Abstract
Objective:
Acute kidney injury (AKI) is a complication of cardiac surgery that is considerably more common in African Americans (1.5-fold). Although homozygous status for apolipoprotein L1 (APOL1) risk alleles is associated with chronic kidney disease in individuals of African ancestry, whether these coding variants confer AKI risk is unknown. The present study examined whether APOL1 homozygous risk allele status is associated with AKI in African Americans after cardiac surgery.
Design:
Retrospective analysis of a cohort.
Setting:
Single-center university hospital.
Participants:
African American patients from the CATHeterization GENetics study cohort who underwent cardiac surgery with cardiopulmonary bypass.
Interventions:
Genotyping of APOL1 alleles.
Measurements and Main Results:
Data from 125 African American patients included 12 APOL1 risk (ie, homozygous for risk alleles) patients and 113 APOL1 control (ie, wildtype or heterozygous for risk alleles) patients. The primary outcome to reflect AKI was peak serum creatinine rise after surgery relative to the preoperative creatinine (%ΔCr). The secondary outcome was Kidney Disease: Improving Global Outcomes (KDIGO) AKI criteria. In the primary analysis, peak creatinine rise was higher in risk compared with control patients in both univariate (%ΔCr 69.1 v 29.6%; p = 0.005) and multivariable regression (%ΔCr 88.5 v 43.7%; p = 0.006) analyses. For the secondary outcome, a trend toward KDIGO AKI development was noted in APOL1 risk patients, but this was not statistically significant.
Conclusions:
African American cardiac surgery patients homozygous for APOL1 chronic kidney disease risk variants averaged a more than 2-fold higher postoperative creatinine rise even after adjustment for other risk factors, suggesting these alleles also are independent risk factors for AKI.
Keywords: acute kidney injury, cardiac surgery, gene polymorphism, apolipoprotein L1
ACUTE KIDNEY injury (AKI) is one of the most serious and common forms of postoperative organ dysfunction.1 Cardiac surgery–associated AKI (CS-AKI) occurs after up to 30% of procedures involving cardiopulmonary bypass (CPB)2–5 and is associated with worse morbidity and mortality, longer intensive care unit stays, and higher rates of hospital readmission and cost.6–8 Moreover, among survivors, long-term mortality rates are higher.9
Although there is much unexplained variability in the development of AKI, race and genetics are important and correlated risk factors.10,11 African Americans generally are at greater AKI risk than are Caucasians,12 including a 1.5-fold greater risk after cardiac surgery.6 In separate cohorts of African American and Caucasian patients,10,11,13 elevated CS-AKI risk previously was reported with the following: specific polymorphisms,13 co-occurrence of polymorphisms,11 and loci in genome-wide association studies.10,14 Because AKI and chronic kidney disease (CKD) share many similar risk factors and injury mechanisms, genetic investigation of CKD often has informed hypotheses in these studies.15
In this regard, apolipoprotein L1 (APOL1) is a 43 kDa protein involved in the autophagy pathway.16 Risk variants in the gene that encodes APOL1 are associated with an almost 2-fold higher rate of CKD and end-stage kidney disease in African American homozygotes (APOL1 risk) compared with heterozygotes/noncarriers (APOL1 controls).17,18 However, the relationship between these risk variants and CS-AKI risk has not been studied. Although candidate polymorphism studies for AKI risk generally have fallen out of favor in lieu of larger unbiased genome-wide association studies,14 the strong and well-validated mechanistic link between APOL1 risk variants and the pathogenesis of CKD supports the selection of this methodology to investigate its relationship with AKI occurrence. Therefore, the authors hypothesized that African American cardiac surgery patients homozygous for APOL1 risk variants would demonstrate greater CS-AKI rates than would APOL1 controls. Identification of patients at highest risk for postoperative renal dysfunction will allow for the adoption of renoprotective measures19 that could alleviate the development of CS-AKI.
Methods
Study Cohort
This analysis was a substudy of the CATHeterization GENetics (CATHGEN) study at the Duke Heart Center.20 The CATHGEN study was approved by the Duke University School of Medicine Institutional Review Board. Informed consent for the present study was obtained from all patients in the CATHGEN cohort, and the study was conducted in adherence to the Declaration of Helsinki. The CATHGEN cohort included 9,249 sequential patients undergoing cardiac catheterization at Duke University Hospital, Durham, NC, between 2001 and 2010.20 For the present study, inclusion criteria included patients of self-reported African American race in the CATHGEN cohort who were genotyped (n = 1,760) and who subsequently underwent cardiac surgery requiring CPB at Duke University Hospital (n = 155). Exclusion criteria included repeat cardiac surgical procedures and patients with end-stage renal disease or CKD stages 3 to 6 at CATHGEN enrollment (Fig. 1).
Fig. 1.
