Abstract
Objective:
To determine the odds of postpartum hemorrhage (PPH) in low-risk women who gave birth vaginally and were exposed to different durations and dosages of oxytocin across a range of labor durations during spontaneous or induced labor.
Design:
A retrospective cross-sectional analysis of data from the Consortium for Safe Labor.
Setting:
Data were gathered from 12 clinical institutions across the United States from 2002 to 2008.
Participants:
After exclusions of high-risk conditions associated with PPH, we examined data from 27,072 women who gave birth vaginally.
Methods:
Postpartum hemorrhage was defined as estimated blood loss of greater than 500 ml at the time of birth and/or a diagnostic code for PPH before hospital discharge. We included covariates were if they were associated with oxytocin use and PPH and did not mediate oxytocin use. We used regression models to determine the likelihood of PPH overall and within induced and spontaneous labor groups separately. We used subgroup analyses within specific durations of labor to clarify the findings.
Results:
The overall rate of PPH was 3.9%. Women with induced labor experienced PPH more frequently than women who labored spontaneously. Labor augmentation was associated with higher adjusted odds for PPH when oxytocin was infused for more than 4 hours. Longer duration of spontaneous labor and second stage labor did not change this association. Oxytocin use during labor induction increased odds for PPH when administered for more than 7 hours. The odds further increased when induction lasted longer than 12 hours and/or the second stage of labor was longer than 3 hours.
Conclusion:
Strategies for judicious oxytocin administration may help mitigate PPH in low-risk women having vaginal birth.
Keywords: Postpartum Hemorrhage; Oxytocin; Labor, Induced
Precis
Oxytocin administration increases women’s odds for postpartum hemorrhage when administered longer than 4 hours during spontaneous labor and longer than 7 hours for labor induction.
Postpartum hemorrhage (PPH) is a key driver of maternal mortality and near miss morbidity globally (Maswime & Buchmann, 2017) and in the United States (Callaghan et al., 2010; Merriam et al., 2018; Wetta et al., 2013). The incidence of PPH that requires blood transfusion or surgical interventions has increased during the last 25 years in the United States (Centers for Disease Control and Prevention [CDC], 2020) and disproportionately affects women of color (Gyamfi-Bannerman et al., 2018). Oxytocin is used to help prevent conditions that may increase risk for PPH such as prolonged labor, intraamniotic infection, and cesarean birth due to labor dystocia (Kernberg & Caughey, 2017; Nyfløt et al., 2017). It is also a primary tool to prevent and treat PPH (Main et al., 2015). However, researchers reported that oxytocin is over used (Bernitz et al., 2014) and may also increase the risk for complications that result in PPH, including postpartum uterine atony, retained placenta, and/or heavy uterine bleeding (Belghiti et al., 2011; Coviello et al., 2015; Davis et al., 2012; Ekin et al., 2015; Endler et al., 2012; Khireddine et al., 2013; Nyfløt et al., 2017; Wetta et al., 2013). Some researchers did not find that intrapartum oxytocin use was a risk factor for PPH; however, these researchers used oxytocin as a dichotomous variable and did not distinguish induction from augmentation (Butwick et al., 2017; Sosa et al., 2011) or the participants were given very low doses of oxytocin (Kearney et al., 2018). Therefore, understanding the precise role of oxytocin use in mitigating or contributing to PPH remains a key area of research.
CALLOUT 1
Researchers studying the relationship between oxytocin use and PPH have found it challenging to separate this relationship from various other factors, including mode of labor onset, labor length/complexity (Erickson et al., 2019; Kearney et al., 2018; Nyfløt et al., 2017) and prophylactic oxytocin to prevent PPH (Belghiti et al.,2011; Davis et al., 2012; Driessen et al., 2012). In addition, studying the relationship between oxytocin use and PPH is further complicated by a host of confounding variables, including antepartum anemia, parity, blood dyscrasias, magnesium sulfate administration, retained placenta, uterine fibroids, intraamniotic infection, macrosomia, uterine distension (polyhydramnios/multiple gestation), genital tract lacerations, instrument assisted vaginal birth, and cesarean birth.
Health care providers require better information on how oxytocin use during labor influences rates of PPH (increased or decreased) in women with a range of labor durations and oxytocin duration/dosages so that they can tailor management to the individual woman. Therefore, the purpose of our study was to determine the odds of PPH in low-risk women who gave birth vaginally and were exposed to different durations and dosages of intrapartum oxytocin across a range of labor durations during spontaneous or induced labor.
Methods
Design
Our study was a retrospective cross-sectional design, and we used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines to generate this report. We gathered data on women’s labor characteristics, including oxytocin administration, from the Consortium for Safe Labor (CSL) dataset (Eunice Kennedy Shriver National Institute of Child Health and Human Development, n.d.). The CSL dataset contains detailed information collected prospectively from 12 clinical centers and includes information describing 228,438 births that occurred in the United States between 2002 and 2008. Originally collected to explore reasons why women end labor with cesarean birth, to describe labor progression in a contemporary group of women, and to define the timing of labor protraction/arrest, the CSL data became available for secondary analyses through the Eunice Kennedy’s National Institute of Child Health and Disease’s Data and Specimen Hub in 2016. Included in the CSL dataset are variables related to maternal demographic characteristics, medical history, reproductive and prenatal history, labor interventions, birth outcomes, and newborn information. Data collected for the CSL study were cleaned, recoded, and validated by the original investigators. Institutional Review Board approval for this analysis was granted by Emory University, and both authors signed standard data use agreements, as required.
Settings
The 12 clinical centers of the CSL were located within nine districts of the United States as defined by the American College of Obstetricians and Gynecologists (2020). Within these 12 clinical centers were a total of 19 hospitals including eight academic, tertiary medical centers; nine community teaching hospitals; and two non-teaching community hospitals.
Participants
Our participants were limited to women who experienced vaginal births at term gestation (37 weeks or later) of a liveborn single fetus with no known congenital anomalies. We excluded cases if the record was a repeat birth for the individual participant in the dataset. We excluded women with missing data for pre-pregnancy body mass index (BMI), estimated blood loss, and/or oxytocin use. We also excluded women who experienced conditions known to increase the risk for bleeding during and after labor, including abnormal placentation (previa, accreta, abruption), antenatal thromboembolic disorders, prolonged third stage of labor (greater than 30 minutes), hypertensive disorders (preeclampsia, eclampsia or gestational hypertension), magnesium sulfate used in labor, presence of severe vaginal or third or fourth degree perineal lacerations, fever, and/or intraamniotic infection. We also excluded three cases for women whose durations of oxytocin administration exceeded the durations of their admissions, which suggested inaccurate data. In cases in which information on the duration of oxytocin duration was missing, we recorded duration as zero (no oxytocin used) if we also had no evidence of oxytocin use after examining induction or augmentation variables. Figure 1 illustrates the selection of the study sample.
