Abstract
Achieving functional repair after peripheral nerve injury (PNI) remains problematic despite considerable advances in surgical technique. Therein, questions lie regarding the variable capacity of peripheral nerves to regenerate based on environmental influence. In-depth analyses of multiple therapeutic strategies have ensued to overcome these natural obstacles. Of these candidate therapies, electrical stimulation has emerged a frontrunner. Extensive animal studies have reported the ability of brief intraoperative electrical stimulation (BES) to enhance functional regeneration after PNI while few trials have been performed in humans. Further clinical studies and introduction of novel delivery platforms may uncover the true regenerative potential of electrical stimulation. In this review, we present the latest clinical trials on electrical stimulation for PNIs in combination with relevant etiologies, treatments and nonclinical findings.
Introduction
A staggering 1 out of every 16 Americans suffer from peripheral nerve injury (PNI) [1]. Most of these individuals are young adults who experienced a traumatic upper limb injury [2]. Severe PNI leads to functional disability and has a disastrous effect on a person’s quality of life [3]. Functional recovery after PNI is influenced by both the location and severity (classification) of the lesion. Improved functional outcomes are seen in distal PNIs compared to proximal due to decreased target reinnervation distance [4] [5]. Neurosurgical guidelines advocate early diagnosis of PNI after trauma is critical for achieving the best possible outcomes [6], while timeline for regeneration and repair (if needed) is dependent on the extent of damage to specific structures within the nerve itself [6] [7]). Comprehension of PNI etiologies and treatment standards is necessary to properly analyze potential strategies for enhancing peripheral nerve regeneration.
Materials and Methods
A systematic search was performed on PubMed, IEEE, and Web of Science databases from database creation to February 2020. PubMed, Ovid, and Cochrane databases were queried using the following keywords: (“electrical” or “stimulation” or “bioelectric” or “electrotherapy” or “electrode”) and (“peripheral” or “nervous” or “system” or “nerve” or “neural”) and (“repair” or “regenerate” or “restore” or “function” or “enhance” or “restore” or “heal”) and (“injury” or “insult” or “lesion” or “trauma” or “laceration” or “compression” or “crush”). References of each manuscript were screened for additional manuscripts of relevance to our study. Two investigators independently filtered each article according to title and abstract and included any article relating to the translation of brief electrical stimulation for enhancing peripheral nerve regeneration after injury. The full text of each selected article was obtained and analyzed. Institutional Review Board approval and Institutional Animal Care and Use Committee approval were not required due to lack of patient information or animals involved in this study.
PNI classifications
The Seddon and Sunderland classification systems are widely used to define PNIs. This review will use the Sunderland system due the added assessment of regenerative ability by addressing continuity of nerve sheath layers [8] [9]. A 1st degree injury is the least severe and defined by focal demyelination (neuropraxia) of the intact nerve with no axon loss. Additional axon loss (axonotmesis) in 2nd degree lesion is the only differentiator from 1st degree, but both will most likely achieve full recovery. Injuries of the 3rd-5th degree are characterized by additional loss of adjacent connective tissue layers from inner to outermost [9] [10]). Damage of the endoneurium in 3rd degree injury disrupts the scaffold by which axons elongate thus impairing regeneration and causing neuroma formation, and the perineurium and fascicular arrangement are preserved. Variable outcomes are seen following 3rd degree lesion. Neuroma formation and inhibited regeneration occur in 4th degree injury due in part to additional perineurial damage which results in poor recovery; however, the epineurium remains intact in 4th degree injury. Unlike 1st-4th degree lesions, 5th degree injury is characterized by complete nerve transection and discontinuity which result in poor functional recovery [9] [10]. Standards of care for both neuroma-in-continuity (3rd and 4th degree) and complete transection (5th degree) include surgical repair to promote regeneration [6].