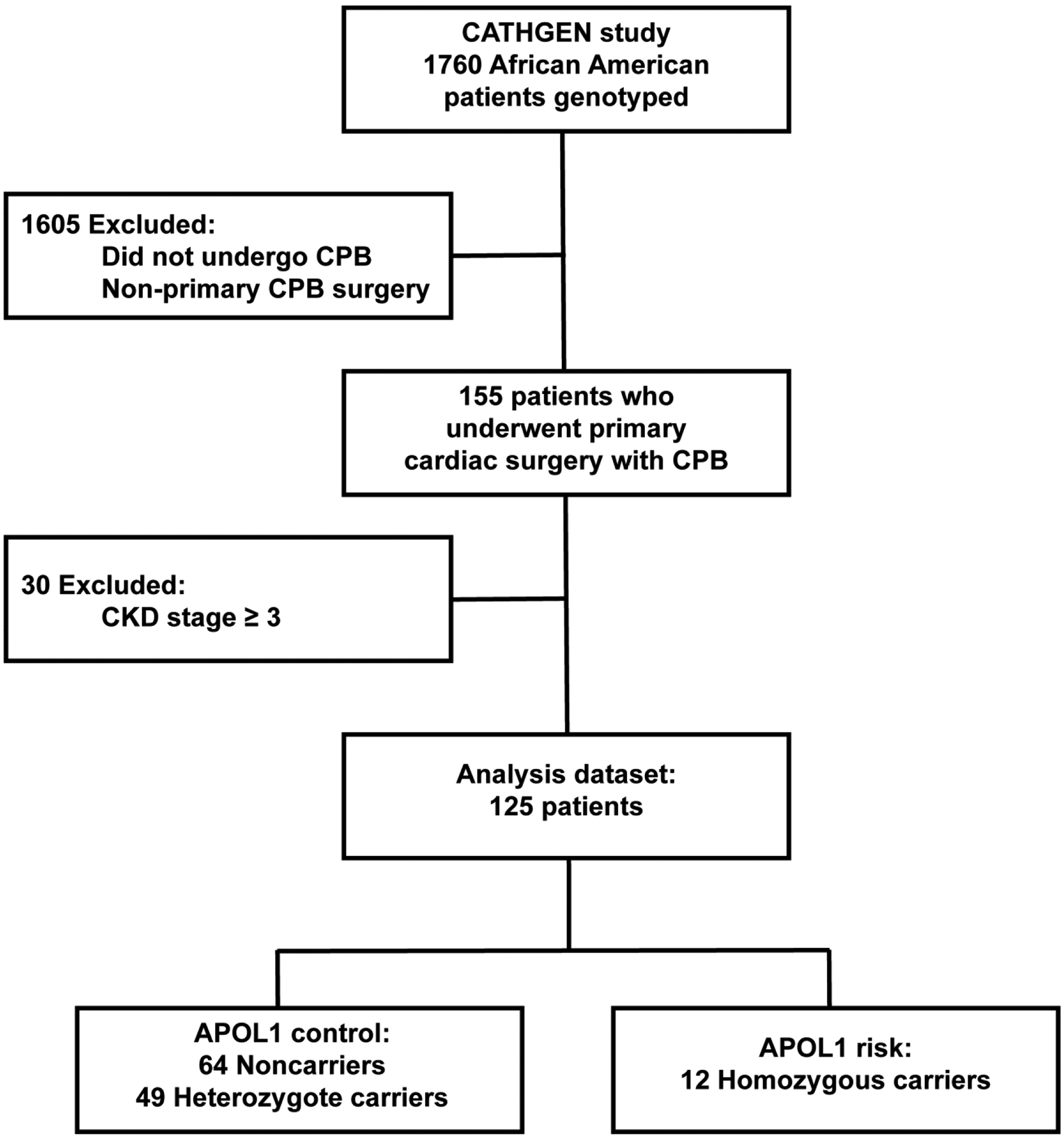
Consort diagram showing selection of patients in analysis dataset. APOL1, apolipoprotein L1; CKD, chronic kidney disease; CPB, cardiopulmonary bypass.
Demographic Variables
Patient and procedural characteristics were sourced from the CATHGEN, Duke Information System for Cardiovascular Care, and Duke Enterprise Data Unified Content Explorer databases and by chart review from the Duke Health Electronic Medical Record. Variables included sex, age at CATHGEN enrollment (in years), body mass index (BMI), history of hypertension, history of congestive heart failure, history of chronic obstructive pulmonary disease, history of diabetes mellitus, previous myocardial infarction, history of peripheral vascular disease (PVD), history of cerebrovascular disease, left ventricular ejection fraction (%), CKD stage (glomerular filtration rate derived by the Chronic the Kidney Disease Epidemiology Collaboration formula [CKD – EPI] and staged by Kidney Disease: Improving Global Outcomes [KDIGO] CKD criteria), history of end-stage renal disease, type of surgery, aortic cross-clamp time, and CPB time.
