Abstract
Dengue viruses 1-4 (DENV1-4) and Zika virus (ZIKV) are closely related flaviviruses transmitted by Aedes mosquitoes that co-circulate in Asia, the Americas, Africa, and Oceania. Here, we review recent and historical literature on in vitro experiments, animal models, and clinical and epidemiological studies to describe how the sequence of DENV1-4 and ZIKV infections modulates subsequent dengue and Zika disease outcome. Overall, we find these interactions are asymmetric. Immunity from a prior DENV infection or a prior ZIKV infection can enhance future severe dengue disease for some DENV serotypes while protecting against other serotypes. Further, prior DENV immunity has not been shown to enhance future uncomplicated or severe Zika and instead appears to be protective. Interestingly, secondary ZIKV infection induces type-specific ZIKV immunity but only weakly cross-neutralizing anti-DENV/ZIKV immunity, consistent with risk of future dengue disease. In contrast, secondary DENV infection induces strongly cross-neutralizing antibodies that protect against subsequent severe dengue disease. These immunologic interactions may be explained by differences in virion structure between DENV1-4 and ZIKV, which modulate thermostability, susceptibility to neutralization, and cell infectivity. Overall, these observations are important for the understanding and prediction of epidemics and development and evaluation of dengue and Zika vaccines.
Keywords: Dengue virus, Zika virus, immunity, virion structure, neutralization, enhancement, epidemiology
INTRODUCTION
Dengue viruses 1-4 (DENV1-4) and Zika virus (ZIKV) are five closely related flaviviruses transmitted by Aedes mosquitoes [1]. Dengue disease ranges from the debilitating but self-limited Dengue Fever to life-threatening Dengue Hemorrhagic Fever/Dengue Shock Syndrome (DHF/DSS) and other complications associated with Severe Dengue [2–4]. ZIKV can also cause life-threatening disease, including ZIKV-associated Guillain-Barre Syndrome (GBS) in adults and congenital defects including microcephaly when infection occurs during pregnancy [5–7]. Over 3 billion people live in dengue-endemic areas in Asia, the Americas, Africa, and Oceania, with the greatest burden in Southeast Asia [8]. Just over 2 million live in areas at risk of Zika, with the highest burden in the Americas; however, the possibility for resurgence of Zika elsewhere remains [9,10]. Travelers to endemic areas are also at high risk of dengue and Zika, and in some instances can introduce these flaviviruses into non-endemic areas [11–13].
The flavivirus envelope (E) protein consists of three β-barrel domains (EDI, EDII, and EDIII). E monomers form head-to-tail homodimers, which are organized in rafts [14]. E is the major target of virus-specific neutralizing antibodies (nAbs) but also contains the immunodominant fusion loop (FL), which is conserved across flaviviruses and is susceptible to binding by antibodies that increase viral infection via antibody-dependent enhancement (ADE) [14–16]. In mature virions, the FL is generally concealed on the homodimer; however, the DENV virion can ‘breathe’, exposing the FL and other ‘cryptic’ epitopes [17,18]. The pre-membrane (pr) protein remains uncleaved from the membrane (M) protein on immature and partially immature virions, and human antibodies targeting prM can enhance DENV in vitro [16,19]. Pre-existing antibodies induced by prior heterotypic DENV infection or ZIKV infection can mediate ADE and enhance future risk of dengue disease, including DHF/DSS [20–23]. There is an urgent need to better understand how DENV1-4 and ZIKV interact immunologically for dengue and Zika vaccine development and evaluation as well as for epidemic preparedness.
In this review, we discuss determinants of protective and disease-enhancing interactions among DENV1-4 and ZIKV, including the sequence of infecting viruses; virus structure, stability, maturation state, and infection mechanisms; and how the epidemiology and pathogenesis of each virus may be differentially affected by pre-existing heterotypic flavivirus immunity. Further, we discuss how interactions between DENV and ZIKV may affect risk of disease and vaccine design and efficacy.
METHODS
We used PubMed to screen the titles and/or abstracts for all articles with the terms ‘dengue’ and ‘Zika’ and one or more of the following terms (serology, seroprevalence, cross protection, cross reactiv*, neutrali*, enhance*, immunity, or structure) in either the title and/or abstract (n=763, May 7, 2020). We identified 117 key studies and emphasize those published since January 2018.
DENV → DENV
Homotypic immunity
Primary DENV infection induces long-lived homotypic protection mediated in part by type-specific nAbs, which target quaternary epitopes displayed on the E homodimer or intact virion [24]. Quaternary type-specific nAbs often target non- or partially overlapping regions on the different serotypes [25]. For some serotypes, multiple distinct quaternary type-specific nAb regions have been identified [26].
Heterotypic immunity
Primary DENV infection also induces weakly-neutralizing anti-DENV antibodies targeting the FL, prM protein, and other epitopes [16,19]. Such antibodies bind heterologous DENV virions and can mediate ADE by promoting virus infection of immune cells via Fcγ receptors, activating the cells, increasing viremia and levels of pathogenic NS1 protein, and initiating an immune cascade that leads to vascular leakage and severe disease [27–30]. Early identification of those at risk of progression to severe dengue disease is urgently needed for patient triage and treatment, but point-of-care biomarkers that reliably predict severe disease remain elusive [31,32]. In contrast, high levels of binding and neutralizing antibody titers are protective against symptomatic secondary dengue [21,22,33,34]. Further, after secondary DENV infection, individuals are at lower risk of subsequent symptomatic disease, although not in all studies [35–37]. Protection after secondary DENV infection has been attributed to the induction of enduring, protective antibodies that target cross-reactive epitopes, including the EDE epitope, the A-strand in EDIII, and the be loop near the FL [38–40].
