Abstract
Pre-existing immunity to dengue virus (DENV) can either protect against or exacerbate, a phenomenon known as antibody dependent enhancement (ADE), a secondary DENV infection. DENV, as an escalating health problem worldwide, has increased the urgency to understand the precise parameters shaping the anti-DENV antibody (Ab) and T cell responses, thereby tipping the balance towards protection versus pathogenesis. Herein, we present the current state of knowledge of about the interplay between the Ab and T cell responses that dictate the outcome of DENV infection and discuss how this newfound knowledge is reshaping strategies for developing safe and effective DENV vaccines.
Keywords: Dengue virus, antibody enhancement of disease, neutralizing Antibodies, crossreactive T cells
Introduction
Dengue virus (DENV) is a member of the Flaviviridae family of positive-sense single-stranded RNA viruses that includes Zika virus (ZIKV), yellow fever virus (YFV), and Japanese encephalitis virus (JEV). The flavivirus RNA genome encodes a large polyprotein that is cleaved into three structural proteins-envelope (E), membrane precursor (prM), and capsid (C), and seven non-structural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). Flavivirus polyproteins exhibit a high degree of sequence homology-68% to 78% among the DENV serotypes and 45% to 56% between DENV and other flaviviruses. As a result, flaviviruses readily elicit cross-reactive antibody (Ab)- and T cell responses. However, whether those immune responses protect against or exacerbate subsequent infections is the subject of intense research.
What is clear is that generation of a subneutralizing Ab response against flaviviruses can facilitate viral entry into Fc receptor-positive cells during a subsequent infection, thereby exacerbating disease. This process, known as Ab-dependent enhancement (ADE), is best illustrated by patients who develop severe dengue disease after recovery from an earlier DENV infection [1,2]. Most cases of severe dengue result from either secondary infection in older children and adults or primary infection of infants born to DENV-immune mothers. Consequently, countries with both a prevalence of flaviviruses and DENV seropositive populations are at increased risk of ADE [3–5]. The DENV vaccines developed to date may at least in theory exacerbate the public health situation, given that most were designed to generate an Ab response-heightening the potential for ADE reactions, if individuals develop poorly neutralizing Abs (nAbs) to the vaccine. Recent studies using non-human primates (NHP) and type 1 interferon receptor (Ifnar)-deficient mouse models validate human studies that suggest that cross-reactive nAb and T cell responses are crucial factors in driving protection vs ADE immune responses. In this review, we highlight recent research on these factors, and how understanding these mechanisms is reshaping DENV vaccine development and testing.
Protective and pathogenic role of antibodies during DENV infection
During a primary DENV infection, the Ab response is predominantly serotype-specific. Of the cross-reactive Abs that are generated during primary DENV infection, the ones that are also neutralizing closely resemble germline-encoded Ab sequences-requiring few somatic mutations to neutralize all four DENV serotypes ex-vivo [6]. Cross-reactive DENV Abs that do not meet the threshold affinity for neutralization need to undergo affinity maturation during secondary infection to increase the breadth of Ab reactivity [7] and enable efficient neutralization [8]. Indeed, after sequential homotypic or heterologous infections (eg, DENV1-DENV1 and JEV-DENV1), cross-neutralizing Abs can be detected against more than one DENV serotype or flavivirus [5].
Anti-flavivirus humoral immune response is largely targeted to various epitopes on the viral envelope (E) protein. These E protein-specific Abs can suppress, enhance, or have no effect on a subsequent infection by a heterologous flavivirus. Flaviviral E proteins consist of three dimer domains (EDI, EDII, EDIII). Highly potent nAbs can be elicited by complex quaternary epitopes, termed E dimer epitopes (EDE) that span EDI and EDIII on mature virions [9]. These EDE-specific Abs neutralize mature virus even at low Ab concentration, by cross-linking E monomers within and between E dimers to fully coat the virion and prevent conformational changes in E protein [10,11]. On the other hand, simple immunodominant epitopes on E monomers that are largely exposed on the immature virion, such as the highly conserved fusion loop (FL) of EDII, drive development of Abs that are highly cross-reactive but subneutralizing [9,12,13]. These subneutralizing Abs can instead increase the likelihood of ADE [1,14,15]. Thus, epitope specificity is one key factor that may impact the protective vs pathogenic potential of the DENV-reactive Ab response.
