Abstract
Purpose:
Topotecan is a camptothecin analogue with potential advantages over irinotecan for transarterial chemoembolization (TACE) of hepatic colorectal metastases including greater anti-neoplastic activity without enzymatic activation. The purpose of this study was to assess safety and tolerability of topotecan-loaded radiopaque microspheres (ROMTOP) administered by TACE in a rabbit model, and to compare the in vitro elution of topotecan from microspheres to irinotecan.
Materials and Methods:
Topotecan was loaded into radiopaque microspheres (70–150 μm, DC Bead LUMI™, Biocompatibles UK Ltd – Boston Scientific Corporation) to the maximum capacity of 80 mg/mL of microspheres. Six healthy New Zealand White rabbits underwent hepatic TACE with ROMTOP under fluoroscopic guidance until angiographic stasis. Assessment of toxicities included regular liver function tests and complete blood counts until euthanasia 28 days post TACE. In vitro topotecan elution from the microspheres was assessed using an open-loop flow-through system and compared to irinotecan.
Results:
The mean bead volume and topotecan dose delivered were 0.086 mL (0.076–0.105 mL) and 1.99 mg/kg (1.51–2.55 mg/kg), respectively. Aspartate aminotransferase and alanine aminotransferase were elevated post-embolization but resolved within two weeks. One rabbit died two days after TACE with pyloric duodenal perforation observed at necropsy, potentially due to non-target embolization. In vitro elution of topotecan from ROMTOP was complete in 10 h compared to 3 h for irinotecan-loaded microspheres.
Conclusion:
Selective embolization with ROMTOP was tolerated at a dose of 2 mg/kg (24 mg/m2) in rabbits. In vitro topotecan elution from microspheres was more prolonged compared to irinotecan.
Keywords: topotecan, drug-eluting beads, microspheres, embolization, irinotecan, radiopaque beads, DEB, toxicity, colorectal metastases, chemoembolization, TACE, rabbit, DEBIRI
Introduction
Transarterial chemoembolization with drug-eluting beads loaded with irinotecan (DEBIRI) provides greater overall survival for patients with colorectal liver metastases compared to systemic administration of folinic acid, fluorouracil, and irinotecan [1]. However, the majority of the anti-neoplastic activity depends on conversion of irinotecan in the liver by carboxylesterase to its more potent form, SN-38, a process that is highly variable among patients [2, 3]. Like irinotecan, topotecan is a camptothecin analogue and topoisomerase I inhibitor. However, topotecan potency does not depend upon enzymatic activation in the liver, which may result in higher local anti-tumor activity [4, 5].
Topotecan has demonstrated activity against tumor cell lines and xenografts derived from human colorectal tumors [6, 7]. However, intravenous administration of topotecan has demonstrated limited activity against advanced colorectal carcinoma and is associated with dose-limiting myelosuppression, as well as nausea, vomiting, alopecia, and fatigue [8–11]. Given its potency and known dose-limiting systemic toxicity, topotecan may be a suitable candidate for local delivery with drug-eluting microspheres. Studies have also suggested that topotecan can inhibit hypoxia inducible factor 1 alpha (HIF-1 alpha), a protein that accumulates under hypoxic conditions and has been implicated in revascularization after embolization [12–15].
Recently, radiopaque drug-eluting microspheres (ROM) were developed that enable real-time visualization of microsphere temporal and spatial distribution using fluoroscopy and CBCT [16–19]. The aim of this in vivo study was to assess the safety of topotecan-loaded radiopaque microspheres (ROMTOP) administered by transarterial chemoembolization in a rabbit model. Additionally, an in vitro experiment was performed comparing the loading and elution of topotecan from radiopaque microspheres to irinotecan.
Materials and Methods
In vitro loading and release
For drug loading, radiopaque microspheres, 70–150 μm in diameter (DC Bead LUMI, Biocompatibles UK Ltd – Boston Scientific Corporation), were incubated with topotecan HCl (Fresenius Kabi, IL) or irinotecan HCl (ScinoPharm, Taiwan) in deionized water (80 mg of topotecan or 50 or 100 mg of irinotecan per milliliter of sedimented microspheres) and agitated for 2 hours. Drug elution from ROMTOP and radiopaque microspheres containing irinotecan (ROMIRI) was assessed in vitro using an open-loop flow-through system that has been previously described [20]. Briefly, this system consisted of a column packed with drug-loaded microspheres which was perfused by elution medium. The concentration of extracted drug was measured via an in-line UV spectrophotometer.