Sequence of infecting DENV serotypes
Pre-existing immunity may be differentially associated with protection and disease-enhancement, depending on the infecting serotypes. Hospital-based studies in Thailand and Nicaragua showed that a greater fraction of symptomatic and severe dengue patients infected with DENV1 and DENV3 are primary infections, whereas nearly all symptomatic and severe dengue patients infected with DENV2 and DENV4 are secondary [41–43]. The exception is severe primary DENV2 in infants, who often experience disease in the presence of maternal antibodies [42]. Further, the sequence of infecting DENV serotypes has long been considered an important epidemiological determinant of subsequent disease severity, especially the sequence DENV1→DENV2 [28,44–46]. However, the sequence DENV2→DENV1 has been associated with severe secondary dengue in French Polynesia [47]. In Vietnam, the order DENV1→DENV2, DENV1→DENV4, DENV2→DENV3, and DENV4→DENV3 were associated with increased risk of experiencing a future symptomatic DENV infection [48]. The level of pre-existing antibodies is also important. While high pre-existing nAb titers against DENV3 were protective against severe DENV3 disease, high titers against DENV2 were not associated with protection against severe disease caused by DENV2 [49]. Further, the postvaccination nAb titer required for protection against DENV2 is higher than that for other serotypes [50]. In support of these observations, we recently found in a cohort study in Nicaragua that high pre-existing anti-DENV antibody titers are protective against symptomatic and severe DENV1 and DENV3, but not DENV2, infections. In contrast, intermediate levels of anti-DENV antibodies are associated with enhancement of symptomatic and severe DENV2 and DENV3 infections, but not DENV1 infections [23] (Figure 1).
Fig. 1. Observed and hypothesized protective and enhancing interactions between DENV1-4 and ZIKV.
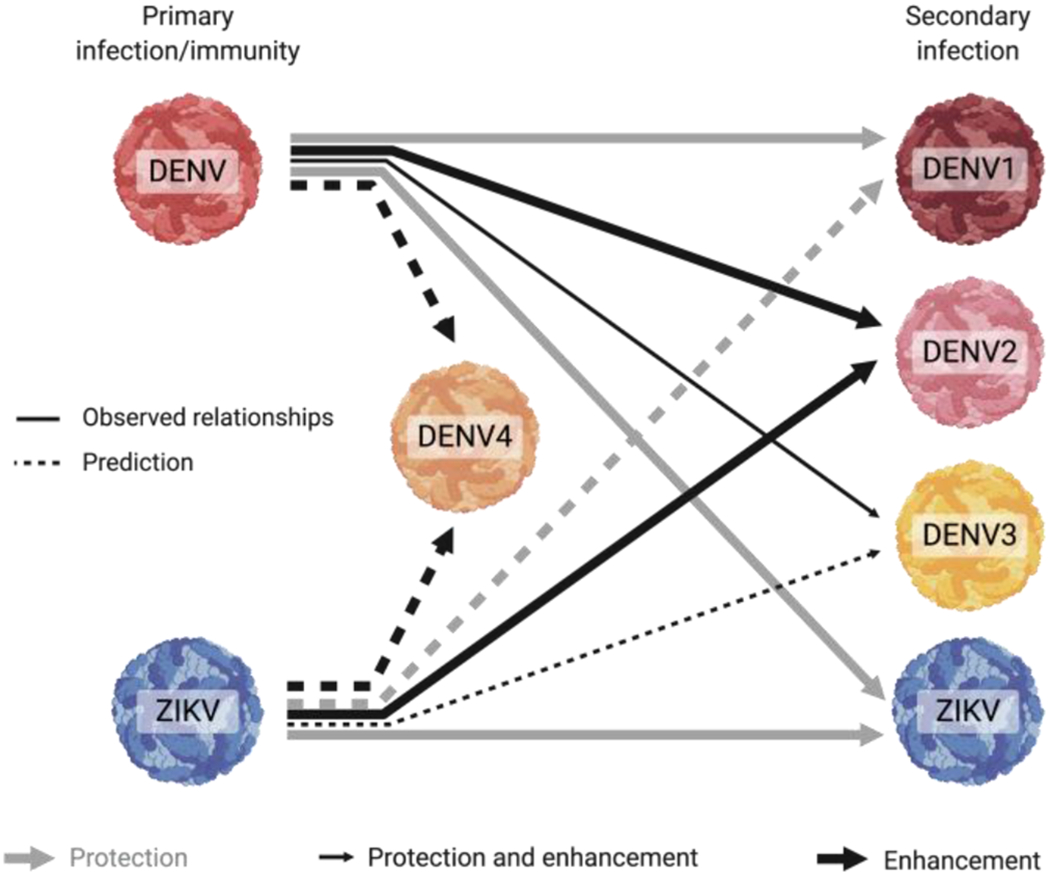
Primary DENV immunity, at high pre-existing antibody concentrations, is observed to protect against secondary symptomatic DENV1, DENV3, and ZIKV infection, but not DENV2 infection. In contrast, intermediate antibody levels generated from primary DENV infection increase symptomatic infection and enhance disease severity caused by DENV3 and DENV2 infection, but not by DENV1 or ZIKV infection. Primary ZIKV infection is also observed to enhance subsequent DENV2 infection and disease severity. We hypothesize that the effect of prior ZIKV infection on secondary infection with each DENV serotype will be similar to how primary DENV infection affects secondary infection with each DENV serotype. Grey arrows indicate protection, large black arrows indicate enhancement, and small black arrows indicate protection and enhancement. Solid lines indicate observed relationships, dotted lines are predicted relationships. The effect of prior DENV or ZIKV immunity on subsequent DENV4 disease has not been fully described.
DENV → ZIKV
In vitro studies and animal models
As ZIKV emerged across the Americas, researchers asked whether pre-existing anti-DENV immunity modified Zika disease. Primary DENV infection induces low or undetectable levels of cross-reactive nAbs against ZIKV, while some secondary DENV infections do generate cross-reactive nAbs to ZIKV [51–53]. After both primary and secondary DENV infection, a large proportion of memory B cells (MBCs) are cross-reactive, but secondary DENV infection induces a broader response that is also ZIKV-cross-reactive [54]. Consistent with these findings, some monoclonal antibodies (mAbs) isolated from patients following secondary DENV infection potently neutralize and protect against ZIKV infection by targeting quaternary epitopes on the virion [51,55–57]. Two such antibodies, the anti-DENV E dimer (EDE) and SIgN-3C mAbs, each have distinct mechanisms of neutralization against DENV and ZIKV [55,58].