Figure 1:
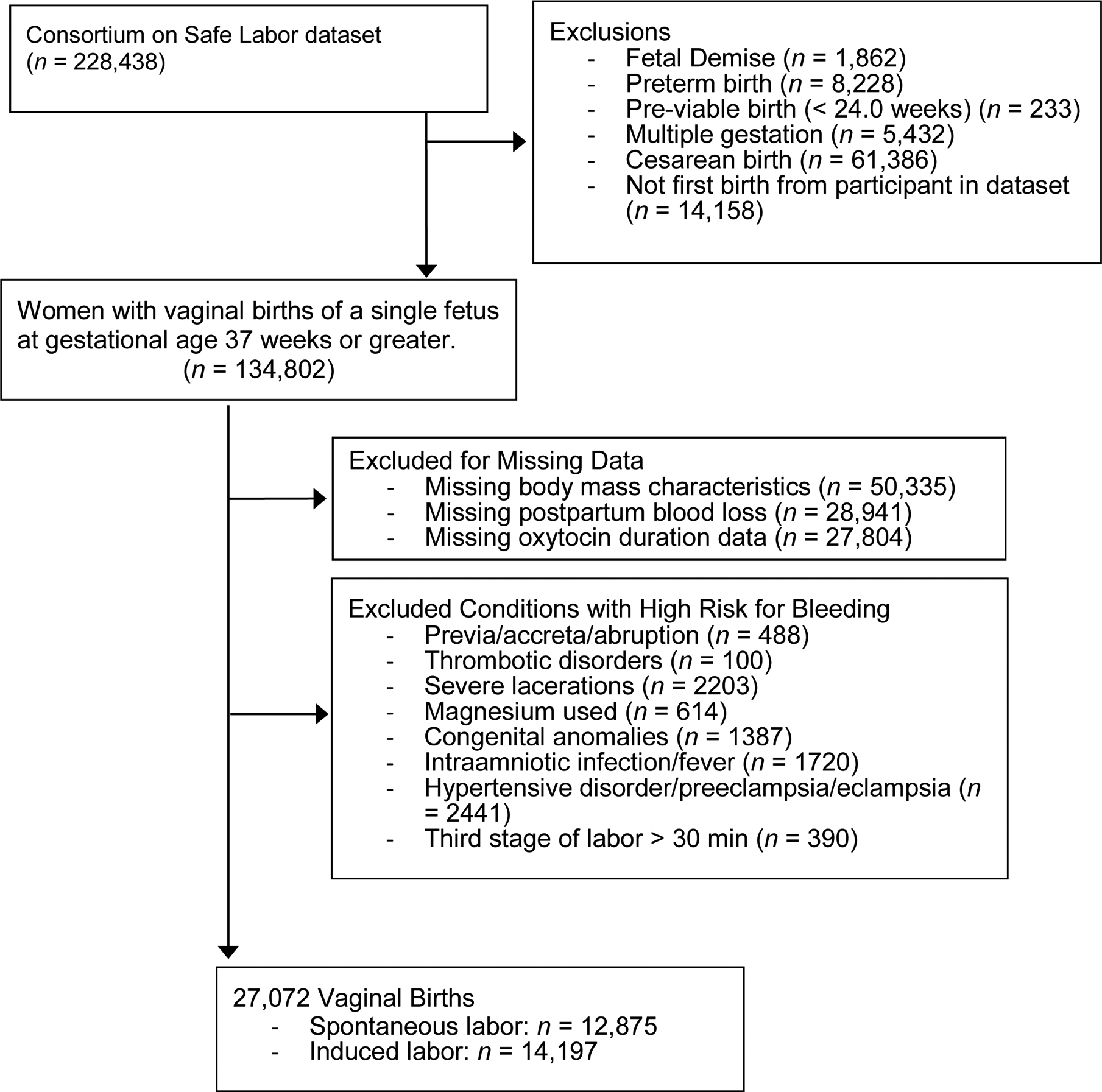
Sample identification procedure for analysis of Consortium for Safe Labor dataset on postpartum hemorrhage following vaginal birth.
Procedures
The PPH outcome was generated by a composite of variables: a recorded blood loss greater than 500 ml on the birth record and/or a diagnosis code indicating that PPH had occurred (Table 1). The definition of PPH for vaginal birth has been updated since data were collected for the CSL study; PPH is now diagnosed at 1,000 ml or more in a woman who has any blood loss accompanied by signs/symptoms of hypovolemia (Sharp et al., 2017). We classified blood loss greater than 500 ml as PPH for this study because this was the definition of PPH for vaginal birth used at the time of data collection. In addition, estimates of blood loss by visualization are prone to underestimation (particularly at the higher end; Natrella et al., 2018). Thus estimated blood loss upwards of 500 ml may have represented higher actual volumes and would qualify as a PPH case even according to contemporary guidelines.
Table 1:
Characteristics of Sample, Postpartum Hemorrhage Frequency following 27,072 Vaginal Births and Use of Oxytocin During Labor from Consortium for Safe Labor Data.
| Variable | PPH Frequency By variable | Maternal and newborn characteristics Frequency by oxytocin use in labor. n (%) |
|||
|---|---|---|---|---|---|
| No Oxytocin in Labor n=5824 |
Augmentation Oxytocin n= 8356 |
Induction Oxytocin n=12892 |
Difference by Oxytocin Use p-value |
||
| Postpartum Hemorrhage | |||||
| Composite Variable | 1,052 (3.9) | 190 (3.3) | 294 (3.5) | 568 (4.4) | <.001 |
| Over 500 mL blood loss | 566 (2.1) | ||||
| ICD-9 Diagnosis Code | 733 (2.7) | ||||
| Maternal/Pregnancy | |||||
| Variables | |||||
| Parity | |||||
| Nulliparous | 540 (5.1)* | 2151 (36.9) | 3688 (44.1) | 4809 (37.3) | <.001 |
| Para 1–3 | 463 (3.1) | 3282 (56.4) | 4273 (51.1) | 7412 (57.5) | |
| Para >=4 | 49 (3.4) | 391 (6.7) | 395 (4.7) | 671 (5.2) | |
| Maternal Race/Ethnicity | |||||
| White | 481 (3.8) | 2829 (50.4) | 3724 (45.9) | 6078 (48.2) | <.001 |
| Black/Non Hispanic | 230 (3.8) | 1268 (22.6) | 1979 (24.4) | 2848 (22.6) | |
| Hispanic | 252 (4.2) | 1106 (19.7) | 1934 (23.8) | 2971 (23.6) | |
| Asian Pacific Islander | 30 (3.9) | 288 (5.1) | 220 (2.7) | 259 (2.1) | |
| Multi-racial/Other | 29 (3.5) | 123 (2.2) | 255 (3.1) | 457 (3.6) | |
| Maternal Age (years) | |||||
| <20 | 154 (4.9)* | 662 (11.4) | 1126 (13.5) | 1354 (10.5) | <.001 |
| 20–34 | 771 (3.7) | 4477 (76.9) | 6462 (77.4) | 10,048 (77.9) | |
| 35 and older | 126 (4.3) | 678 (11.7) | 766 (9.2) | 1487 (11.5) | |
| Maternal Body Mass Index (Pre-pregnancy) | <.001 | ||||
| Underweight | 51 (3.5) | 370 (6.4) | 483 (5.8) | 625 (4.9) | |
| Normal | 575 (3.8) | 3332 (57.2) | 4794 (57.4) | 6985 (54.2) | |
| Overweight | 249 (4.0) | 1277 (21.9) | 1822 (21.8) | 3067 (23.8) | |
| Obese | 177 (4.1) | 845 (14.5) | 1257 (15.0) | 2215 (17.2) | |
| Gestational Age (weeks) | |||||
| 37–38.9 | 346 (3.9)* | 1921 (32.9) | 3205 (38.4) | 3611 (28.0) | <.001 |
| 39–40 | 443 (3.5) | 2862 (49.1) | 3453 (41.3) | 6306 (48.9) | |
| 40.1–41 | 214 (4.6) | 894 (15.4) | 1442 (17.3) | 2308 (17.9) | |
| >41 | 49 (4.6) | 147 (2.5) | 256 (3.1) | 667 (5.2) | |
| Gestational Weight Gain | |||||
| Institute of Medicine | <.001 | ||||
| Met guidelines | 289 (3.5)* | 1758 (30.2) | 2508 (30.0) | 3937 (30.5) | |
| Under guidelines | 210 (3.4) | 1377 (23.6) | 1946 (23.3) | 2810 (21.8) | |
| Exceeded guidelines | 553 (4.3) | 2689 (46.2) | 3902 (46.7) | 6145 (47.7) | |
| Labor/Birth Variables | |||||
| Labor Onset | |||||
| Induction of Labor | 625 (4.4)* | 1305 (22.4) | 0 (0) | 12,892 (100) | <.001 |
| Spontaneous Labor | 427 (3.3) | 4519 (77.6) | 8356 (100) | 0 (0) | |
| Cervical Dilation on Admission (centimeters) | <.001 | ||||
| 3 or less | 582 (4.3)* | 1875 (40.3) | 3582 (53.5) | 8047 (77.6) | |
| 3.5–5.5 | 240 (3.8) | 1833 (39.4) | 2418 (36.1) | 2082 (20.1) | |
| 6 or higher | 45 (2.4) | 943 (20.3) | 694 (10.4) | 247 (2.4) | |
| Duration Full Dilation | |||||
| 30 minutes or less | 400 (3.1)* | 2895 (58.6) | 3755 (49.6) | 6261 (55.3) | <.001 |
| 31–59 minutes | 180 (3.7) | 938 (18.9) | 1608 (21.3) | 2372 (20.9) | |
| >= 1 hour < 3 hours | 275 (5.3) | 954 (19.3) | 1880 (24.9) | 2374 (20.9) | |
| >=3 hours < 6 hours | 54 (6.8) | 153 (3.1) | 323 (4.3) | 314 (2.8) | |
| Oxytocin Duration | |||||
| No oxytocin used | 190 (3.3)* | 5824 (100) | 0 | 0 | <.001 |
| <= 2 hours | 155 (2.9) | 0 | 3214 (38.5) | 2159 (16.8) | |
| >2 to 4 hours | 161 (3.3) | 0 | 2250 (26.9) | 2669 (20.7) | |
| >4 to 7 hours | 230 (4.3) | 0 | 1720 (20.6) | 3653 (28.3) | |
| >7 to 12 hours | 183 (4.8) | 0 | 864 (10.3) | 2926 (22.7) | |
| >12 hours | 133 (7.4) | 0 | 308 (3.7) | 1485 (11.5) | |
| Newborn | |||||
| Macrosomic (4000g+) | 92 (5.0) | 387 (6.8) | 502 (6.0) | 961 (7.5) | <.001 |
| Not macrosomic | 955 (3.8)* | ||||
Note.