Obstacles to functional nerve repair
For many PNIs, surgical intervention is necessary to allow functional regeneration. Substantial advances have been made in peripheral nerve surgery over the years with addition of techniques including microsurgery, nerve grafting and intraoperative electrophysiology. Clinical results initially improved but have since plateaued. Indeed, promising new strategies, such as nerve transfer, have provided surgeons the ability to overcome previous limitations in treatment [11]. Reports showing inadequate functional recovery after surgical repair of various PNIs remain prevalent despite these advances. A 2005 meta-analysis was performed to identify predictors for motor and sensory recovery after microsurgical repair of median and ulnar nerve transection lesions [5]. Identical to early reports by Sunderland from his 40-years on nerve repair, meta-analysis revealed the three significant predictors of successful motor recovery include patient age, site of lesion and delay in repair. Only patient age and delayed repair were found to be significant predictors of sensory recovery. Meta-analysis of median and ulnar nerve transection repair interestingly found only 51.6% and 42.6% of repairs achieve satisfactory motor and sensory recovery, respectively [5]. Despite extensive elucidation of the mechanisms for PNI and regeneration, a lack of dependable therapeutic strategies to fully restore function after injury still persists.
BES to enhance peripheral nerve regeneration
Countless therapies including neurotrophic factors, nerve conduits, neural scaffolds, gene therapy, stem cells and more continue to be investigated for their potential to restore nerve function after PNI. Of these strategies, electrical stimulation (ES) has emerged a frontrunner. The safety of ES on peripheral nerves has been established in humans [12], while sensory feedback in upper limb amputation and neuropathic pain relief with ES have been effective [13] [14]. In 1952, the first animal study on electrical stimulation of peripheral nerves by Hoffman et al. revealed accelerated axon sprouting into partially denervated muscle. Investigation by others would later show the ability of electrical stimulation on the proximal nerve stump to enhance target reinnervation [15] [16] [17] [18] and/or axon regeneration [19] [18]. Similar combined efforts elucidated effective ES durations and frequencies for nonharmful treatment in animals. A single dose of brief electrical stimulation (BES) of injured nerves at 20 Hz for 1 hr was found as effective in accelerating sensorimotor regeneration than stimulation with higher frequency and/or longer duration [19] [20] [21] [22]. These reports are upheld by the many studies on BES enhancement of nerve regeneration in animals which have followed [23] [4]. The mechanism of action contributing to the success of BES involves an ES-induced increased production of neurotrophic factors and upregulation of their receptors [19, 22, 24]. Specifically, increased production of brain-derived neurotrophic factor (BDNF) and regeneration-associated factors (RAFs) including Talpha-1 tubulin and GAP-43 may increase neural plasticity and accelerate axonal regeneration [19, 22, 24]. In addition, ES has been shown to upregulate neurotrophin 4/5, which has been correlated with increased nerve regrowth in mice [25]. Despite encouraging results in animals and established safety in humans, only a few studies on BES to enhance peripheral nerve regeneration have been performed in a clinical setting. In this review, we present the latest BES clinical trials in combination with a background to relevant etiologies, standards of care and BES animal studies.
Clinical Study 1: BES accelerates target reinnervation after chronic nerve compression
1.1. Intro to compression injury
Compressive neuropathies are among the most prevalent nerve lesions seen within a clinical setting (source). Compression injuries commonly occur where nerves pass through narrow anatomical openings resulting in focal demyelination (1st degree injury) [26] [10]). Unlike acute compression, untreated chronic compression may progress to axonal damage or axonotmesis (2nd degree injury) causing denervation atrophy of target muscle, as seen in severe carpal tunnel syndrome (CTS) [26] [7]).
1.2. BES after delayed repair in rats
Initial studies in rats revealed chronic denervation of distal stump and target muscle impair functional regeneration [7]. Two different studies have investigated the potential of BES to enhance axonal regeneration after chronic axotomy repair in rats. Huang et al. and Elzinga et al. concluded that BES accelerates axon regeneration and increases target reinnervation of motor and sensory neurons after a 4- and 3-month delay, respectively, in axotomy repair [27] [28]. In addition, Huang reported the same treatment was effective in promoting functional recovery after a 4-month delay in repair of chronically axotomized neurons [27]. These novel findings reveal the therapeutic potential of BES to stimulate functional recovery after chronic axonotmesis. However, while much of the current literature investigates delayed repair following acute compression, additional studies are required to better understand the implications of chronic compression.