Early studies during the Zika pandemic focused on the ability of anti-DENV antibodies to enhance ZIKV infection (Figure 2). DENV-immune serum from pregnant and non-pregnant individuals as well as mAbs isolated following primary and secondary dengue enhanced ZIKV infection in FcγRII-expressing K562 cells, PBMC-derived CD14+ monocytes, and monocyte-derived immature dendritic cells [59–63]. DENV-immune serum also enhanced ZIKV infection and reduced induction of pro-inflammatory cytokines and antiviral responses in primary human macrophages, placental macrophages (Hofbauer cells), and other cells in placental explants [64– 66]. Further, DENV-immune serum and mAbs targeting EDI/II enhanced ZIKV infection and morbidity in Stat2−/− and AG129 mice [63,67]. However, in three independent studies in rhesus macaques previously infected with DENV1, DENV2, DENV3, or DENV4, enhancement was not observed in magnitude or kinetics of ZIKV viral load nor in the immune cell response compared to naive animals [68–70]. Additionally, anti-DENV CD8+ and CD4+ T cells can protect against ZIKV in mice [71–73]. Thus while in vitro and mouse models suggest DENV can protect against and enhance ZIKV, ADE is not observed in non-human primate (NHP) studies.
Fig. 2. In vitro, animal models, and clinical studies showing protective and enhancing interactions between DENV → DENV, DENV → ZIKV, and ZIKV → DENV.

Columns show studies conducted in in vitro assays, mouse models, non-human primate challenge studies, human epidemiological and clinical studies, and human pregnancy/infant studies. Rows show the sequence of infecting viruses: primary DENV followed by secondary DENV infection with a different serotype, primary DENV infection followed by secondary ZIKV infection, and primary ZIKV infection followed by secondary DENV infection. Green “P” indicates protection, red “E” indicates enhancement, and question marks indicate unknown relationships.
Clinical and epidemiological studies
Consistent with findings in NHPs, ZIKV viremia, cytokine expression, innate immune profile, and/or clinical score did not differ between DENV-exposed and DENV-naive patients with Zika in Brazil and Nicaragua [74,75]. In Puerto Rico, secondary DENV2 and DENV3 infections had significantly higher viral load than primary DENV2 and DENV3 infections, but no significant difference was observed in viremia of primary and secondary Zika cases [76]. Instead, in a Nicaraguan pediatric cohort, prior DENV infection and higher pre-existing anti-DENV and anti-ZIKV antibody levels were associated with reduced risk of uncomplicated Zika when adjusting for age, sex, and recent DENV infection [23,77]. Further, a study in Brazil showed that the level of pre-existing anti-NS1 DENV antibodies was protective against ZIKV infection and disease [78]. To date, studies of infant outcomes of maternal ZIKV infection do not differ significantly by maternal disease severity, viremia, or acute-phase anti-DENV IgG antibody titers [79]. Higher PRNT titers to ZIKV at birth are seen in women with infants born with microcephaly compared to controls, likely due to greater ZIKV viral load, but prior DENV status did not distinguish cases and controls [80,81]. At the population level, one study observed that areas with large dengue epidemics within the last six years had lower rates of microcephaly, suggesting a protective role of recent DENV infection, while areas with major epidemics >6 years ago were at greater risk for microcephaly epidemics [82]. Finally, case-control studies showed that ZIKV exposure but not prior DENV exposure was strongly associated with Guillain-Barre Syndrome [6]. Thus, to date, ADE has not been observed in clinical or epidemiological studies of uncomplicated ZIKV nor in studies of severe Zika, including congenital Zika syndrome.
ZIKV → DENV
In the first two years after the Zika epidemic, there was little DENV transmission in the Americas. This phenomenon was linked to transient cross-protection against dengue epidemics as a result of the Zika epidemic [83]. However, several years later, large epidemics of dengue returned as predicted, and the question arose as to whether ZIKV may enhance subsequent risk of dengue disease (Figure 2) [83].
Early B cell and antibody responses
During and soon after ZIKV infection, those with prior DENV infection (secondary ZIKV infection) displayed distinct adaptive immune responses from ZIKV-infected DENV-naïve individuals (primary ZIKV infection). While anti-ZIKV IgM and IgA antibodies expanded at similar rates, anti-ZIKV IgG antibody responses were stronger and occurred earlier in secondary ZIKV infection in humans and NHPs, consistent with an anamnestic response [68,74]. In secondary ZIKV infection, plasmablasts were clonally related and somatically mutated, DENV/ZIKV cross-reactive but with stronger binding to DENV (especially the FL), and capable of enhancing DENV2 and/or ZIKV, overall pointing to a bias toward DENV [84,85]. In contrast, plasmablasts in primary ZIKV infection were clonally unrelated, displayed much less somatic hypermutation, were ZIKV-specific, targeted the whole virion and not ZIKV E protein (suggesting binding to quaternary epitopes), and were not capable of enhancing DENV2 [84,85]. At 14 days post-symptom onset, those with a history of DENV also had a stronger DENV-ZIKV cross-reactive MBC responses compared with those experiencing primary ZIKV infection [86].
Late B cell and antibody responses
By late convalescence (5 to 8 months post-infection), potent, type-specific anti-ZIKV nAbs and MBCs were produced in humans and NHPs following both primary and secondary ZIKV infection [53,68,74,86]. These type-specific nAbs were unaffected by depletion of anti-DENV1-4 antibodies [52,86]. Even in secondary ZIKV infection, potent anti-ZIKV nAbs were derived from MBCs with somatic mutation levels consistent with primary ZIKV infection [84].
In contrast, neither primary nor secondary ZIKV infection consistently produced broadly neutralizing cross-reactive anti-DENV-ZIKV B cell responses. In secondary ZIKV infection, the most highly mutated MBCs had higher affinity for ZIKV E than the ZIKV-specific potently neutralizing mAbs (derived from MBCs), but mAbs from these affinity matured MBCs only weakly neutralized DENV1-4 and ZIKV and predominantly targeted the FL [84]. Following primary and secondary ZIKV infection, mAbs toward EDI/II were highly DENV-ZIKV cross-reactive and capable of ADE in vitro and in a DENV mouse model [67]. Further, the degree of DENV-ZIKV MBC cross-reactivity was similar for ZIKV-infected individuals with 0, 1, or >1 prior DENV infections [86]. Finally, even in secondary ZIKV infection, almost none of the ZIKV serum nAb response is attributable to DENV-ZIKV cross-neutralizing antibodies, whereas after secondary DENV infection, the majority of the neutralizing antibody response to DENV1-4 was cross-reactive [86,87]. Thus, secondary ZIKV infection in humans appears to produce a cross-reactive polyclonal MBC and antibody response that is poorly neutralizing and may be capable of ADE.