Another major factor that influences neutralization vs enhancement capacity of the flaviviral Ab response is the concentration of virus-reactive Abs, which is a function of the robustness of the initial Ab response and subsequent decay. A longitudinal pediatric cohort study revealed that an intermediate titer of DENV-specific Abs (vs low and high titers) associates with an increased probability of DENV ADE [1]. Thus, high titers of cross-reactive anti-DENV nAb can protect against secondary DENV infections that occur shortly after primary infection (Figure 1A). As these nAb titers decay over time, they reach an intermediate concentration that can drive ADE and eventually drop below a functional range to have no effect on infection outcome (Figure 1B). In line with this schematic, a recent Ab transfer study demonstrated that low concentrations of Abs from both asymptomatic and symptomatic human ZIKV infection but not naïve patients could induce ADE in mice infected with ZIKV [16]. This evidence implies that ADE can occur in the context of sequential infection with the same flavivirus. Consistent with this implication, reinfection with the same DENV serotype has been documented in humans [17,18]. However, an exact timeline for the Ab decay after a flaviviral infection likely varies by individual. A recent study established a diagnostic timepoint 1-year after DENV infection to define a minimum nAb titer that would correlate with continued protection against DENV ADE in humans [19].
Figure 1. Effects of nAb decay on secondary infection outcome.
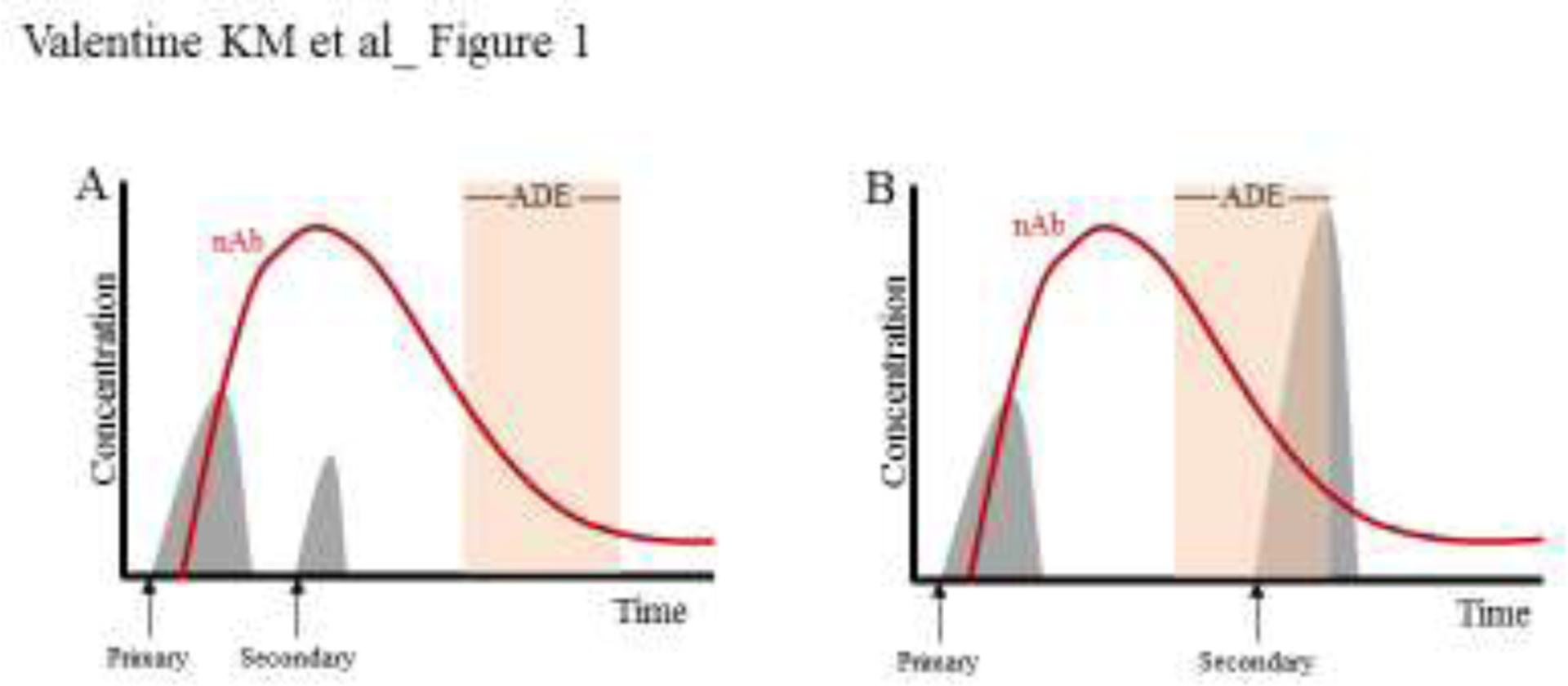
Primary DENV infection induces a nAb responses (red line) that rises and then decays, eventually reaching a titer range that can induce ADE (orange rectangle). The clinical outcome of a secondary DENV infection (grey histograms) depends on whether the challenge occurs A) prior to the ADE-inducing titer range, leading to a protective response, or B) after, leading to a large spike in virus and potentially ADE.