1.3. Chronic CTS diagnosis and standard of care
Carpal tunnel syndrome (CTS) is the most prevalent compression lesion and initially presents as recurrent nocturnal paresthesias and dysesthesias [6]. Progression of CTS may cause loss of sensation and, ultimately, thenar motor function in moderate to severe cases. For a CTS patient with mild symptoms and no axonal loss (1st degree injury), nonsurgical treatment options are standard. In late stage CTS (2nd degree injury), symptoms of axonal loss are present thus necessitating surgical release of the transverse carpal ligament [6]. Carpal tunnel release surgery (CTRS) is known to be highly effective, but lack of symptom improvement and prolonged recovery may occur due to extensive axonal loss prior to treatment [7] [6]. Therapeutic strategies to accelerate or increase median nerve regeneration in chronic CTS would undoubtedly have profound implications.
1.4. BES accelerates median nerve regeneration after CTRS
While surgical intervention of CTS may ameliorate symptoms, many individuals continue to experience sensorimotor impairment thereafter [6]. Given the variation in etiologies and outcomes of CTS, further studies are needed to explore combinations of both diagnostic and therapeutic modalities [29] [30]. In a 2007 clinical trial, it was reported that patients receiving BES after carpal tunnel release surgery (CTRS) had higher estimated numbers of intact motor units compared to unstimulated patients after 6 months and up to 12 months [31]. These differences between treatment and control groups were indeed significant and suggest BES leads to accelerated regeneration and target reinnervation after chronic median nerve entrapment. A follow up study was performed in 2010 to further determine the efficacy of BES in treating severe CTS [32]. Despite no observed difference in functional outcomes between groups, this trial confirmed that BES immediately following decompression surgery in patients suffering from severe CTS results in early and complete reinnervation of thenar muscles by regenerating axons. Individuals undergoing CTRS alone did not show significant muscle reinnervation. It should be noted the lack of difference in motor performance may have been due to inadequate characterization of thenar motor function by tests performed in this study [33]. Further investigation into whether BES improves motor function in severe CTS is warranted. These findings endorse the use of BES as an adjunct therapy after CTRS for accelerating median nerve regeneration [32]. A contemporary study conducted by Power et al. in 2020 extended these findings from CTRS to cubital tunnel syndrome and found that postsurgical ES may be effective in enhancing muscle reinnervation and recovery following surgery[34]. Further research is required to fully understand the limits of ES in patients with compressive neuropathies.
Clinical Study 2: BES accelerates recovery of sensory function after complete nerve transection
2.1. Intro to transection injury
Poor functional outcomes in peripheral nerve repair continue to persist in the face of surgical advancement. This phenomenon is largely due to dramatic variation in the regenerative abilities of specific nerve lesions. The presence of an intact endoneurial tube, as seen in 2nd degree lesions, provides an optimal environment for axonal regrowth which leads to better outcomes in nerve regeneration [26]. Transection injury or neurotmesis is characterized by complete discontinuation of the peripheral nerve (5th degree injury) and commonly occurs due to laceration from knives, glass, bullets and etc. [26] in the setting of an open injury [10]. Prompt recognition and surgical repair of transection injuries are critical for achieving the best possible outcomes. Despite early intervention, outcomes may be negatively impacted by factors outside the surgeon’s control including patient age, target reinnervation distance and time from injury to presentation [6]. Increases in each of the aforementioned parameters have shown to adversely affect nerve regeneration [10].
