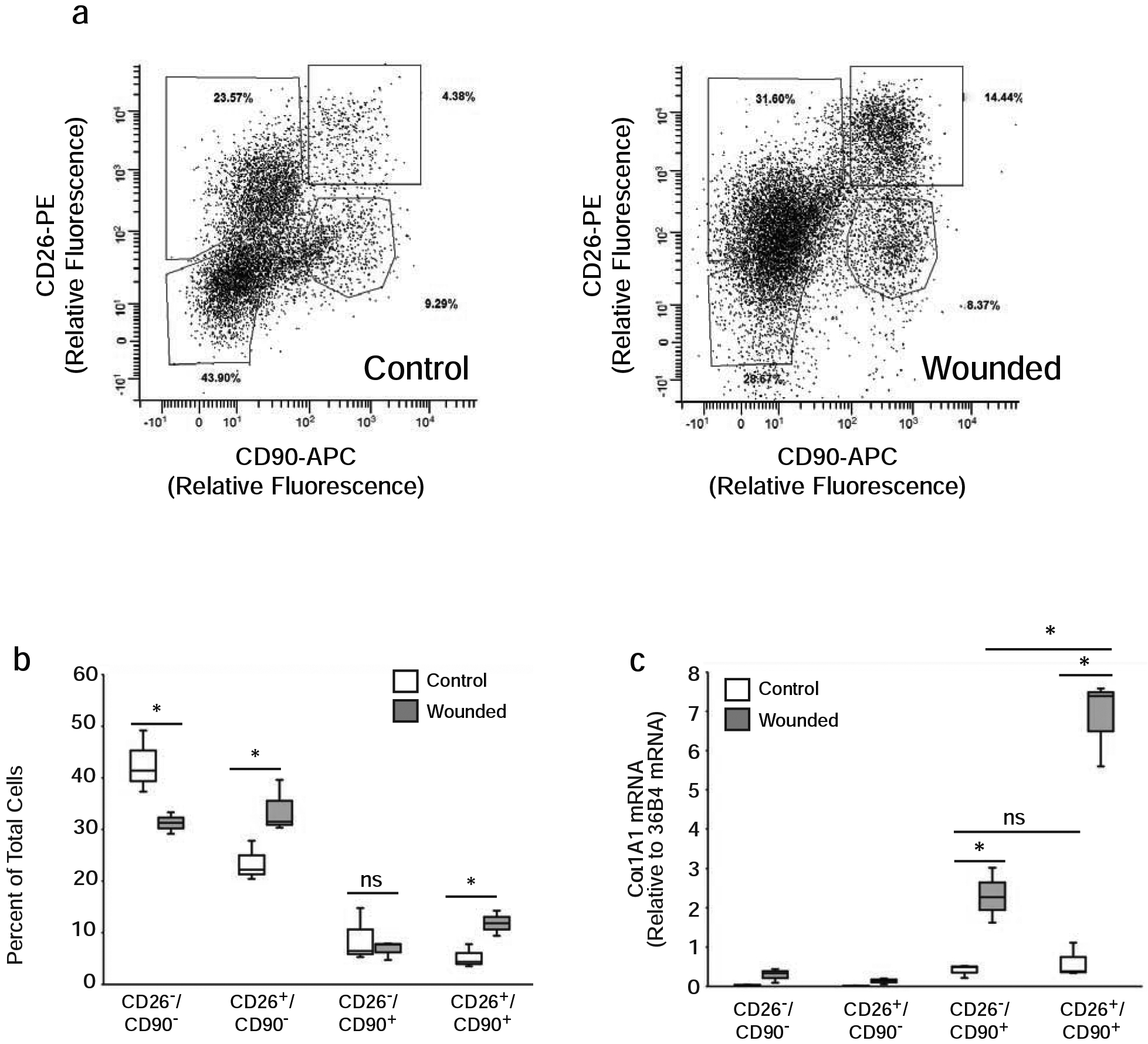
Partial thickness wounds were generated on buttocks skin by ablative CO2 laser treatment. A) Samples from mock-treated (Control) and CO2 laser-treated (Wounded) skin were obtained three weeks after wounding and dermal cells were analyzed by flow cytometry. 12,000 cells are displayed in each plot for comparison. B) Percentages of CD26/CD90 positive and negative dermal cells in control and wounded skin from five subjects. Results are means+SEM, *p<0.05. C) Three weeks after wounding dermal cells were sorted by FACS and COL1A1 gene expression was determined by real-time PCR, as described in Figure 2A legend. Results are means+SEM, n=5, *p<0.05.
We next investigated the impact of wounding on the levels of COL1A1 gene expression in the four populations (CD26−/CD90−, CD26+/CD90−, CD26−/CD90+ and CD26+/CD90+) of dermal cells. Subject-matched skin samples from non-wounded and wounded skin were obtained three weeks post wounding and dermal cell populations were isolated by FACS. Levels of COL1A1 gene expression in the isolated cell populations were determined by real-time PCR. Wounding induced significant elevations of COL1A1 gene expression in both CD26− (5.6-fold increase) and CD26+ (11.2-fold increase) CD90+ fibroblasts. In contrast, wounding did not alter COL1A1 gene expression in the CD90− cell populations (CD26−/CD90− and CD26+/CD90−) (Figure 3C). COL1A1 gene expression was near the limit of detection in both non-wounded and wounded skin in these cell populations. Importantly, in the CD90+ cell populations, wounding induced a significant 3-fold increase of COL1A1 gene expression in CD26+ fibroblasts compared to CD26− fibroblasts (Figure 3C). According to these data, CD26+ fibroblasts contribute the majority of increased COL1A1 expression during wound healing.