The role of nAb titers in determining flavivirus ADE vs protection has been most clearly demonstrated in parent-offspring studies. Infants born to DENV-exposed mothers acquire the maternal anti-DENV Abs via the placenta and are protected for first several months (typically 6 months), then become at risk to severe dengue disease for the next several months (between 6–9 months of age) but are immunologically naïve by 12 months of age [20]. Accordingly, some of the strongest data for the role of DENV Abs in dengue pathogenesis comes from the observations that 1) severe dengue disease has rarely been observed in infants born to DENV-naïve mothers, and 2) as the maternally transferred DENV Ab levels decrease, there is an increase in the probability of ADE in the offspring [21,22]. Consistent with these human findings, our recent work demonstrated that maternal Abs induced DENV ADE in mouse pups born to ZIKV-immune mothers and infected with DENV 4 to 5 weeks post-birth (ie, post-weaning) [23]. Reciprocally, two different groups showed that DENV-immune mothers infected with ZIKV during pregnancy increased ZIKV infection and resorption of fetuses [24,25]. More recently, we reported that ZIKV ADE could occur in mouse pups born to JEV-immune mothers [26]. In line with these mouse studies, A formaldehyde and UV-inactivated tetravalent dengue vaccine sensitized monkeys to enhanced viremia during challenge with DENV [27]. These recent studies lend further support to the concept that ADE may be observed when the nAb titer wanes and reaches below a protective threshold concentration--this feature of the flaviviral nAb response highlights a limitation in Ab-centric vaccines against DENV.
Protective role of T cells against DENV infection
Increasing evidence suggests that T cells provide protection against DENV infection, even in situations where Ab titers wane to levels conducive for ADE. DENV- or ZIKV-specific CD4 and CD8 T cells target different immunodominant epitopes: CD4 T cells largely target epitopes in structural proteins C and E and some variable NS epitopes, while CD8 T cells predominately target NS proteins including NS3 and NS5 and some additional E epitopes [28–32]. Recent reviews also describe a number of factors that might contribute to the formation of immunodominance patterns, including T cell subsets analyzed, the infecting virus type, and whether primary or secondary immune responses [33,34]. Early evidence of T cell activation, and especially IFN-γ production, are inversely associated with viremia and disease severity in primary DENV infection [35]. In secondary infections, DENV and ZIKV viral burden can be limited by cross-reactive CD4 and CD8 T cells specific for a broad range of conserved NS epitopes [36,37]. Then, type-specific and cross-reactive T cells can persist through convalescence after DENV infection in humans [38]. These memory T cells can be reactivated by DENV or ZIKV to promote target cell killing [38]. Thus, the presence of functional flavivirus-specific T cells during acute, convalescent, and resolved stages of DENV or ZIKV infections illustrate a potentially important protective role for T cells during natural infection or vaccination.
CD4 T cells:
T cells expand during acute flavivirus viral infection in humans [37,39], and with either primary DENV or ZIKV infections they adopt a T helper (Th) 1 phenotype and produce the associated cytokines IFN-γ, TNF and IL-2 [40,41]. In addition to Th1 responses, DENV and ZIKV lead to the expansion of populations of CXCR5+ T follicular helper (Tfh) cells and Foxp3+ T regulatory (Treg) cells [29,39,40]. Tfh cells are important for germinal center and neutralizing Ab development [29,42]. The contribution of Treg during DENV or ZIKV clearance has not been fully characterized, but they may be involved in limiting T cell responses [34].
During primary viral infections, CD4 T cells play a non-redundant indirect role to promote Ab production and CD8 T cell function in controlling systemic and tissue specific viral load [31]. Recent studies using mouse models of ZIKV infection have shown that, in the absence of CD4 T cells at specific infection sites, such as intravaginal ZIKV infections, viral infection is uncontrolled [29]. Although CD4 T cells may mediate some direct viral control, reduced Ab titers in the absence of CD4 T cells likely contributes to increased ZIKV burden [29]. However, in another study, memory CD4 T cells optimally protected Ifnar1−/− mice against ZIKV in combination with CD8 T cells [42], highlighting CD4 T cells as potent support for cytotoxic immune responses to aid control of infection.