Denotes significantly different (p <.05) rate of postpartum hemorrhage associated with variable.
We examined the duration of oxytocin used in hours by quartile and used chi-square tests to evaluate significant differences in the frequency of PPH by duration quartile. To develop the appropriate panel of covariates for our analysis, we considered each variable’s place within the causal pathway to PPH using the directed acyclic graph (DAG) method as advocated by Tilden and Snowden (2018) among others. In this approach, variables identified as mediators (rather than covariates) are not used as controls in the regression, which limits over-adjustment bias (Tilden & Snowden, 2018). In our study, mediators were variables that may have been caused or influenced by oxytocin use during intrapartum events leading to the PPH. We determined that neuroaxial analgesia use, instrument assisted vaginal birth, genital lacerations, and duration of third stage of labor were mediators and thus were not used as covariates in adjusted analyses. By contrast, we identified and retained for analyses covariates, defined as characteristics significantly associated with oxytocin use and PPH. The covariates we examined for inclusion in the analyses were maternal age, parity, gestational age, gestational weight gain, dilation of cervix at the time of labor admission, duration of full dilation, maternal pre-pregnancy BMI, maternal race/ethnicity, and newborn macrosomia.
Statistical Analyses
We evaluated the relationship of the covariates with the PPH outcome overall and according to use of oxytocin during women’s labors (no oxytocin, augmentation of spontaneous labor, induction of labor) using chi-square tests with a significance of p <.05 (Table 1). We conducted regression analyses using the composite PPH variable as the outcome and oxytocin duration (by quartile) as the exposure. We adjusted the full sample regression models for parity, maternal age, gestational age, level of weight gain, cervical dilation on admission, duration of full dilation, and macrosomia. Pre-pregnancy BMI and maternal race/ethnicity were associated with oxytocin use but not associated with PPH outcomes, thus were not retained in the analysis. We repeated the regression (unadjusted and adjusted) within groups of women who had induced or augmented labors separately. In the adjusted analysis, we labeled women with the shortest oxytocin exposure (less than 2 hours) as the reference group (Table 2). We also examined factors associated with PPH in the subgroup of the sample that had no oxytocin administration during spontaneous or induced labors (Table 3).
Table 2:
Odds of postpartum hemorrhage following vaginal birth by duration of oxytocin used during labor augmentation or induction of labor
| All Oxytocin Use (n = 21,248) | Augmentation Only (n = 8,356) | Induction Only (n = 12,892) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) Augmentation Only | aOR (95% CI) Augmentation Only | OR (95% CI) Induction Only | aOR (95% CI) Induction Only | |
| Oxytocin Duration | ||||||
| <=2 hours | Reference | Reference | Reference | Reference | Reference | Reference |
| >2 hours to 4 hours | 1.14 (0.91–1.42) | 1.27 (0.97–1.66) | 1.10 (0.78–1.55) | 1.19 (0.80–1.77) | 1.06 (0.79–1.44) | 1.24 (0.85–1.79) |
| >4 hours to 7 hours | 1.51 (1.22–1.85)** | 1.69 (1.32–2.17)** | 2.17 (1.59–2.96)** | 2.36 (1.64–3.40)** | 1.09 (0.82–1.45) | 1.29 (0.90–1.83) |
| >7 hours to 12 hours | 1.71 (1.37–2.12)** | 1.72 (1.30–2.27)** | 2.26 (1.56–3.28)** | 1.86 (1.16–2.99)* | 1.33 (1.0– 1.76) | 1.51 (1.05–2.19)* |
| >12 hours | 2.69 (2.13–3.42))** | 2.29 (1.63–3.20)** | 3.09 (1.90–5.04)** | 2.29 (1.19–4.41)* | 2.18 (1.62–2.94)** | 2.08 (1.37–3.17)** |
Note. Regression analyses adjusted for parity, age, gestational age, level of weight gain, cervical dilation on admission, macrosomia and duration of full dilation. Maternal pre-pregnancy BMI and maternal race predicted oxytocin use but not postpartum hemorrhage in bivariate analyses and were thus not included in the analysis.
p <.001
p <.05
Table 3:
Multivariable Regression of Characteristics Associated with PPH in Labors Without Oxytocin
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Parity | ||
| Nulliparous | 1.34 (0.86–2.10) | .19 |
| Para 1–3 | Reference | |
| Para >=4 | 0.77 (0.30–1.97) | .60 |
| Maternal Age (years) | ||
| <20 | 1.26 (0.74–2.13) | .40 |
| 20–34 | Reference | |
| 35 and older | 1.32 (0.78–2.25) | .30 |
| Gestational Age (weeks) | ||
| 37–38.9 | 1.24 (0.85–1.82) | .27 |
| 39–40 | Reference | |
| 40.1–41 | 1.04 (0.62–1.75) | .88 |
| >41 | 0.37 (0.05–2.72) | .33 |
| Gestational Weight Gain | ||
| Institute of Medicine | ||
| Met guidelines | Reference | |
| Under guidelines | 1.25 (0.76–2.04) | .38 |
| Exceeded guidelines | 1.19 (0.78–1.80) | .42 |
| Labor/Birth Variables | ||
| Cervical Dilation on Admission (centimeters) | ||
| 3 or less | 1.12 (0.69–1.82) | .65 |
| 3.5–5.5 | 0.97 (0.60–1.59) | .90 |
| 6 or higher | Reference | |
| Duration Full Dilation | ||
| 30 minutes or less | Reference | |
| 31–59 minutes | 0.92 (0.55–1.55) | .77 |
| >= 1 hour < 3 hours | 1.46 (0.90–2.35) | .13 |
| >=3 hours < 6 hours | 1.73 (0.77–3.91) | .19 |
| Newborn | ||
| Macrosomia (4000g+) | 1.50 (0.79–2.80) | .21 |
We conducted an additional sub-group multivariable regression analysis to examine the role of oxytocin administration within groups of women according to duration of labor they experienced following hospital admission and also within groups having a similar second stage labor duration to further clarify the relationship between oxytocin use and PPH. Finally, we assessed the maximum dose of oxytocin reached within the duration categories using a Mann-Whitney non-parametric test and compared maximum dose reached within the oxytocin duration category between cases with/without PPH.
Results
The final sample size after exclusions included 27,072 vaginal births. Most participants were parous and identified as women of color or of multi-racial background. Most participants were between 20 to 34 years of age and gave birth at 37 to 40 weeks gestation. Induction of labor occurred in 52.4% (n = 14,197) of births and 62.2% of participants entered the hospital with cervical dilation of 3 cm or less (n=13,504).
The total frequency of PPH in the sample was 3.9% (n = 1,052). Oxytocin duration ranged from 0 hours (n = 5,824) to 107.4 hours during labor. The mean duration of use among those who received oxytocin was 5.6 hours (SD 5.5 hours). Among participants who started labor spontaneously n = 12,875 (47.6%), 65% received oxytocin to augment labor, with a mean duration of 3.9 hours (SD 4.2). Nearly all (91%, n = 12,892) women whose labors were induced received oxytocin with a mean duration of 6.5 hours (SD 5.8 hours).
The frequency of PPH was greatest among participants who underwent induction of labor with oxytocin (4.41%) compared to those who received no oxytocin (3.3%) or those who received oxytocin for augmentation following spontaneous onset of labor (3.5%; χ2 = 21.3, p <.011). Differences in PPH frequencies and use of oxytocin by sample characteristics are presented in Table 1.
CALLOUT 2
Postpartum Hemorrhage in Women with Different Exposure to Oxytocin During Labor
Among participants with augmented and induced labor, the duration of oxytocin infusion was associated with development of PPH at distinct time points (Table 1). For example, in participants with spontaneous onset of labor, rates of PPH significantly increased when they received augmentation for more than 4 hours (5.1%) compared to rates for women with no oxytocin exposure (2.9%; p <.001; Figure 2). Moreover, when spontaneous labor was followed by 12 or more hours of oxytocin augmentation, the rate of PPH increased to 7.1% (p <.001 vs. no oxytocin).
Figure 2.