Aging does not alter the proportion of CD26+/ CD90+ fibroblasts
Dermal collagen production declines during aging (Varani et al., 2006). This decline is a major cause of skin thinning, fragility and poor wound healing often seen in the elderly. Given that the CD26+/CD90+ double positive fibroblast population is the major source of collagen production during wound healing, we examined whether this fibroblast subpopulation is preferentially reduced during aging. We analyzed dermal cell populations in skin samples from individuals 20 to 90 years of age by flow cytometry. Interestingly, the relative abundance of CD26−/CD90+ and CD26+/CD90+ double positive fibroblasts does not appear to change with age (Figure 4A). Indeed, there were few differences in the distribution of any of the dermal cell populations between aged and young skin (Supplemental Figure 2A). In addition, the progenitor cell marker CD34 was present on nearly all the CD26+/CD90+ double positive fibroblasts from all ages (Figure 4B, Supplemental Figure 2B). These data indicate that reduced collagen production in aged skin is not due to selective alterations in the proportions of CD26/CD90 fibroblast subpopulations.
Figure 4. Aging does not alter the relative abundance of CD26/CD90 dermal cells in human skin.
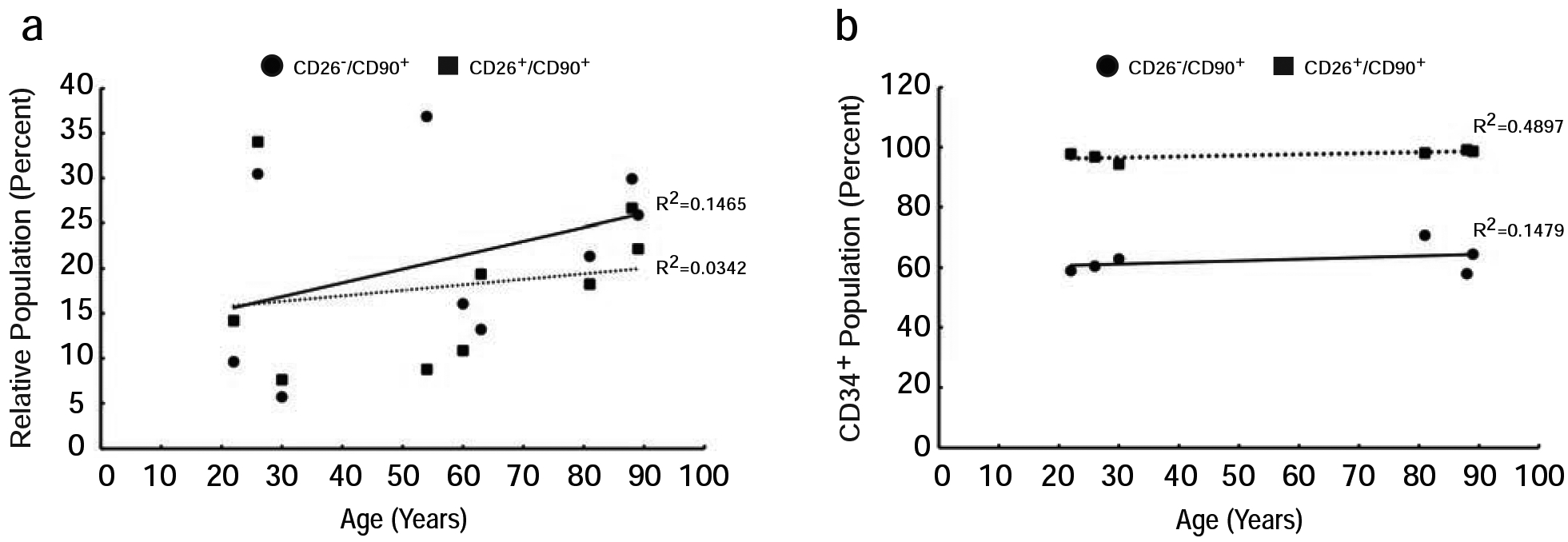
Dermal cells from buttocks skin of individuals ranging from 20 to 90 years old were analyzed by flow cytometry as described in the Figure 1 legend. A) Scatter plot of the percentages of CD26−/CD90+ (circles, solid line) and CD26+/CD90+ (squares, dotted line) dermal cell populations as a percent of viable single cells. n=9. B) Percentages of the CD26−/CD90+ (circles, solid line) and CD26+/CD90+ (squares, dotted line) dermal cell populations that were positive for CD34. N=6. Correlation coefficients for all cell populations vs. age were not statistically significant.
