Abstract
High fat, low carbohydrate ketogenic diets (KD) have been in use for the treatment of epilepsy for almost a hundred years. Remarkably, seizures that are resistant to conventional anti-seizure drugs can in many cases be controlled by the KD therapy, and it has been shown that many patients with epilepsy become seizure free even after discontinuation of the diet. These findings suggest that KD combine anti-seizure effects with disease modifying effects. In addition to the treatment of epilepsy, KDs are now widely used for the treatment of a wide range of conditions including weight reduction, diabetes, and cancer. The reason for the success of metabolic therapies is based on the synergism of at least a dozen different mechanisms through which KDs provide beneficial activities. Among the newest findings are epigenetic mechanisms (DNA methylation and histone acetylation) through which KD exerts long-lasting disease modifying effects. Here we review mechanisms through which KD can affect neuroprotection in the brain, and how a combination of those mechanisms with epigenetic alterations can attenuate and possibly reverse the development of epilepsy.
Keywords: ketogenic diet, epilepsy, epigenetics, neuroprotection, antiepileptogenesis
Graphical abstract
1. Introduction
Therapeutically, ketogenic diets (KD) are best known for their efficacy in stopping seizures. In addition to stopping seizures, multiple lines of evidence suggest that a KD can be antiepileptogenic. In this review, we highlight the mechanisms mediated by the KD which confer neuroprotection and antiepileptogenesis. The antiepileptogenic effects of the KD were first reported anecdotally in the 1920’s (Wilder, 1921), and follow-up studies describe a consistent subset (10–20%) of patients that remain seizure-free even after discontinuation of the diet (Bergqvist et al., 2005; Caraballo et al., 2006; Coppola et al., 2010; DiMario and Holland, 2002; Hassan et al., 1999; Hemingway et al., 2001; Kang et al., 2007; Kossoff et al., 2010; Nordli Jr. et al., 2001; Panico et al., 2000; Sharma et al., 2009; Suo et al., 2013; Vining et al., 1998). The clinical investigation of antiepileptogenic mechanisms of KD ideally requires the implementation of KD therapy immediately after the initial diagnosis of epilepsy. Since KD therapy is often used as a last resort and only in highly refractory patients, obtaining a clear mechanistic understanding of the antiepileptogenic potential of the KD in a clinical setting is challenging. Given the promising and consistent subset of seizure-free cases following KD therapy, we rely on animal studies to unravel the antiepileptogenic mechanisms of the diet. In line with the clinical observations, experimental studies confirm the antiepileptogenic properties of the diet in various animal models: (1) kindling models (Hu et al., 2011; Jiang et al., 2012), (2) chemically-induced post status epilepticus (SE) models (Muller-Schwarze et al., 1999; Su et al., 2000), and (3) spontaneous seizure models - EL mice (Todorova et al., 2000).
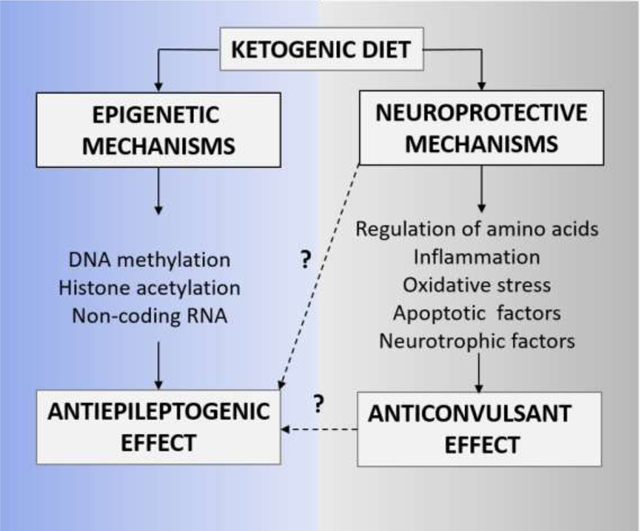
Detailed reviews of the proposed anti-seizure and disease-modifying mechanisms of the KD (D’Andrea Meira et al., 2019, Youngson et al., 2017, Boison, 2017; Masino and Rho, 2012) illustrate that a combination of several mechanisms likely contribute to potent seizure suppression including alterations in gut microbiome (Newell et al., 2016), regulation of kynurenine metabolism (Heischmann et al., 2018, Żarnowska et al., 2019) and rescued mitochondrial respiration and restored glucose metabolism (Kumar et al., 2016, Kim et al., 2015) and are not described in this review. The anti-seizure effects of KD is attributed to two key broader groups of mechanisms which are described by Elamin and Masino in this special issue (Elamin and Masino, 2020), and include (i) increase in free fatty acids, polyunsaturated fatty acids, chronic ketosis and the production of ketones such as beta-hydroxybutyrate (BHB), and (ii) reduced glucose mediated increases in adenosine and KATP channel activity (Lutas and Yellen, 2013; Masino et al., 2011a; Masino and Rho, 2012; Rho, 2015). While we have reasonable evidence of the anti-seizure effects of the KD therapy (Masino et al., 2011, Masino and Rho, 2012) but whether these mechanisms are implicated in epileptogenesis remain elusive. The antiepileptogenic effects of the KD is most commonly attributed to (i) adenosine metabolism and epigenetic mechanisms such as DNA methylation, histone acetylation and non-coding RNAs (ncRNA) and (ii) neuroprotective mechanisms such as regulation of neurotransmitters (GABA and glutamate) (Yudkoff et al., 2006, Yudkoff et al., 2008), inhibition of neuronal apoptosis (Gimenez-Cassina et al., 2012), increase in growth factors (Vizuete et al., 2013, Marosi et al., 2016) and regulation of inflammatory mediators (Dupuis et al., 2015). The distinction between mechanisms ascribed to the antiepileptogenic and antiseizure effects is not straightforward and an overlap is conceivable. Hence, in this review we specifically focus on KD-induced epigenetic and neuroprotective mechanisms which mediate long-lasting therapeutic outcomes even after discontinuation of the diet (Figure 1).
Figure 1: Epigenetic and Neuroprotective mechanisms of KD therapy.