Cross-reactive CD4 T cell responses that can promote neutralizing Abs and help CD8 T cells then represent a promising vaccine target that may address complications due to ADE. In humans, JEV-vaccination generates limited cross-reactive CD4 T cells, mostly against ZIKV and to a lesser extent DENV and YFV [5]. Ideally, robust cross-reactive CD4 T cells with broad epitope specificity would be elicited to convey lasting protection against multiple DENV serotypes and flaviviruses. To this end, our lab immunized Ifnar1−/− HLA-DRB1*0101 mice with DENV/ZIKV cross-reactive CD4 T cell epitopes in E, NS2A, NS4B and NS5. Immunization with these cross-reactive CD4 T cell peptides enhanced CD4 T cell responses and reduced viral burden after ZIKV challenge [41]. Thus, vaccine strategies that combine epitopes enhancing CD4 T cell help with epitopes driving cross-reactive B cells or CD8 T cells may provide robust cross-protection against different DENV serotypes and flaviviruses.
CD8 T cells:
Eliciting CD8 T cells that directly clear viral infection independent of Ab is likely important for lasting protection against reoccurring flavivirus infections. CD8 T cells isolated from patients during acute DENV or ZIKV infections express IFN-γ and adopt an activated cytolytic phenotype after ex vivo stimulation [32,43]. DENV- and ZIKV-specific CD8 T cells are frequently polyfunctional and when stimulated by viral peptides can co-produce IFN-γ with CD107a, granzyme B, or TNF [28,30,37,44,45]. In some individuals, DENV-specific CD8 T cells can persist with T effector memory (Tem) or T effector memory expressing CD45RA (Temra) phenotypes that display robust activation ex vivo after stimulation with DENV peptide [46]. In animal models, activated DENV- or ZIKV-specific CD8 T cells are essential for control of primary infection [44,47]. CD8 T cell depletion prior to primary DENV or ZIKV infection dramatically reduces host survival, with evidence suggesting direct CD8 T cell lysis of infected targets is largely responsible for viral clearance [28,30,42,48]. Even in the absence of CD4 T cell help, CD8 T cells can be induced and control the severity of DENV and ZIKV infection in Ifnar1−/− and LysMCre+Ifnar1fl/fl mouse models [29,42,49].
Adoptive transfer studies with cross-reactive CD8 T cells from DENV-immune mice have further illustrated protection against ZIKV challenge and vice versa [44,50]. Importantly, these primed CD8 T cells are potent enough to control viral burden even in the presence of immune sera that otherwise can induce ADE [51]. Even in pregnancy models of ZIKV infection, DENV-elicited CD8 T cells efficiently controlled ZIKV virus [49], negating cross-reactive Ab responses that could promote ADE [24,25,52]. DENV-immune CD8 T cells not only reduced viremia within the maternal spleen and placenta but also conveyed protection at the maternal-fetal interface and rescued fetal weight and size [49]. Although the exact mechanism of cross-reactive CD8 T cell protection is not fully defined, the efficient induction of these cross-reactive memory CD8 T cells is then an avenue for promoting lasting protection even in situations where ADE might normally result from prior flavivirus immunity (Figure 2A). This has been the focus of recent vaccine-related studies. Our lab tested a peptide-immunization strategy with DENV immunodominant CD8 T cell epitopes. In this setting, DENV2-immune CD8 T cells were efficiently induced in Ifnar−/− HLA-B*0702 and HLA-A*0101 transgenic mice and engendered cross-reactive protection against a subsequent ZIKV infection [53]. This lends further support to the concept that cross-reactive CD8 T cell recall responses generated by emerging vaccine strategies may contribute to protection against DENV and ZIKV even when the pre-existing Abs could result in ADE (Figure 2B).
Figure 2. T cells provide lasting protection even in the presence of ADE promoting nAb.