Assessment of Renal Function
Serum creatinine was determined for clinical use using the Jaffe method (UniCel DxC 600/800; Beckman Coulter, Inc, Brea, CA), with a normal range of 0.6 to 1.3 mg/dL for men and 0.4 to 1.0 mg/dL for women. As per standard care protocols, available serum creatinine data included preoperative within 30 days of surgery (baseline) and daily postoperative for the first 10 postoperative days. When more than 1 creatinine determination occurred on the same day, the first was used.
As in prior genetic studies,10,11,13,21,22 peak serum creatinine rise after surgery relative to the preoperative creatinine (%ΔCr) was selected as an AKI biomarker over standard dichotomous criteria because of the enhanced sensitivity and power of the former to identify risk variants. As a continuous variable, this primary study outcome (ie, %ΔCr) relates peak serum creatinine rise after surgery to baseline preoperative to reflect glomerular filtration impairment. Notably, even small increases in postoperative serum creatinine are associated with additional morbidity and mortality,5 important variability that is missed by dichotomous AKI criteria.10 In addition, dichotomous AKI status using KDIGO consensus criteria (excluding urine output criteria) was selected as an outcome for secondary analysis.23 Noninclusion of oliguria in the KDIGO criteria in cardiac surgical studies is common9–11,13,24,25 because of the following reasons: (1) perioperative factors with limited relationship to AKI risk often cause low urine output (eg, general anesthesia, major regional anesthesia/analgesia, vasodilator therapy, hemofiltration during CPB) and (2) AKI diagnosed using low urine output criteria in this setting is not associated with other typically related major adverse outcomes (eg, mortality, renal replacement therapy, delayed discharge).26
APOL1 Genotyping
APOL1 genotyping was performed as previously described.27 Because there are 2 relevant variant/risk alleles, G1 (S342G and I384M) and G2 (N388del:Y389del), in the APOL1 gene, the authors refer to noncarriers as subjects carrying only wildtype (WT) alleles and neither of the G1 and G2 risk alleles (WT/WT); heterozygous carriers as subjects carrying 1 type of risk allele (G1/WT or G2/WT); and homozygote carriers as subjects carrying 2 risk alleles (G1/G1, G1/G2, G2/G2).18
Statistical Analysis
As with prior APOL1 CKD studies,17,18 for the present study a recessive genetic model was used for analysis, in which a genotype is coded as 1 for homozygous carriers (“risk” group) and 0 for others (“control” group [ie, including heterozygous individuals and noncarriers]). Demographic and patient characteristics were compared among the 3 APOL1 genotype groups using the chi-square, Kruskal Wallis, and Fisher exact tests, as appropriate. Univariate and multivariable linear regression models were used to test APOL1 association with the primary outcome (%ΔCr) and secondary outcome (KDIGO AKI status). Considering the primary outcome (%ΔCr), which was a continuous measure, a linear regression model was used for association tests for APOL1. A univariable linear regression model was performed for demographic and procedural variables known to be renal risk factors from previous studies.10 The final multivariable linear regression model was determined on the basis of backward selection for an initial set of variables selected from univariate analysis (p < 0.15). To examine the collinearity of variables in the final model, the variance inflation factor also was estimated. For the binary outcome of KDIGO AKI status, a logistic regression model was used. Similarly, the final multivariable logistic regression model for KDIGO AKI status involved backward selection of variables (p < 0.15) from the univariate results. The diagnosis of final multivariable logistic regression model was assessed using the Hosmer-Lemeshow goodness-of-fit test for variables in which nonsignificant results were expected. All analyses were performed using SAS9.4 (SAS Inc, Cary, NC).
Results
Description of Clinical Dataset
Of 1,760 genotyped African American participants in the CATHGEN study, 155 underwent cardiac surgery within a mean (standard deviation) of 21.0 (38.0) months after enrollment. After additional exclusion criteria, 125 participants remained and formed the base of the present study’s population. Of the 125 eligible African American cardiac surgery patients, 12 were APOL1 risk subjects (homozygotes; 10% total) and 113 were APOL1 control subjects (heterozygotes, n = 49 [39%]; noncarriers, n = 64 [51%]) (see Fig. 1). Overall demographic and procedural variables were similar between groups, including baseline renal function, although the incidence of PVD was greater in the APOL1 risk group (p = 0.007) (Table 1).
Table 1.