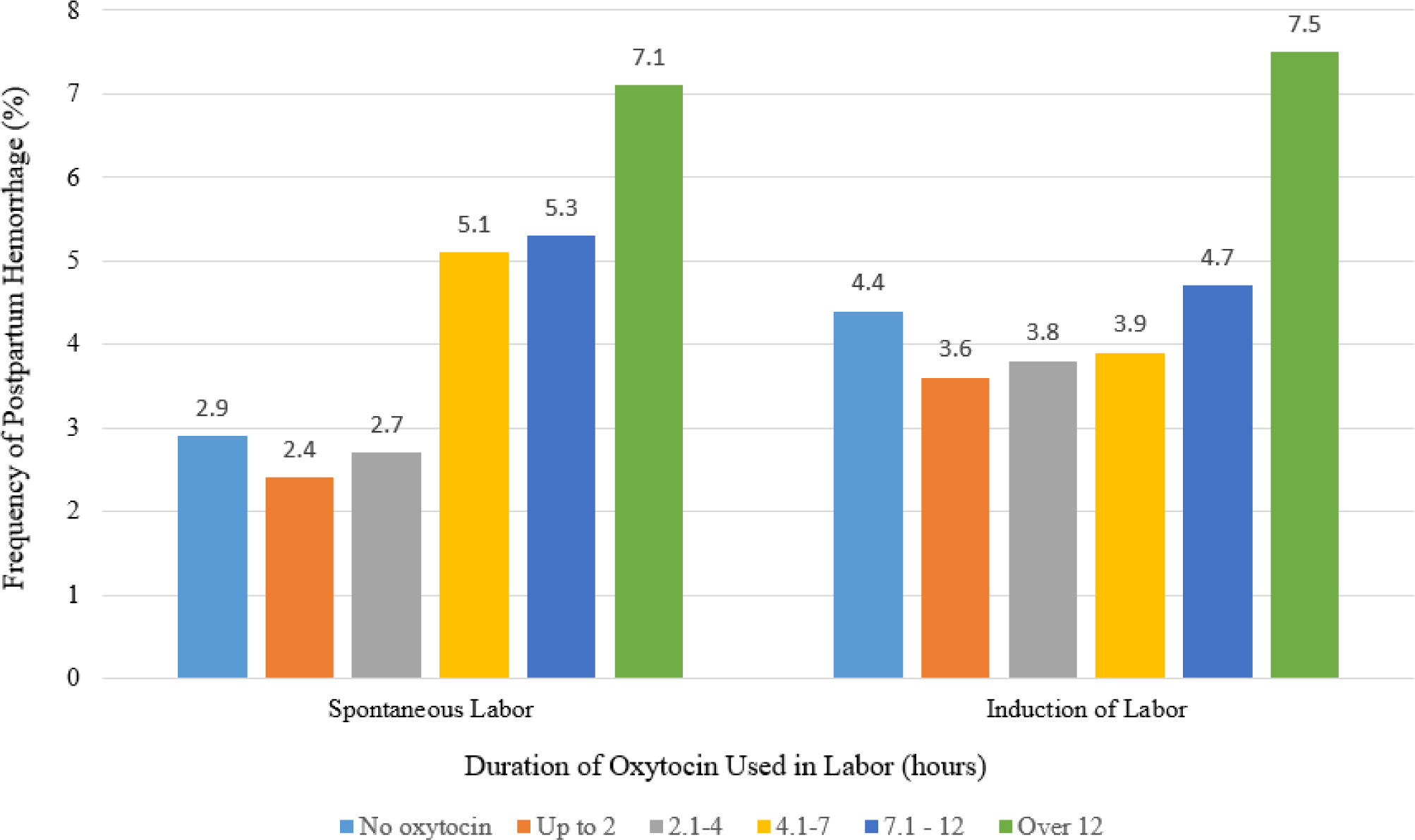
Frequency of postpartum hemorrhage following vaginal birth by duration of oxytocin used in labor by mode of labor onset. Data from Consortium for Safe Labor. Difference in PPH frequency by duration of oxytocin during spontaneous labor (n = 12,875, Chi Square p<0.001) and by induced labor (n = 14,197, Chi Square p<0.001).
Participants with induced labor who did not require oxytocin (n = 1305) had a greater overall frequency of PPH (4.4%) than those with spontaneous labor (2.9%, n = 4519, p = .01). The rate of PPH among participants who were induced was not significantly greater until the duration of oxytocin exposure exceeded 7 hours (PPH rate 4.7%) compared oxytocin use for 2 hours or fewer (3.6%, p=.02). Infusion of oxytocin for longer than 12 hours was associated with a frequency of PPH of 7.5%, more than double the frequency for infusion for 2 or fewer hours (3.6%, p <.001).
Spontaneous labor.
In multivariable analyses adjusting for parity, age, gestational age, level of weight gain, cervical dilation on admission, macrosomia, and duration of second stage labor, we found that women with spontaneous onset of labor (n= 1720) who were augmented with 4 to 7 hours of oxytocin were 2.36 times as likely to have PPH (95% CI [1.64–3.40]) than women who had oxytocin infused for 2 hours or fewer (n = 3214). Also in adjusted analyses, longer durations of oxytocin augmentation beyond 7 hours among women with spontaneous labor were associated with PPH occurrence in a dose-response fashion (Table 2). Furthermore, in this model of oxytocin use during spontaneous labor, we found a 23% increased likelihood of PPH with every additional hour of full dilation (duration of second stage of labor; OR 1.23 95% CI [1.10–1.37]). No other covariates (maternal age, parity, gestational age, gestational weight gain, dilation of cervix at the time of labor admission, or newborn macrosomia) were significantly associated with PPH occurrence. Additionally, among participants who had no oxytocin exposure, none of the covariates in the model were statistically associated with PPH (Table 3).
Induced labor.
In adjusted analyses, the odds of PPH among women with induced labor increased significantly after 7 hours of oxytocin infusion after adjustment for maternal demographic, pregnancy, and labor factors. Women who had their labors induced with 7 to 12 hours of oxytocin were 51% more likely to have PPH (OR 1.51 95% CI [1.05–2.19]) compared to women who were induced with less than 2 hours of oxytocin. We found a dose-response relationship between increasing durations of intrapartum oxytocin infusions during induction and PPH. For example, infusion of oxytocin during induction for longer than 12 hours was associated with the highest odds of PPH (OR 2.08 95% CI [1.37–3.17]) compared to less than 2 hours of administration. Among the covariates examined in this model, only duration of the second stage of labor was significantly associated with PPH (OR 1.20, 95% CI [1.09–1.33]). These findings suggest that for women undergoing labor induction who otherwise appeared to be low risk, the duration of oxytocin use and the second stage of labor were useful factors with which to predict the likelihood of PPH.
Postpartum Hemorrhage in Women with Different Durations of Labor following Hospital Admission
Because prolonged labor duration is thought to increase risk of PPH (Nyfløt et al., 2017), we also analyzed the relationship between oxytocin use and PPH by the duration of time in the hospital before vaginal birth. In these subgroup analyses, we again controlled for maternal age, gestational age, BMI, weight gain, cervical dilation, and macrosomia. Figure 3 shows four categories of admission to birth duration (less than 6 hours, 6–12 hours, 12–18 hours, and greater than 18 hours). First, we calculated the likelihood of PPH among women who started labor spontaneously when they were given oxytocin for more than 4 hours compared to 0 to 4 hours during some part of labor. In the adjusted models, women who had moderate durations from admission to birth (6–12 or 12–18 hours) had greater odds of PPH if they were augmented with oxytocin for longer than 4 hours compared to fewer than 4 hours (4.6%–6.8% vs. 2.7%–3.8%) or no augmentation (3.0%–3.8%). Among women with moderate durations from admission to birth, we found no difference in PPH rates between women with no oxytocin and those with less than 4 hours of oxytocin. Although women with spontaneous labor onset with the longest duration from admission to birth (more than18 hours) and no oxytocin augmentation had greater rates of PPH (9.1%) than similar women whose labors were augmented (6.8%–6.9%), this increase was not statistically significant in bivariate or adjusted regression analyses. Thus, these findings suggest that oxytocin augmentation following spontaneous labor onset may increase risk for PPH if oxytocin infusions during labor continue for longer than 4 hours.