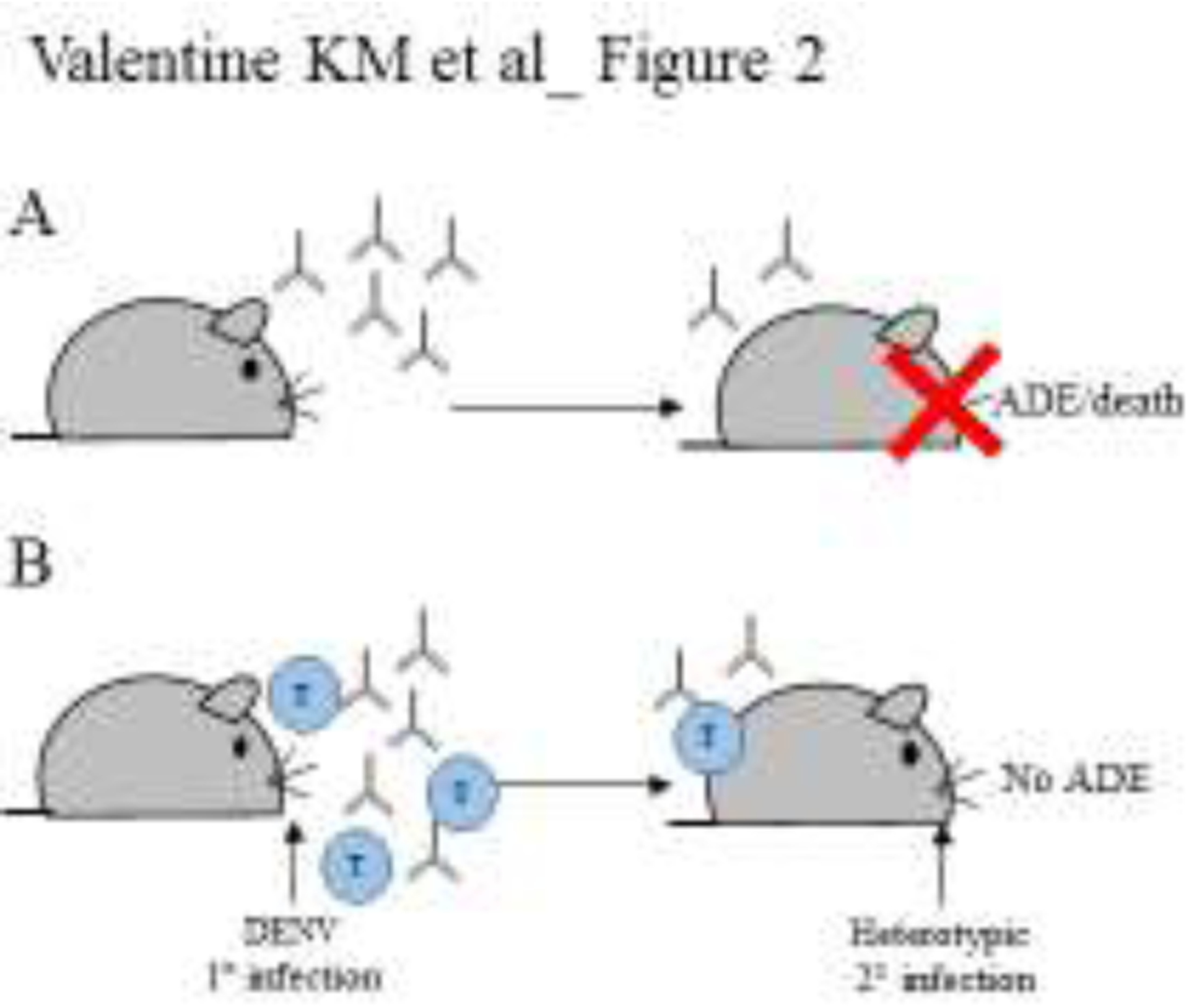
Exploiting mouse models permissive for DENV and ZIKV infection. A) After a primary DENV infection that induces only Ab responses, a secondary challenge is likely to promote ADE. B) Whereas simultaneous Ab and T cell induction prevents ADE.
Balancing Ab and T cell responses to change DENV infection outcomes.
The effort to develop a DENV vaccine is 70 years old. Accumulating evidence indicates that traditional, nAb-inducing vaccines are likely to generate ADE complications. In fact, Dengvaxia, the only DENV vaccine approved for human use is not approved for use in children less than 9-years-old or naive individuals, since it appears to prime these patient populations for more severe DENV infection. To develop safe and effective DENV vaccines that induce sustained immunity requires understanding the factors and mechanisms driving protective vs pathogenic immune responses. In this regard, extensive longitudinal Ab surveys of endemic regions, characterization of Ab interactions, and evaluation of T cell characteristics, have begun to define potential mechanisms of immune protection against DENV.