In vivo chemoembolization
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The study protocol was approved by the Animal Care and Use Committee of the NIH Clinical Center. Rabbits received meloxicam (0.1 mg/kg) prior to surgical procedures as well as buprenorphine SR (0.12–0.2 mg/kg) post embolization for analgesia. In order to minimize phlebotomy-related trauma and facilitate blood collection, a jugular venous catheter was placed seven days in advance of embolization according to a previously published report [21]. Catheters were flushed twice daily with taurolidine-citrate catheter solution (Access Technologies, Skokie, Illinois) in order to avoid line blockage.
In preparation for the embolization procedure, six healthy female New Zealand White rabbits with median weight of 3.5 kg (range: 2.9–4.0 kg) underwent anesthesia induced using ketamine (20 mg/kg) and acepromazine (0.75 mg/kg) and maintained with isoflurane by face mask. The right or left femoral artery was surgically exposed and cannulated using a 3-F vascular sheath (Cook, Inc., Bloomington, Indiana). The left hepatic artery was selectively catheterized using a 2.4-F microcatheter (Progreat; Terumo Medical Corporation, Somerset, NJ) under fluoroscopic guidance (Allura Xper FD20; Philips, Best, Netherlands). ROMTOP were suspended at a dilution of 1 part microspheres to 19 parts 100% Omnipaque 350 contrast media (GE Healthcare, Oslo, Norway) and injected slowly into the left hepatic artery until blood flow stasis was achieved. A multidetector CT scan (Philips Brilliance MX8000 IDT 16-section Detector CT, Philips, Cleveland, OH, USA) was performed (120 kVp, 2-mm section thickness, 1-mm intervals) 1–3 hours after embolization. Rabbits were placed in an incubator for initial recovery under controlled temperature and humidity. They were subsequently housed for 28 days and then euthanized by administration of a combination of pentobarbital sodium 390 mg/mL and phenytoin sodium 50 mg/mL (Euthasol 1 mL/10 lb; Virbac Animal Health, Fort Worth, TX).
Rabbits were observed daily for physical manifestations of toxicities including persistent loss of appetite, weight loss, lethargy, behavior changes, and tachypnea. Blood was collected for liver function tests (LFT) and complete blood counts (CBC) immediately prior to embolization and 1, 2, 4, 7, 14, 21, and 28 days post embolization. Hematologic signs of toxicities included persistent liver enzyme elevations and sustained marrow suppression/leukopenia.
Statistics