Figure 3:
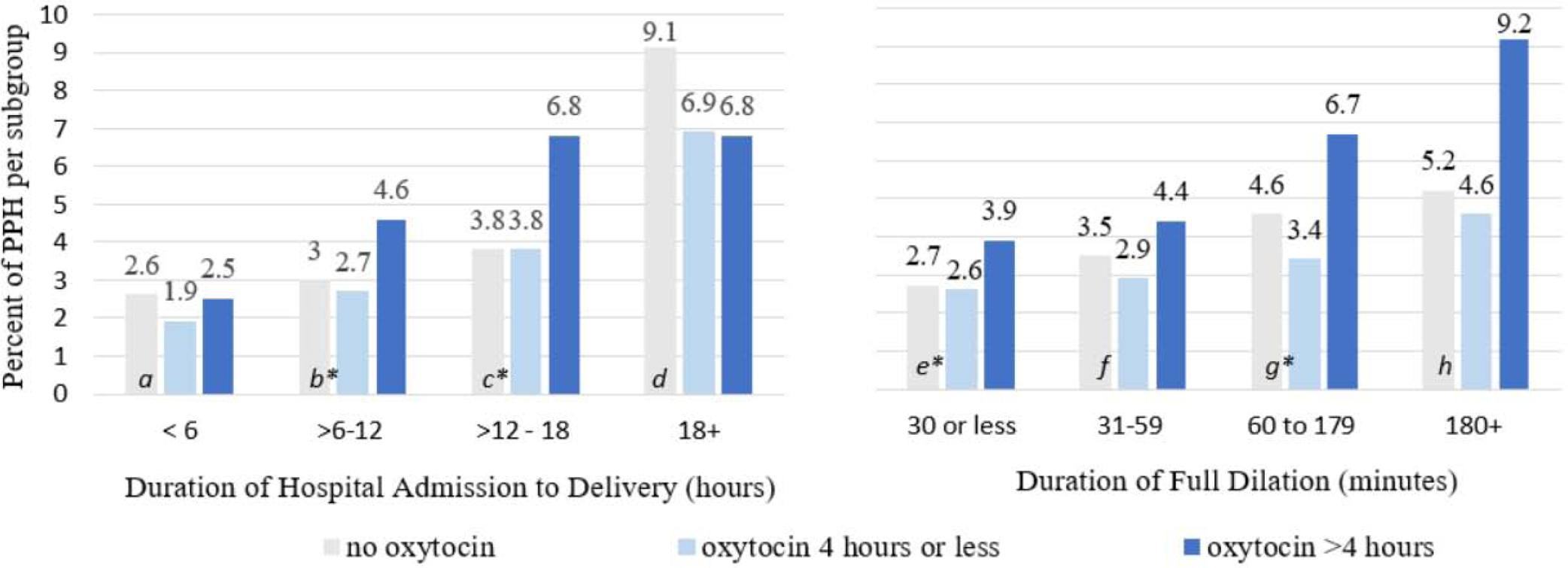
Duration of interval of hospital admission to delivery and duration of full dilation relative to oxytocin augmentation used during spontaneous labor and postpartum hemorrhage following vaginal birth (n = 12,875). * = p<0.05. Adjusted Odds Ratio (95% Confidence Interval) for PPH are as follows for Hospital Admission to Delivery Time: a=1.11 (0.44–2.78), b= 1.98 (1.41–2.80), c= 1.81 (1.13–2.87), d= 0.72 (0.34–1.68); Duration of Full Dilation: e = 2.41 (1.32–3.48), f = 1.72 (0.97–3.14), g =2.32 (1.44–3.74), h =1.51 (0.55–4.17). Adjusted for parity, maternal age, gestational age, excess gestational weight gain and cervical dilation on admission. n=1460 missing duration of full dilation, n = 188 missing duration admission. Odds Ratio is for oxytocin >4 hours compared to use of oxytocin for less than 4 hours or no oxytocin used. No odd ratios were significant for the comparison of no oxytocin used to 4 hours or less used. Data from Consortium for Safe Labor.
For women whose labors were induced (Figure 4), exposure to oxytocin for more than 7 hours was associated with increased odds of PPH compared to exposure to oxytocin for less than 7 hours (OR 1.46, 95% CI [1.14–1.88]) when the time from admission to birth lasted longer than 12 hours. If women’s labor inductions lasted less than 12 hours, this difference in oxytocin infusion duration and PPH was not seen. Thus, longer durations of intrapartum oxytocin administration during labor induction protocols did not appear to increase women’s risk for PPH if their labor induction process was relatively brief (less than 12 hours).
Figure 4.
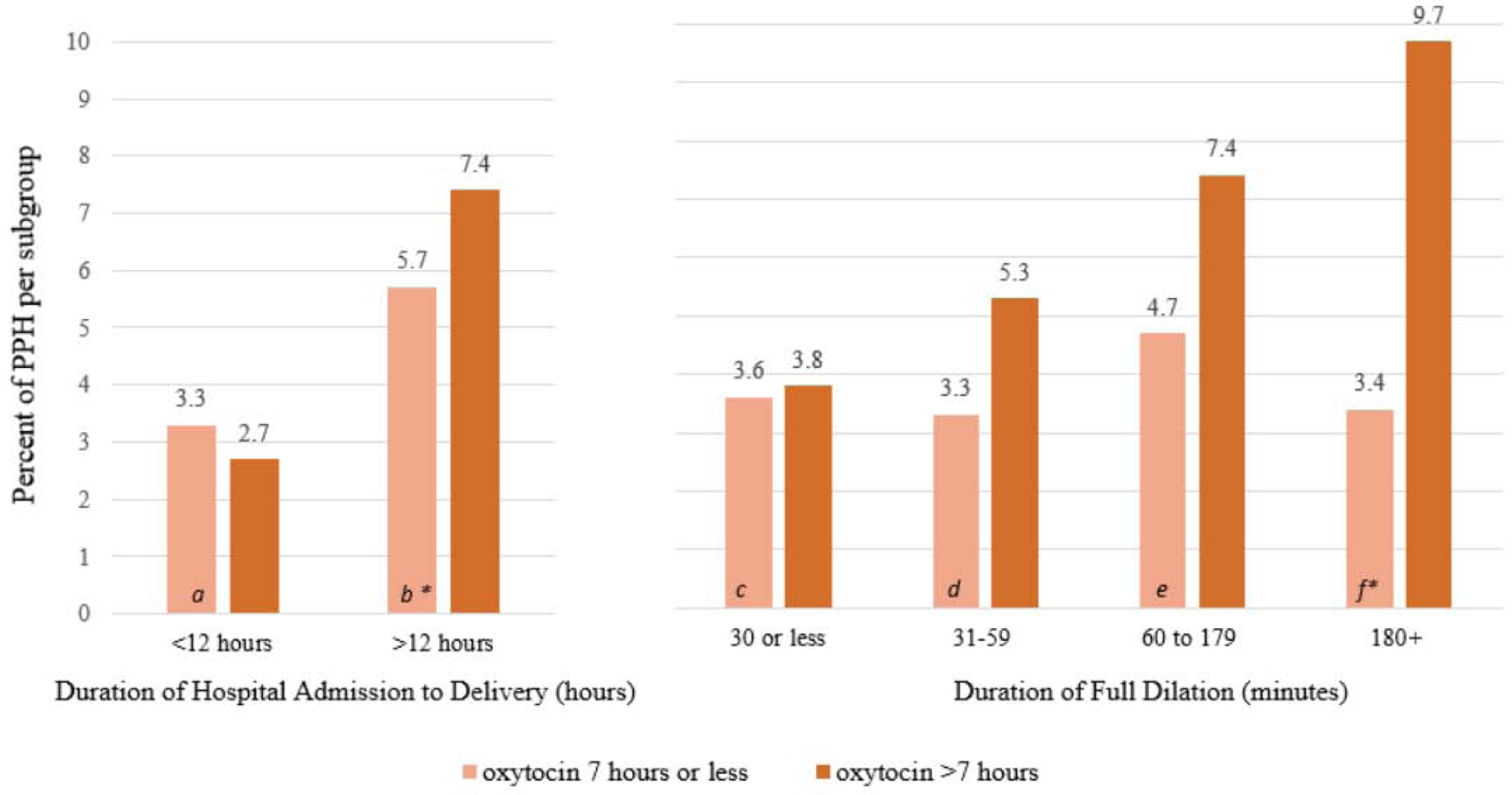
Duration of interval of hospital admission to delivery and duration of full dilation relative to oxytocin administration used during induced labor and postpartum hemorrhage following vaginal birth (n = 14,197). * = p<0.05. Adjusted Odds Ratio (95% Confidence Interval) for PPH are as follows for Hospital Admission to Delivery Time: a=0.77 (0.52–1.14), b= 1.46 (1.14–1.88); Duration of Full Dilation: c = 1.19 (0.85–1.67), d = 1.40 (0.87–2.26), e =1.37 (0.94–2.00), f =3.15 (1.19–8.29). Adjusted for parity, maternal age, gestational age, excess gestational weight gain and cervical dilation on admission. n=1785 missing duration of full dilation, n = 45 missing duration of admission. Odds Ratio is for oxytocin >7 hours compared to use of oxytocin for less than 7 hours. Data from Consortium for Safe Labor.