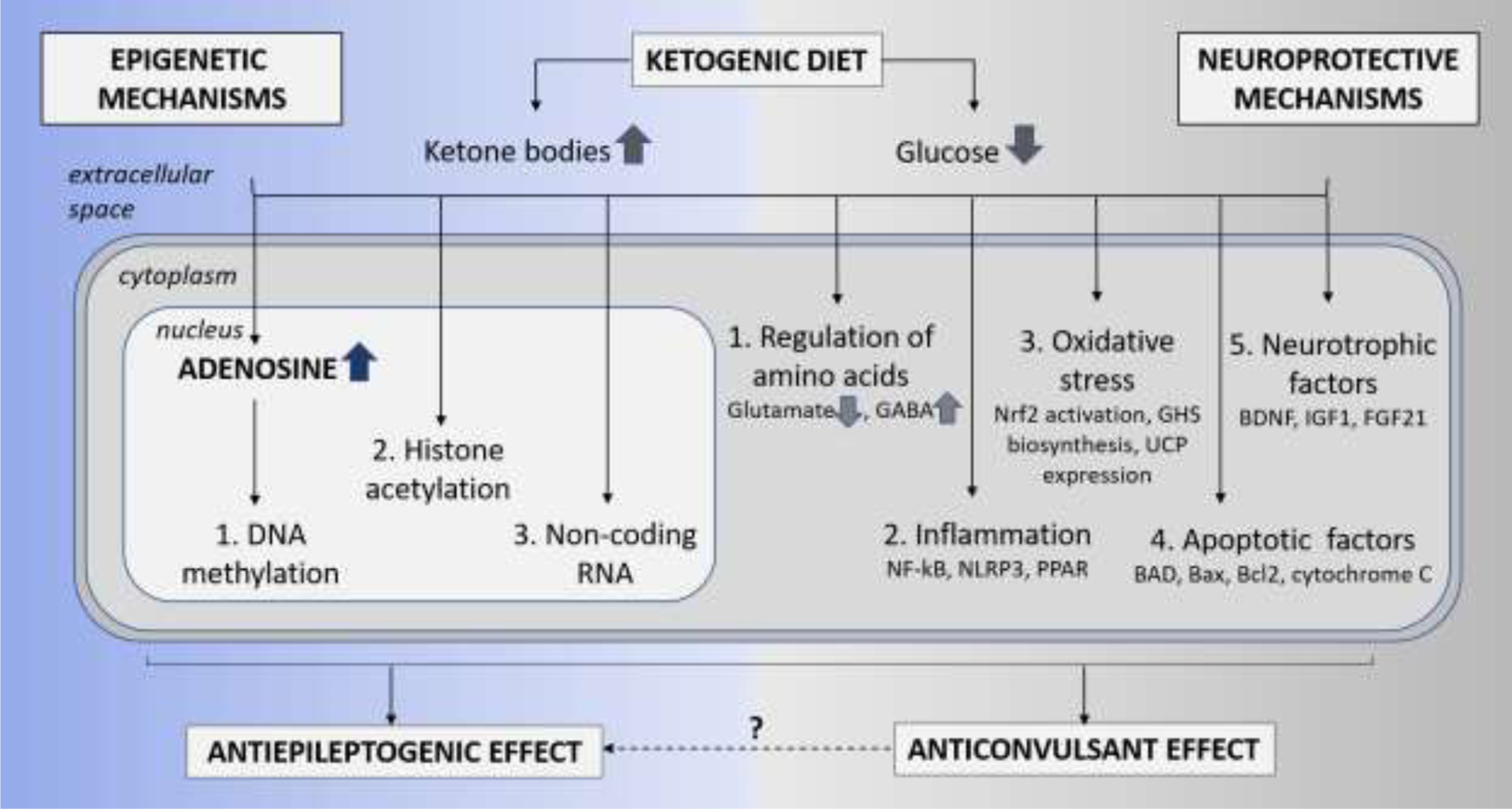
The schematic is a simplistic representation of the two broader mechanisms thought to play a critical role in the neuroprotective and antiepileptogenesis functions of the KD therapy. The epigenetic mechanisms include restoration of (1) DNA methylation, (2) histone acetylation, and (3) non-coding RNA. Whereas, the neuroprotective mechanisms include (1) regulation of amino acids resulting in reduced glutamate and increased GABA neurotransmitters, (2) reduction in inflammation and oxidative stress via activation of mediators such as NF-kB, NLRP3 and PPAR, (3) reduction in oxidative stress via activation of Nrf2 pathway, glutathione (GHS) biosynthesis and expression of uncoupling proteins (UCPs), (4) inhibition of apoptotic factors such as BAD, Bax, and cytochrome C, and (5) release of neurotrophic factors such as BDNF and FGF21.
1. Epigenetic Mechanisms of Epileptogenesis
Epileptogenesis, most commonly triggered by injuries to the brain, is a battery of plastic changes that lead to the development of spontaneous recurrent seizure activity in a previously healthy brain (Klein et al., 2018; Pitkanen et al., 2009; Pitkanen and Lukasiuk, 2009; Wu et al., 2013). Although the underlying mechanisms remain elusive, neuroinflammation, neurodegeneration, and epigenetic changes, are well-accepted contributors to the progression of epileptogenesis (Klein et al., 2018; Pitkanen et al., 2009; Wu et al., 2013). Epigenetic modifications are highly plastic changes to the genome without alterations in the DNA sequence or genetic code. These epigenetic changes are extremely powerful since they can preserve short-lived cellular signals and/or changes in neuronal activity as long-lasting influence on gene expression (Henshall and Kobow, 2015; Qureshi and Mehler, 2014). An increasing number of studies report that epigenetic processes such as DNA methylation, histone acetylation, and ncRNA expression are significantly altered in the epigenome of an epileptic brain (Boison and Rho, 2020; Debski et al., 2016; Graff et al., 2011; Jaenisch and Bird, 2003; Kiefer, 2007; Sweatt, 2013). Since epigenetic modifications play a crucial role in the regulation of gene expression, these mechanisms can affect the expression of several genes simultaneously and can represent risk factors for epilepsy. Further, unlike genetic mutations, epigenetic changes are potentially reversible and may constitute a novel target for therapeutic intervention. In this section, we highlight the emerging antiepileptogenic potential of epigenetic modulators, specifically those regulated by the KD (Figure 2).
Figure 2: Epigenetic mechanisms regulated by ketogenic diet.
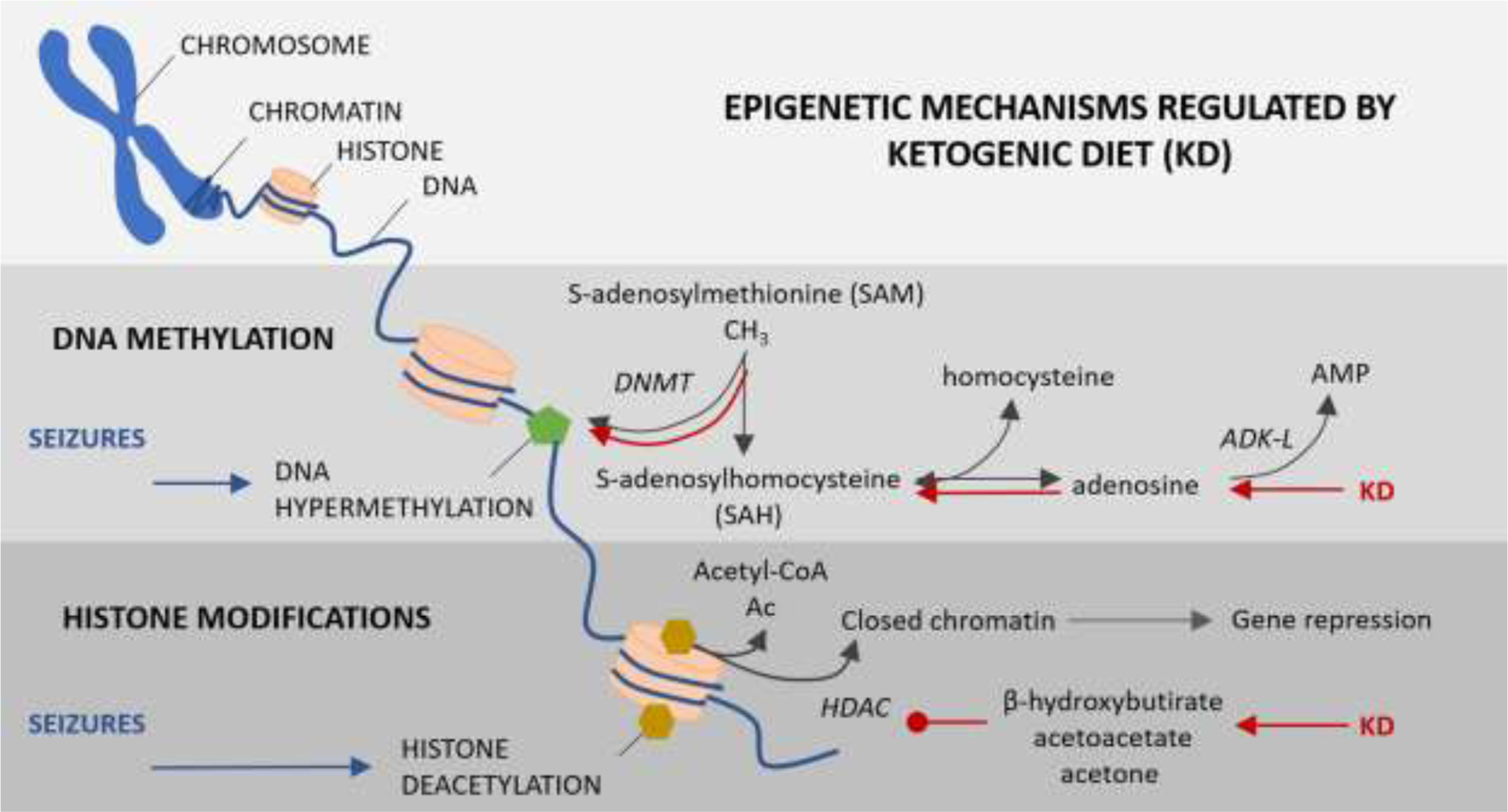
The diagram represents the two major epigenetic alterations noted in patients and animal models of temporal lobe epilepsy and the mechanisms regulated by KD. DNA methylation: Global DNA hypermethylation induced by seizures is restored by KD via adenosine augmentation, shift in the SAH and SAM homeostasis and aiding DNA methylation by DNA methyltransferases (DNMT). Histone acetylation: Seizure-induced histone deacetylation is catalyzed by histone deacetylase (HDACs) resulting in closed chromatin structure and transcriptional gene repression. KD-induced increase in ketone bodies such as β-hydroxybutyrate, acetoacetate and acetone reverses histone deacetylation by inhibiting HDACs.