Patient, Procedural, and Renal Characteristics of 125 African American Patients in Analysis Dataset Who Underwent Primary Surgery Requiring Cardiopulmonary Bypass by APOL1 Genotype
| Noncarriers (n = 64; 51%) | Heterozygous Carriers (n = 49; 39%) | Homozygous Carriers (n = 12; 10%) | p Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Demographics | ||||
| Male, n (%) | 38 (59.4) | 28 (57.1) | 7 (58.3) | 0.97* |
| Age, mean (SD) | 54.4 (11.7) | 58.1 (9.9) | 53.6 (10.8) | 0.15† |
| BMI (kg/m2), mean (SD) | 30.2 (7.0) | 32.6 (9.4) | 32.1 (8.1) | 0.50† |
| History | ||||
| HTN | 42 (65.6%) | 33 (67.4%) | 10 (83.3%) | 0.48* |
| CHF | 31 (49.2) | 26 (54.2) | 6 (50.0) | 0.87* |
| COPD | 3 (4.7) | 1 (2.0) | 0 (0.0) | 0.76‡ |
| Diabetes mellitus | 27 (42.2) | 19 (38.8) | 4 (33.3) | 0.83‡ |
| Previous MI | 18 (28.1) | 11 (22.5) | 2 (16.7) | 0.62* |
| PVD | 4 (6.3) | 0 (0.0) | 3 (25.0) | 0.007‡ |
| CVD | 8 (12.5) | 5 (10.2) | 2 (16.7) | 0.72‡ |
| Ejection fraction, % (SD) | 47.8 (17.7) | 50.7 (14.7) | 51.9 (13.9) | 0.78† |
| Procedure characteristics | ||||
| Type | 0.29* | |||
| CABG, n (%) | 41 (64.1%) | 23 (46.9%) | 9 (75.0%) | |
| Valve, n (%) | 15 (23.4%) | 16 (32.7%) | 2 (16.7%) | |
| Other, n (%) | 8 (12.5%) | 10 (20.4%) | 1 (8.3%) | |
| Cross-clamp time (min), mean (SD) | 90.8 (56.4) | 86.9 (40.9) | 78.0 (15.4) | 0.83† |
| CPB time, mean (SD) | 158.5 (65.4) | 146.2 (50.4) | 153.3 (58.3) | 0.85† |
| Renal characteristics | ||||
| Serum creatinine (mg/dL) | ||||
| Baseline, mean (SD) | 1.2 (0.4) | 1.2 (0.4) | 1.4 (0.4) | 0.36† |
| Peak, mean (SD) | 1.5 (0.4) | 1.5 (0.4) | 2.3 (1.6) | 0.52† |
| %ΔCr, % (SD) | 30.3 (32.2) | 28.6 (27.0) | 69.1 (117.5) | 0.94† |
| KDIGO AKI, n (%) | 34 (53.1%) | 32 (65.3%) | 8 (66.7%) | 0.38‡ |
| Postoperative dialysis, n (%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 0.10‡ |
Abbreviations: APOL1, apolipoprotein L1; %ΔCr, peak serum creatinine rise after surgery relative to preoperative creatinine; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; CVD, cerebrovascular disease; HTN, hypertension; KDIGO, Kidney Disease: Improving Global Outcomes; MI, myocardial infarction; PVD, peripheral vascular disease; SD, standard deviation.
Chi-square test.
Kruskal Wallis test.
Fisher exact test.
Association of APOL1 Genotypes and %ΔCr
Univariate analysis demonstrated that the average peak rise in serum creatinine after surgery relative to baseline was significantly greater in APOL1 risk homozygotes than in controls (%ΔCr 69.1% [95% confidence interval {CI} 41.9–96.3] v 29.6% [95% CI 21.1–38.0]; p = 0.005) (Tables 1 and 2). The initial multivariable model included sex, PVD, cross-clamp time, CPB time, and ejection fraction because these variables met p < 0.15 in the univariate analysis for %ΔCr. However, in the backward selection process, PVD and CPB time were not significantly associated and therefore were not included in the final multivariable model. Multivariable regression analysis demonstrated significantly increased %ΔCr in patients with APOL1 risk alleles compared with APOL1 controls after adjustment for sex, cross-clamp time, and ejection fraction. The %ΔCr mean difference between APOL1 risk and APOL1 controls was 44.8% [95% CI 13.3–76.3]; p = 0.006) (see Table 2 and Fig. 2). Furthermore, model diagnosis showed no collinearity among predictors in the model with a variance inflation factor near 1 (see Table 2).
Table 2.
Association of APOL1 Risk Genotype With %ΔCr in the Analysis Dataset
| Model | Variable | Mean Difference (SE) | 95% CI | p Value | VIF |
|---|---|---|---|---|---|
| Univariate | APOL1 risk (recessive) | 39.5 (13.7) | 12.28–66.71 | 0.005 | --- |
| Multivariable | APOL1 risk (recessive) | 44.8 (15.8) | 13.29–76.27 | 0.006 | 1.02 |
| Sex (male) | −17.6 (8.1) | − 33.72 to −1.51 | 0.03 | 1.01 | |
| Cross-clamp time | 0.11 (0.1) | − 0.02 to 0.31 | 0.09 | 1.08 | |
| Ejection fraction | − 0.5 (0.3) | −0.93 to 0.14 | 0.15 | 1.07 |
NOTE. Because the outcome is %ΔCr and APOL1 is coded as a recessive model, the mean difference is the beta-coefficient estimate of APOL1. The interpretation of the APOL1 effect is that the rare homozygous APOL1 risk genotype has an increase of 44.8%ΔCr compared with the APOL1 control genotype.