Postpartum Hemorrhage in Women with Different Durations of Second Stage of Labor
Because duration of the second stage of labor is thought to increase women’s risk of PPH (Dionne et al., 2015), we also analyzed the relationship between oxytocin use and PPH within subgroups of women whose second stages of labor lasted for increasing amounts of time. Although it was not possible to quantify exactly when oxytocin was infused during these women’s labors (earlier vs. later in labor), this analysis nevertheless helps describe the relationship between oxytocin use and PPH risk in women with different second stage labor durations.
Figure 3 shows that within each category representing the amount of time women in spontaneous labor spent at full dilation (≤30 minutes, 31–59 minutes, 60–179 minutes, 180+minutes), use of oxytocin for more than 4 hours was associated with increased rates of PPH compared to use of oxytocin for less than 4 hours. However, in analyses adjusted for maternal demographic, pregnancy, and labor factors, only women with short (less than 30 minutes) or moderate (60–179 minutes) second stages of labor had increased odds of PPH when they received oxytocin augmentation for more than 4 hours compared to less than 4 hours. In these adjusted analyses, maternal demographics, pregnancy, and labor characteristics were not significantly associated with PPH, regardless of the duration of second stage of labor. Only when the duration of the second stage was longer than 3 hours, one covariate, neonatal macrosomia, was significantly associated with PPH (OR 3.60, 95% CI [1.13–11.5]) in women with oxytocin augmentation for any length of time. Thus, for women in this sample who experienced second stage of labor that lasted less than 3 hours, oxytocin use for more than 4 hours during labor augmentation was a more significant predictor of PPH than infant macrosomia, gestational weight gain, or a host of other maternal and pregnancy factors.
In contrast, for women with labor induction (Figure 4), PPH rates did not differ following use of oxytocin for longer than 7 hours vs. less than 7 hours until the second stage of labor persisted for at least 3 hours (9.7% in women with more than 7 hours oxytocin vs. 3.4% in women with less than 7 hours of oxytocin, p=0.01). These findings were confirmed in multivariable models after adjusting for maternal age, gestational age, BMI, weight gain, cervical dilation, and macrosomia. In these adjusted analyses, maternal demographic, pregnancy, and labor characteristics were not significantly associated with PPH, regardless of the duration of the second stage of labor. Thus, in women in this sample, prolonged exposure to oxytocin during induction of labor (more than 7 hours) was not a strong contributing factor to PPH if the second stage of labor concluded in less than 3 hours. Maternal demographic, pregnancy, and labor characteristics did not appear to change this relationship between oxytocin duration and PPH risk.
Postpartum Hemorrhage in Women with Exposure to Different Oxytocin Dosages During Labor
Because oxytocin infusions are titrated upwards over time, women with longer oxytocin infusion also tend to receive the highest titrations. However, in some women, health care providers may administer oxytocin infusions using a more measured approach whereby oxytocin titrations are increased very gradually over time. To examine more closely the relationship between oxytocin titration and PPH in this sample, we compared PPH rates in women by the maximum oxytocin titration (dose/time in mU/min) to which they were exposed during labor. We found that among women with spontaneous labor onset whose oxytocin infusions lasted less than 4 hours, the maximum oxytocin titration was similar regardless of PPH outcome (M 7.63 mU/min, SD 4.39). However, when labor augmentation lasted longer than 4 hours, women with PPH reached a mean maximum oxytocin dose of 14.47 mU/min (SD 7.79) compared to a mean of 12.93 mU/min (SD 8.46) among similar women who did not have PPH (p=0.01).
For women whose labors were induced, we found higher maximum oxytocin doses among those who experienced PPH in the shorter and longer duration of administration subgroups. Women with oxytocin infusions that lasted less than 7 hours and experienced a PPH, had a mean maximal dose of 14.32 (SD 7.70) mU/min compared to the mean of 12.72 (SD 6.86) mU/min (p <.001) for those without PPH. When oxytocin lasted more than 7 hours and the woman experienced a PPH, the mean maximal dose reached 21.10 mU/min (SD 11.17) compared to a mean of 18.42 mU/min (SD 9.48) (p <.001) in women without PPH. These findings indicate that during labor induction, the duration of oxytocin infusion and the peak titration may be important factors in the development of PPH. In this sample, the safest combination of oxytocin dose and duration to avoid PPH was less than 12 mU/min for less than 7 hours.
Discussion
The purpose of our study was to determine the odds of PPH in low-risk women who gave birth vaginally and received different durations and dosages of oxytocin across a range of labor durations during spontaneous or induced labor. All women in this sample had term, singleton fetuses and gave birth vaginally. Our findings help to clarify the durations and doses of oxytocin infusion during labor that are associated with increased odds of PPH. We found that when health care providers administered oxytocin for longer than 4 hours in women with spontaneous labor onset, PPH was more likely to occur regardless of the duration of time in hospital from admission to birth or of second stage labor. Although women in this sample whose labors were induced experienced PPH at greater rates overall than women in spontaneous labor, oxytocin durations of longer than 7 hours during induction were significantly associated with increased odds for PPH after controlling for relevant covariates. This relationship between longer oxytocin use and PPH was even stronger when induced labor lasted longer than 12 hours or the second stage of labor was longer than 3 hours. Our findings add to the literature on the risk of PPH in women with spontaneous or induced labor. In addition, we evaluated the influence of overall duration of labor and duration of the second stage of labor on risk of PPH within the context of duration of oxytocin titration. Finally, we clarified that among women without any oxytocin in this cohort, none of the covariates were significantly related to PPH.
CALLOUT 3
Postpartum Hemorrhage and Intrapartum Oxytocin Literature
Many researchers reported greater rates of PPH among women who received oxytocin during labor for augmentation or induction (Belghiti et al., 2011; Coviello et al., 2015; Davis et al., 2012; Ekin et al., 2015; Endler et al., 2012; Khireddine et al., 2013; Nyfløt et al., 2017; Wetta et al., 2013). For example, in a case-controlled study from France, Belghiti et al. (2011) found that women with severe PPH had nearly 1.5 more hours of oxytocin infusion than matched women who did not hemorrhage (4.4 [IQR 2.5–6.8] hours vs. 3.0 [IQR 1.6–4.9]). In another case-controlled study from Sweden, Endler et al. (2012) found that women’s odds of experiencing retained placenta and prolonged third stage of labor were associated in a dose-response manner to the duration of oxytocin infusion they received before birth. Compared to no oxytocin use, women who received 3.25 to 6.9 hours of oxytocin were twice as likely to experience retained placenta at vaginal birth (OR 2.0, 95% CI [1.20–3.34]), and women who received 6.9 hours of oxytocin were 6.55 times as likely to have retained placenta (OR 6.55, 95% CI [3.42–12.54]). Although we excluded women with third stages of labor that lasted longer than 30 minutes, our finding that women with oxytocin infusions exceeding 4 hours (augmentation) or 7 hours (induction) were at higher risk of PPH appear to be in line with previous findings from Endler et al. Our results are also supported by those of Grotegut and colleagues (2011). In their study, women with PPH involving uterine atony received a mean of 10.45 (SD 9.56) hours of oxytocin compared to a mean of 4.9 (SD 7.8) hours among women without uterine atony and PPH. Although the durations of oxytocin infusions associated with PPH in the study by Grotegut et al. (2011) were lengthier than those from our study, their sample was not stratified by mode of labor onset.
Implications
As revealed by animal-model research and foundational human studies, oxytocin secretion appears to increase naturally during active labor, through the second and third stages of labor and culminates in separation of the placenta (Carter, 2018; Kenkel et al., 2014; Uvnäs-Moberg et al., 2019). When uterine myocytes are exposed to oxytocin, receptor down-regulation and desensitization occurs relative to the dose and duration of exposure (Balki et al., 2013, 2016). Once desensitization occurs, uterine myocyte cells may require up to 4 hours to replenish downregulated receptors, which allows the tissue to again respond to oxytocin by increasing the strength and frequency of uterine contractions (Conti et al., 2009). Corroborating these findings, clinical researchers recently noted that “oxytocin rest” of at least 8 hours during labor induction might be effective to improve uterine function (vaginal birth rates; McAdow et al., 2020).
In the context of PPH, it is therefore logical to assume that women who are exposed to longer or higher doses of oxytocin during labor are more likely to experience downregulation of oxytocin receptors. After birth, their uteruses are less responsive to natural and synthetic oxytocin and impeded from the heavy contractile work required during the third stage of labor to prevent excess bleeding (Balki et al., 2016). Our findings that women exposed to oxytocin for more than 4 hours (spontaneous labor onset) or 7 hours (induced labor) had greater odds of PPH even after accounting for the influence of known PPH risk factors such as fetal size, maternal demographic factors, and cervical dilation at labor admission fit within the larger body of research on oxytocin receptor desensitization.