ZIKV neutralizing epitopes
Potent type-specific anti-ZIKV mAbs target epitopes on EDIII as well as other quaternary epitopes [67,84,85,88,89]. A recent study showed that a chimeric virus with ZIKV EDIII introduced into the DENV4 E protein did not become susceptible to neutralization by human ZIKV-immune serum, suggesting that key epitope(s) targeted by ZIKV nAbs are outside EDIII [90]. Other potent anti-ZIKV nAbs have been identified, including intradimer epitopes in EDI/EDII, EDII, and EDI/EDIII, and an interdimer epitope in EDII -- some of which have been shown to be protective against ZIKV in mouse models [88,89]. Notably, some studies have identified DENV/ZIKV cross-neutralizing epitopes, including for DENV1/ZIKV (EDIII lateral ridge), DENV2/ZIKV (EDI/EDIII linker region), and DENV3/ZIKV (unknown epitope), suggesting that broad neutralization of DENV/ZIKV may be possible [84,86,91,92].
ADE in skin explants and mouse models
Primary ZIKV infection induces anti-DENV antibodies capable of enhancing DENV2 infection in various models. One study used a skin explant model to show that primary anti-ZIKV human serum mediates ADE of DENV2 infection in FcγR-b earing cells in the skin, including macrophages, dendritic cells, and Langerhans cells. More FcγR-bearing cells were infected and produced a greater quantity of DENV2 in the presence of ZIKV-immune serum than cells infected in the presence of naive serum. Adding anti-FcγRI and anti-FcγRII antibodies fully blocked the enhancing effect. In contrast, epidermal keratinocytes, which lack FcγR, were similarly infected by DENV2, with or without heterotypic serum. The authors also showed that similar enhancement effects were observed for DENV2 and ZIKV infection in the presence of anti-DENV3 antibodies [93]. In addition, ZIKV infection and inactivated ZIKV vaccines induce cross-reactive antibodies to DENV in mice that enhance DENV2 infection and disease [94,95]. LysMCre+Ifnar1fl/fl mice (lacking the IFNα/β receptor in myeloid cells) born to ZIKV-infected mothers and naive mice that received passively transferred anti-ZIKV maternal antibodies displayed elevated viral load, clinical severity, and mortality when challenged with DENV2 [96]. In contrast, in the reverse experiment, mice born to DENV2-immune mothers did not experience greater disease severity upon challenge with ZIKV than naive mice. While ZIKV induced antibodies that bound but did not neutralize DENV2, DENV2 infection did not induce antibodies that bound to ZIKV, helping explain the asymmetric enhancing interactions [96].
Non-human primate and human studies
Prior ZIKV infection can also enhance DENV viremia in NHPs [70,97], although not in all studies [98]. One study showed that macaques with prior ZIKV infection had increased viral load, thrombocytopenia, leukopenia, and neutropenia following DENV2 challenge [97]. A follow-up study showed IgG1 antibodies were associated with DENV2 enhancement [99]. Another study showed that macaques previously infected with ZIKV had elevated viremia but not disease following DENV2 challenge compared to controls [70]. However, one study in macaques did not observe that ZIKV infection led to ADE of DENV2 [98].
We have found that children with one prior ZIKV infection are at significantly greater risk of symptomatic and severe dengue disease caused by DENV2 in pediatric cohorts in Nicaragua [23]. Additionally, children with one prior DENV plus one prior ZIKV infection are also at elevated risk of symptomatic and severe DENV2 infection, consistent with previous studies of MBCs and serum antibodies showing that secondary ZIKV infection does not induce broadly neutralizing anti-DENV antibodies [84–86]. Further, intermediate pre-existing levels of anti-DENV or anti-ZIKV antibodies are associated with enhancement of symptomatic and severe dengue. However, individuals with two or more prior DENV infections with or without a subsequent ZIKV infection and those with high anti-DENV or anti-ZIKV antibodies are at much lower risk of symptomatic and severe DENV2, with rates similar to DENV-naïve individuals.
T cell responses
Differences in T cell specificity also likely play a role in protective and possibly pathogenic interactions between DENV and ZIKV, as reviewed in detail elsewhere [100,101]. Anti-DENV CD8+ T cells predominantly target non-structural proteins (NS3, NS4B, and NS5) with the exception of DENV3, which also targets structural proteins [102]. Anti-DENV CD4+ T cells target structural (C, E) and non-structural 5 (NS5) proteins [100]. Notably, a large proportion of CD8+ and CD4+ T cells from DENV vaccine recipients and blood donors in Nicaragua and Sri Lanka, all collected before the emergence of Zika, were stimulated by ZIKV peptides from the whole ZIKV proteome. Some CD8+ and CD4+ cells reacted with epitopes on ZIKV non-structural proteins that were fully or mostly conserved with DENV1-4 [103]. Consistent with these findings, anti-DENV CD8+ T cells as well as anti-DENV CD4+ T cells have been shown to be protective against ZIKV in mice and in a pregnant mouse model [71–73].
As for studies of B cells, secondary ZIKV infection induced earlier and higher magnitude T cell responses that were declining by convalescence, while primary ZIKV T cell responses were increasing at convalescence. Further, secondary ZIKV induced CD8+ T cells that upregulated granzyme B and PD1 [103]. However, a later study showed that both primary and secondary ZIKV infection induced multifunctional ZIKV-specific CD8+ T cells by late convalescence [104]. In contrast to DENV, ZIKV CD4+ T cells mostly target structural proteins, and following primary and secondary ZIKV infection, a low proportion of the CD4+ T cell response was reactive to DENV E protein [67,103]. Some studies suggest ZIKV may skew the CD4+ T cell response and bias future DENV infections toward pathogenesis in mice and severe outcomes, including microcephaly, in humans [105,106].
Structural differences between DENV1-4 and ZIKV
Structural differences between ZIKV and DENV1-4 have been observed to modify exposure of epitopes targeted by enhancing antibodies and potently neutralizing antibodies and to modulate virion binding to key cell receptors and attachment factors. Such differences may help explain asymmetric immune interactions between DENV1-4 and ZIKV (Figure 3).
Fig. 3. Key structural differences between ZIKV and DENV.