Abbreviations: APOL1, apolipoprotein L1; %ΔCr, peak serum creatinine rise after surgery relative to preoperative creatinine; CI, confidence interval; SE, xxxx; VIF, variance inflation factor.
Fig. 2.
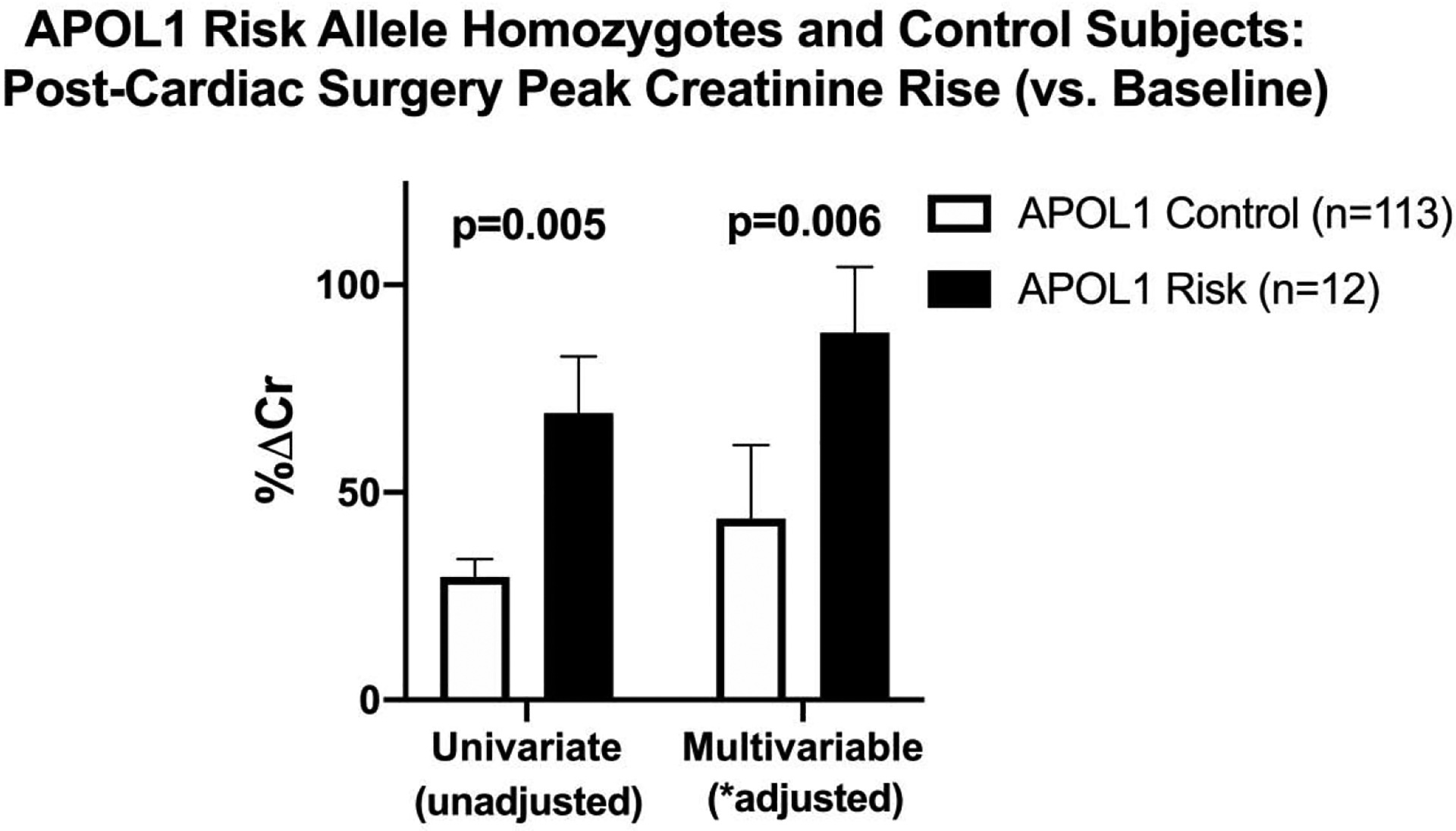
Comparison of postcardiac surgery creatinine rise (v baseline) between subjects with apolipoprotein L1 risk and control alleles.*Multivariable analysis adjusted for sex, aortic cross-clamp duration, and ejection fraction. APOL1, apolipoprotein L1; %ΔCr, peak serum creatinine rise after surgery relative to preoperative creatinine.