Clinicians might consider optimizing their management of oxytocin throughout labor to achieve safe vaginal birth and minimize risk of PPH. For example, oxytocin exposure during labor induction might be reduced by continuation of cervical ripening interventions (prostaglandins, transcervical catheters) until Bishop scores reach 6 or higher for multiparous or 8 higher for nulliparous women, as suggested in recent induction guidelines (California Maternal Quality Care Collaborative. (2018). Another strategy to optimize labor stimulation may be a trial of oxytocin discontinuation for women in the active phase of labor (Hernández-Martínez et al., 2019).
Although international (World Health Organization, 2012) and interdisciplinary (Main et al., 2015) agencies promote oxytocin administration in the postpartum period to reduce PPH, the influence of oxytocin duration and dose in labor on subsequent postpartum uterine contractility has not been a focus of PPH prevention guidelines. For example, the PPH risk-screening tool from the California Maternal Quality Care Collaborative (Gabel et al., 2015) listed “prolonged oxytocin” use during labor as a risk factor but does not quantify “prolonged.” On a risk assessment table that is part of the Safe Motherhood Initiative via ACOG (2019) also listed prolonged oxytocin, defined as greater than 24 hours of administration, as a medium risk factor for PPH. In the PPH toolkit, the Association of Women’s Health, Obstetric and Neonatal Nurses (2015) lists any induction or augmentation with oxytocin as a medium risk factor for PPH. In our study, oxytocin durations far shorter than 24 hours were associated with PPH in otherwise low-risk women; thus, we recommend that future researchers reproduce and clarify these results in other populations of women.
Women who receive oxytocin during labor augmentation are known to require greater quantities of oxytocin prophylaxis during the third stage of labor than women whose labors are not augmented (Belghiti et al., 2011; Foley et al., 2018; Lavoie et al., 2015). Moreover, women who do not receive oxytocin during labor (physiologic birth) may not appreciate the same benefits of postpartum prophylactic oxytocin for minimizing PPH compared to women with oxytocin infusion in labor (Davis et al., 2012; Erickson et al., 2017, 2019; Kearney et al., 2018). Thus, oxytocin exposure during labor appears to influence the occurrence of PPH and the efficacy of oxytocin prophylaxis in the third stage of labor. Women with no intrapartum synthetic oxytocin exposure (no oxytocin receptor downregulation) may require little or no supplemental oxytocin to prevent PPH, while women exposed to oxytocin during labor (oxytocin receptor downregulation) may require higher doses of prophylactic oxytocin in third stage labor. Refinement of PPH risk assessment tools to more accurately quantify the minimum doses and durations of oxytocin that should trigger health care providers to increase awareness for potential PPH would be a useful strategy to help them more effectively support women to avoid PPH.
Limitations
Our study is limited in ways common to other retrospective and cross-sectional studies, including unmeasured confounding caused by lack of randomization of women to comparison groups. In addition, although our intent was to limit this analysis to a cohort of women at low risk for PPH, there were some PPH risk factors (e.g., fibroids, history of PPH) that were not detailed in the CSL dataset, thus residual confounding variables remain a possibility. The CSL dataset may not be representative of all U.S. women, as most data came from women in participating tertiary care centers. Missing data on oxytocin duration, maternal BMI, and blood loss also reduced the sample size for this analysis. For example, we were unable to calculate the duration of oxytocin infusion in different phases of labor due to a lack of variables revealing relative timelines in the CSL. Finally, we were not able to analyze the influence of prophylaxis techniques such as active management of the third stage of labor on PPH in CSL medical centers because information was not collected for this dataset.
Conclusion
We found that even short durations of oxytocin infusion during labor are associated with increased likelihood of PPH in low risk women who have vaginal births. During labor augmentation, oxytocin infusions longer than 4 hours increased risk of PPH. During labor induction, odds of PPH increased when oxytocin infusion lasted longer than 7 hours. Even shorter durations of augmentation (less than 4 hours) were not protective against PPH compared to no oxytocin infusion in women who experienced similar durations of labor. Although oxytocin is an important obstetric tool, effective uterine function after the birth of the newborn may be hindered by even short oxytocin infusions during labor.
Callouts.
Use of oxytocin for labor management is ubiquitous, however, the precise duration of exposure that increases adverse outcomes such as postpartum hemorrhage is not known.
Augmentation with oxytocin did not reduce the likelihood of postpartum hemorrhage when administered for less than 4 hours.
Maternity care providers should consider strategies to promote effective labor without prolonged use of oxytocin when possible to help reduce the risk of postpartum hemorrhage.
Acknowledgement
Dr. Erickson was supported as a scholar in the Oregon BIRCWH K12 Program funded by NICHD, NIH, under award number K12HD043488. Dr. Carlson was supported by the NINR, NIH, under award number K01NR016984.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflicts of interest or relevant financial relationships.
Contributor Information
Elise N. Erickson, Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Fellow, Oregon Health and Science University School of Nursing, Portland, OR..
Nicole S. Carlson, Emory University School of Nursing, Atlanta, GA..
References
- American College of Obstetricians and Gynecologists. (2020). Districts and sections. https://www.acog.org/community/districts-and-sections
- American College of Obstetricans and Gynecologists. (2019). Obstetric hemorrhage risk assessment tables. https://www.acog.org/-/media/project/acog/acogorg/files/forms/districts/smi-ob-hemorrhage-bundle-risk-assessment-ld-admin-intrapartum.pdf
- Association of Women’s Health, Obstetric and Neonatal Nurses. (2015). Postpartum hemorrhage (PPH) risk assessment table 1.0 https://mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH_Risk_Assessment_Table-7-17-15.pdf
- Balki M, Erik-Soussi M, Kingdom J, & Carvalho JCA (2013). Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology, 119(3), 552–561. 10.1097/ALN.0b013e318297d347 [DOI] [PubMed] [Google Scholar]
- Balki M, Ramachandran N, Lee S, & Talati C (2016). The recovery time of myometrial responsiveness after oxytocin-induced desensitization in human myometrium in vitro. Anesthesia and Analgesia, 122(5), 1508–1515. 10.1213/ANE.0000000000001268 [DOI] [PubMed] [Google Scholar]
- Belghiti J, Kayem G, Dupont C, Rudigoz RC, Bouvier-Colle MH, & Deneux-Tharaux C (2011). Oxytocin during labour and risk of severe postpartum haemorrhage: A population-based, cohort-nested case-control study. BMJ Open. 10.1136/bmjopen-2011-000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernitz S, Øian P, Rolland R, Sandvik L, & Blix E (2014). Oxytocin and dystocia as risk factors for adverse birth outcomes: A cohort of low-risk nulliparous women. Midwifery, 30(3), 364–370. 10.1016/j.midw.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Butwick AJ, Ramachandran B, Hegde P, Riley ET, El-Sayed YY, & Nelson LM (2017). Risk factors for severe postpartum hemorrhage after cesarean delivery: Case-control studies. Anesthesia and Analgesia, 125(2), 523–532. 10.1213/ANE.0000000000001962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Maternal Quality Care Collaborative. (2018). Appendix R: Induction of labor algorithm. https://www.cmqcc.org/resource/appendix-r-induction-labor-algorithm
- Callaghan WM, Kuklina EV, & Berg CJ (2010). Trends in postpartum hemorrhage: United States, 1994–2006. American Journal of Obstetrics and Gynecology, 202(4), 353.e1–353.e6. 10.1016/j.ajog.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Carter CS (2018). Oxytocin and human evolution. Current Topics in Behavioral Neurosciences, 35, 291–319. 10.1007/7854_2017_18 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020). Severe maternal morbidity in the United States. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html#anchor_trends