2.1. DNA methylation regulated by adenosine
DNA methylation is the most prominently investigated epigenetic mechanism. The methylation of DNA is catalyzed by DNA methyltransferase (DNMT) and typically results in transcriptional repression of genes. Changes in global DNA methylation has been observed in epileptic hippocampus in both clinical and experimental settings. In human TLE samples from resected hippocampus, gene targets with both increased and decreased methylation were identified. Of note, 146 protein-coding genes exhibit altered DNA methylation in temporal lobe epilepsy hippocampus when compared to control of which approximately 80% of these gene promoters display hypermethylation, a common and prominent biomarker of sclerotic hippocampal tissue (Miller-Delaney et al., 2015). In rodent models of TLE, similar patterns of hypermethylation in the epileptic hippocampus have been demonstrated in kainic acid induced status epilepticus (KASE) models (Ryley Parrish et al., 2013; Williams-Karnesky et al., 2013a) and in pilocarpine induced status epilepticus models (Kobow et al., 2013; Lusardi et al., 2015). In line with this, adenosine was identified to be a key regulator of DNA methylation (Williams-Karnesky et al., 2013a). Adenosine has a mass effect on biochemical enzyme reactions and is an obligatory end-product of the S-adenosylmethionine (SAM) dependent transmethylation pathway, necessary for the transfer of methyl groups onto DNA (Boison et al., 2002; Williams-Karnesky et al., 2013). As predicted by this biochemical pathway, exogenous application of either adenosine or its complementary end product homocysteine inhibited the reaction and reduced DNA methylation, whereas addition of the methyl group donor SAM increased DNA methylation in the naive rodent brain (Williams-Karnesky et al., 2013a). In support of this, pharmacological augmentation of adenosine using 5-iodotubercidin (5-ITU), a pharmacological inhibitor of adenosine kinase (ADK) reduced hippocampal DNA methylation by 50%.
In an epileptic brain, the expression of the adenosine metabolizing enzyme ADK is upregulated particularly in astrocytes and causes adenosine deficiency in epileptogenic sclerotic tissue in a variety of rodent models of epilepsy (Gouder et al., 2004; Pignataro et al., 2008) as well as in human specimens resected from patients with temporal lobe epilepsy and hippocampal sclerosis (Li et al., 2008; Aronica et al., 2011). Hence, it is believed that lowered adenosine levels in the epileptic brain shift the equilibrium of the S-adenosylhomocysteine (SAH) hydrolase reaction away from the formation of SAH (Mandaviya et al., 2014), an inhibitor of DNA methyltransferase activity (James et al., 2002), thereby increasing the flux of DNA methylation reactions in the epileptic brain. Seizures resulting from the proconvulsant L-methionine-dl-sulfoximine, which increases the methylation flux by increasing the SAM/SAH ratio, can be blocked by adenosine and homocysteine (Gill and Schatz, 1985; Schatz et al., 1983; Sellinger et al., 1984). In the rat systemic KASE model, the direct ventricular administration of adenosine for 10 days significantly reduced epilepsy disease progression, including the progressive increase of spontaneous convulsive seizures and additional mossy fiber sprouting, and restored global DNA methylation to control levels, lasting well after the conclusion of the adenosine delivery (Williams-Karnesky et al., 2013). These findings show that global DNA methylation levels are under the direct control of adenosine, and that disruption of adenosine homeostasis (due to ADK upregulation at the epileptogenic focus) affected DNA methylation levels and altered gene expression in the epileptic brain. The KD augments adenosine signaling and can affect epileptogenesis through adenosine receptor-independent mechanisms via the interference with the transmethylation pathway (Lusardi et al., 2015, Williams-Karnesky et al., 2013), in addition to the adenosine receptor- dependent mechanisms (Masino et al., 2011). In line with this, a study showed that KD, but not a conventional antiepileptic drug (valproic acid), suppressed kindling induced epileptogenesis, an effect that persisted even after a return to a standard lab diet, while the conventional antiepileptogenic drug, valproic acid attenuated only the seizures without blocking the epileptogenic process. These data demonstrate persistent effects of the KD that are not merely due to seizure suppression (Lusardi et al., 2015). When fed to rats following status epilepticus, the KD not only reduced spontaneous seizure development but also reduced DNA methylation levels both during diet administration and after a return to standard diet (Kobow et al., 2013; Lusardi et al., 2015). Though a direct link between the KD and DNA methylation levels must still be demonstrated, taken collectively, these studies indicate that the lasting effects of the KD may be conferred via adenosine regulation of the DNA methylome, supporting a key mechanism implicated in epilepsy and epileptogenesis.
2.2. Histone acetylation
Histones are important proteins that maintain the chromatin structure in eukaryotic cells and regulate gene expression. Histone modifications such as acetylation and deacetylation are essential parts of gene regulation and are mediated by the enzymes histone acetyltransferase and histone deacetylase (HDAC), respectively (Simeone et al., 2017a). Altered histone acetylation has been noted in epilepsy patients as well as in animal models of epilepsy and is thought to be associated with epileptogenesis (Boison and Rho, 2020; Hartman and Rho, 2014; Hauser et al., 2018). Epileptic seizures triggered the deacetylation of histone H4 at the GluR2 locus (Huang et al., 2002; Tsankova et al., 2004), which is associated with increased neuronal excitability and the initiation of epileptogenesis (Tanaka et al., 2000). Experimental findings support the idea that KD, as well as ketone bodies formed from fatty acid oxidation, such as BHB, acetoacetate (ACA), and acetone may have antiepileptogenic potential by inhibiting HDACs (Boison and Rho, 2020; Hartman and Rho, 2014; Hauser et al., 2018; Simeone et al., 2017b; Tanaka et al., 2000). Thus, inhibition of HDAC activity by chronic administration of butyrate retarded the development of limbic epileptogenesis and prevented epileptogenic mossy fiber axonal sprouting in a mouse hippocampal kindling model of TLE (Reddy et al., 2018). Another study used Tuberous Sclerosis Complex genetically modified mice (TSC2+/− mice), a mouse model with characteristic developmental deficits including cognitive defects, autism and epilepsy. This study showed that altered mTORC1 signaling led to aberrant hippocampal synaptic plasticity, which was prevented by the inhibition of HDAC using trichostatin A (Basu et al., 2019). These studies confirm that KD reverses seizure-induced histone deacetylation primarily via the BHB-HDAC axis contributing to antiepileptogenesis.