Percent weight change, LFT, and CBC were assessed using repeated-measures ANOVA models, which produced least squares means estimates and corresponding 95% confidence intervals. The models included a dose effect (continuous) and a day effect (categorical). For LFT and CBC, measurements on day 0 were also included as a covariate in order to account for potential differences in baseline values. However, for the purpose of comparing daily LFT or CBC values to baseline, measurements on day 0 were included in the model as an observation (part of the dependent variable) instead of as a covariate. P-values were not adjusted for multiple comparisons.
Results
In vitro loading and release
The maximum topotecan loading capacity in ROMTOP was 80 mg/mL of microspheres. Drug loading occurred within 15 minutes for ROMTOP and ROMIRI at 80 mg/mL of microspheres and 50 mg/mL of microspheres, respectively, and within 45 minutes for ROMIRI at 100 mg/mL of microspheres with agitation of the loading solution. Complete drug release from ROMTOP and ROMIRI at their maximum drug loading capacity occurred within 10 and 3 hours, respectively, using a continuous flow elution system (Figure 1).
Fig. 1.
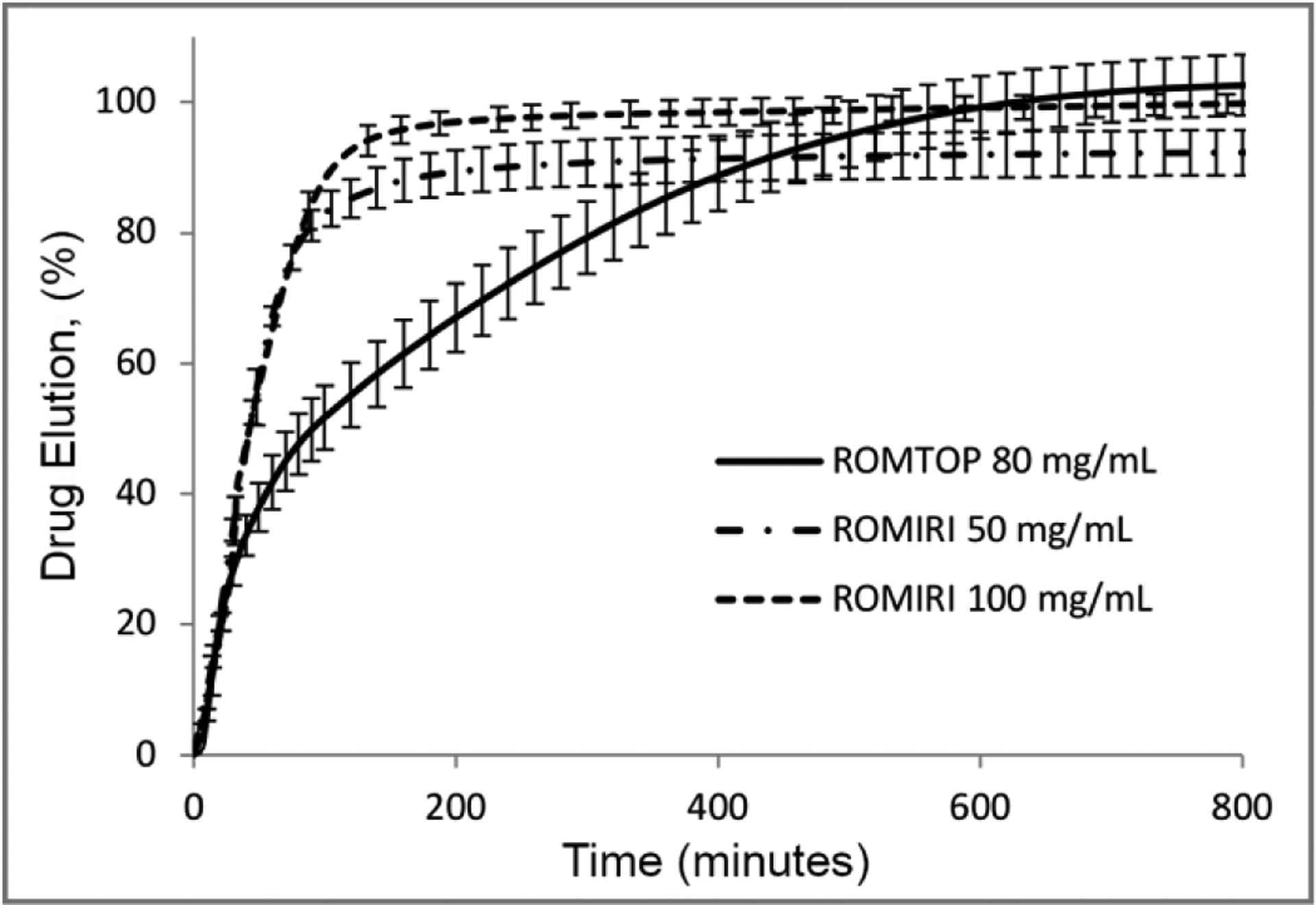
Elution profiles from 70–150 μm radiopaque microspheres containing topotecan (ROMTOP) and irinotecan (ROMIRI) as a percentage of total amount of drug loaded. Error bars represent standard deviation of the mean. Elution profiles are shown for each drug at their maximum loading capacity in the microspheres (80 and 100 mg/mL for topotecan and irinotecan, respectively). The release profile for irinotecan loaded at 50 mg/mL of microspheres is also shown, reflecting the concentration typically administered clinically
In vivo chemoembolization
Six rabbits underwent successful chemoembolization and received a mean topotecan dose of 1.99 mg/kg (range: 1.51–2.55 mg/kg). Rabbit characteristics and technical outcomes of embolization with ROMTOP are summarized in Table 1. ROMTOP delivery was monitored with fluoroscopy and could be visualized after the procedure using unenhanced multidetector CT (Figure 2). One rabbit died two days after chemoembolization. At necropsy, the cause of death was attributed to pyloric duodenal perforation. While this condition is known to occur spontaneously in rabbits [22], the temporal relationship to the embolization procedure suggests the likelihood of non-target embolization as a cause.
Table 1.
Rabbit characteristics and technical outcomes.
| Characteristic | N = 6 |
|---|---|
| Age (wks), median (range) | 43.9(21.4–59.4) |
| Weight (kg), median (range) | 3.5 (2.9–4.0) |
| Microsphere volume delivered (mL), mean (range) | 0.086 (0.076–0.105) |
| Topotecan dose delivered (mg/kg), mean (range) | 1.99(1.51–2.55) |
| 28-Day survival | 5/6 |
Fig. 2.