Most dermal fibroblasts express CD26 in primary culture
The above data demonstrate that CD90+ fibroblasts produce the majority of collagen in dermal fibroblasts. The CD90+ fibroblasts can be divided into two nearly equal populations based on expression of CD26. We next investigated the behavior of the CD26− and CD26+ fibroblast populations in primary culture. We initially performed flow cytometry analysis of primary fibroblast cultures which had undergone three passages since isolation from adult human skin (approximately 9 population doublings). It is of interest that these fibroblasts were almost entirely CD26+ (CD26+/CD90+ double positive) (Figure 5A, Supplemental Figure 1 for contour plot). Lack of CD26− fibroblasts in culture could result from failure of this subpopulation to survive and/or convert from CD26− to CD26+ fibroblasts in culture. To investigate these possibilities, freshly isolated dermal cells were placed in culture for three days to enrich for adherent cells (i.e. mostly fibroblasts) and subsequently separated by FACS based on CD26 and CD90 expression. As expected, the majority (64.5%) of total cells were CD90+, and 49.6% were CD26+ (Figure 5B, Supplemental Figure 1 for contour plot). Of the CD90+ cells, 38.1% were CD26− and 61.9% were CD26+. The sorted cells from this experiment were immediately cultured in similar numbers (105) of CD26− and CD26+ fibroblasts, and re-analyzed by flow cytometry after 4, 7, and 14 days.
Figure 5. Primary dermal fibroblasts express CD26 in culture.
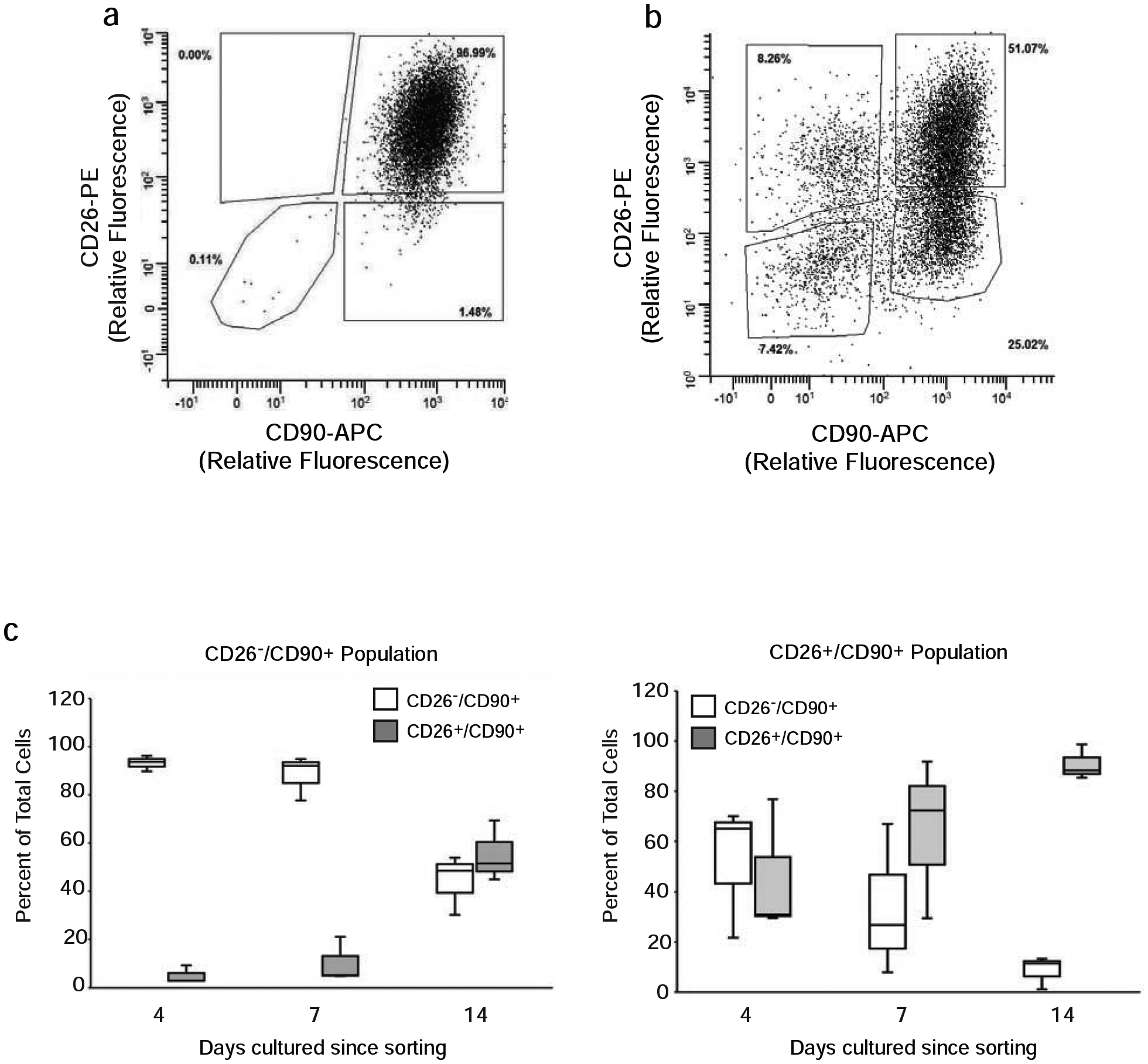
A) Dermal cells from buttocks skin were grown in culture through three passages and then analyzed by flow cytometry as described in the Figure 1 legend. Results are representative of five subjects. B) Freshly isolated dermal cells from buttocks skin were placed in culture for three days, and adherent cells were analyzed and sorted by FACS, as described in Figure 2 legend. Results are representative of five subjects. C) Following FACS sorting as in (B), 105 CD26− and CD26+ fibroblasts (CD90+) were separately cultured. Cultures were analyzed for CD26 and CD90, at the indicated times in culture by flow cytometry. Graphs are the average of five different subjects.