Circles, from the top: (1) ZIKV is more thermally stable and undergoes less virion ‘breathing’ than DENV. (2) The ZIKV EDIII CD loop contains an additional amino acid at position 346 compared to DENV, which modifies virion thermostability. (3) The ZIKV glycan loop at N154 helps mediate homodimer formation and covers the FL, making ZIKV nonsusceptible to anti-FL antibodies, whereas anti-FL antibodies can weakly neutralize and enhance DENV. (4) DENV contains a glycan at position 67 that is not present on ZIKV and alters susceptibility to broadly neutralizing antibodies. (5) The furin cleavage site may differ in efficiency between DENV1-4 and ZIKV, altering virion maturation state.
Virion breathing
Differences between strains may alter virion ‘breathing’, i.e. the structural conformations sampled by the virion that can influence stoichiometry of antibody binding. Notably, ZIKV is more thermally stable than DENV but not WNV [107,108]. Further support for increased stability of ZIKV is that a mutation in the E protein at amino acid position 198 alters breathing of DENV and WNV, but not ZIKV [109]. A nearby mutation on DENV at E position 204 also modifies breathing of DENV1 strains, and this modulates exposure of EDIII and susceptibility to an anti-EDIII DENV1/ZIKV cross-neutralizing mAb [17,110]. However, how breathing modifies infection and susceptibility to antibodies in vivo is unknown.
CD-loop on EDIII
Virion stability is also reinforced by a feature in the CD-loop of EDIII. The E protein of neurotropic flaviviruses, including ZIKV, contains an extended CD-loop compared to non-neutrotropic flaviriruses such as DENV1-4, and this has been directly associated with ZIKV thermostability [111]. Deletion of the extra amino acid (Δ346) destabilized the ZIKV virion, even at room temperature [111].
Glycan loop glycan at N153 or N154
Differences in the glycan loop in EDI (N154 for ZIKV, N153 for DENV) are also important for virion stability and susceptibility to neutralization. On mature ZIKV virions, as compared to DENV, the loop containing the glycosylation site N154 extends toward DII on adjacent E proteins, improving E homodimer formation, covering the FL, and eliminating susceptibility to weakly-neutralizing, often enhancing anti-DENV FL antibodies [107,112,113]. Artificial mutation of positions in the N154 environment modify the early stage of ZIKV infection, with some mutations increasing fusion with the endosomal membrane, possibly due to increased exposure of the FL, and others blocking glycosylation and eliminating virion binding to cells expressing DC-SIGNR [114,115]. Further, the ZIKV glycan at N154 contains a more complex sugar than DENV, which improves binding to DC-SIGNR-expressing cells including placental villi [115,116].
N67 glycan
Unlike ZIKV and other flaviviruses, the DENV E protein also contains an N glycan at position 67. The N67 glycan has high affinity for DC-SIGN, which is expressed on dendritic cells and a subset of macrophages. Improved binding to DC-SIGN leads to greater virus tropism for dendritic cells and monocyte/macrophage-derived cells [117–119]. Interaction with DC-SIGN is related to high-mannose glycan, a simple sugar, whereas complex sugars improve binding to DC-SIGNR. WNV and JEV do not naturally contain N67, but substitution with this glycosylation site increases WNV and JEV interaction with DC-SIGN [119]. Further, potently neutralizing EDE mAbs neutralize ZIKV differently from DENV2 because of the N67 glycan. EDE1 mAbs are N67-independent and potently neutralize ZIKV and DENV2. In contrast, EDE2 mAbs depend on the N67 glycan for binding, resulting in potent neutralization of DENV2 and lack of neutralization but rather enhancement of ZIKV [55,60].
Virion maturation
Anti-prM and anti-FL antibodies are able to bind immature and partially immature DENV virions and facilitate ADE [120]. Maturation state is determined by the degree of furin cleavage, which can vary by cell type and the sequence of pr-M furin cleavage site. Although DENV1-4 virions are partially immature when grown in common cell lines, DENV1 virions in blood of patients with primary DENV infection were mature in vivo and not susceptible to binding by prM antibodies [121]. However, the virion maturation state of other DENV strains and serotypes and the maturation state during secondary infection are unknown. Of interest, DENV1-4 display amino acid variation near the pr-M cleavage site that is not present in other flaviviruses and that modulates the production of immature virions [122]. The evolutionary and functional importance of prM for DENV1-4 and ZIKV and its role modulating maturation state is of great interest.
Dengue and Zika vaccines
Immunological interactions among DENV1-4 and ZIKV are a critical concern for dengue and Zika vaccines. The only licensed dengue vaccine, Dengvaxia®, was introduced in mass vaccination programs and then confirmed to elevates incidence of DHF/DSS in those without prior DENV infection and thus is only recommended for individuals with a documented history of DENV infection [123,124]. Several other DENV vaccines are in Phase 3 clinical trials and may have different efficacy and safety profiles. However, two tetravalent dengue vaccine candidates have shown serotype-specific differences in protection [50,125], which may be attributable to differences in vaccine formulation and T cell responses, but also to differences in how anti-DENV immunity modulates disease caused by each serotype. Further, leading DENV vaccines were developed before the introduction of ZIKV. How DENV vaccines perform in the context of ZIKV immunity requires further study.
Dozens of candidate Zika vaccines were rapidly developed during the epidemic to protect at-risk populations. The possibility that a ZIKV vaccine could enhance DENV has been a prominent concern for many ZIKV vaccine developers, and approaches to avoid inducing ADE include ZIKV EDIII vaccines, stabilized ZIKV dimer vaccines, and ZIKV NS1 vaccines [89]. The finding that natural ZIKV infection can enhance future dengue disease severity further highlights the importance finding safe approaches to ZIKV vaccination [23]. Notably, a recent study has shown even ZIKV-specific protection differs dramatically across constructs: ZIKV vaccine candidates that induce potent nAbs to mature ZIKV virions, and not just high nAb titers overall, are most protective in challenge studies [126]. This finding suggests that methods for measuring immune correlates that take into account key structural features of ZIKV, and flaviviruses in general, will be most valuable for evaluating clinical studies.