Association of APOL1 Genotype With KDIGO-Defined AKI
For the secondary outcome, KDIGO AKI rates were compared with APOL1 status. Because KDIGO AKI status was a binary outcome, the same set of covariates as for the primary continuous outcome were not used, and instead, only variables that were significant by univariate comparison (ie, age, ejection fraction, BMI, and diabetes mellitus) were used. BMI was excluded from the final model during the backward variable selection process. The Hosmer-Lemeshow test demonstrated that the final model met the goodness of fit criteria (p = 0.48). In this secondary analysis comparing KDIGO AKI rates between APOL1 risk and control subjects, only trends toward significance were observed (odds ratio 2.80, 95% CI 0.64–12.13; multivariable p = 0.17) (Tables 1 and 3).
Table 3.
Association of APOL1 Risk Genotype With KDIGO-Defined AKI in the Analysis Dataset
| Model | Variable | OR | 95% CI | p Value |
|---|---|---|---|---|
| Univariate | APOL1 risk (recessive) | 1.42 | 0.41–5.01 | 0.58 |
| Multivariable | APOL1 risk (recessive) | 2.80 | 0.64–12.13 | 0.17 |
| Age | 1.05 | 1.01–1.09 | 0.02 | |
| Ejection fraction | 0.96 | 0.93–0.99 | 0.006 | |
| Diabetes | 2.60 | 1.09–6.19 | 0.03 |
Abbreviations: APOL1, apolipoprotein L1; CI, confidence interval; KDIGO, Kidney Disease: Improving Global Outcomes; OR, odds ratio.
Discussion
In a cohort of African American cardiac surgery patients with good renal function before surgery, a postoperatively higher (2-fold) average peak creatinine rise was demonstrated in those homozygous for APOL1 risk genotypes compared with controls, reflecting a statistically significant difference even after accounting for known renal risk factors. In a similar analysis using the less granular dichotomous KDIGO consensus AKI criteria, a trend toward higher CS-AKI rates in APOL1 risk patients also was observed, but this did not reach statistical significance. Taken together, these findings suggest that homozygous status for APOL1 risk alleles is associated with CS-AKI development.
Two previous clinical studies examined AKI risk and APOL1 gene polymorphisms. An AKI case control genome-wide association study in a cohort of 1,429 critically ill patients identified 2 single-nucleotide polymorphisms at a risk locus on chromosome 4 near the APOL1-regulator IRF2,28 a mediator of TLR-3–mediated upregulation of APOL1 production.29 Notably, in the context of CKD, upregulation of APOL1 is believed to contribute to disease progression.29 Although not limited to African American patients, the study by Zhao et al. did adjust for ethnic structure and, like the present analysis, used a sensitive marker for AKI.28 In contrast, a second community-based investigation used a nonstandard criterion (ie, billing codes) to assess AKI rates in 2,240 African American subjects and did not find an association with APOL1 CKD risk alleles, but those authors readily acknowledged the limitations of their AKI definition.12 Hence, even though no equivalent analyses are available to directly compare with the present study, these previous investigations do not conflict with the present study’s main finding, that APOL1 CKD risk alleles are associated with AKI risk.
Speculation is warranted on implications and potential explanatory mechanisms for the present study’s findings. The greater incidence of CS-AKI in African American cardiac surgery patients may be explained partly by the high prevalence of APOL1 risk allele homozygote status in this group. The 2 APOL1 coding variants (G1 and G2) were first related to human disease as a result of their ability to lyse trypanosomes responsible for the acute form of sleeping sickness (Trypanosoma brucei rhodesiense). Heterozygotes are resistant to this parasitic protozoan infection, which is endemic in 13 eastern and southern African countries.17,30,31 The benefit of enhanced innate immunity from these variants presumably explains their prevalence in African Americans (10%−15%), and they are not seen in Caucasians.17 However, homozygote status has since been recognized to increase CKD risk, which partially may explain the 4- to 5-fold higher CKD rates in African Americans compared with Caucasians.32 Furthermore, patients with risk allele–associated CKD have been noted to have more interstitial fibrosis.33 It is plausible that the APOL1-related AKI risk noted in the present study also is associated with increased fibrosis. If confirmed, this effect could contribute to the well-described link between AKI frequency and severity and subsequent CKD development.34,35 Finally, CKD risk in homozygotes occurs in a pattern of incomplete penetrance, as seen in the setting of hypertensive end-stage renal disease, focal and segmental glomerulosclerosis, and human immunodeficiency virus–associated nephropathy.16–18,29 Evidence from human immunodeficiency virus infection and patients receiving interferon therapy suggests homozygotes also require a “second hit,” such as inflammation, to cause kidney damage.16,29,36 In cell culture, toll-like receptor agonists (mediated through TLR-3–dependent pathways) and interferons increase APOL1 production up to 200-fold.29 Inflammation-mediated upregulation of APOL1 production is believed to be an important inducer of CKD in homozygotes.29 The major inflammatory insult of CPB in the setting of cardiac surgery is well-described37,38 and may be pertinent to APOL1-homozygote–related AKI risk (if a second hit is similarly required) and a potentially interesting renoprotective target in African Americans.