2.2. BES after complete nerve transection
Despite the elucidation of mechanisms for nerve injury and regeneration, a significant lack of reliable therapy for enhancing nerve regeneration still remains. Early in the new millennium, Al-Majed et al. determined that a single delivery of continuous 20 Hz electric stimulation for 1 hour (brief electrical stimulation) after nerve repair is equally as effective in accelerating motoneuron regeneration as stimulation for 2 weeks duration. Later studies demonstrated how BES treatment is equally effective in accelerating sensory regeneration as stimulation with higher frequency and/or longer duration [20] [21] [22]. The ability of such a simple, acute therapy to enhance both motor and sensory regeneration in rats reveals an exciting potential strategy for accelerating nerve regeneration in humans.
2.3. Nerve transection diagnosis and standard of care
Early management of traumatic nerve injury is essential for restoration of nerve function. Patients with traumatic nerve injury usually present with a motor, sensory or pain deficit immediately following the inciting event. While historical information may provide insight to mechanism of injury, detailed documentation of initial neurological examination is critical to establishing a comparative baseline [6]. Traumatic injuries are generally classified as open or closed [10]. Open injuries commonly result in neurotmesis, but timing of surgical intervention is dependent on presence of either sharp or ragged transection [6]. Primary repair of ragged transections from blast, gunshot, fracture or crush injury should be delayed for approximately 3 weeks to allow demarcation of nerve ends. Sharp transections from knives or razors should be surgically repaired within 3 days of trauma [6] [10]. Due to the limited time frame for functional repair of sharp transection, delayed intervention often leads to incomplete motor and sensory recovery [10]. Therapeutic enhancement of regeneration may provide a larger window for repair and functional restoration after peripheral nerve transection.
2.4. BES enhances sensory function after digital nerve transection repair
The potential for BES to improve sensory regeneration after repair of transected digital nerves was assessed in humans. Wong et al. recruited 36 patients with complete digital nerve transection to participate in a randomized, double-blind placebo-controlled trial to determine whether BES after surgery results in improved digit tip sensation and functional outcomes compared to surgery alone. Within 14 days of injury, patients in the treatment group underwent epineural nerve repair immediately followed by BES. Sensory function and functional disability were assessed monthly up to 6 months after surgery. Disability from the nerve injury was monitored and graded using the Disability of Arm, Shoulder and Hand (DASH) Questionnaire, while sensory function was analyzed via 3 different modalities including temperature threshold, tactile discrimination and pressure detection. A trend of greater functional improvements in the BES group was seen though it was not statistically significant likely due to small sample size. Unlike the control group, BES-treated patients experienced significant and near complete recovery of all sensory modalities by 5 to 6 months compared to baseline [35]. BES enhancement of sensory regeneration in patients with complete digital nerve transection argues for further clinical application after complete nerve transection repair.
Clinical Study 3: Intraoperative brief ES of CN XI preserves shoulder function in patients undergoing oncologic neck dissection
3.1. Intro to crush injury
Crush injuries usually occur as a result of acute compressive trauma by blunt objects such as a bat, clamps or etc. Extent of nerve damage and classification grade in crush injury are highly variable and dependent on the amount of compressive force. Thus, crush injuries may be classified at any level of the Sunderland system except 5th degree due to lack of transection in crush injury [26]. Since crush lesions involve structural damage ranging from focal demyelination to neuroma-in-continuity, these lesions are difficult to manage and require extensive follow-up before proper treatment can be determined [6] [36]. Even so, multiple factors including delayed repair, proximal lesion location and age may negatively impact functional outcomes.
3.2. BES after acute crush injury in rats
Many traumatic nerve lesions do not completely transect the nerve but leave the nerve in continuity with poor functional recovery due to inadequate regeneration and reinnervation. To address this concern, ability of BES to improve outcomes in a rat sciatic neuroma-in-continuity (NIC) and facial nerve crush lesion were assessed. A significant improvement in locomotor function was seen in BES-treated rats after NIC lesion at 4-, 6- and 8-weeks post-surgery, tibialis anterior compound muscle action potential (CMAP) at 8 weeks was significantly greater in the BES group. By 12 weeks, there was no significant behavioral or electrophysiologic difference between the two groups [36]. Single-session BES effect on facial nerve function and synkinesis after acute crush injury was assessed in rats. Amplitude of whisking after injury was used to assess motor function. Target-specific muscle reinnervation was measured by injecting neurotracers into buccal and marginal mandibular branches of facial nerve at 3 months postinjury. Although no difference of double-labeled motoneurons was seen, BES significantly improved whisking capacity 2- and 4-weeks post-crush injury. Similar to previous reports, no difference in functional outcomes was seen over the long term. Although short regeneration distances in rat models ultimately lead to similar outcomes in BES and control groups, the stated findings support the role of BES in accelerating functional recovery after acute crush injury. Were BES found to expedite restoration of sensorimotor function in humans it would an invaluable adjuvant therapy.