During the first four days in culture, both CD26− and CD26+ fibroblasts attached to the culture plates and began to proliferate. The cultures continued to proliferate at similar rates and reached confluency after 14 days. After 4 days of culture, the composition of the CD26− cultures remained essentially unchanged, with greater than 95% of cells being CD26−/CD90+. During the next 10 days in culture, the cells proliferated and the proportion of CD26+ fibroblasts significantly increased from less than 5% to 60% (Figure 5C, left panel). Four days after sorting and plating, the CD26+ cultures contained approximately equal numbers of CD26− and CD26+ fibroblasts. During the subsequent 10 days in culture, the proportion of CD26+ fibroblasts increased to 95% (Figure 5C, right panel). These data indicate that CD26− fibroblasts proliferate and become mostly CD26+ fibroblasts in primary culture.
DISCUSSION
In this report, we have characterized the presence and utility of cell surface protein CD26 to improve our understanding of fibroblast subpopulations in human skin. CD26, also named dipeptidyl peptidase 4 (DPP4), is an intrinsic membrane glycoprotein with serine exopeptidase activity, which preferably cleaves dipeptides from proteins that have proline or alanine residues in the NH2-terminal region. It plays diverse roles in a variety of cell types and processes including T-cell activation, incretin hormone signaling and fibrosis (Dang et al., 1990, Drucker, 2006, Mah et al., 2017, Mentlein et al., 1993). It functions via binding to ECM and cell surface proteins, such as caveolin, as well as through its peptidase activity (Gorrell et al., 2001), which plays an important role in regulating the activities of several chemokines (Metzemaekers et al., 2016). Inhibition of its peptidase activity appears to decrease its fibrogenic role (Kaji et al., 2014, Min et al., 2014, Rinkevich et al., 2015, Xin et al., 2017). CD26 is most highly expressed in the lower gastrointestinal tract, while expression in human skin is relatively low (Fagerberg et al., 2014).
CD90 is a GPI-anchored glycoprotein, originally identified in thymocyte cells and termed Thy-1. It is considered a marker for bone marrow-derived stem cells and is expressed in a variety of immune cells and other cell types including neurons, epithelial cells and fibroblasts. In humans, expression of CD90 is highest in brain, kidney and bladder (Fagerberg et al., 2014). In comparison to these organs, CD90 expression in skin is relatively low (Fagerberg et al., 2014). We found that CD90+ cells represent approximately 10–15% of all cells that are recovered by collagenase digestion of full thickness human dermis. We also found that the CD90+ cell population accounts for the vast majority of COL1A1 gene expression. The CD90+ cell population displays typical fibroblast morphology and expresses high levels of COL1A1 in culture. These data indicate that CD90 is a useful marker of human dermal fibroblasts, consistent with other reports (Lupatov et al., 2015, Vaculik et al., 2012).
In mice, CD90 expression has been reported on thymocytes, T-cells, neurons, myoblasts, fibroblasts, bone marrow stem cells, and epidermal cells (Saalbach et al., 1999). In contrast, human CD90 expression has only been shown on a subset of CD34+ blood stem cells, neuronal cells, and fibroblasts, despite wide expression in mice (Haeryfar and Hoskin, 2004, Ishizu et al., 1997, Leyton et al., 2001, Romero et al., 1997, Saalbach et al., 1998, Saalbach et al., 1999). In this study, we found that approximately half of the CD90+ fibroblast population in human skin expresses CD26. Our reported values for the relative sizes of the CD90 positive fibroblast populations are slightly inflated due to the presence of immune cells, endothelial cells, and erythrocyte precursors. To address this issue, we quantified the levels of these cell types in the CD90+ cell populations, by multi-color flow cytometry. We found that together the levels of these cells account for less than 5% of the CD90+ populations (Supplemental Figure 3). Therefore, the CD90+ fibroblast populations in dermal cell suspensions are largely free from the presence of hematopoietic and endovascular cells.
Immunohistology of human skin revealed abundant dermal cells with fibroblast morphology that co-express CD26 and the collagen chaperone HSP-47. Thus, CD26+ fibroblasts comprise a substantial subpopulation of fibroblasts in adult human skin. Interestingly, essentially all CD26+ fibroblasts co-expressed CD34. CD34+ stromal cells have been reported to serve as a source of mesenchymal cells (Diaz-Flores et al., 2014). These findings suggest that CD26+ dermal fibroblasts may possess some properties associated with progenitor or mesenchymal stem cells.