Two additional mechanisms through which APOL1 may promote CKD also could have relevance to AKI pathophysiology. First, APOL1 is present in podocytes,39 and podocytes expressing APOL1 variants may be deficient in autophagy and more prone to the injurious effects of toxic aggregates and oxidative stress.40,41 The renoprotective role of autophagy is recognized,42–47 and defective APOL1-mediated autophagy may facilitate AKI development. Second, APOL1 is present in antioxidative HDL3 particles, and dysfunctional HDL with altered antioxidative properties may add to renal risk associated with APOL1 coding variants.48 Because higher levels of protective HDL are associated with protection from AKI,49 it is possible that the presence of APOL1 risk alleles changes the ratio of protective and dysfunctional HDL to contribute to AKI.
Although the CATHGEN database is one of the largest of its kind in which to study the effects of APOL1 risk phenotype on CS-AKI development, an important limitation of the present study is the small sample size of the APOL1 risk group in the analysis dataset (12 patients). In addition, the standard deviation for %ΔCr was larger in the APOL1 risk group, at least partly attributable to 1 patient. Furthermore, the retrospective nature of the present study adds a risk for unrecognized bias. Nonetheless, the primary finding relating homozygous status for APOL1 CKD risk genotypes to elevated AKI risk is statistically significant and aligns with the pattern of CKD risk that formed the basis of the present study’s hypothesis. Along these lines, a recessive risk allele approach (ie, G1/G1, G1/G2, G2/G2) was selected for analysis because of the known renal risk of these genotypes in the CKD setting; however, an additive risk allele model also may have renal risk (ie, G1/WT, G2/WT, G1/G1, G1/G2, G2/G2). Therefore, a secondary analysis was performed to assess an additive model, which was nonsignificant in univariate and multivariable analyses (p = 0.07 and p = 0.16, respectively). Lastly, a nontraditional approach to investigate AKI was used, comparing the peak rise in serum creatinine in the several days after surgery to the baseline value before surgery (%ΔCr). Notably %ΔCr allows for a linear characterization of AKI and has been widely accepted as a more sensitive measure of reductions in glomerular filtration that has been used in numerous previous studies investigating the genetic basis of AKI risk after cardiac surgery10,11,13,22 (ie, %ΔCr reflects a spectrum of injury to the kidneys that often does not meet dichotomous AKI threshold criteria). Indeed, the secondary analysis demonstrated only a trend in multivariable association of risk alleles with the less-sensitive dichotomous AKI definition (KDIGO) that did not meet statistical significance. Taken together, the present study’s findings require validation in larger datasets.
Conclusion
In summary, the present study demonstrated that African American cardiac surgery patients with high APOL1-related CKD risk profiles sustained greater kidney injury as reflected by a greater rise in serum creatinine relative to baseline, a sensitive marker for AKI development. The results implicate APOL1 coding variants in AKI development, much like their well-studied association with CKD. These findings contribute insights into both racial and genetic contributions to CS-AKI. Furthermore, investigations are warranted to assess the value of APOL1 variants as a preoperative renal risk stratification tool for African American cardiac surgery patients and as a novel target for renoprotective therapies.