3.3. Acute crush injury diagnosis and standard of care
Proper diagnosis of traumatic nerve injury requires early assessment with a detailed neurological examination and thorough history [6]. Crush injury due to blunt trauma may present in either an open or closed setting. After confirmation of in-continuity injury, crush injuries are followed for a period of 3 to 6 months before potential treatment, unlike transection, during which physical examinations and electrodiagnostic studies may document recovery [10]. Delayed treatment is necessary to allow either neuroma formation so damaged nerve can be properly resected at the time of repair or spontaneous recovery of crushed nerve. It should be noted the therapeutic window for surgical repair is narrow, and waiting too long before intervention may lead to suboptimal outcomes [10]. Considered with the regenerative variability seen among crush lesions, avoidance of surgical repair by promoting spontaneous recovery of crushed nerves with BES may be a valuable therapeutic strategy to improve outcomes.
3.4. BES improves functional outcomes after head and neck cancer dissection
Head and neck cancer (HNC) commonly occurs in the third or fourth decade of life, thus treatment options should take functional preservation into account for the many potential work years remaining for HNC patients. Treatment options for advanced HNC include primary surgical resection involving oncologic Level IIB with or without Level V depending on presence of malignancy in adjacent lymph nodes. In Level IIB dissection, the spinal accessory nerve (SAN) is manipulated and often devascularized leading to impaired nerve function and subsequent regeneration [37]. HNC postoperative shoulder pain and dysfunction due to SAN axonal injury are well-documented and have detrimental effects on quality of life [38] [39].
In this double-blind randomized control trial, Barber et al. analyzed the efficacy of BES of the SAN in reducing shoulder dysfunction of HNC patients following Level IIb +/− Level V dissection. The well-established Constant-Murley Score (CMS) was used to assess both objective and subjective measures of shoulder function, including pain, activities of daily living, range of motion and strength [40]. Primary outcomes were measured using CMS scores at 12 months post-dissection compared to baseline. It should be noted that spontaneous recovery after crush lesion appears between 3 and 6 months after injury. Secondary outcomes were assessed by measuring changes in Neck Dissection Impairment Index (NDII) and compound muscle action potential amplitude (CMAP) scores over the same timeline. Primary outcomes but not secondary outcomes were significantly greater in BES-treated patients compared to patient baseline. It is possible improved outcomes were seen due to BES promoting spontaneous recovery of injured nerves following HNC resection; however, additional trials are needed to confirm this. This study reveals the potential of BES to preserve shoulder function after oncologic neck dissection [40].
However, the role of BES in oncology continues to be highly debated and variable, further validation studies are required to fully understand the role of BES in patient management. Additional multi-center, randomized, double-blinded studies involving BES for a variety of cancer subtypes is required to fully demonstrate the efficacy of BES in oncological patient management.