A better approach than Ab-centric DENV vaccines is to incorporate Ab vaccination strategies with those that also capitalize on the protective effects of memory T cell responses. The NIH DENV vaccine TV005, which is a combination of attenuated DENV1, 3, and 4 with a DENV2/4 chimeric viruses, induces CD4 and CD8 T cells responses to different DENV serotypes [54]. Another live-attenuated DENV vaccine, TAK-003, is a recombinant tetravalent platform incorporating DENV2 backbone with prM and E genes from DENV1, 3, and 4, and this vaccine elicits CD8 T cells with cross-reactive cytokine and proliferative function against all four DENV serotypes [55]. Results of the ongoing phase 3 trial data on TV005 and TAK-003 vaccines should provide critical insights into the potential role of vaccine-induced T cells in conferring protection against DENV. Based on recent human and animal model data, DENV vaccines that induce robust nAb responses and robust memory CD4 and CD8 T cell responses will be key. Achieving a proper balance of these Ab and T cell responses may be challenging with live-attenuated vaccines due to potential dominant effects of one serotype over the other three serotypes. However, new advances in the development of subunit vaccine platforms offer promising avenues for designing next generation DENV vaccines that induce robust and balanced Ab and T cell responses.
Funding sources:
This work was supported by the NIH/La Jolla Institute for Immunology Training grant T32 AI125179 awarded to KMV and NIH grants AI116813, AI140063, and NS106387 awarded to SS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure:
The authors have declared that no conflict of interest exists.
References:
- 1.**.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E: Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors combine inhibitory ELISAs and statistical modeling of DENV infection severity in a Nicaraguan pediatric cohort to define clinical parameters associated with DENV ADE. This study defines a distinct intermediate Ab titer that predicts the likelihood of ADE during secondary infection.
- 2.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. : Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 2018, 379:327–340. [DOI] [PubMed] [Google Scholar]
- 3.Inziani M, Adungo F, Awando J, Kihoro R, Inoue S, Morita K, Obimbo E, Onyango F, Mwau M: Seroprevalence of yellow fever, dengue, West Nile and chikungunya viruses in children in Teso South Sub-County, Western Kenya. Int J Infect Dis 2019, 91:104–110. [DOI] [PubMed] [Google Scholar]
- 4.Montecillo-Aguado MR, Montes-Gomez AE, Garcia-Cordero J, Corzo-Gomez J, Vivanco-Cid H, Mellado-Sanchez G, Munoz-Medina JE, Gutierrez-Castaneda B, Santos-Argumedo L, Gonzalez-Bonilla C, et al. : Cross-Reaction, Enhancement, and Neutralization Activity of Dengue Virus Antibodies against Zika Virus: A Study in the Mexican Population. J Immunol Res 2019, 2019:7239347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.*.Saron WAA, Rathore APS, Ting L, Ooi EE, Low J, Abraham SN, St John AL: Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci Adv 2018, 4:eaar4297.v [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors evaluate the role of prior flavivirus (JEV, YFV, and DENV) immunity on cross-reactive and CD4 T cell responses. Memory CD4 T cells isolated from JEV-vaccinated patients can be reactivated against not only JEV but also DENV, YFV and ZIKV.
- 6.Hu D, Zhu Z, Li S, Deng Y, Wu Y, Zhang N, Puri V, Wang C, Zou P, Lei C, et al. : A broadly neutralizing germline-like human monoclonal antibody against dengue virus envelope domain III. PLoS Pathog 2019, 15:e1007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.*.Andrade P, Narvekar P, Montoya M, Michlmayr D, Balmaseda A, Coloma J, Harris E: Primary and secondary dengue virus infections elicit similar memory B cell responses but breadth to other serotypes and cross-reactivity to Zika virus is higher in secondary dengue. J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluates differences in memory B cell responses between primary and secondary DENV infections . The authors dmonstrate that prior DENV immunity enahnces cross-reactivity of memory B cell responses suggesting that sequential DENV infections informs B cell maturation.