CONCLUSION
Overall, the literature to date indicates that DENV1-4 and ZIKV can reciprocally modify disease outcomes. However, these interactions are asymmetric (Figure 1). Some sequences of infection have been mostly associated with cross-protection, including DENV1-4 infection followed by ZIKV infection. Other sequences have been observed to result in enhanced disease, including ZIKV infection followed by DENV2 infection. It has long been observed that specific DENV serotypes differentially modulate subsequent DENV disease severity in a serotype-dependent manner, and recent studies suggest such differences extend to ZIKV. Further studies of the structural and immunological differences between DENV1-4, ZIKV, and possibly other flaviviruses, and how immunity to one modulates disease with another, will provide important insights for safe and effective flavivirus vaccine development and use as well as preparation for future epidemics.
Acknowledgments
We thank all those who have conducted dengue and Zika studies around the world for their contributions to understanding the epidemiology, immunology, and virology of DENV1-4 and ZIKV.
Funding: This work was supported by National Institutes of Health grant P01AI106695. LCK was supported in part by the Global Health Equity Scholar FIC/NIH training grant D43TW010540. The funders had no involvement in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi P, Vasilakis N: Zika virus: History, emergence, biology, and prospects for control. Antiviral Res 2016, 130:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB, Cohen SN: Dengue Hemorrhagic Fever at 60 Years: Early Evolution of Concepts of Causation and Treatment. Microbiol Mol Biol Rev 2015, 79:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization: Dengue: Guidelines for diagnosis, treatment, prevention and control. 2009. [PubMed]
- 4.World Health Organization: Dengue haemorrhagic fever: diagnosis, treatment, prevention, and control. 2nd edition 1997. [Google Scholar]
- 5.Zorrilla CD, Garcia Garcia I, Garcia Fragoso L, De La Vega A: Zika Virus Infection in Pregnancy: Maternal, Fetal, and Neonatal Considerations. J Infect Dis 2017, 216:S891–S896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. : Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016, 387:1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vouga M, Chiu Y-C, Pomar L, de Meyer S V, Masmejan S, Genton B, Musso D, Baud D, Stojanov M: Dengue, Zika and chikungunya during pregnancy: pre- and post-travel advice and clinical management. J Travel Med 2019, 26:taz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. : The global distribution and burden of dengue. Nature 2013, 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina JP, Kraemer MUG, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, Golding N, Ruktanonchai CW, Gething PW, Cohn E, et al. : Mapping global environmental suitability for Zika virus. Elife 2016, 5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamer DH, Chen LH: Zika in Angola and India. J Travel Med 2019, 26:taz012. [DOI] [PubMed] [Google Scholar]
- 11.Halstead S, Wilder-Smith A: Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med 2019, 26:taz062. [DOI] [PubMed] [Google Scholar]
- 12.Wilder-Smith A, Chang CR, Leong WY: Zika in travellers 1947–2017: a systematic review. J Travel Med 2018, 25:tay044. [DOI] [PubMed] [Google Scholar]
- 13.Redondo-Bravo L, Ruiz-Huerta C, Gomez-Barroso D, Sierra-Moros MJ, Benito A, Herrador Z: Imported dengue in Spain: a nationwide analysis with predictive time series analyses. J Travel Med 2019, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, et al. : Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002, 108:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roehrig JT: Antigenic structure of flavivirus proteins. Adv Virus Res 2003, 59:141—175. [DOI] [PubMed] [Google Scholar]
- 16.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al. : Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowd KA, DeMaso CR, Pierson TC: Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. MBio 2015, 6:e01559–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin SK, Dowd K a., Shrestha B, Nelson C a., Edeling M a., Johnson S, Pierson TC, Diamond MS, Fremont DH: Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog 2012, 8:el002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Alwis R, Williams KL, Schmid MA, Lai C-YY, Patel B, Smith SA, Crowe JE, Wang W-KK, Harris E, de Silva AM: Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog 2014, 10:e1004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen SN, Halstead SB: Shock associated with dengue infection. J Pediatr 1966, 68:448–456. [DOI] [PubMed] [Google Scholar]
- 21.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E: Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salje H, Cummings DAT, Rodriguez-Barraquer I, Katzelnick LC, Lessler J, Klungthong C, Thaisomboonsuk B, Nisalak A, Weg A, Ellison D, et al. : Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 2018, 557:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzelnick LC, Narvaez C, Arguello S, Lopez Mercado B, Collado D, Ampie O, Elizondo D, Miranda T, Bustos Carillo F, Mercado JC, et al. : Zika virus infection enhances future risk of severe dengue disease. Science 2020, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (**) Children in a cohort study with a history of one prior ZIKV infection or one DENV infection followed by ZIKV infection were at significantly increased risk of symptomatic and severe DENV2 infection compared to naive children and have similar risk to children with primary DENV immunity.
- 24.Gallichotte EN, Baric RS, de Silva AM: The Molecular Specificity of the Human Antibody Response to Dengue Virus Infections. Adv Exp Med Biol 2018, 1062:63–76. [DOI] [PubMed] [Google Scholar]
- 25.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WMPB, White LJ, Diamond MS, Baric RS, Crowe JE, et al. : Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci 2012, 109:7439–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young E, Carnahan RH, Andrade DV., Kose N, Nargi RS, Fritch EJ, Munt JE, Doyle MP, White L, Baric TJ, et al. : Identification of Dengue Virus Serotype 3 Specific Antigenic Sites Targeted by Neutralizing Human Antibodies. Cell Host Microbe 2020, 27:710–724.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halstead S: Recent advances in understanding dengue. F1000Research 2019, 8:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halstead SB: Neutralization and Antibody-Dependent Enhancement of Dengue Viruses. Adv Virus Res 2003, 60:421–467. [DOI] [PubMed] [Google Scholar]
- 29.Waggoner JJ, Katzelnick LC, Burger-Calderon R, Gallini J, Moore RH, Kuan G, Balmaseda A, Pinsky BA, Harris E: Antibody-Dependent Enhancement of Severe Disease Is Mediated by Serum Viral Load in Pediatric Dengue Virus Infections. J Infect Dis 2020, 221:1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*) Measurement of viral load in patients with DHF DSS and dengue fever revealed that viral load mediates the relationship between pre-existing anti-DENV antibodies and dengue disease severity.