2.3. Non-coding RNAs
The non-coding RNAs including short microRNA (miRNA) and long non-coding RNA (lncRNA), are capable of acting as epigenetic modulators, whereby they affect the protein levels of the target mRNAs without modifying the gene sequences. For instance, certain miRNAs such as miR-9, miR-124a, and miR-132 were identified to target NRSF/RE1-silencing transcription factor (REST), proteins with direct roles in epigenetics (Wu and Xie, 2006). Interestingly, each of these miRNAs were also altered in epilepsy (Jimenez-Mateos et al., 2011; Peng et al., 2013; Pichardo-Casas et al., 2012). A recent study showed global changes in miRNA expression in pediatric epilepsy patients after KD therapy (Olaso-González et al., 2018). However, many of these miRNAs were involved in antioxidant pathways, suggesting that KD-induced changes in miRNA expression might be involved in the prevention of oxidative stress and therefore be neuroprotective (Cannataro et al., 2019; Olaso-González et al., 2018). The array of miRNA changes induced by KD therapy, particularly those involved in epigenetic modifications, need to be further examined.
Currently, there is no direct evidence linking epigenetic mechanisms controlled by lncRNA in the therapeutic efficacy of KD, however research in other areas suggests that important roles will emerge and warrants further investigation. For instance, lncRNA Malat1 regulates dendritic spine density (Bernard et al., 2010) and loss of the lncRNA BC1 reduced convulsive thresholds (Wang et al., 2017; Zhong et al., 2009). Moreover, lncRNAs have been implicated in the regeneration of GABAergic neurons (Qureshi and Mehler, 2013). Also, a de novo mutation in an lncRNA (BX118339) was also recently implicated in a patient with West syndrome suggestive of key roles for these lncRNAs in epilepsy progression and prevention (Vandeweyer et al., 2012).
2. Neuroprotection
Neurodegeneration and the selective loss of certain neuron populations, in particular GABAergic interneurons, is a pathological hallmark of acquired epilepsies, and thought to be a driving factor for the development and progression of epilepsy. Several mechanisms of the KD have neuroprotective properties. KD induced neuroprotection may be one of the mechanisms underlying the antiepileptogenic properties of the diet. In addition to the epigenetic influence, changes in adenosine metabolism regulated by the KD are also neuroprotective. The neuroprotective effects of adenosine are mediated by the activation of pre- and postsynaptic adenosine A1 receptors (Gouder et al., 2003, Sun et al., 2005). The adenosine receptor-mediated anticonvulsant mechanisms have been extensively reviewed previously and are therefore excluded from this review (Cunha et al., 2005, Masino et al., 2014, Boison et al., 2010, Boison et al., 2012). In this section we discuss the regulation of amino acids, cytokines, neurotrophic and apoptotic factors by KD in the context of antiepileptogenesis.
3.1. Regulation of amino acids
One of the major mechanisms for the neuroprotective effect of KD is based on the regulation of amino acid levels in the brain. Of particular interest is glutamate metabolism, the main excitatory transmitter in the central nervous system. The switch from glucose to ketone bodies as a fuel alters brain amino acid metabolism, by reducing transamination of glutamate to aspartate and favoring decarboxylation of glutamate to form GABA (Daikhin and Yudkoff, 1998; Erecinska et al., 1996; Yudkoff et al., 1997; Yudkoff et al., 2001). In addition, ketosis in astrocytes favors the reduction of extracellular glutamate. This is achieved by efficient removal of excitatory glutamate from the synaptic cleft and conversion to glutamine by the enhanced astrocyte-based glutamine synthetase pathway (Yudkoff et al., 2005). In a rat model of pentylenetetrazole (PTZ)-induced seizures, enhanced disposal of brain glutamate prevented seizures and conferred neuroprotection following a relatively brief (24 h) period of calorie restriction (Yudkoff et al., 2006). Further, during ketosis, less glutamate is metabolized and more glutamine becomes available for the purpose of GABA synthesis (Yudkoff et al., 2008).
3.2. Inflammation and oxidative stress
Inflammation plays a major role in the pathophysiology of epilepsy, particularly epileptogenesis (Arena et al., 2019; Martinc et al., 2012; Terrone et al., 2020). The KD is inherently anti-inflammatory in nature and some of the main mechanisms are described below.
KD therapy exerts anti-inflammatory activity independent of polyunsaturated fatty acids (Dupuis et al., 2015) and is mediated by inhibiting nuclear factor kappa-B (NF-kB) activation and NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome activation (Pinto et al., 2018). Another possible mechanism is via the activation of transcription factor peroxisome proliferator activated receptors (PPAR) (Boison, 2017). PPARα is activated by X-box binding protein 1 (XBP1), which is activated by hepatic serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 (IRE1). It is noteworthy that IRE1α regulates fasting-induced metabolic adaptive programs through the XBP1-PPARα signaling axis (Shao et al., 2014). Decanoic acid, a fatty acid that is elevated in the plasma following medium chain triglyceride (MCT) ketogenic diet (Haidukewych, 1982), is known to exert anti-seizure activity in several ex vivo models of epilepsy, likely through the direct inhibition of AMPA receptors (Chang et al. 2016). In vitro experiments using SH-SY5Y neuronal cultures suggests that decanoic acid can increase the number of mitochondria by activation of PPARγ (Hughes et al. 2014) and might therefore mediate the anti-inflammatory and anti-oxidant properties of the KD. In line with this, GW9662, a PPARγ antagonist abrogated KD-induced seizure protection in Kv1.1 knockout mice, a spontaneously epileptic mouse strain responsive to KD therapy, whereas, a PPARγ agonist conferred seizure protection (Simeone et al., 2017a). These findings suggest that PPARγ may contribute to the anti-seizure effects of KD therapy.
Recent studies show that dampening of inflammation is anti-epileptogenic. For instance, the use of the non-steroidal anti-inflammatory drug etoricoxib, a selective COX-2 inhibitor, reduced the development of absence seizures in WAG/Rij rats, a recognized animal model of absence epilepsy and epileptogenesis (Citraro et al. 2015). Similarly, a drug cocktail targeting multiple inflammatory signaling pathways including IL-1r and COX-2 reduced the development of spontaneous recurrent seizures and limited the extent of mossy fiber sprouting in a lithium-pilocarpine model status epilepticus in rats (Kwon et al. 2013). However, whether anti-inflammatory property of the KD confers antiepileptogenesis needs further investigation.
3.3. Oxidative stress
KD is known to decrease oxidative stress and improve mitochondrial respiratory complex activity (Greco et al. 2016; Sullivan et al. 2004). In particular, the KD exerts neuroprotective effects by diminishing ROS production through activation of mitochondrial uncoupling protein (Sullivan et al. 2004). The anti-oxidant properties of the KD are mainly mediated by BHB, the most studied ketone body. BHB reduces the production of reactive oxygen species by improving mitochondrial respiration. More specifically, it stimulates the cellular endogenous antioxidant system by activation of nuclear factor erythroid-derived 2-related factor 2 (Nrf2). The activation of Nrf2 modulates the ratio between the oxidized and reduced forms of nicotinamide adenine dinucleotide (NAD+/NADH) and increases the efficiency of electron transport chain through the expression of uncoupling proteins (Pinto et al., 2018). The ability of the KD to influence the Nrf2 pathway is of particular interest owing to its neuroprotective role in epilepsy (Mazzuferi et al. 2013; Pauletti et al. 2019; Shekh-Ahmad et al. 2019). The chronic Nrf2 overexpression attained by gene therapy after epilepsy onset reduces spontaneous recurrent seizures evoked by pilocarpine injection in mice (Mazzuferi et al. 2013). The activation of Nrf2 by the KD also mediates glutathione biosynthesis, enhances mitochondrial antioxidant status, and protects mitochondrial DNA from oxidant- induced damage (Milder et al., 2010; Jarrett et al. 2008). Whether activation of the Nrf2 pathway, and thus glutathione biosynthesis, contributes to the anticonvulsant or epileptogenic effects of the KD remains to be determined. The KD also mediates cytochrome P450 4A-dependent ω- and ω−1-hydroxylation of reactive lipid species, a novel mechanism that might contribute to the anti-inflammatory properties of KD therapy (Jin et al. 2014).