In normal human skin, the CD26−/CD90+ single positive and CD26+/CD90+ double positive fibroblasts expressed similar levels of COL1A1 mRNA. In contrast, following wounding, CD26+ fibroblasts displayed significantly greater increases in COL1A1 gene expression than CD26− fibroblasts (normalized to cell number). In addition, the relative size of the CD26+ fibroblast population doubled following wounding. Taking into account both increases in population size and levels of expression, the CD26+ fibroblast population accounted for the majority (approximately 85%) of COL1A1 gene expression at three weeks post wounding, in human skin. Preferential enhancement of collagen production by CD26+ fibroblasts after wounding has also been observed in mice (Rinkevich et al., 2015) and in a red Duroc pig model (Mah et al., 2017). Importantly, neither of these reports describe CD26 expression in wounded human skin. The primary focus of Mah et al. was to contrast the relative CD26 expression and response to wounding between skin and gingiva, in a red Duroc pig model. Our study describes CD26 expression in human skin, in response to wounding and aging. Furthermore, while Mah et al. report that an increased abundance of CD26 expression in pig skin is associated with an increase in collagen expression, it is implied that the entire CD26+ population is responsible for this response. In our study, we have directly shown that, both CD26+/CD90+ fibroblasts and CD26−/CD90+ fibroblasts significantly contribute to collagen production in wounded human skin.
Furthermore, in contrast to the CD26−/CD90+ cell population, the CD26+/CD90− cell population expresses negligible collagen. Thus, we demonstrate clear functional difference between CD26 positive cell populations in the wound response, in human skin. Furthermore, while CD26+ cells were shown by others to contribute to the wound healing response, we found that CD26+/CD90− cells that lack collagen production comprise a significant portion of the CD26+ population. Our findings have therefore revealed that a subpopulation of CD26+ cells are responsible for the bulk of wound healing. Importantly, these data demonstrate the importance of investigating functional subpopulations of CD26+ cells in the study of wound healing.
Selective increases in the CD26+ fibroblast population after wounding, with negligible change in the size of the CD26− fibroblast population, could result from conversion of CD26− fibroblasts to CD26+ fibroblasts and/or selective expansion of the CD26+ fibroblast population by proliferation. To address this issue, we examined the properties of isolated CD26− and CD26+ fibroblasts in culture. Both cell populations proliferated at similar rates and the CD26− population became largely CD26+ within two weeks in culture. These data suggest that expansion of the CD26+ fibroblast population after wounding may result from both conversion of CD26− fibroblasts to CD26+ fibroblasts as well as proliferation of CD26+ fibroblasts.
Dermal fibroblasts in human skin are responsible for the production and turnover of the dermal ECM. These critical functions are regulated by the dermal ECM microenvironment via feedback mechanisms involving integrin-mediated adhesion of fibroblasts to collagen fibrils, other ECM proteins and mechano-sensing signaling pathways. During aging, the dermal microenvironment undergoes deleterious alterations due to fragmentation of dermal collagen fibrils and reduction of collagen production (Mah et al., 2017, Varani et al., 2006). This reduction manifests as reduced dermal mass, skin fragility and weakened wound healing in the elderly. Based on our finding that CD26+ fibroblasts generate the majority of collagen during wound healing, we investigated whether aging results in preferential loss of this fibroblast subset. Our results indicate that the proportion of CD26+ fibroblasts in aged and young skin are similar, although small differences may exist. These data suggest that age-related changes of the ECM microenvironment, rather than a disproportionate reduction of CD26+ fibroblasts, are largely responsible for the reduced collagen production observed in fibroblasts during aging. This conclusion is consistent with our previous observation that fibroblasts in aged skin produce abundant collagen in response to dermal filler, which enhances structural support within the dermis (Quan et al., 2013).
As mentioned above, CD26 possesses serine exopeptidase activity. CD26 breaks down incretins, which act to lower blood glucose levels after eating (Kim and Egan, 2008). Inhibition of CD26 activity increases the half-life of incretins and thereby improves glucose homeostasis. CD26 inhibitors have been used clinically for the treatment of type II diabetes (Kim et al., 2015). More recently, activities of CD26+ cells that relate to tissue repair and fibrosis have been recognized (Kaji et al., 2014, Min et al., 2014, Rinkevich et al., 2015, Xin et al., 2017). This recognition has led to investigations into the efficacy of CD26 inhibitors for the improvement of wound healing in diabetic skin. Studies of CD26 inhibitors in diabetic mouse models (Schurmann et al., 2012) and humans (Long et al., 2018, Marfella et al., 2012) have shown significantly improved healing and reduced scarring, in part by promoting migration and epithelial-mesenchymal transition of keratinocytes. The knowledge gained in this report, concerning the role of CD26+ fibroblasts in human wound healing, has the potential to reveal novel insights into how CD26 inhibitors lead to improved healing in the clinic.
MATERIALS AND METHODS
Human subjects and tissue procurement
All procedures involving human subjects were approved by the University of Michigan Institutional Review Board and all subjects provided written informed consent. Subjects were recruited from the Department of Dermatology, University of Michigan. Skin samples from sun-protected buttocks skin were obtained by full-thickness punch biopsy (4 – 6 mm diameter) under local anesthesia (1% lidocaine), as previously described (Rittie et al., 2006).