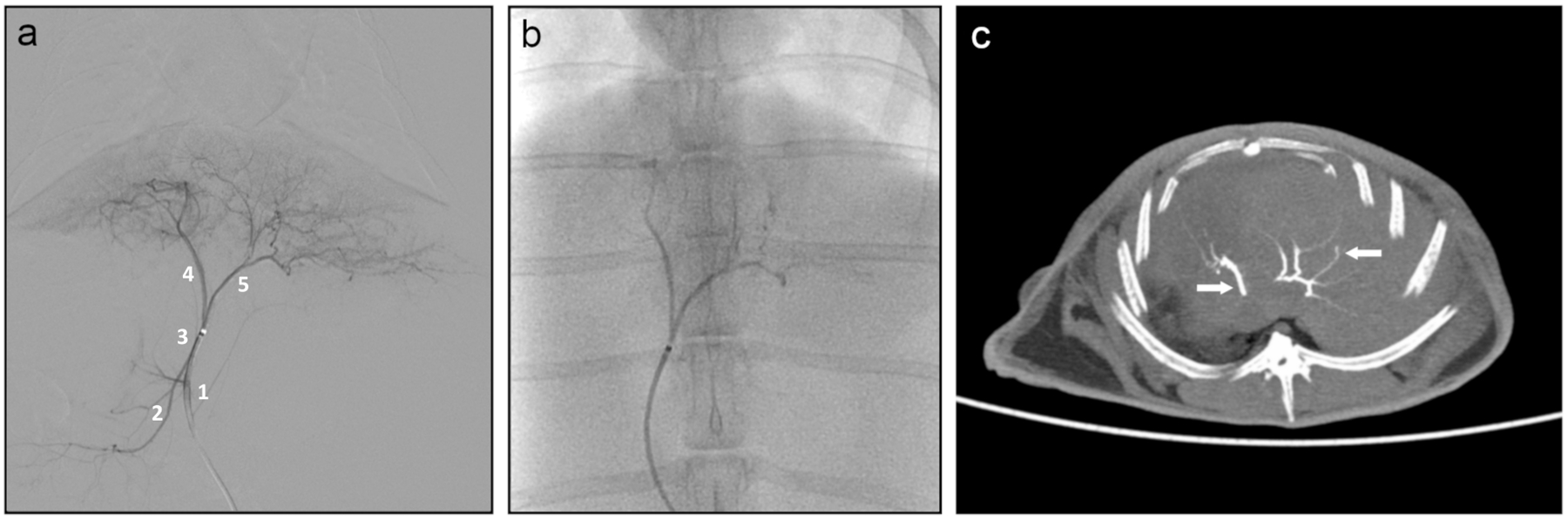
Hepatic embolization. (A) Digital subtraction angiography during contrast injection into the left hepatic artery (3) showing opacification of the medial (4) and lateral (5) branches as well as reflux into the proper hepatic artery (1) and right hepatic artery (2); (B) Fluoroscopic image during embolization with ROMTOP; (C) Unenhanced multi detector CT of rabbit liver, axial slice (10 mm thick slice maximum intensity projection), showing conspicuity of ROMTOP (arrows) in the hepatic arteries post embolization
Moderate elevation in pain was observed but resolved by day 5. Otherwise, no physical manifestations of toxicity, including weight loss (p-value for overall dose effect = 0.5608) (Figure 3), were observed following embolization with ROMTOP. A summary of p values for overall dose and day effects for liver function tests and complete blood counts is found in Table 2. Elevated ALT levels were found post embolization on day 1 (p=<0.0001), 2 (p=<0.0001), and 7 (p=0.0124) (Figure 4 A). AST was elevated on day 1 (p=<0.0001) relative to pre-procedural baseline values. WBC elevation occurred on day 1 (p=0.0016), 4 (p=0.0410), 7 (p=0.0007), and 14 (p=0.0248) compared to pre-procedural baseline values (Figure 4 B). Platelet counts were elevated post embolization on day 1 (p=0.0261), 2 (p=0.0053), 7 (p=<0.0001), and 14 (p=<0.0001) (Figure 4 C). RBC elevation was observed on day 1 (p=0.0032) compared to preprocedural baseline values (Figure 4 D).
Fig. 3.