- 30.Glasner DR, Puerta-Guardo H, Beatty PR, Harris E: The Good, the Bad, and the Shocking: The Multiple Roles of Dengue Virus Nonstructural Protein 1 in Protection and Pathogenesis. Ann Rev Virol 2018, 5:227–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YH, Leong W-Y, Wilder-Smith A: Markers of dengue severity: a systematic review of cytokines and chemokines. J Gen Virol 2016, 97:3103–3119. [DOI] [PubMed] [Google Scholar]
- 32.Vuong NL, Le Duyen HT, Lam PK, Tam DTH, Vinh Chau N Van, Van Kinh N, Chanpheaktra N, Lum LCS, Pleités E, Jones NK, et al. : C-reactive protein as a potential biomarker for disease progression in dengue: A multi-country observational study. BMC Med 2020, 18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buddhari D, Aldstadt J, Endy TP, Srikiatkhachorn A, Thaisomboonsuk B, Klungthong C, Nisalak A, Khuntirat B, Jarman RG, Fernandez S, et al. : Dengue virus neutralizing antibody levels associated with protection from infection in Thai cluster studies. PLoS Negl Trop Dis 2014, 8:e3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E: Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci 2016, 113:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST: Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 2013, 208:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP, Srikiatkhachorn A: Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 2007, 77:910–913. [PubMed] [Google Scholar]
- 37.Montoya M, Gresh L, Mercado JC, Williams KL, Jose M, Gutierrez G, Kuan G, Gordon A, Balmaseda A, Harris E, et al. : Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis 2013, 7:e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, et al. : The Potent and Broadly Neutralizing Human Dengue Virus-Specific Monoclonal Antibody 1C19 Reveals a Unique Cross-Reactive Epitope on the be Loop of Domain II of the Envelope Protein. MBio 2013, 4:e00873-13-e00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney M-C, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, et al. : Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520:109–113. [DOI] [PubMed] [Google Scholar]
- 40.Tsai W-Y, Lai C-Y, Wu Y-C, Lin H-E, Edwards C, Jumnainsong A, Kliks S, Halstead S, Mongkolsapaya J, Screaton GR, et al. : High-Avidity and Potently Neutralizing Cross-Reactive Human Monoclonal Antibodies Derived from Secondary Dengue Virus Infection. J Virol 2013, 87:12562–12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clapham HE, Cummings DAT, Johansson MA: Immune status alters the probability of apparent illness due to dengue virus infection: Evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl Trop Dis 2017, 11:e0005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nisalak A, Lessler JT, Klungthong C, Yoon I, Macareo LR, Thaisomboonsuk B, Clapham HE, Cummings DAT, Reiser J, Kalayanarooj S, et al. : Forty Years of Dengue Surveillance at a Tertiary Pediatric Hospital in Bangkok, Thailand, 1973–2012. Am J Trop Med Hyg 2016, 94:1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balmaseda A, Hammond SN, Pérez L, Tellez Y, Saborío SI, Mercado JC, Cuadra R, Rocha J, Pérez MA, Silva S, et al. : Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg 2006, 74:449–456. [PubMed] [Google Scholar]
- 44.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB: Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol 1984, 120:653–669. [DOI] [PubMed] [Google Scholar]
- 45.Guzmán MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Delgado I, Halstead SB: Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am J Epidemiol 2000, 152:793–9. [DOI] [PubMed] [Google Scholar]
- 46.OhAinle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborío S, Nuñez A, Lennon NJ, Birren BW, Gordon A, et al. : Dynamics of Dengue Disease Severity Determined by the Interplay Between Viral Genetics and Serotype-Specific Immunity. Sci Transl Med 2011, 3:114ra128–114ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubert B, Halstead SB: Dengue 1 virus and dengue hemorrhagic fever, French Polynesia, 2001. Emerg Infect Dis 2009, 15:1265–1270. [DOI] [PubMed] [Google Scholar]
- 48.Aguas R, Dorigatti I, Coudeville L, Luxemburger C, Ferguson NM: Cross-serotype interactions and disease outcome prediction of dengue infections in Vietnam. Sci Rep 2019, 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH: Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis 2004, 189:990–1000. [DOI] [PubMed] [Google Scholar]
- 50.Moodie Z, Juraska M, Huang Y, Zhuang Y, Fong Y, Carpp LN, Self SG, Chambonneau L, Small R, Jackson N, et al. : Neutralizing Antibody Correlates Analysis of Tetravalent Dengue Vaccine Efficacy Trials in Asia and Latin America. J Infect Dis 2018, 217:742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS: Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. MBio 2016, 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, de Silva AM: Lack of durable cross-neutralizing antibodies against zika virus from dengue virus infection. Emerg Infect Dis 2017, 23:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montoya M, Collins M, Dejnirattisai W, Katzelnick LC, Puerta-Guardo H, Jadi R, Schildhauer S, Supasa P, Vasanawathana S, Malasit P, et al. : Longitudinal Analysis of Antibody Cross-neutralization Following Zika Virus and Dengue Virus Infection in Asia and the Americas. J Infect Dis 2018, 218:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrade P, Narvekar P, Montoya M, Michlmayr D, Balmaseda A, Coloma J, Harris E: Primary and Secondary Dengue Virus Infections Elicit Similar Memory B-Cell Responses, but Breadth to Other Serotypes and Cross-Reactivity to Zika Virus Is Higher in Secondary Dengue. J Infect Dis 2020, 222:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. : Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536:48–53. [DOI] [PubMed] [Google Scholar]
- 56.Abbink P, Larocca RA, Dejnirattisai W, Peterson R, Nkolola JP, Borducchi EN, Supasa P, Mongkolsapaya J, Screaton GR, Barouch DH: Therapeutic and protective efficacy of a dengue antibody against Zika infection in rhesus monkeys. Nat Med 2018, 24:721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kam Y-W, Lee CY-P, Teo T-H, Howland SW, Amrun SN, Lum F-M, See P, Kng NQ-R, Huber RG, Xu M-H, et al. : Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI Insight 2017, 2:e92428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang S, Loy T, Ng TS, Lim XN, Chew SYV, Tan TY, Xu M, Kostyuchenko VA, Tukijan F, Shi J, et al. : A Human Antibody Neutralizes Different Flaviviruses by Using Different Mechanisms. Cell Rep 2020, 31:107584. [DOI] [PubMed] [Google Scholar]; (*) Anti-DENV monoclonal antibody SIgN-3C potently neutralizes DENV1-4 and ZIKV by distinct mechanisms: SIgN-3C prevents DENV2 from fusing with the endosomal membrane, while SIgN-3C blocks ZIKV by causing virus aggregation.