Fibroblast cultures and flow cytometry
Skin samples were cut into 1 – 2 mm pieces with scissors and treated with 0.5% bacterial collagenase (collagenase-A, Worthington Biochemical Corp) at 37°C overnight to release dermal cells from the ECM. Epidermis and appendages were removed from the collagenase-treated tissue by filtration through 110-micron nylon mesh (03–110/47, ELKO Filtering Co.). The resulting dermal rich skin cell suspensions were used for generation of fibroblast cultures or flow cytometry/FACS.
For culture, dermal rich skin cell suspensions were pelleted by centrifugation, resuspended in culture medium (MEM-α Glutamax + 10% FBS with Pen/Strep and Amphotericin B) and transferred to 60mm tissue culture plates. After two days, non-adherent cells were washed away and adherent cells, which are mostly fibroblasts (Rittie, 2005), were grown to 70% confluency and expanded by serial passage.
For flow cytometry/FACS, isolated dermal cells were washed in HBSS before red blood cell lysis according to manufacturer’s instructions (00-4300-54, ThermoFisher-eBioscience). Cells were washed with buffer (PBS+2% FBS) and incubated with relevant antibodies (see below) on ice for 1 hour. Cells were then washed and analyzed by flow cytometry or FACS. All flow cytometry/FACS experiments were gated using forward scatter vs side scatter, double discrimination using forward scatter area vs forward scatter height then a second pass of double discrimination using side scatter area vs side scatter height. DAPI or 7AAD positive cells were excluded using DAPI/7AAD vs side scatter area. Experiments were then performed using DAPI/7AAD negative single cell isolates. Single stain APC/PE controls and isotype controls are displayed in Supplemental Figure 4. Staining for CD45 was negligible in CD26+ and CD90+ cells and was checked as it marks bone-marrow derived cells not removed with red blood cell lysis. Additionally, the CD90+ cells showed very limited expression of markers (Korosec et al., 2019) for erythrocyte precursors (CD235), bone marrow-derived cells (CD45), and endothelial cells (CD31) (Supplemental Figure 3). Flow cytometry was done using a BD LSRII cytometer. FACS analyses were performed using Synergy Head and Sony MA900 FACS sorters with the help of the University of Michigan Biomedical Research Core Facilities Flow Cytometry Core. Winlist 3D was used to analyze and compile data shown. At least 10,000 events were recorded per sample, although up to 50,000 events were recorded for samples with small subpopulations. Unless otherwise indicated, percentages of DAPI/7AAD negative single cells were compared in figures.
qPCR
Total RNA was extracted from isolated fibroblast populations directly after sorting. Cells were centrifuged and lysed in FACS tubes before extraction with RNeasy mini kit (Qiagen), according to manufacturer instructions. cDNA was made from 100ng of total RNA using the standard protocol of TaqMan reverse transcription kit (Applied Biosystems). qPCR was run using SYBR green on an ABI 7300 Real-Time PCR System (Applied Biosystems). Unless otherwise noted, five biological replicates and two technical replicates were used.
Antibodies
Flow cytometry of dermal cells was done using CD26-PE (340423, BD Biosciences, and 302705, BioLegend), CD90-APC (130-095-402, Miltenyi Biotech, and A15726, Thermo Fisher Scientific), CD34-FITC (8011-0349-120, ThermoFisher-eBioscience), CD45-APC Cy7 (304014, Biolegend), CD45 BV421 (304031, Biolegend), isotype-PE (558595, BD Bioscience), CD31-FITC (11-0319-42, ThermoFisher-eBioscience), CD235-PE Cy7 (306619, Biolegend), isotype-APC, isotype-FITC (400110, Biolegend) and isotype-APC Cy-7 (557873, BD Biosciences). Antibodies used for immunostaining were CD26 (AF1180, R&D Systems) and HSP-47 (ADISPA-470-D, Enzo Life Sciences).
Immunofluorescence
Immunofluorescence was performed on frozen sections (10μm thick) from buttocks skin samples. Skin samples, which were obtained as described above, were embedded in Tissue-Tek OCT compound (VWR), snap frozen in liquid nitrogen and stored at −80°C until processing. Skin samples were sectioned by cryostat microtome, fixed in paraformaldehyde, incubated in primary antibodies for 3 hours at room temperature or overnight at 4°C degrees for CD26, followed by incubation with secondary antibodies at room temperature for 1 hour.
CO2 laser treatment
CO2 laser treatment was performed as previously described (Orringer et al., 2012, Rittie et al., 2016). Briefly, buttocks skin was locally anesthetized by injection of 1% lidocaine. Partial-thickness, standard sized (6 mm square) wounds were made using three passes of a fractional CO2 laser (UltraPulse Encore, Lumenis) used in continuous wave mode and set at 100mJ, 60 W, with settings of 3/5/6. Wounds were gently rinsed with water and covered with semipermeable dressing (Tegaderm, 3M). Full-thickness punch biopsies (4 – 6 mm diameter) were taken under local anesthesia (1% lidocaine) from wounded and adjacent non-wounded skin three weeks after laser treatment. Four 6 mm diameter skin samples, 1 – 2 cm apart, were taken from the same individual. No surrounding uninjured skin was included in wounded samples.