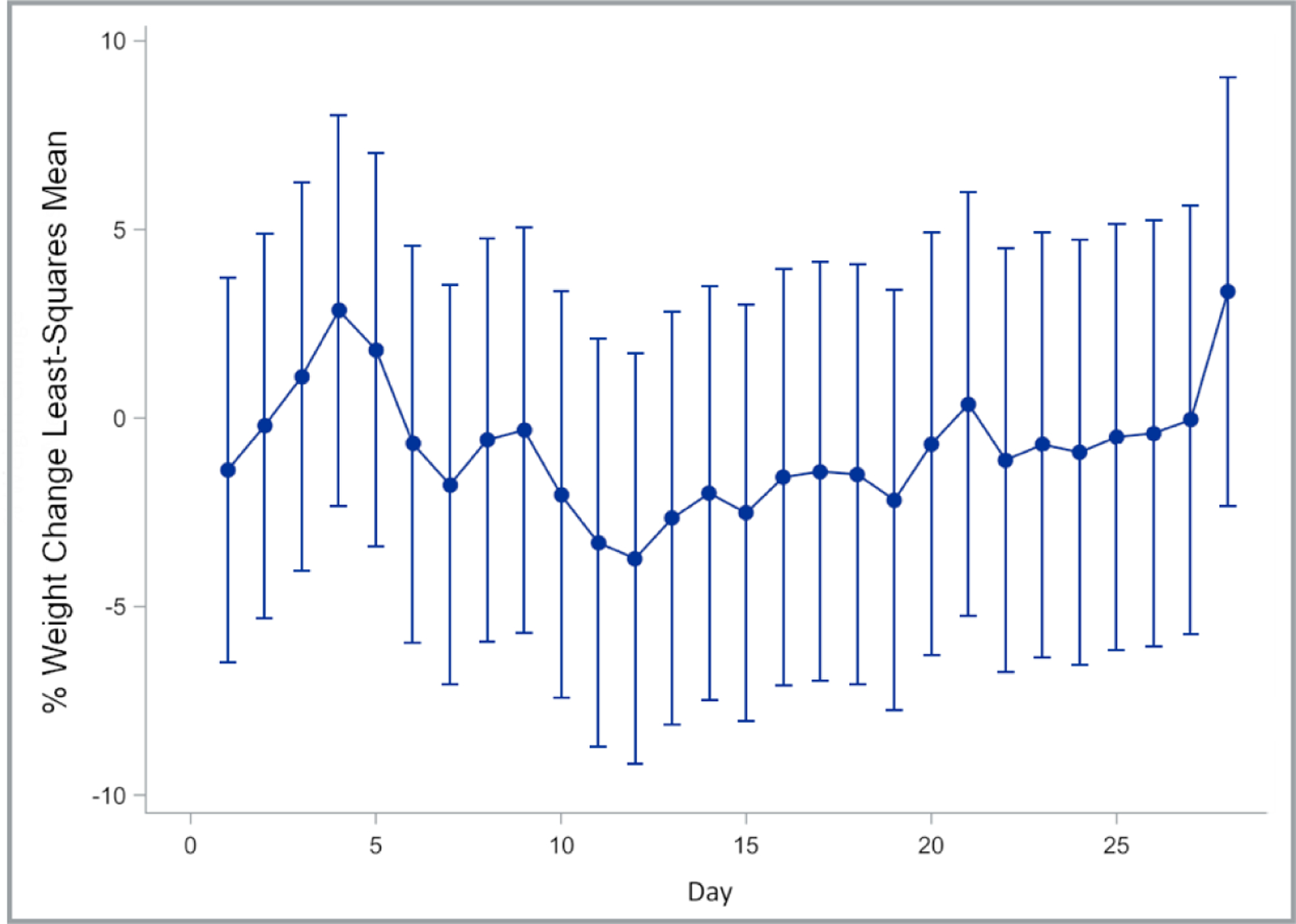
Least squares means and corresponding 95% confidence intervals of percent change in body weight relative to weight measured on the day of embolization (day 0)
Table 2.
Number of observations and p-values for overall dose and day effects for liver function tests and complete blood counts.
| LFT and CBC components | Total # of observations analyzed (# rabbits) | p-value for overall dose effect* | p-value for overall day effect |
|---|---|---|---|
| AST | 25 (5) | 0.0957 | 0.0074 |
| ALT | 25 (5) | 0.0535 | 0.0001 |
| Neutrophils | 30(5) | 0.0537 | <0.0001 |
| WBC | 33 (6) | 0.5200 | 0.0107 |
| RBC | 34 (6) | 0.1729 | 0.1187 |
| Platelets | 34 (6) | 0.5064 | <0.0001 |
The dose effect in the statistical model refers to the combined effects of microspheres and drug
Fig. 4.