3.3. Inhibition of apoptotic factors
The KD prevents neuronal apoptosis in animal models of traumatic brain injury (TBI) (Hu et al., 2009a; Hu et al., 2009b). A study noted a significant reduction in cytochrome c release and cellular apoptosis following TBI (Hu et al., 2009a). The same group showed that KD administration also reduces TBI-induced brain edema and cellular apoptosis which correlated with the levels of Bax, a well-known mediator of apoptosis (Hu et al., 2009b). Another mediator of apoptosis of particular interest is BAD because of its alternative role in glucose metabolism. The genetic modification of BAD designed to reduce glucose metabolism produces an increase in the activity of neuronal KATP channels and resistance to seizures in vivo (Gimenez-Cassina et al., 2012). However, these effects involve phosphoregulation of BAD which are independent of its apoptotic function (Gimenez-Cassina et al., 2012). These studies suggest that in addition to reducing inflammation, KD might prevent neuronal apoptosis and neurodegeneration.
3.4. Neurotrophic factors
3.4.1. Brain derived neurotrophic factor (BDNF)
Brain derived neurotrophic factor (BDNF) is associated with AMP kinase and is thought to be implicated in epileptogenesis (Heinrich et al., 2011; Lahteinen et al., 2004). Interestingly, KD causes a circadian shift in the expression of BDNF in brain and liver (Genzer et al., 2016). Another study confirmed that the KD reduced BDNF levels in striatum, but not hippocampus of healthy rats (Vizuete et al., 2013). Further, BHB induces the expression of BDNF in the cerebral cortex as a possible neuroprotective mechanism against excitotoxicity and oxidative stress (Marosi et al., 2016). Although the significance of KD-mediated alteration in BDNF expression is not clear, it is conceivable that it may play a key role in the neuroprotective effect of the KD in subjects with epilepsy.
3.4.2. Insulin-like growth factor (IGF1)
Another neuroprotective mechanism mediated by the KD is thought to be mediated by insulin-like growth factor (IGF) and its associated signaling mechanisms. Since IGF1 is a key regulator of glucose transport and utilization in the developing murine brain (Cheng et al., 2000), it is possible that KD may enhance IGF1 activity, thereby improving energy utilization and confer protection from seizures. Juvenile rats fed with calorie restricted KD for 7 days demonstrate an increase in IGF1 receptor (IGF1R) and glutamate transporter (GLUT1) gene expression (Cheng et al., 2003). While GLUT1 deficiency resulted in a seizure disorder that is highly responsive to KD (Nordli Jr. et al., 2001), its overexpression protected against seizure-induced neuron loss (Gupta et al., 2001). Taken together, IGF1, IGF1R and GLUT1 might be implicated in the beneficial mechanisms conferred by KD.
3.4.3. Fibroblast Growth Factor (FGF21)
In addition to carbohydrate restriction, the KD also restricts protein levels. Protein restriction is known to enhance the production of the endocrine signal fibroblast growth factor 21(FGF21) which in turn reduces blood glucose levels (Laeger et al., 2014) and may supplement to the neuroprotective effects of KD.
3. Consequences of epigenetics and neuroprotection for epilepsy prevention
The KD is a highly effective alternative treatment option for patients with intractable epilepsy. Although the anti-seizure effects of the diet are understood to a certain extent, our understanding of the neuroprotective and antiepileptogenic potential of the KD is still in its infancy. The studies presented in this review article suggests that the KD could play a neuroprotective role by modulating neurotransmitter levels, reducing inflammation, decreasing oxidative stress, maintaining energy metabolism, in addition to the regulation of epigenetic mechanisms. These mechanisms together might confer the diet’s antiepileptogenic effect, namely, seizure free conditions seen in patients even after cessation of the diet (Kossoff and Rho, 2009; Masino and Rho, 2012). An interesting observation in these studies is the variable nature of the number of responders to the KD. Hence, understanding the underlying mechanisms, particularly an alteration in the epigenetic markers or “epigenetic signature” (Hwang et al., 2013) might help differentiate the reason for complete success (seizure-free), partial success (reduction in seizure frequency) and no success in patients undergoing KD therapy. It is important that future studies take into consideration these important mechanisms and carefully design their investigations in order to shed light on the antiepileptogenic effects of the KD.
4. Conclusion
With up to 35% of persons with epilepsy considered to be refractory to treatment, and no therapies available that prevent epilepsy or its progression, the novel epigenetic functions of the KD therapy discussed here might be of significant therapeutic value. In addition to relieving the seizure burden in patients with epilepsy, it is also capable of modifying the development of epilepsy with its sequelae of drug resistance and the development of epilepsy-associated comorbidities. These aspects make the KD a powerful adjunct therapy to existing pharmacologic and surgical approaches to seizure relief. Renewed interest in the KD has led to refinements in diet formulation and administration (Kossoff et al., 2009; Wibisono et al., 2015) including an improved understanding of the potential positive and negative interactions with conventional antiepileptic drugs (Morrison et al., 2009; van der Louw et al., 2015), improving compliance and seizure suppression rates. However, the diet requires close monitoring by physicians and dietitians, and seemingly minor deviations from the ketogenic regimen can negate its beneficial effects. While a “ketogenic diet in a pill” may be unlikely, ongoing studies to understand the biochemical mechanisms of the KD are an essential step in the continued refinement of anti-seizure and antiepileptogenic therapies. The neuroprotective and epigenetic mechanisms of the KD are varied, and diet efficacy may rely on their combined influences. Among the metabolites regulated by the KD, adenosine has both a direct relevance to seizure suppression by A1R activation and an indirect influence on epilepsy and epileptogenesis via regulation of DNA methylation. A clearer understanding of how KD therapy affects adenosine metabolism and its epigenetic sequelae may help us understand adenosine dysregulation in epilepsy, and may guide the development of therapies designed to directly restore adenosine homeostasis, with the goal of developing a novel class of antiepileptogenic drugs.
Highlights.
Ketogenic diet (KD) is beneficial in patients with medically refractory epilepsy.
Antiseizure versus antiepileptogenic mechanisms of KD need to be distinguished.
Antiepileptogenic effects are attributed to neuroprotection and epigenetic mechanisms.
KD alters DNA methylation and histone acetylation, and thereby promotes antiepileptogenesis.
Abbreviations
- 5-ITU
5-iodotubercidin
- A1R
Adenosine receptor
- ACA
Acetoacetate
- ADK
Adenosine kinase
- BDNF
Brain derived neurotrophic factor
- BHB
beta-hydroxybutyrate
- DNMT
DNA methyltransferase
- FGF
Fibroblast growth factor
- GLUT1
Glutamate transporter 1
- HDAC
Histone deacetylase
- IGF
Insulin-like growth factor
- IGF1R
Insulin-like growth factor 1 receptor
- IRE1
Inositol-requiring enzyme 1
- KASE
Kainic acid induced status epilepticus
- KD
Ketogenic diet
- lncRNA
Long non-coding RNA
- miRNA
microRNA
- ncRNA
Noncoding RNA
- NF-kB
Nuclear factor –kappa B
- NLRP3
Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
- Nrf2
Nuclear factor erythroid-derived 2-related factor 2
- PPAR
Peroxisome proliferator activated receptors
- PTZ
Pentylenetetrazole
- REST
RE1-silencing transcription factor
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- TBI
Traumatic brain injury
- TLE
Temporal lobe epilepsy
- XBP1
X-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Sources of Funding:
The authors disclose that this manuscript was written without any commercial or financial associations that could be construed as a conflict of interest. DB acknowledges research funding support provided by the NIH (NS065957, NS103740) and Citizens United for Research in Epilepsy (DB, CURE Catalyst Award).