Future Studies
The therapeutic potential of electrical stimulation to enhance peripheral nerve repair is evident, but additional clinical trials are necessary to validate possible benefits. A single-dose BES of peripheral nerve proximal to the repair site has shown to accelerate and improve functional regeneration. Although reports have been positive, a serious limitation of previous studies has been confinement of therapy application to the intraoperative period. In response, a wireless, bioresorbable electrical peripheral nerve stimulator has been introduced [3]. This programmable platform allows electrical stimulation of injured nerves to extend beyond the intraoperative period. Data reveals chronic electrical stimulation at 20 Hz for 1 hr per day for 6 days after rat sciatic nerve transection repair achieved significantly greater functional recovery than those treated with BES. Although these results conflict with prior studies, which demonstrated that multiple periods of electrical stimulation demonstrated no benefit, similar clinical studies have not been performed and future prospective trials are required to fully understand the efficacy of multiple ES periods on improving nerve growth. Thus, the use of wireless bioresorbable electronics opens new doors to applying electrical stimulation for peripheral nerve regeneration [3].
In contrast to BES, long-term peripheral nerve stimulation (PNS) has been shown to drastically improve patient outcomes in those experiencing painful nerve injuries. A 2004 study conducted by Eisenberg et al. demonstrated that up to 78% of patients treated with long-term PNS following nerve injury may report pain relief with 3-16 year follow-up[41]. Contemporary studies continue to support the findings of the aforementioned paper, and a 2015 study conducted by Lee et al. found that long-term PNS may reduce pain scores in patients with chronic intractable headaches by more than one-third[42].
Further, non-invasive ES, such as transcutaneous electrical nerve stimulation (TENS), have gained popularity over the last decade in treating neuropathic pain. TENS is often administered at high intensities close to the site of pain with hopes of achieving pain relief[43]. However, studies evaluating the efficacy of TENS are few and lack the power necessary to fully establish this technique as a route for management of chronic pain.
Lastly, as BES studies translate from animal experiments to human experimentation, several limitations may become clear. First, the difference in nerve length and diameter between the rats/mice and humans may present difficulties in translation and require additional experimentation to locate the optimum site for ES. Furthermore, stimulation at 20Hz for 1 hour may be suboptimal for human subjects, requiring prospective research.
Conclusions
In laboratory animals and humans, electrical stimulation has been repeatedly shown to accelerate the regeneration of injured axons and reinnervation of target tissues. Several clinical trials performed have yielded encouraging results that place BES as a candidate adjunctive therapy but more data is necessary to determine application frequency for optimum effectiveness. Additional human studies are needed to properly assess recovery of thenar motor function after CTRS and potency of electrical stimulation after delayed repair. The true capacity of electrical stimulation to help overcome limitations in peripheral nerve regeneration remains promising and to be uncovered.
Figure 1.
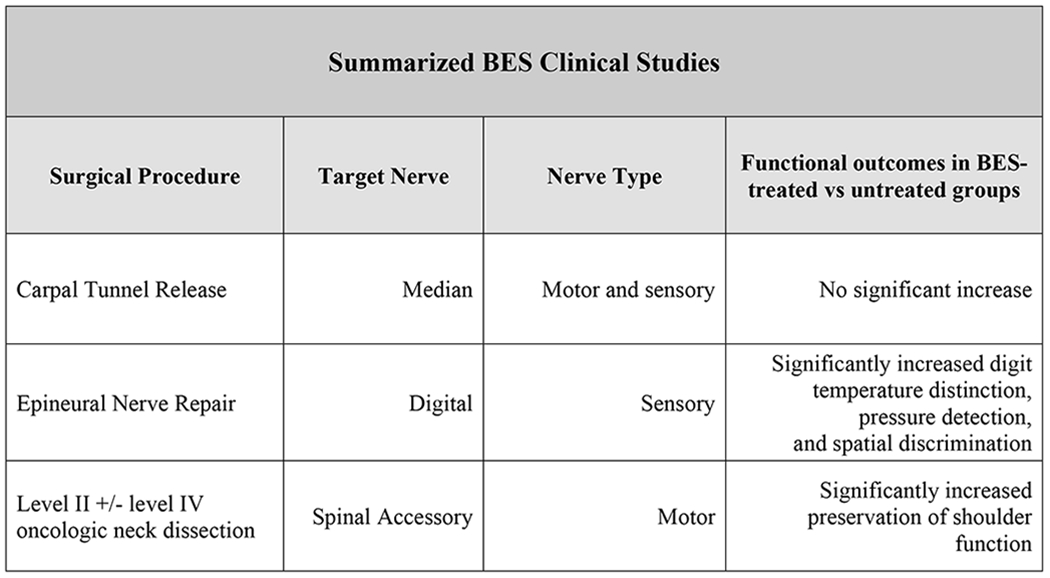
Comparative functional outcomes of 3 clinical trials using brief intraoperative electrical stimulation (BES) at 20 Hz for 1 hr immediately following standard procedure [37] [32] [7].