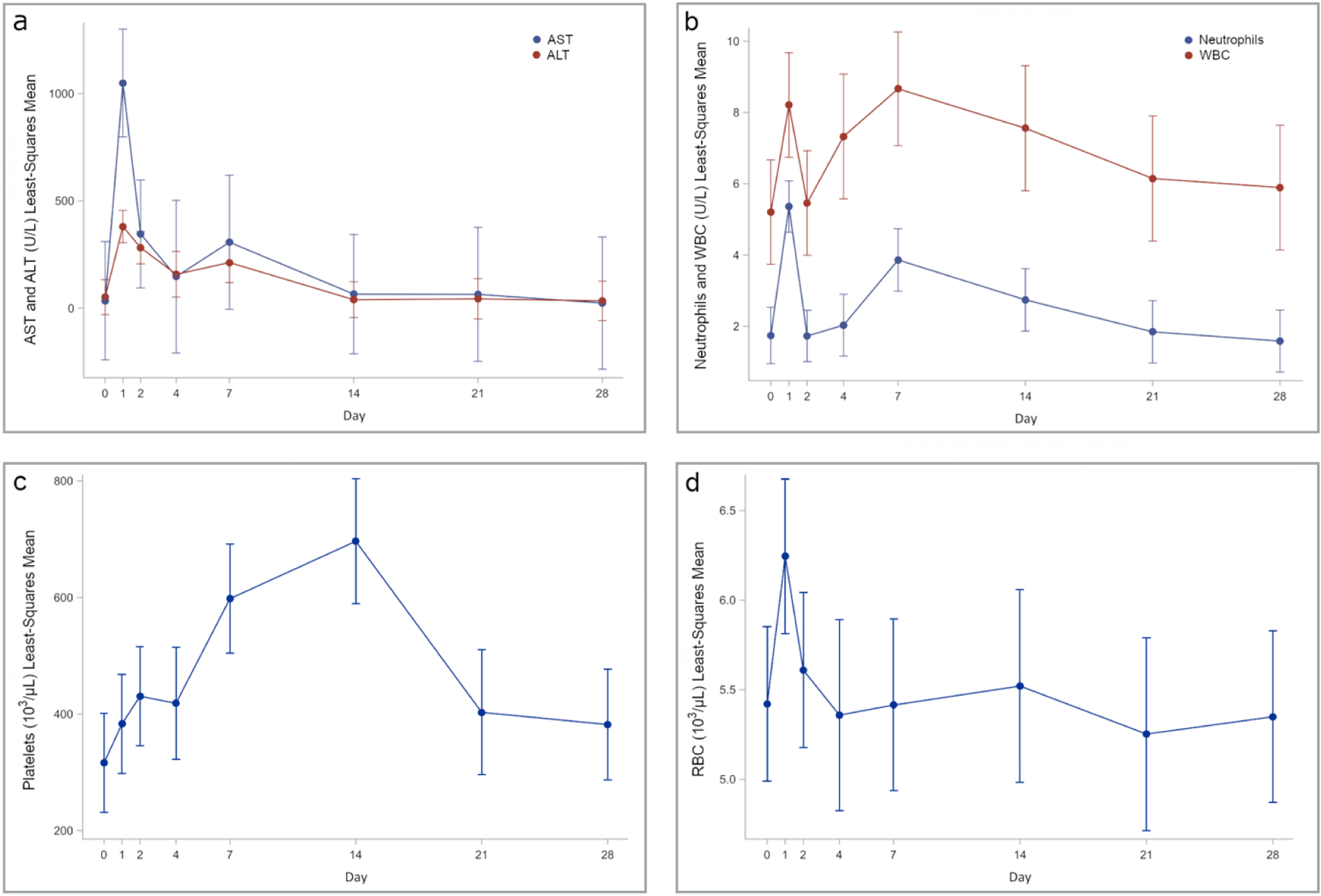
Least squares mean values for hematology and serum biochemistry. A) aspartate aminotransferase (AST) and alanine aminotransferase (ALT), B) neutrophils and white blood cell count (WBC), C) platelets, and D) red blood cell count (RBC). Bars represent 95% confidence intervals
Discussion
The primary objective of this preclinical study was to assess the safety and tolerability of TACE with topotecan-eluting radiopaque microspheres in non-tumor-bearing rabbits. The topotecan dose was determined by the drug loading capacity and total administered volume of the microspheres. ROMTOP was well tolerated at the maximum microsphere loading capacity of 80 mg/mL of microspheres and a mean administered topotecan dose of 2 mg/kg.
Topotecan has been approved by the FDA for treatment of advanced or recurrent cervical cancer, recurrent ovarian cancer, and relapsed small-cell lung carcinoma. Topotecan doses typically range from 0.75–1.5 mg/m2 by intravenous infusion over 30 minutes daily for 3–5 consecutive days every three weeks [23]. Myelosuppression is the principle dose-limiting toxicity of topotecan administered via intravenous infusion [8]. Other dosing regimens, such as continuous infusions and low dose metronomic administration, have been explored [24, 25]. In this study, local delivery of topotecan (2 mg/kg) was performed by selective, intraarterial delivery of topotecan-loaded microspheres to the liver. Based on FDA guidance on animal-to-human dose conversion [26], this dose of topotecan, normalized to body surface area, was 24 mg/m2. This exceeds the clinical doses administered to humans by intravenous infusion (0.75–1.5 mg/m2).
The rabbits experienced moderate pain in the days immediately post embolization but no other physical manifestation. Typical hematologic toxicities generally associated with i.v administration of topotecan were not observed in this study following delivery of ROMTOP. This is consistent with reduced systemic drug exposure and increased local drug concentration associated with DEBIRI [27, 28]. Toxicity related to liver embolization with ROMTOP included alanine aminotransferase (ALT) and aspartate transaminase (AST) elevations that resolved within 14 days and are consistent with measurements following embolization with doxorubicin-loaded microspheres in rabbits [29]. These findings suggest good tolerance for lobar therapy with ROMTOP.