Acknowledgments
This work was supported by 5R01-HL095987-06 and 1R01-HL127009-02 to SHS. Research reported in this publication was supported by the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health (award number P30DK096493).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- 1.Harris DG, Koo G, McCrone MP, et al. Acute kidney injury in critically ill vascular surgery patients is common and associated with increased mortality. Front Surg 2015;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel RM, Buckley MJ, Austen WG, et al. Etiology, incidence, and prognosis of renal failure following cardiac operations. Results of a prospective analysis of 500 consecutive patients. J Thorac Cardiovasc Surg 1976;71:323–33. [PubMed] [Google Scholar]
- 3.Swaminathan M, Shaw A, Phillips-Bute B, et al. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med 2007;35:2286–91. [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan M, Hudson CC, Phillips-Bute BG, et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg 2010;89:1098–104. [DOI] [PubMed] [Google Scholar]
- 5.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 2004;15:1597–605. [DOI] [PubMed] [Google Scholar]
- 6.Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrol Dialy Transplant 1999;14:1158–62. [DOI] [PubMed] [Google Scholar]
- 7.Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 1998;128:194–203. [DOI] [PubMed] [Google Scholar]
- 8.Brown JR, Parikh CR, Ross CS, et al. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg 2014;97:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444–53. [DOI] [PubMed] [Google Scholar]
- 10.Stafford-Smith M, Li YJ, Mathew JP, et al. Genome-wide association study of acute kidney injury after coronary bypass graft surgery identifies susceptibility loci. Kidney Int 2015;88:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stafford-Smith M, Podgoreanu M, Swaminathan M, et al. Association of genetic polymorphisms with risk of renal injury after coronary bypass graft surgery. Am J Kidney Dis 2005;45:519–30. [DOI] [PubMed] [Google Scholar]
- 12.Grams ME, Matsushita K, Sang Y, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol 2014;25:1834–41.24722442 [Google Scholar]
- 13.Chew STH, Newman MF, White WD, et al. Preliminary report on the Association of Apolipoprotein E Polymorphisms, with postoperative peak serum creatinine concentrations in cardiac surgical patients. Anesthesiology 2000;93:325. [DOI] [PubMed] [Google Scholar]
- 14.Larach DB, Engoren MC, Schmidt EM, et al. Genetic variants and acute kidney injury: A review of the literature. J Crit Care 2018;44:203–11. [DOI] [PubMed] [Google Scholar]
- 15.He L, Wei Q, Liu J, et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int 2017;92:1071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruzel-Davila E, Wasser WG, Aviram S, et al. APOL1 nephropathy: From gene to mechanisms of kidney injury. Nephrol Dial Transplant 2015;31:349–58. [DOI] [PubMed] [Google Scholar]
- 17.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010;329:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013;369:2183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med 2017;43:1551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus WE, Granger CB, Sketch MH Jr, et al. A guide for a cardiovascular genomics biorepository: The CATHGEN experience. J Cardiovasc Transl Res 2015;8:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köttgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nature Genet 2009;41:712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AD, Stafford-Smith M, White WD, et al. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med 2008;358:784–93. [DOI] [PubMed] [Google Scholar]
- 23.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kid Dis 2013;61:649–72. [DOI] [PubMed] [Google Scholar]
- 24.Robert AM, Kramer RS, Dacey LJ, et al. Cardiac surgery-associated acute kidney injury: A comparison of two consensus criteria. Ann Thorac Surg 2010;90:1939–43. [DOI] [PubMed] [Google Scholar]
- 25.Englberger L, Suri RM, Li Z, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care 2011;15:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIlroy DR, Argenziano M, Farkas D, et al. Incorporating oliguria into the diagnostic criteria for acute kidney injury after on-pump cardiac surgery: Impact on incidence and outcomes. J Cardiothorac Vasc Anesth 2013;27:1145–52. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Pun PH, Kwee L, et al. Apolipoprotein L1 genetic variants are associated with chronic kidney disease but not with cardiovascular disease in a population referred for cardiac catheterization. Cardiorenal Med 2016;7:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao B, Lu Q, Cheng Y, et al. A genome-wide association study to identify single-nucleotide polymorphisms for acute kidney injury. Am J Respir Crit Care Med 2016;195:482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int 2015;87:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 2003;422:83–7. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005;309:469–72. [DOI] [PubMed] [Google Scholar]
- 32.Friedman DJ, Kozlitina J, Genovese G, et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 2011;22:2098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp JB, Winkler CA, Zhao X, et al. Clinical features and gistology of apolipoprotein L1- associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol 2015;26:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 35.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson C, Hudson J, Swaminathan M, et al. Emerging concepts in acute kidney injury following cardiac surgery. Semin Cardiothorac Vasc Anesth 2008;12:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: Current understanding and future directions. Crit Care (London, England) 2016;20:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu CA, Klopfer EI, Ray PE. Human apolipoprotein L1 (ApoL1) in cancer and chronic kidney disease. FEBS Lett 2012;586:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhaorigetu S, Wan G, Kaini R, et al. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy 2008;4:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan G, Zhaorigetu S, Liu Z, et al. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 2008;283:21540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi A, Kimura T, Takabatake Y, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol 2012;180:517–25. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Hartleben B, Kretz O, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 2012;8:826–37. [DOI] [PubMed] [Google Scholar]
- 44.Kimura T, Takabatake Y, Takahashi A, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 2011;22:902–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang M, Wei Q, Dong G, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 2012;82:1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isaka Y, Kimura T, Takabatake Y. The protective role of autophagy against aging and acute ischemic injury in kidney proximal tubular cells. Autophagy 2011;7:1085–7. [DOI] [PubMed] [Google Scholar]
- 47.Hsiao HW, Tsai KL, Wang LF, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock 2012;37:289–96. [DOI] [PubMed] [Google Scholar]
- 48.Fornoni A, Merscher S, Kopp JB. Lipid biology of the podocyte—new perspectives offer new opportunities. Nature reviews. Nephrology 2014;10:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith LE, Smith DK, Blume JD, et al. High-density lipoprotein cholesterol concentration and acute kidney injury after cardiac surgery. J Am Heart Assoc 2017(6). [DOI] [PMC free article] [PubMed] [Google Scholar]