Statistics
Data are presented as means ± SEM. Comparisons between treatment groups were assessed using the paired t-test. An overall α-level of 0.05 was used to determine statistical significance, and all tests were two-sided. Data were analyzed using SAS v9.2 (SAS Institute, Cary, NC). Analysis of variance and Tukey’s test were used to analyze the significance of differences between groups of samples.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Suzan Rehbine and Bethany Ruffino for procurement of tissue, Diane Fiolek for administrative and graphic assistance, as well as services of the Flow Cytometry Core, University of Michigan Medical School, which is supported by the National Cancer Institute of the National Institutes of Health under award number P30CA046592. The authors thank Joel Maust for providing writing/editorial support. This research was supported by the Department of Dermatology, University of Michigan.
Funding sources: Training grant T32AR07197
Abbreviations used:
- ECM
Extracellular matrix
- COL1A1
type I collagen
- HSP-47
heat shock protein-47
- DPP4
CD26, also named dipeptidyl peptidase 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement: No datasets were generated or analyzed during the current study.
CONFLICT OF INTEREST
The authors state no conflict of interest
REFERENCES
- Abbott CA, Baker E, Sutherland GR, McCaughan GW. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics 1994;40(5):331–8. [DOI] [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Deusch K, Schlossman SF, Morimoto C. Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. The Journal of Immunology 1990;144(11):4092–100. [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Garcia MP, Saez FJ, Diaz-Flores L Jr., Valladares F, et al. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol 2014;29(7):831–70. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013;504(7479):277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends in cell biology 2015;25(2):92–9. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell metabolism 2006;3(3):153–65. [DOI] [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res 2007;329(3):503–14. [DOI] [PubMed] [Google Scholar]
- Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 2014;13(2):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Rittié L. Restoration of the basement membrane after wounding: a hallmark of young human skin altered with aging. Journal of cell communication and signaling 2018;12(1):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol 1994;72(3):283–92. [DOI] [PubMed] [Google Scholar]
- Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol 2001;54(3):249–64. [DOI] [PubMed] [Google Scholar]
- Haeryfar SMM, Hoskin DW. Thy-1: More than a Mouse Pan-T Cell Marker. The Journal of Immunology 2004;173(6):3581–8. [DOI] [PubMed] [Google Scholar]
- Ishizu A, Ishikura H, Nakamaru Y, Kikuchi K, Koike T, Yoshiki T. Interleukin-1alpha regulates Thy-1 expression on rat vascular endothelial cells. Microvascular research 1997;53(1):73–8. [DOI] [PubMed] [Google Scholar]
- Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Douhara A, et al. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol 2014;49(3):481–91. [DOI] [PubMed] [Google Scholar]
- Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis 2015;74(11):1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008;60(4):470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselbach L, Merges M, Bossie A, Boyd A. CD90 Expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology 2009;59(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemann C, Wagner L, Stephan M, von Horsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin Exp Immunol 2016;185(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosec A, Frech S, Lichtenberger BM. Isolation of Papillary and Reticular Fibroblasts from Human Skin by Fluorescence-activated Cell Sorting. Journal of visualized experiments : JoVE 2019(147). [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tajima S. HSP47 is a useful marker for skin fibroblasts in formalin-fixed, paraffin-embedded tissue specimens. J Cutan Pathol 2004;31(3):241–6. [DOI] [PubMed] [Google Scholar]
- Leyton L, Schneider P, Labra CV, Ruegg C, Hetz CA, Quest AF, et al. Thy-1 binds to integrin beta(3) on astrocytes and triggers formation of focal contact sites. Current biology : CB 2001;11(13):1028–38. [DOI] [PubMed] [Google Scholar]
- Long M, Cai L, Li W, Zhang L, Guo S, Zhang R, et al. DPP-4 Inhibitors Improve Diabetic Wound Healing via Direct and Indirect Promotion of Epithelial-Mesenchymal Transition and Reduction of Scarring. Diabetes 2018;67(3):518–31. [DOI] [PubMed] [Google Scholar]
- Lupatov AY, Vdovin AS, Vakhrushev IV, Poltavtseva RA, Yarygin KN. Comparative analysis of the expression of surface markers on fibroblasts and fibroblast-like cells isolated from different human tissues. Bull Exp Biol Med 2015;158(4):537–43. [DOI] [PubMed] [Google Scholar]
- Mah W, Jiang G, Olver D, Gallant-Behm C, Wiebe C, Hart DA, et al. Elevated CD26 Expression by Skin Fibroblasts Distinguishes a Profibrotic Phenotype Involved in Scar Formation Compared to Gingival Fibroblasts. The American journal of pathology 2017;187(8):1717–35. [DOI] [PubMed] [Google Scholar]