References
- Acharya MM, Hattiangady B, Shetty AK, 2008. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol 84, 363–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena A, Zimmer TS, van Scheppingen J, Korotkov A, Anink JJ, Muhlebner A, Jansen FE, van Hecke W, Spliet WG, van Rijen PC, Vezzani A, Baayen JC, Idema S, Iyer AM, Perluigi M, Mills JD, van Vliet EA, Aronica E, 2019. Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: molecular insights into their interdependence. Brain Pathol 29, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Crino PB, 2011. Inflammation in epilepsy: clinical observations. Epilepsia 52 Suppl 3, 26–32. [DOI] [PubMed] [Google Scholar]
- Bao Y, Chen X, Wang L, Zhou J, Fu X, Wang X, Xiao Z, 2018. RASgrf1, a Potential Methylatic Mediator of Anti-epileptogenesis? Neurochem Res 43, 2000–2007. [DOI] [PubMed] [Google Scholar]
- Basu T, O’Riordan KJ, Schoenike BA, Khan NN, Wallace EP, Rodriguez G, Maganti RK, Roopra A, 2019. Histone deacetylase inhibitors restore normal hippocampal synaptic plasticity and seizure threshold in a mouse model of Tuberous Sclerosis Complex. Sci Rep 9, 5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist AGC, Schall JI, Gallagher PR, Cnaan A, Stallings VA, 2005. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia 46, 1810–1819. [DOI] [PubMed] [Google Scholar]
- Bernard D, Prasanth KV, Tripathi V, Colasse S, Nakamura T, Xuan Z, Zhang MQ, Sedel F, Jourdren L, Coulpier F, Triller A, Spector DL, Bessis A, 2010. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J 29, 3082–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB, 2010. Adenosine signaling and function in glial cells. Cell Death Differ 17, 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, 2012. Adenosine dysfunction in epilepsy. GLIA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, 2017. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 30, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Rho JM, 2020. Epigenetics and epilepsy prevention: The therapeutic potential of adenosine and metabolic therapies. Neuropharmacology 167, 107741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, Brandner S, Mohler H, 2002. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA 99, 6985–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Steinhauser C, 2018. Epilepsy and astrocyte energy metabolism. Glia 66, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannataro R, Caroleo MC, Fazio A, La Torre C, Plastina P, Gallelli L, Lauria G, Cione E, 2019. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo RH, Cersosimo RO, Sakr D, Cresta A, Escobal N, Fejerman N, 2006. Ketogenic diet in patients with myoclonic-astatic epilepsy. Epileptic Disord. 8, 151–155. [PubMed] [Google Scholar]
- Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, Hardege JD, Chen PE, Walker MC, Williams RS, 2016. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 139, 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CM, Kelley B, Wang J, Strauss D, Eagles DA, Bondy CA, 2003. A ketogenic diet increases brain insulin-like growth factor receptor and glucose transporter gene expression. Endocrinology 144, 2676–2682. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy CA, 2000. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci U S A 97, 10236–10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro R, Leo A, Marra R, De Sarro G, Russo E, 2015. Antiepileptogenic effects of the selective COX-2 inhibitor etoricoxib, on the development of spontaneous absence seizures in WAG/Rij rats. Brain Res Bull 113, 1–7. [DOI] [PubMed] [Google Scholar]
- Coppola G, Verrotti A, Ammendola E, Operto FF, della_Corta R, Signoriello G, Pascotto A, 2010. Ketogenic diet for the treatment of catastrophic epileptic encephalopathies in childhood. Eur. J. Paediatr. Neurol 14, 229–234. [DOI] [PubMed] [Google Scholar]
- Cunha RA, 2005. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal 1, 111–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea Meira I, Romao TT, Pires do Prado HJ, Kruger LT, Pires MEP, da Conceicao PO, 2019. Ketogenic Diet and Epilepsy: What We Know So Far. Front Neurosci 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikhin Y, Yudkoff M, 1998. Ketone bodies and brain glutamate and GABA metabolism. Dev Neurosci 20, 358–364. [DOI] [PubMed] [Google Scholar]
- Debski KJ, Pitkanen A, Puhakka N, Bot AM, Khurana I, Harikrishnan KN, Ziemann M, Kaspi A, El-Osta A, Lukasiuk K, Kobow K, 2016. Etiology matters - Genomic DNA Methylation Patterns in Three Rat Models of Acquired Epilepsy. Sci Rep 6, 25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario FJ, Holland J, 2002. The ketogenic diet: a review of the experience at Connecticut children’s medical center. Pediatr. Neurol 26, 288–292. [DOI] [PubMed] [Google Scholar]
- Dupuis N, Curatolo N, Benoist JF, Auvin S, 2015. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia 56, e95–98. [DOI] [PubMed] [Google Scholar]
- Elamin M, Masino S, 2020. KD Mechanisms. Epilepsy Research This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M, 1996. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem 67, 2325–2334. [DOI] [PubMed] [Google Scholar]
- Gano LB, Patel M, Rho JM, 2014. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res 55, 2211–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL, 2006. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol 17, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzer Y, Dadon M, Burg C, Chapnik N, Froy O, 2016. Effect of dietary fat and the circadian clock on the expression of brain-derived neurotrophic factor (BDNF). Mol Cell Endocrinol 430, 49–55. [DOI] [PubMed] [Google Scholar]
- Gill MW, Schatz RA, 1985. The effect of diazepam on brain levels of S-adenosyl-L-methionine and S-adenosyl-L-homocysteine: possible correlation with protection from methionine sulfoximine seizures. Res Commun Chem Pathol Pharmacol 50, 349–363. [PubMed] [Google Scholar]
- Gimenez-Cassina A, Martinez-Francois JR, Fisher JK, Szlyk B, Polak K, Wiwczar J, Tanner GR, Lutas A, Yellen G, Danial NN, 2012. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron 74, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouder N, Fritschy JM, Boison D, 2003. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia 44, 877–885. [DOI] [PubMed] [Google Scholar]
- Graff J, Kim D, Dobbin MM, Tsai LH, 2011. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol Rev 91, 603–649. [DOI] [PubMed] [Google Scholar]
- Gupta A, Ho DY, Brooke S, Franklin L, Roy M, McLaughlin J, Fink SL, Sapolsky RM, 2001. Neuroprotective effects of an adenoviral vector expressing the glucose transporter: a detailed description of the mediating cellular events. Brain Res 908, 49–57. [DOI] [PubMed] [Google Scholar]
- Haidukewych D, Forsythe WI, Sills M, 1982. Monitoring octanoic and decanoic acids in plasma from children with intractable epilepsy treated with medium-chain triglyceride diet. Clin Chem 28, 642–645. [PubMed] [Google Scholar]
- Hartman AL, 2012. Neuroprotection in metabolism-based therapy. Epilepsy Res 100, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Rho JM, 2014. The New Ketone Alphabet Soup: BHB, HCA, and HDAC. Epilepsy Curr 14, 355–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AM, Keene DL, Whiting SE, Jacob PJ, Champagne JR, Humphreys P, 1999. Ketogenic diet in the treatment of refractory epilepsy in childhood. Pediatr. Neurol 21, 548–552. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Henshall DC, Lubin FD, 2018. The Epigenetics of Epilepsy and Its Progression. Neuroscientist 24, 186–200. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Lahteinen S, Suzuki F, Anne-Marie L, Huber S, Haussler U, Haas C, Larmet Y, Castren E, Depaulis A, 2011. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis 42, 35–47. [DOI] [PubMed] [Google Scholar]
- Heischmann S, Gano LB, Quinn K, Liang LP, Klepacki J, Christians U, Reisdorph N, Patel M, 2018. Regulation of kynurenine metabolism by a ketogenic diet. J Lipid Res 59, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway C, Freeman JM, Pillas DJ, Pyzik PL, 2001. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics 108, 898–905. [DOI] [PubMed] [Google Scholar]
- Henshall DC, Kobow K, 2015. Epigenetics and Epilepsy. Cold Spring Harb Perspect Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X-L, Cheng X, Fei J, Xiong Z-Q, 2011. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia 52, 1609–1616. [DOI] [PubMed] [Google Scholar]