Acknowledgments
Source of funding: RS is funded in part by an MSTP Grant from the NIH (T32-GM08620).
Footnotes
Financial interests: None
Conflict of interest: None
References
- [1].Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kouyoumdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006. December;34(6):785–8. [DOI] [PubMed] [Google Scholar]
- [3].Koo J, MacEwan MR, Kang SK, Won SM, Stephen M, Gamble P, et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat Med. 2018. December;24(12):1830–6. [DOI] [PubMed] [Google Scholar]
- [4].Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013. December;9(12):668–76. [DOI] [PubMed] [Google Scholar]
- [5].Ruijs AC, Jaquet JB, Kalmijn S, Giele H, Hovius SE. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005. August;116(2):484–94; discussion 95-6. [DOI] [PubMed] [Google Scholar]
- [6].Spinner RJ, Hanna AS, Maldonado AA, Wilson TJ. Peripheral Nerve. Oper Neurosurg (Hagerstown). 2019. August 1;17(Suppl 2):S229–s55. [DOI] [PubMed] [Google Scholar]
- [7].Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011. April 6;31(14):5325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].SEDDON HJ. THREE TYPES OF NERVE INJURY. Brain. 1943;66(4):237–88. [Google Scholar]
- [9].Sunderland SS, Williams HB. Nerve Injuries and Their Repair: A Critical Appraisal. Plastic and Reconstructive Surgery. 1992;89(6):1170. [Google Scholar]
- [10].Houdek MT, Shin AY. Management and complications of traumatic peripheral nerve injuries. Hand Clin. 2015. May;31(2):151–63. [DOI] [PubMed] [Google Scholar]
- [11].Spinner RJ, Shin AY, Bishop AT. Advances in the Repair of Peripheral Nerve Injury. Neurosurgery. 2015. August;62 Suppl 1:146–51. [DOI] [PubMed] [Google Scholar]
- [12].Gunter C, Delbeke J, Ortiz-Catalan M. Safety of long-term electrical peripheral nerve stimulation: review of the state of the art. J Neuroeng Rehabil. 2019. January 18;16(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reddy CG, Flouty OE, Holland MT, Rettenmaier LA, Zanaty M, Elahi F. Novel technique for trialing peripheral nerve stimulation: ultrasonography-guided StimuCath trial. Neurosurg Focus. 2017. March;42(3):E5. [DOI] [PubMed] [Google Scholar]
- [14].Schiefer M, Tan D, Sidek SM, Tyler DJ. Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J Neural Eng. 2016. February;13(1):016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nix WA, Hopf HC. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983. August 1;272(1):21–5. [DOI] [PubMed] [Google Scholar]
- [16].Pockett S, Gavin RM. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci Lett. 1985. August 30;59(2):221–4. [DOI] [PubMed] [Google Scholar]
- [17].Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000. April 1;20(7):2602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002. August 1;22(15):6631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000. December;12(12):4381–90. [PubMed] [Google Scholar]
- [20].Gordon T, Udina E, Verge VM, de Chaves EI. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009. October;13(4):412–41. [DOI] [PubMed] [Google Scholar]
- [21].Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005. July;194(1):221–9. [DOI] [PubMed] [Google Scholar]
- [22].Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007. June;205(2):347–59. [DOI] [PubMed] [Google Scholar]
- [23].Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2016. February;43(3):336–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol. 2004. June;24(3):379–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].English AW, Schwartz G, Meador W, Sabatier MJ, Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007. February 1;67(2):158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013. August;29(3):317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang J, Zhang Y, Lu L, Hu X, Luo Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur J Neurosci. 2013. December;38(12):3691–701. [DOI] [PubMed] [Google Scholar]