- Conti F, Sertic S, Reversi A, & Chini B (2009). Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a Rab4/Rab5 “short cycle.” American Journal of Physiology Endocrinology and Metabolism, 296(3), E532–42. 10.1152/ajpendo.90590.2008 [DOI] [PubMed] [Google Scholar]
- Coviello EM, Grantz KL, Huang C-C, Kelly TE, & Landy HJ (2015). Risk factors for retained placenta. American Journal of Obstetrics and Gynecology. 10.1016/j.ajog.2015.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Baddock S, Pairman S, Hunter M, Benn C, Anderson J, Dixon L, & Herbison P (2012). Risk of severe postpartum hemorrhage in low-risk childbearing women in New Zealand: Exploring the effect of place of birth and comparing third stage management of labor. Birth: Issues in Perinatal Care, 39(2), 98–105. 10.1111/j.1523-536X.2012.00531.x [DOI] [PubMed] [Google Scholar]
- Dionne MD, Deneux-Tharaux C, Dupont C, Basso O, Rudigoz RC, Bouvier-Colle MH, & Ray C. Le. (2015). Duration of expulsive efforts and risk of postpartum hemorrhage in nulliparous women: A population-based study. PLoS ONE, 10(11), e0142171 10.1371/journal.pone.0142171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen M, Bouvier-Colle MH, Dupont C, Khoshnood B, Rudigoz RC, & Deneux-Tharaux C (2011). Postpartum hemorrhage resulting from uterine atony after vaginal delivery: Factors associated with severity. Obstetrics and Gynecology, 117(1), 21–31. 10.1097/AOG.0b013e318202c845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekin A, Gezer C, Solmaz U, Taner CE, Dogan A, & Ozeren M (2015). Predictors of severity in primary postpartum hemorrhage. Archives of Gynecology and Obstetrics, 292(6), 1247–1254. 10.1007/s00404-015-3771-5 [DOI] [PubMed] [Google Scholar]
- Endler M, Grünewald C, & Saltvedt S (2012). Epidemiology of Retained Placenta. Obstetrics & Gynecology, 119(4), 801–809. 10.1097/AOG.0b013e31824acb3b [DOI] [PubMed] [Google Scholar]
- Erickson EN, Lee CS, & Emeis CL (2017). Role of prophylactic oxytocin in the third stage of labor: physiologic versus pharmacologically influenced labor and birth. Journal of Midwifery and Women’s Health, 62(4), 418–424. 10.1111/jmwh.12620 [DOI] [PubMed] [Google Scholar]
- Erickson EN, Lee CS, Grose E, & Emeis C (2019). Physiologic childbirth and active management of the third stage of labor: A latent class model of risk for postpartum hemorrhage. Birth: Issues in Perinatal Care, 46(1), 69–79. 10.1111/birt.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eunice Kennedy Shriver National Institute of Child Health and Human Development. (n.d.). Data and specimen hub. Consortium on Safe Labor (CSL) - 3.13 GB https://dash.nichd.nih.gov/study/2331
- Foley A, Gunter A, Nunes KJ, Shahul S, & Scavone BM (2018). Patients undergoing cesarean delivery after exposure to oxytocin during labor require higher postpartum oxytocin doses. Anesthesia and Analgesia, 126(3), 920–924. 10.1213/ANE.0000000000002401 [DOI] [PubMed] [Google Scholar]
- Gabel K, Lyndon A, & Main E Risk factor assessment. file:///C:/Users/angha/Downloads/OB%20Hem%20Risk%20Factor%20Assessment.pdf
- Grotegut CA, Paglia MJ, Johnson LNC, Thames B, & James AH (2011). Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. American Journal of Obstetrics and Gynecology, 204(1), 56.e1–56.e6. 10.1016/j.ajog.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi-Bannerman C, Srinivas SK, Wright JD, Goffman D, Siddiq Z, D’Alton ME, & Friedman AM (2018). Postpartum hemorrhage outcomes and race. American Journal of Obstetrics and Gynecology, 219(2), 185.e1–185.e10. 10.1016/J.AJOG.2018.04.052 [DOI] [PubMed] [Google Scholar]
- Hernández-Martínez A, Arias-Arias A, Morandeira-Rivas A, Pascual-Pedreño AI, Ortiz-Molina EJ, & Rodriguez-Almagro J (2019). Oxytocin discontinuation after the active phase of induced labor: A systematic review. Women and Birth, 32(2), 112–118. 10.1016/j.wombi.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Kearney L, Kynn M, Reed R, Davenport L, Young J, & Schafer K (2018). Identifying the risk: A prospective cohort study examining postpartum haemorrhage in a regional Australian health service. BMC Pregnancy and Childbirth, 18(1), 214 10.1186/s12884-018-1852-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Yee JR, & Carter CS (2014). Is oxytocin a maternal-foetal signalling molecule at birth? Implications for development. Journal of Neuroendocrinology, 26(10), 739–749. 10.1111/jne.12186 [DOI] [PubMed] [Google Scholar]
- Kernberg A, & Caughey AB (2017). Augmentation of labor: a review of oxytocin augmentation and active management of labor. Obstetrics and Gynecology Clinics of North America, 44(4), 593–600. 10.1016/j.ogc.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Khireddine I, Le Ray C, Dupont C, Rudigoz R-C, Bouvier-Colle M-H, & Deneux-Tharaux C (2013). Induction of labor and risk of postpartum hemorrhage in low risk parturients. PLoS ONE, 8(1), e54858 http://dx.plos.org/10.1371/journal.pone.0054858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie A, McCarthy RJ, & Wong CA (2015). The ED90 of prophylactic oxytocin infusion after delivery of the placenta during cesarean delivery in laboring compared with nonlaboring women: An up-down sequential allocation dose-response study. Anesthesia and Analgesia, 121(1), 159–164. 10.1213/ANE.0000000000000781 [DOI] [PubMed] [Google Scholar]
- Main EK, Goffman D, Scavone BM, Low LK, Bingham D, Fontaine PL, Gorlin JB, Lagrew DC, & Levy BS (2015). National Partnership for Maternal Safety: Consensus bundle on obstetric hemorrhage. Journal of Obstetric, Gynecologic, & Neonatal Nursing, 44(4), 462–470. 10.1111/1552-6909.12723 [DOI] [PubMed] [Google Scholar]
- Maswime S, & Buchmann E (2017). A systematic review of maternal near miss and mortality due to postpartum hemorrhage. International Journal of Gynecology and Obstetrics, 137(1), 1–7. 10.1002/ijgo.12096 [DOI] [PubMed] [Google Scholar]
- McAdow M, Xu X, Lipkind H, Reddy UM, & Illuzzi JL (2020). Association of oxytocin rest during labor induction of nulliparous women with mode of delivery. Obstetrics & Gynecology, 135(3), 569–575. 10.1097/AOG.0000000000003709 [DOI] [PubMed] [Google Scholar]
- Merriam AA, Wright JD, Siddiq Z, D’Alton ME, Friedman AM, Ananth CV, & Bateman BT (2018). Risk for postpartum hemorrhage, transfusion, and hemorrhage-related morbidity at low, moderate, and high volume hospitals. Journal of Maternal-Fetal and Neonatal Medicine, 31(8), 1025–1034. 10.1080/14767058.2017.1306050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrella M, Di Naro E, Loverro M, Benshalom-Tirosh N, Trojano G, Tirosh D, Besser L, Loverro MT, & Mastrolia SA (2018). The more you lose the more you miss: Accuracy of postpartum blood loss visual estimation. A systematic review of the literature. Journal of Maternal-Fetal and Neonatal Medicine, 31(1), 106–115. 10.1080/14767058.2016.1274302 [DOI] [PubMed] [Google Scholar]
- Nyfløt LT, Stray-Pedersen B, Forsén L, & Vangen S (2017). Duration of labor and the risk of severe postpartum hemorrhage: A case-control study. PLoS ONE, 12(4), e0175306–10. 10.1371/journal.pone.0175306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp HT, Johnson JV, Lemieux LA, & Currigan SM (2017). Executive summary of the revitalize initiative: Standardizing gynecologic data definitions. Obstetrics & Gynecology, 129(4), 603–607. 10.1097/AOG.0000000000001939 [DOI] [PubMed] [Google Scholar]
- Sosa CG, Althabe F, Belizan JM, & Buekens P (2011). Use of oxytocin during early stages of labor and its effect on active management of third stage of labor. American Journal of Obstetrics and Gynecology, 204(3), 238.e1–238.e5. 10.1016/j.ajog.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilden EL, & Snowden JM (2018). The causal inference framework: A primer on concepts and methods for improving the study of well-woman childbearing processes. Journal of Midwifery and Women’s Health, 63(6), 700–709. 10.1111/jmwh.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Ekström-Bergström A, Berg M, Buckley S, Pajalic Z, Hadjigeorgiou E, Kotłowska A, Lengler L, Kielbratowska B, Leon-Larios F, Magistretti CM, Downe S, Lindström B, & Dencker A (2019). Maternal plasma levels of oxytocin during physiological childbirth - A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy and Childbirth, 19(1). 10.1186/s12884-019-2365-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, & Tita ATN (2013). Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. American Journal of Obstetrics and Gynecology, 209(1), 51.e1–51.e6. 10.1016/j.ajog.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2012). WHO recommendations for the prevention and treatment of postpartum haemorrhage. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548502/en/ [PubMed]