- Hu ZG, Wang HD, Jin W, Yin HX, 2009a. Ketogenic diet reduces cytochrome c release and cellular apoptosis following traumatic brain injury in juvenile rats. Ann Clin Lab Sci 39, 76–83. [PubMed] [Google Scholar]
- Hu ZG, Wang HD, Qiao L, Yan W, Tan QF, Yin HX, 2009b. The protective effect of the ketogenic diet on traumatic brain injury-induced cell death in juvenile rats. Brain Inj 23, 459–465. [DOI] [PubMed] [Google Scholar]
- Huang Y, Doherty JJ, Dingledine R, 2002. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci 22, 8422–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O’Donnell M, Cross JH, Rahman S, Eaton S, Heales SJ, 2014. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem 129, 426–433. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Aromolaran KA, Zukin RS, 2013. Epigenetic mechanisms in stroke and epilepsy. Neuropsychopharmacology 38, 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A, 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33 Suppl, 245–254. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA, 2002. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr 132, 2361S–2366S. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Milder JB, Liang LP, Patel M, 2008. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem 106, 1044–1051. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yang Y, Wang S, Ding Y, Guo Y, Zhang M-M, Wen S-Q, Ding M-P, 2012. Ketogenic diet protects against epileptogenesis as well as neuronal loss in amygdaloid-kindling seizures. Neurosci. Lett 508, 22–26. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Bray I, Sanz-Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T, Saugstad JA, Simon RP, Stallings RL, Henshall DC, 2011. miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol 179, 2519–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Berthiaume JM, Li Q, Henry F, Huang Z, Sadhukhan S, Gao P, Tochtrop GP, Puchowicz MA, Zhang GF, 2014. Catabolism of (2E)-4-hydroxy-2-nonenal via omega- and omega-1-oxidation stimulated by ketogenic diet. J Biol Chem 289, 32327–32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H-C, Lee HS, You SJ, Kang DC, Ko T-S, Kim HD, 2007. Use of a modified Atkins diet in intractable childhood epilepsy. Epilepsia 48, 182–186. [DOI] [PubMed] [Google Scholar]
- Kiefer JC, 2007. Epigenetics in development. Dev Dyn 236, 1144–1156. [DOI] [PubMed] [Google Scholar]
- Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM, 2015. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol 78, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D, Brodie MJ, Brooks-Kayal AR, Engel J Jr., Forcelli PA, Hirsch LJ, Kaminski RM, Klitgaard H, Kobow K, Lowenstein DH, Pearl PL, Pitkanen A, Puhakka N, Rogawski MA, Schmidt D, Sillanpaa M, Sloviter RS, Steinhauser C, Vezzani A, Walker MC, Loscher W, 2018. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia 59, 37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobow K, Kaspi A, Harikrishnan KN, Kiese K, Ziemann M, Khurana I, Fritzsche I, Hauke J, Hahnen E, Coras R, Mühlebner A, El-Osta A, Blümcke I, 2013. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathologica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Bosarge JL, Miranda MJ, Wiemer-Kruel A, Kang HC, Kim HD, 2010. Will seizure control improve by switching from the modified Atkins diet to the traditional ketogenic diet? Epilepsia 51, 2496–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Rho JM, 2009. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics 6, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Helen Cross J, Dahlin MG, Donner EJ, Klepper J, Jehle RS, Kim HD, Christiana Liu YM, Nation J, Nordli DR Jr., Pfeifer HH, Rho JM, Stafstrom CE, Thiele EA, Turner Z, Wirrell EC, Wheless JW, Veggiotti P, Vining EP, Charlie Foundation, P.C.o.t.C.N.S., Practice Committee of the Child Neurology, S., International Ketogenic Diet Study, G., 2009. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia 50, 304–317. [DOI] [PubMed] [Google Scholar]
- Kumar MG, Rowley S, Fulton R, Dinday MT, Baraban SC, Patel M, 2016. Altered Glycolysis and Mitochondrial Respiration in a Zebrafish Model of Dravet Syndrome. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R, 2013. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD, 2014. FGF21 is an endocrine signal of protein restriction. J Clin Invest 124, 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahteinen S, Pitkanen A, Knuuttila J, Toronen P, Castren E, 2004. Brain-derived neurotrophic factor signaling modifies hippocampal gene expression during epileptogenesis in transgenic mice. Eur J Neurosci 19, 3245–3254. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D, 2008. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest 118, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusardi TA, Akula KK, Coffman SQ, Ruskin DN, Masino SA, Boison D, 2015. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 99, 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutas A, Yellen G, 2013. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci 36, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaviya PR, Stolk L, Heil SG, 2014. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab 113, 243–252. [DOI] [PubMed] [Google Scholar]
- Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP, 2016. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem 139, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinc B, Grabnar I, Vovk T, 2012. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr Neuropharmacol 10, 328–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D, 2011. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Inv 121, 2679–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Rho JM, 2012. Mechanisms of Ketogenic Diet Action, in: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (Eds.), Jasper’s Basic Mechanisms of the Epilepsies, 4th ed, Bethesda (MD). [PubMed] [Google Scholar]
- Masino SA, Kawamura M Jr., Ruskin DN, 2014. Adenosine receptors and epilepsy: current evidence and future potential. Int Rev Neurobiol 119, 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuferi M, Kumar G, van Eyll J, Danis B, Foerch P, Kaminski RM, 2013. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann Neurol 74, 560–568. [DOI] [PubMed] [Google Scholar]
- Milder JB, Liang LP, Patel M, 2010. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis 40, 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Delaney SF, Bryan K, Das S, McKiernan RC, Bray IM, Reynolds JP, Gwinn R, Stallings RL, Henshall DC, 2015. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 138, 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PF, Pyzik PL, Hamdy R, Hartman AL, Kossoff EH, 2009. The influence of concurrent anticonvulsants on the efficacy of the ketogenic diet. Epilepsia 50, 1999–2001. [DOI] [PubMed] [Google Scholar]
- Muller-Schwarze AB, Tandon P, Liu Z, Lang Y, Holmes GL, Stafstrom CE, 1999. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. NeuroReport 10, 1517–1522. [DOI] [PubMed] [Google Scholar]
- Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J, 2016. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordli DR Jr., Kuroda MM, Carroll J, Keonigsburger DY, Hirsch LJ, Bruner HJ, Seidel WT, De Vivo DC, 2001. Experience with the ketogenic diet in infants. Pediatrics 108, 129–133. [DOI] [PubMed] [Google Scholar]
- Olaso-González G, Serna E, Herrero JR, Martínez C, Gómez-Cabrera MC, Pedrón C, Vina J, 2018. MiRNome of epileptic children suggests the involvement of antioxidant pathways in the neuroprotective role of ketogenic diet. Free Radical Biology and Medicine 120. [Google Scholar]
- Panico LR, Ríos VG, Demartini MG, Carniello MA, 2000. [The electroencephalographic evolution of a group of patients on a ketonic diet]. Rev. Neurol 30, 8–15. [PubMed] [Google Scholar]
- Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, Pastore A, Pascente R, Liang LP, Villa BR, Balosso S, Abramov AY, van Vliet EA, Del Giudice E, Aronica E, Patel M, Walker MC, Vezzani A, 2019. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 142, e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F, 2013. Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci 50, 291–297. [DOI] [PubMed] [Google Scholar]