- [28].Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015. July;269:142–53. [DOI] [PubMed] [Google Scholar]
- [29].Leit ME, Weiser RW, Tomaino MM. Patient-reported outcome after carpal tunnel release for advanced disease: a prospective and longitudinal assessment in patients older than age 70. J Hand Surg Am. 2004. May;29(3):379–83. [DOI] [PubMed] [Google Scholar]
- [30].Urits I, Gress K, Charipova K, Orhurhu V, Kaye AD, Viswanath O. Recent Advances in the Understanding and Management of Carpal Tunnel Syndrome: a Comprehensive Review. Curr Pain Headache Rep. 2019. August 1;23(10):70. [DOI] [PubMed] [Google Scholar]
- [31].Gordon T, Brushart TM, Amirjani N, Chan KM. The potential of electrical stimulation to promote functional recovery after peripheral nerve injury--comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3–11. [DOI] [PubMed] [Google Scholar]
- [32].Gordon T, Amirjani N, Edwards DC, Chan KM. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010. May;223(1):192–202. [DOI] [PubMed] [Google Scholar]
- [33].Chan KM, Curran MW, Gordon T. The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice. J Physiol. 2016. July 1;594(13):3553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Power HA, Morhart MJ, Olson JL, Chan KM. Postsurgical Electrical Stimulation Enhances Recovery Following Surgery for Severe Cubital Tunnel Syndrome: A Double-Blind Randomized Controlled Trial. Neurosurgery. 2020. June 1;86(6):769–77. [DOI] [PubMed] [Google Scholar]
- [35].Wong JN, Olson JL, Morhart MJ, Chan KM. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann Neurol. 2015. June;77(6):996–1006. [DOI] [PubMed] [Google Scholar]
- [36].Shapira Y, Sammons V, Forden J, Guo GF, Kipp A, Girgulis J, et al. Brief Electrical Stimulation Promotes Nerve Regeneration Following Experimental In-Continuity Nerve Injury. Neurosurgery. 2019. July 1;85(1):156–63. [DOI] [PubMed] [Google Scholar]
- [37].Celik B, Coskun H, Kumas FF, Irdesel J, Zarifoglu M, Erisen L, et al. Accessory nerve function after level 2b-preserving selective neck dissection. Head Neck. 2009. November;31(11):1496–501. [DOI] [PubMed] [Google Scholar]
- [38].van Wilgen CP, Dijkstra PU, van der Laan BF, Plukker JT, Roodenburg JL. Shoulder complaints after nerve sparing neck dissections. Int J Oral Maxillofac Surg. 2004. April;33(3):253–7. [DOI] [PubMed] [Google Scholar]
- [39].Chepeha DB, Taylor RJ, Chepeha JC, Teknos TN, Bradford CR, Sharma PK, et al. Functional assessment using Constant’s Shoulder Scale after modified radical and selective neck dissection. Head Neck. 2002. May;24(5):432–6. [DOI] [PubMed] [Google Scholar]
- [40].Barber B, Seikaly H, Ming Chan K, Beaudry R, Rychlik S, Olson J, et al. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: a double-blinded, randomized controlled trial. J Otolaryngol Head Neck Surg. 2018. January 23;47(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Eisenberg E, Waisbrod H, Gerbershagen HU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin J Pain. 2004. May-Jun;20(3):143–6. [DOI] [PubMed] [Google Scholar]
- [42].Lee PB, Horazeck C, Nahm FS, Huh BK. Peripheral Nerve Stimulation for the Treatment of Chronic Intractable Headaches: Long-term Efficacy and Safety Study. Pain Physician. 2015. Sep-Oct;18(5):505–16. [PubMed] [Google Scholar]
- [43].Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother. 2011. May;11(5):735–53. [DOI] [PubMed] [Google Scholar]