ROMTOP offers several potential treatment advantages over DEBIRI. The release rate of topotecan from microspheres in vitro is slower than irinotecan, which may provide longer drug exposure time in embolized tumors [4]. Initial clinical experience suggests that the ability to see radiopaque microspheres on intraprocedural fluoroscopy and CBCT as well as multidetector CT may provide additional information to refine definition of endpoints of embolization or identify tumor regions at risk of undertreatment [18]. Due to the relative colocalization of microspheres and drug in embolized tissues, microsphere radiopacity on CT has the potential to serve as a surrogate marker of local drug dose in the liver [30].
This study had several limitations. Flow stasis was selected as the treatment endpoint rather than absolute drug dose in order to minimize potential differences in ischemic effects among animals which could confound the evaluation of drug-related toxicities. Variation in the volume of microspheres required to achieve flow stasis, and corresponding drug dose, was inevitable. Treatment effects reported in this study reflect the combined effects of ischemia and drug cytotoxicity since the volume of microspheres and drug dose delivered were inextricably linked. Finally, limitations in the volume of microspheres that could be delivered, as well as the amount of topotecan that could be loaded into the microspheres, restricted administration of higher doses.
Conclusion
Selective embolization with ROMTOP was tolerated at a dose of 2 mg/kg (24 mg/m2) with no evidence of myelosuppression, the dose limiting side effect of topotecan. This was the maximum dose that could be administered due to the maximum loading capacity of the microspheres. Overall, topotecan is an attractive candidate for local delivery via TACE with radiopaque drug-eluting microspheres. Further evaluation of the pharmacokinetics of this route of administration may be required prior to clinical translation.
Funding
This work was supported by the Center for Interventional Oncology in the Intramural Research Program of the National Institutes of Health (NIH) by intramural NIH Grants NIH Z01 1ZID BC011242 and CL040015. The NIH and Biocompatibles BTG UK have a Cooperative Research and Development Agreement which provided funding for the conduct of the study. Biocompatibles BTG UK had no control over the conduct of the study, the inclusion of any data, data analysis and interpretation, manuscript preparation and decisions on submission for publication. NIH may hold intellectual property in the field.
The content of this manuscript does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services. The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as an actual or implied endorsement of such products by the United States government.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The study protocol was approved by the Animal Care and Use Committee of the NIH Clinical Center.
References
- 1.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V, & Coschiera P. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: Final results of a phase III study. Anticancer Res. 2012;32(4):1387–95 [PubMed] [Google Scholar]
- 2.Xu G, Zhang W, Ma MK, McLeod HL. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(8):2605–11. [PubMed] [Google Scholar]
- 3.Guichard S, Terret C, Hennebelle I, Lochon I, Chevreau P, Fretigny E, et al. CPT-11 converting carboxylesterase and topoisomerase activities in tumour and normal colon and liver tissues. British Journal of Cancer. 1999;80(3–4):364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forster RE, Small SA, Tang Y, Heaysman CL, Lloyd AW, Macfarlane W, et al. Comparison of DC Bead-irinotecan and DC Bead-topotecan drug eluting beads for use in locoregional drug delivery to treat pancreatic cancer. J Mater Sci Mater Med. 2010;21(9):2683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis AL, Hall B. Toward a better understanding of the mechanism of action for intra-arterial delivery of irinotecan from DC Bead (DEBIRI). Future Oncol. 2019;15(17):2053–68. [DOI] [PubMed] [Google Scholar]
- 6.Burris HA, Hanauske AR, Johnson RK, Marshall MH, Kuhn JG, Hilsenbeck SG, et al. Activity of topotecan, a new topoisomerase i inhibitor, against human tumor colony-forming units in vitro. J Natl Cancer Inst. 1992;84(23):1816–20. [DOI] [PubMed] [Google Scholar]
- 7.Houghton PJ, Cheshire PJ, Myers L, Stewart CF, Synold TW, Houghton JA. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol. 1992;31(3):229–39. [DOI] [PubMed] [Google Scholar]
- 8.Rowinsky EK, Grochow LB, Hendricks CB, Ettinger DS, Forastiere AA, Hurowitz LA, et al. Phase I and pharmacologic study of topotecan: A novel topoisomerase I inhibitor. J Clin Oncol. 1992;10(4):647–56. [DOI] [PubMed] [Google Scholar]