- Pichardo-Casas I, Goff LA, Swerdel MR, Athie A, Davila J, Ramos-Brossier M, Lapid-Volosin M, Friedman WJ, Hart RP, Vaca L, 2012. Expression profiling of synaptic microRNAs from the adult rat brain identifies regional differences and seizure-induced dynamic modulation. Brain Res 1436, 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D, 2008. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab 28, 17–23. [DOI] [PubMed] [Google Scholar]
- Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R, 2018. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Immonen RJ, Grohn OH, Kharatishvili I, 2009. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia 50 Suppl 2, 21–29. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Lukasiuk K, 2009. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav 14 Suppl 1, 16–25. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF, 2013. Long non-coding RNAs: novel targets for nervous system disease diagnosis and therapy. Neurotherapeutics 10, 632–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF, 2014. Epigenetic mechanisms underlying the pathogenesis of neurogenetic diseases. Neurotherapeutics 11, 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SD, Clossen BL, Reddy DS, 2018. Epigenetic Histone Deacetylation Inhibition Prevents the Development and Persistence of Temporal Lobe Epilepsy. J Pharmacol Exp Ther 364, 97–109. [DOI] [PubMed] [Google Scholar]
- Rho JM, 2015. How does the ketogenic diet induce anti-seizure effects? Neurosci Lett. [DOI] [PubMed] [Google Scholar]
- Ryley Parrish R, Albertson AJ, Buckingham SC, Hablitz JJ, Mascia KL, Davis Haselden W, Lubin FD, 2013. Status epilepticus triggers early and late alterations in brain-derived neurotrophic factor and NMDA glutamate receptor Grin2b DNA methylation levels in the hippocampus. Neuroscience 248C, 602–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz RA, Wilens TE, Tatter SB, Gregor P, Sellinger OZ, 1983. Possible role of increased brain methylation in methionine sulfoximine epileptogenesis: effects of administration of adenosine and homocysteine thiolactone. J Neurosci Res 10, 437–447. [DOI] [PubMed] [Google Scholar]
- Sellinger OZ, Schatz RA, Porta R, Wilens TE, 1984. Brain methylation and epileptogenesis: the case of methionine sulfoximine. Ann Neurol 16 Suppl, S115–120. [DOI] [PubMed] [Google Scholar]
- Shao M, Shan B, Liu Y, Deng Y, Yan C, Wu Y, Mao T, Qiu Y, Zhou Y, Jiang S, Jia W, Li J, Li J, Rui L, Yang L, Liu Y, 2014. Hepatic IRE1alpha regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARalpha axis signalling. Nat Commun 5, 3528. [DOI] [PubMed] [Google Scholar]
- Sharma S, Gulati S, Kalra V, Agarwala A, Kabra M, 2009. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy - Indian experience. Seizure 18, 446–449. [DOI] [PubMed] [Google Scholar]
- Shekh-Ahmad T, Lieb A, Kovac S, Gola L, Christian Wigley W, Abramov AY, Walker MC, 2019. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol 26, 101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Matthews SA, Samson KK, Simeone KA, 2017a. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol 287, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Simeone KA, Rho JM, 2017b. Ketone Bodies as Anti-Seizure Agents. Neurochem Res 42, 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SW, Cilio MR, Sogawa Y, Silveira DC, Holmes GL, Stafstrom CE, 2000. Timing of ketogenic diet initiation in an experimental epilepsy model. Dev. Brain Res 125, 131–138. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhong XL, Zong Y, Wu ZC, Zhang Q, Yu JT, Tan L, 2015. Activation of adenosine receptor potentiates the anticonvulsant effect of phenytoin against amygdala kindled seizures. CNS Neurol Disord Drug Targets 14, 378–385. [DOI] [PubMed] [Google Scholar]
- Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, Cao D, Huang T, Li B, Li C, 2013. Efficacy and safety of the ketogenic diet in Chinese children. Seizure 22, 174–178. [DOI] [PubMed] [Google Scholar]
- Sweatt JD, 2013. The emerging field of neuroepigenetics. Neuron 80, 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Grooms SY, Bennett MV, Zukin RS, 2000. The AMPAR subunit GluR2: still front and center-stage. Brain Res 886, 190–207. [DOI] [PubMed] [Google Scholar]
- Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A, 2020. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 167, 107742. [DOI] [PubMed] [Google Scholar]
- Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN, 2000. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia 41, 933–940. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ, 2004. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci 24, 5603–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Louw EJ, Desadien R, Vehmeijer FO, van der Sijs H, Catsman-Berrevoets CE, Neuteboom RF, 2015. Concomitant lamotrigine use is associated with decreased efficacy of the ketogenic diet in childhood refractory epilepsy. Seizure 32, 75–77. [DOI] [PubMed] [Google Scholar]
- Vandeweyer G, Van der Aa N, Ceulemans B, van Bon BW, Rooms L, Kooy RF, 2012. A de novo balanced t(2;6)(p15;p22.3) in a patient with West Syndrome disrupts a lnc-RNA. Epilepsy Res 99, 346–349. [DOI] [PubMed] [Google Scholar]
- Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, Shinnar S, Shuman R, Trevathan E, Wheless JW, 1998. A multicenter study of the efficacy of the ketogenic diet. Arch. Neurol 55, 1433–1437. [DOI] [PubMed] [Google Scholar]
- Vizuete AF, de Souza DF, Guerra MC, Batassini C, Dutra MF, Bernardi C, Costa AP, Goncalves CA, 2013. Brain changes in BDNF and S100B induced by ketogenic diets in Wistar rats. Life Sci 92, 923–928. [DOI] [PubMed] [Google Scholar]
- Wang A, Wang J, Liu Y, Zhou Y, 2017. Mechanisms of Long Non-Coding RNAs in the Assembly and Plasticity of Neural Circuitry. Front Neural Circuits 11, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibisono C, Rowe N, Beavis E, Kepreotes H, Mackie FE, Lawson JA, Cardamone M, 2015. Ten-year single-center experience of the ketogenic diet: factors influencing efficacy, tolerability, and compliance. J Pediatr 166, 1030–1036 e1031. [DOI] [PubMed] [Google Scholar]
- Wilder RM, 1921. High fat diets in epilepsy. Mayo Clin. Bull 2, 308. [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D, 2013a. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. Journal of Clinical Investigation 123(8), 3552–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xie X, 2006. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol 7, R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS, 2013. Estrous cycle regulation of extrasynaptic delta-containing GABA(A) receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther 346, 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson NA, Morris MJ, Ballard JWO, 2017. The mechanisms mediating the antiepileptic effects of the ketogenic diet, and potential opportunities for improvement with metabolism-altering drugs. Seizure 52, 15–19. [DOI] [PubMed] [Google Scholar]
- Younus I, Reddy DS, 2017. Epigenetic interventions for epileptogenesis: A new frontier for curing epilepsy. Pharmacol Ther 177, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I, 2008. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia 49 Suppl 8, 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Grunstein R, Nissim I, 1997. Effects of ketone bodies on astrocyte amino acid metabolism. J Neurochem 69, 682–692. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S, Nissim I, 2005. Response of brain amino acid metabolism to ketosis. Neurochem Int 47, 119–128. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A, Nissim I, 2006. Short-term fasting, seizure control and brain amino acid metabolism. Neurochem Int 48, 650–656. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I, 2001. Ketogenic diet, amino acid metabolism, and seizure control. J Neurosci Res 66, 931–940. [DOI] [PubMed] [Google Scholar]
- Zhong J, Chuang SC, Bianchi R, Zhao W, Lee H, Fenton AA, Wong RK, Tiedge H, 2009. BC1 regulation of metabotropic glutamate receptor-mediated neuronal excitability. J Neurosci 29, 9977–9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska I, Wrobel-Dudzinska D, Tulidowicz-Bielak M, Kocki T, Mitosek-Szewczyk K, Gasior M, Turski WA, 2019. Changes in tryptophan and kynurenine pathway metabolites in the blood of children treated with ketogenic diet for refractory epilepsy. Seizure 69, 265–272. [DOI] [PubMed] [Google Scholar]