- 8.Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, et al. : Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 2017, 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. : Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536:48–53. [DOI] [PubMed] [Google Scholar]
- 10.Fibriansah G, Tan JL, Smith SA, de Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, de Silva AM, Crowe JE, et al. : A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat Commun 2015, 6:6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan SS, Miller A, Sapparapu G, Fernandez E, Klose T, Long F, Fokine A, Porta JC, Jiang W, Diamond MS, et al. : A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat Commun 2017, 8:14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripoll DR, Wallqvist A, Chaudhury S: Molecular Simulations Reveal the Role of Antibody Fine Specificity and Viral Maturation State on Antibody-Dependent Enhancement of Infection in Dengue Virus. Front Cell Infect Microbiol 2019, 9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel B, Longo P, Miley MJ, Montoya M, Harris E, de Silva AM: Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl Trop Dis 2017, 11:e0005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang WH, Urbina AN, Wu CC, Lin CY, Thitithanyanont A, Assavalapsakul W, Lu PL, Chen YH, Wang SF: An epidemiological survey of the current status of Zika and immune interaction between dengue and Zika infection in Southern Taiwan. Int J Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, Tan NWW, Chan KWK, Vasudevan SG: Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J Infect Dis 2019, 219:223–233. [DOI] [PubMed] [Google Scholar]
- 16.Shim BS, Kwon YC, Ricciardi MJ, Stone M, Otsuka Y, Berri F, Kwal JM, Magnani DM, Jackson CB, Richard AS, et al. : Zika Virus-Immune Plasmas from Symptomatic and Asymptomatic Individuals Enhance Zika Pathogenesis in Adult and Pregnant Mice. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forshey BM, Reiner RC, Olkowski S, Morrison AC, Espinoza A, Long KC, Vilcarromero S, Casanova W, Wearing HJ, Halsey ES, et al. : Incomplete Protection against Dengue Virus Type 2 Re-infection in Peru. PLoS Negl Trop Dis 2016, 10:e0004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messer WB, Yount B, Hacker KE, Donaldson EF, Huynh JP, de Silva AM, Baric RS: Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS Negl Trop Dis 2012, 6:e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salje H, Cummings DAT, Rodriguez-Barraquer I, Katzelnick LC, Lessler J, Klungthong C, Thaisomboonsuk B, Nisalak A, Weg A, Ellison D, et al. : Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 2018, 557:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP: Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis 2002, 8:1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guignard A, Haguinet F, Wery S, Kerdpanich P: Prevalence and Persistence of Maternal Dengue Neutralizing Antibodies in Infants From Central and Southern Thailand: A Retrospective Cohort Study. Asia Pac J Public Health 2019, 31:288–295. [DOI] [PubMed] [Google Scholar]
- 22.Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Lien le B, Quy NT, Hieu NT, Hien TT, et al. : Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis 2007, 196:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.*.Fowler AM, Tang WW, Young MP, Mamidi A, Viramontes KM, McCauley MD, Carlin AF, Schooley RT, Swanstrom J, Baric RS, et al. : Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 2018, 24:743–750 e745. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is one of the first to define ZIKV ADE induced by maternally trasnfered Ab in mice. The authors demonstrate that pups post-weaning from ZIKV-immune mothers but not ZIKV-naïve mothers develop severe TNF-dependent dengue.
- 24.Brown JA, Singh G, Acklin JA, Lee S, Duehr JE, Chokola AN, Frere JJ, Hoffman KW, Foster GA, Krysztof D, et al. : Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy. Immunity 2019, 50:751–762 e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathore APS, Saron WAA, Lim T, Jahan N, St John AL: Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv 2019, 5:eaav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Duan Z, Zhou W, Zou W, Jin S, Li D, Chen X, Zhou Y, Yang L, Zhang Y, et al. : Japanese encephalitis virus-primed CD8+ T cells prevent antibody-dependent enhancement of Zika virus pathogenesis. J Exp Med 2020, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borges MB, Marchevsky RS, Carvalho Pereira R, da Silva Mendes Y, Almeida Mendes LG, Diniz-Mendes L, Cruz MA, Tahmaoui O, Baudart S, Freire M, et al. : Detection of post-vaccination enhanced dengue virus infection in macaques: An improved model for early assessment of dengue vaccines. PLoS Pathog 2019, 15:e1007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, Shresta S: Mapping and Role of the CD8(+) T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elong Ngono A, Young MP, Bunz M, Xu Z, Hattakam S, Vizcarra E, Regla-Nava JA, Tang WW, Yamabhai M, Wen J, et al. : Correction: CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog 2019, 15:e1007821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassert M, Harris MG, Brien JD, Pinto AK: Identification of Protective CD8 T Cell Responses in a Mouse Model of Zika Virus Infection. Front Immunol 2019, 10:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassert M, Wolf KJ, Schwetye KE, DiPaolo RJ, Brien JD, Pinto AK: CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog 2018, 14:e1007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, et al. : Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013, 87:2693–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elong Ngono A, Shresta S: Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development. Front Immunol 2019, 10:1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y, Grifoni A, Sette A, Weiskopf D: Human T Cell Response to Dengue Virus Infection. Front Immunol 2019, 10:2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijeratne DT, Fernando S, Gomes L, Jeewandara C, Ginneliya A, Samarasekara S, Wijewickrama A, Hardman CS, Ogg GS, Malavige GN: Quantification of dengue virus specific T cell responses and correlation with viral load and clinical disease severity in acute dengue infection. PLoS Negl Trop Dis 2018, 12:e0006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgado FG, Torres KI, Castellanos JE, Romero-Sánchez C, Simon-Lorière E, Sakuntabhai A, Roth C: Improved Immune Responses Against Zika Virus After Sequential Dengue and Zika Virus Infection in Humans. Viruses 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grifoni A, Pham J, Sidney J, O’Rourke PH, Paul S, Peters B, Martini SR, de Silva AD, Ricciardi MJ, Magnani DM, et al. : Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrera BB, Tsai WY, Brites C, Luz E, Pedroso C, Drexler JF, Wang WK, Kanki PJ: T Cell Responses to Nonstructural Protein 3 Distinguish Infections by Dengue and Zika Viruses. mBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haltaufderhyde K, Srikiatkhachorn A, Green S, Macareo L, Park S, Kalayanarooj S, Rothman AL, Mathew A: Activation of Peripheral T Follicular Helper Cells During Acute Dengue Virus Infection. J Infect Dis 2018, 218:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S: CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 2010, 185:5405–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen J, Wang YT, Valentine KM, Dos Santos Alves RP, Xu Z, Regla-Nava JA, Ngono AE, Young MP, Ferreira LCS, Shresta S: CD4(+) T Cells Cross-Reactive with Dengue and Zika Viruses Protect against Zika Virus Infection. Cell Rep 2020, 31:107566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas CGO, Kitoko JZ, Ferreira FM, Suzart VG, Papa MP, Coelho SVA, Cavazzoni CB, Paula-Neto HA, Olsen PC, Iwasaki A, et al. : Critical role of CD4(+) T cells and IFNgamma signaling in antibody-mediated resistance to Zika virus infection. Nat Commun 2018, 9:3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grifoni A, Costa-Ramos P, Pham J, Tian Y, Rosales SL, Seumois G, Sidney J, de Silva AD, Premkumar L, Collins MH, et al. : Cutting Edge: Transcriptional Profiling Reveals Multifunctional and Cytotoxic Antiviral Responses of Zika Virus-Specific CD8(+) T Cells. J Immunol 2018, 201:3487–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Li S, Zhang Y, Han X, Jia B, Liu H, Liu D, Tan S, Wang Q, Bi Y, et al. : CD8(+) T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardy RD, Rajah MM, Condotta SA, Taylor NG, Sagan SM, Richer MJ: Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog 2017, 13:e1006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian Y, Babor M, Lane J, Seumois G, Liang S, Goonawardhana NDS, De Silva AD, Phillips EJ, Mallal SA, da Silva Antunes R, et al. : Dengue-specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J Clin Invest 2019, 130:1727–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngono AE, Shresta S: Immune Response to Dengue and Zika. Annu Rev Immunol 2018, 36:279–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S: A protective role for dengue virus-specific CD8+ T cells. J Immunol 2009, 182:4865–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.**.Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh AT, Wang YT, Nguyen AT, Salgado R, Mamidi A, Kim K, Diamond MS, et al. : Cross-reactive Dengue virus-specific CD8(+) T cells protect against Zika virus during pregnancy. Nat Commun 2018, 9:3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen J, Elong Ngono A, Regla-Nava JA, Kim K, Gorman MJ, Diamond MS, Shresta S: Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat Commun 2017, 8:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a novel pregnancy model of ZIKV infection the authors demonstrate a role for prior DENV immunity in ZIKV pathogensis. These results are the first to show a protective role for CD8 T cell despite conditions that would otherwise induce DENV ADE.
- 51.Zellweger RM, Eddy WE, Tang WW, Miller R, Shresta S: CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J Immunol 2014, 193:4117–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. : Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24:731–742 e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen J, Tang WW, Sheets N, Ellison J, Sette A, Kim K, Shresta S: Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8(+) T cells. Nat Microbiol 2017, 2:17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grifoni A, Voic H, Dhanda SK, Kidd CK, Brien JD, Buus S, Stryhn A, Durbin AP, Whitehead S, Diehl SA, et al. : T cell responses induced by attenuated flavivirus vaccination are specific and show limited cross-reactivity with other flavivirus species. J Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waickman AT, Victor K, Li T, Hatch K, Rutvisuttinunt W, Medin C, Gabriel B, Jarman RG, Friberg H, Currier JR: Dissecting the heterogeneity of DENV vaccine-elicited cellular immunity using single-cell RNA sequencing and metabolic profiling. Nat Commun 2019, 10:3666. [DOI] [PMC free article] [PubMed] [Google Scholar]