- 9.Creemers GJ, Wanders J, Gamucci T, Vallentin S, Dirix LY, Schoffski P, et al. Topotecan in colorectal cancer: a phase II study of the EORTC early clinical trials group. Ann Oncol. 1995;6(8):844–6. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald JS, Benedetti JK, Modiano M, Alberts DS. Phase II evaluation of topotecan in patients with advanced colorectal cancer. A Southwest Oncology Group trial (SWOG 9241). Invest New Drugs. 1997;15(4):357–9. [DOI] [PubMed] [Google Scholar]
- 11.Rowinsky EK, Baker SD, Burks K, O’Reilly S, Donehower RC, Grochow LB. High-dose topotecan with granulocyte-colony stimulating factor in fluoropyrimidine-refractory colorectal cancer: a phase II and pharmacodynamic study. Ann Oncol. 1998;9(2):173–80. [DOI] [PubMed] [Google Scholar]
- 12.Kummar S, Raffeld M, Juwara L, Horneffer Y, Strassberger A, Allen D, et al. Multihistology, target-driven pilot trial of oral topotecan as an inhibitor of hypoxia-inducible factor-1alpha in advanced solid tumors. Clin Cancer Res. 2011;17(15):5123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapisarda A, Zalek J, Hollingshead M, Braunschweig T, Uranchimeg B, Bonomi CA, et al. Schedule-dependent inhibition of hypoxia-inducible factor-1alpha protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 2004;64(19):6845–8. [DOI] [PubMed] [Google Scholar]
- 14.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64(4):1475–82. [DOI] [PubMed] [Google Scholar]
- 15.Guerin E, Raffelsberger W, Pencreach E, Maier A, Neuville A, Schneider A, et al. In vivo topoisomerase I inhibition attenuates the expression of hypoxia-inducible factor 1 alpha target genes and decreases tumor angiogenesis. Mol Med. 2012;18:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis AL, Willis SL, Dreher MR, Tang Y, Ashrafi K, Wood BJ, et al. Bench-to-clinic development of imageable drug-eluting embolization beads: finding the balance. Future oncology (London, England). 2018;14(26):2741–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashrafi K, Tang Y, Britton H, Domenge O, Blino D, Bushby AJ, et al. Characterization of a novel intrinsically radiopaque drug-eluting bead for image-guided therapy: DC Bead LUMI. J Control Release. 2017;250:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy EB, Krishnasamy VP, Lewis AL, Willis S, Macfarlane C, Anderson V, et al. First human experience with directly image-able iodinated embolization microbeads. Cardiovasc Interv Radiol. 2016;39(8):177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negussie AH, Dreher MR, Johnson CG, Tang Y, Lewis AL, Storm G, et al. Synthesis and characterization of image-able polyvinyl alcohol microspheres for image-guided chemoembolization. J Mater Sci Mater Med. 2015;26(6):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaine T, Tang Y, Garcia P, John J, Waters LJ, Lewis AL. Evaluation of ion exchange processes in drug-eluting embolization beads by use of an improved flow-through elution method. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2016;93:351–9. [DOI] [PubMed] [Google Scholar]
- 21.Walsh TJ, Bacher J, Pizzo PA. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Laboratory animal science. 1988;38(4):467–71. [PubMed] [Google Scholar]
- 22.Hinton M Gastric ulceration in the rabbit. J Comp Pathol. 1980;90(3):475–81. [DOI] [PubMed] [Google Scholar]
- 23.McCarley RL. Redox-responsive delivery systems. Annual Review of Analytical Chemistry, 2012; p. 391–411. [DOI] [PubMed] [Google Scholar]
- 24.Morris R, Munkarah A. Alternate dosing schedules for topotecan in the treatment of recurrent ovarian cancer. Oncologist. 2002;7 Suppl 5:29–35. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Man S, Xu P, Cruz-Munoz W, Tang T, Kumar R, et al. Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Molecular cancer therapeutics. 2010;9(4):996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. Guidance for Industry – Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Center for Drug Evaluation and Research (CDER). 1995. [Google Scholar]
- 27.Lewis AL, Holden RR, Chung ST, Czuczman P, Kuchel T, Finnie J, et al. Feasibility, safety and pharmacokinetic study of hepatic administration of drug-eluting beads loaded with irinotecan (DEBIRI) followed by intravenous administration of irinotecan in a porcine model. J Mater Sci Mater Med. 2013;24(1):115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy EB, Peer C, Sissung TM, Venkatesan A, Pandalai P, Greten T, et al. Pilot Study Comparing Systemic and Tissue Pharmacokinetics of Irinotecan and Metabolites after Hepatic Drug-Eluting Chemoembolization. J Vasc Interv Radiol. 2019;30(1):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gholamrezanezhad A, Mirpour S, Geschwind JF, Rao P, Loffroy R, Pellerin O, et al. Evaluation of 70–150-mum doxorubicin-eluting beads for transcatheter arterial chemoembolization in the rabbit liver VX2 tumour model. European radiology. 2016;26(10):3474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikhail AS, Pritchard WF, Negussie AH, Krishnasamy VP, Amchin DB, Thompson JG, et al. Mapping drug dose distribution on CT images following transarterial chemoembolization with radiopaque drug-eluting beads in a rabbit tumor model. Radiology. 2018;289(2):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]