- Marfella R, Sasso FC, Rizzo MR, Paolisso P, Barbieri M, Padovano V, et al. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diabetes Res 2012;2012:892706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh E, Gonzalez DG, Lathrop EA, Boucher J, Greco V. Positional Stability and Membrane Occupancy Define Skin Fibroblast Homeostasis In Vivo. Cell 2018;175(6):1620–33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheeussen V, Baerts L, De Meyer G, De Keulenaer G, Van der Veken P, Augustyns K, et al. Expression and spatial heterogeneity of dipeptidyl peptidases in endothelial cells of conduct vessels and capillaries. Biol Chem 2011;392(3):189–98. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. European Journal of Biochemistry 1993;214(3):829–35. [DOI] [PubMed] [Google Scholar]
- Metzemaekers M, Van Damme J, Mortier A, Proost P. Regulation of Chemokine Activity - A Focus on the Role of Dipeptidyl Peptidase IV/CD26. Front Immunol 2016;7:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE. Revitalizing Aging Skin through Diet. Cell 2018;175(6):1461–3. [DOI] [PubMed] [Google Scholar]
- Min HS, Kim JE, Lee MH, Song HK, Kang YS, Lee MJ, et al. Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction. Lab Invest 2014;94(6):598–607. [DOI] [PubMed] [Google Scholar]
- Orringer JS, Kang S, Johnson TM, Karimipour DJ, Hamilton T, Hammerberg C, et al. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Archives of dermatology 2004;140(11):1326–32. [DOI] [PubMed] [Google Scholar]
- Orringer JS, Sachs DL, Shao Y, Hammerberg C, Cui Y, Voorhees JJ, et al. Direct quantitative comparison of molecular responses in photodamaged human skin to fractionated and fully ablative carbon dioxide laser resurfacing. Dermatol Surg 2012;38(10):1668–77. [DOI] [PubMed] [Google Scholar]
- Philippeos C, Telerman SB, Oules B, Pisco AO, Shaw TJ, Elgueta R, et al. Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. The Journal of investigative dermatology 2018;138(4):811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 2009;4(10):e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Shao Y, He T, Voorhees JJ, Fisher GJ. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. The Journal of investigative dermatology 2010;130(2):415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Wang F, Shao Y, Rittie L, Xia W, Orringer JS, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. The Journal of investigative dermatology 2013;133(3):658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science (New York, NY) 2015;348(6232):aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittie L, Farr EA, Orringer JS, Voorhees JJ, Fisher GJ. Reduced cell cohesiveness of outgrowths from eccrine sweat glands delays wound closure in elderly skin. Aging Cell 2016;15(5):842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittie L, Fisher GJ. Isolation and Culture of Skin Fibroblasts In: Varga J, Brenner DA, Phan SH, editor. Fibrosis Research: Methods and Protocols. Methods in Molecular Medicine. 117 Totowa, New Jersey: Humana Press; 2005. [DOI] [PubMed] [Google Scholar]
- Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. The Journal of investigative dermatology 2006;126(4):732–9. [DOI] [PubMed] [Google Scholar]
- Romero LI, Zhang DN, Herron GS, Karasek MA. Interleukin-1 induces major phenotypic changes in human skin microvascular endothelial cells. Journal of cellular physiology 1997;173(1):84–92. [DOI] [PubMed] [Google Scholar]
- Saalbach A, Aust G, Haustein UF, Herrmann K, Anderegg U. The fibroblast-specific MAb AS02: a novel tool for detection and elimination of human fibroblasts. Cell Tissue Res 1997;290(3):593–9. [DOI] [PubMed] [Google Scholar]
- Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res 1998;290(7):360–6. [DOI] [PubMed] [Google Scholar]
- Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell and tissue research 1999;298(2):307–15. [DOI] [PubMed] [Google Scholar]
- Salzer MC, Lafzi A, Berenguer-Llergo A, Youssif C, Castellanos A, Solanas G, et al. Identity Noise and Adipogenic Traits Characterize Dermal Fibroblast Aging. Cell 2018;175(6):1575–90.e22. [DOI] [PubMed] [Google Scholar]
- Schurmann C, Linke A, Engelmann-Pilger K, Steinmetz C, Mark M, Pfeilschifter J, et al. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. The Journal of pharmacology and experimental therapeutics 2012;342(1):71–80. [DOI] [PubMed] [Google Scholar]
- Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014;32(6):1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol 2009;276:161–214. [DOI] [PubMed] [Google Scholar]
- Tabib T, Morse C, Wang T, Chen W, Lafyatis R. SFRP2/DPP4 and FMO1/LSP1 Define Major Fibroblast Populations in Human Skin. The Journal of investigative dermatology 2018;138(4):802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaculik C, Schuster C, Bauer W, Iram N, Pfisterer K, Kramer G, et al. Human dermis harbors distinct mesenchymal stromal cell subsets. The Journal of investigative dermatology 2012;132(3 Pt 1):563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. The American journal of pathology 2006;168(6):1861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel A, Chavakis T, Preissner KT, Sticherling M, Haustein UF, Anderegg U, et al. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Immunol 2004;172(6):3850–9. [DOI] [PubMed] [Google Scholar]
- Xie T, Wang Y, Deng N, Huang G, Taghavifar F, Geng Y, et al. Single-Cell Deconvolution of Fibroblast Heterogeneity in Mouse Pulmonary Fibrosis. Cell reports 2018;22(13):3625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, Wang X, Zhu M, Qu M, Bogari M, Lin L, et al. Expansion of CD26 positive fibroblast population promotes keloid progression. Exp Cell Res 2017;356(1):104–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