- 59.Li M, Zhao L, Zhang C, Wang X, Hong W, Sun J, Liu R, Yu L, Wang J, Zhang F, et al. : Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS One 2018, 13:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau V-M, Malasit P, Rey FA, et al. : Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat Immunol 2016, 17:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. : Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 2016, 113:7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castanha PMS, Nascimento EJM, Braga C, Cordeiro MT, De Carvalho OV., De Mendonça LR, Azevedo EAN, França RFO, Dhalia R, Marques ETA: Dengue virus-specific antibodies enhance Brazilian Zika virus infection. J Infect Dis 2017, 215:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]; (**) Anti-ZIKV and anti-DENV3 serum antibodies, as compared to naïve serum, mediate ADE of DENV2 infection in FcγR-bearing cells in a skin explant model.
- 63.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. : Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Londono-Renteria B, Troupin A, Cardenas JC, Hall A, Perez OG, Cardenas L, Hartstone-Rose A, Halstead SB, Colpitts TM: A relevant in vitro human model for the study of Zika virus antibody-dependent enhancement. J Gen Virol 2017, 98:1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmerman MG, Quicke KM, O’Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. : Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 2018, 24:731–742.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermanns K, Göhner C, Kopp A, Schmidt A, Merz WM, Markert UR, Junglen S, Drosten C: Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg Microbes Infect 2018, 7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. : Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353:823–6. [DOI] [PubMed] [Google Scholar]
- 68.McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, et al. : Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLOS Pathog 2017, 13:e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pantoja P, Pérez-Guzmán EX, Rodríguez IV., White LJ, González O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, et al. : Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 2017, 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breitbach ME, Newman CM, Dudley DM, Stewart LM, Aliota MT, Koenig MR, Shepherd PM, Yamamoto K, Crooks CM, Young G, et al. : Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLOS Pathog 2019, 15:e1007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh AT, Wang YT, Nguyen AVT, Salgado R, Mamidi A, Kim K, Diamond MS, et al. : Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat Commun 2018, 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen J, Elong Ngono A, Regla-Nava JA, Kim K, Gorman MJ, Diamond MS, Shresta S: Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat Commun 2017, 8:1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen J, Wang Y-T, Valentine KM, Dos Santos Alves RP, Xu Z, Regla-Nava JA, Ngono AE, Young MP, Ferreira LCS, Shresta S: CD4+ T Cells Cross-Reactive with Dengue and Zika Viruses Protect against Zika Virus Infection. Cell Rep 2020, 31:107566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tonnerre P, Melgaço JG, Torres-Cornejo A, Pinto MA, Yue C, Blümel J, de Sousa PSF, de Mello V da M, Moran J, de Filippis AMB, et al. : Evolution of the innate and adaptive immune response in women with acute Zika virus infection. Nat Microbiol 2020, 5:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michlmayr D, Kim E-Y, Rahman AH, Raghunathan R, Kim-Schulze S, Che Y, Kalayci S, Gümüş ZH, Kuan G, Balmaseda A, et al. : Comprehensive Immunoprofiling of Pediatric Zika Reveals Key Role for Monocytes in the Acute Phase and No Effect of Prior Dengue Virus Infection. Cell Rep 2020, 31:107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santiago GA, Sharp TM, Rosenberg E, Sosa Cardona II, Alvarado L, Paz-Bailey G, Muñoz-Jordán JL: Prior Dengue Virus Infection Is Associated With Increased Viral Load in Patients Infected With Dengue but Not Zika Virus. Open Forum Infect Dis 2019, 6:ofz320. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*) Patients with secondary as compared to primary DENV2 and DENV3 infections have significantly higher viral loads, while those with primary and secondary ZIKV infections have similar viral loads.
- 77.Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez N, Mercado JC, Chowell G, Lopez B, Elizondo D, Coloma J, et al. : Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua. PLOS Med 2019, 16:e1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez-Barraquer I, Costa F, Nascimento EJM, Castanha PMS, Sacramento GA, Cruz J, Carvalho M, Olivera D De, Adhikarla H, Azar SR, et al. : Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. 2019, 610:607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halai UA, Nielsen-Saines K, Moreira ML, De Sequeira PC, Pereira JP, De Araujo Zin A, Cherry J, Gabaglia CR, Gaw SL, Adachi K, et al. : Maternal Zika virus disease severity, virus load, prior dengue antibodies, and their relationship to birth outcomes. Clin Infect Dis 2017, 65:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreira-Soto A, Sarno M, Pedroso C, Netto EM, Rockstroh A, Luz E, Feldmann M, Fischer C, Bastos FA, Kummerer BM, et al. : Evidence for Congenital Zika Virus Infection from Neutralizing Antibody Titers in Maternal Sera, Northeastern Brazil. J Infect Dis 2017, 216:1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castanha PMS, Souza WV, Braga C, Araújo TVB de, Ximenes RAA, Albuquerque M de FPM, Montarroyos UR, Miranda-Filho DB, Cordeiro MT, Dhalia R, et al. : Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil. PLoS Negl Trop Dis 2019, 13:e0007246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carvalho MS, Freitas LP, Cruz OG, Brasil P, Bastos LS: Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci Rep 2020, 10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borchering RK, Huang AT, Mier-y-Teran-Romero L, Rojas DP, Rodriguez-Barraquer I, Katzelnick LC, Martinez SD, King GD, Cinkovich SC, Lessler J, et al. : Impacts of Zika emergence in Latin America on endemic dengue transmission. Nat Commun 2019, 10:5730. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*) A period of atypically low dengue followed the Zika epidemic in Brazil and Colombia, and simulations showed that partial cross-protection by ZIKV infection would result in low dengue incidence followed by a later resurgence.
- 84.Rogers TF, Goodwin EC, Briney B, Sok D, Beutler N, Strubel A, Nedellec R, Le K, Brown ME, Burton DR, et al. : Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol 2017, 2:eaan6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhaumik S, Priyamvada L, Kauffman R, Lai L, Natrajan M, Cho A, Rouphael N, Suthar M, Mulligan M, Wrammert J: Pre-Existing Dengue Immunity Drives a DENV-Biased Plasmablast Response in ZIKV-Infected Patient. Viruses 2018, 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrade P, Gimblet-Ochieng C, Modirian F, Collins M, Cárdenas M, Katzelnick LC, Montoya M, Michlmayr D, Kuan G, Balmaseda A, et al. : Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika. Nat Commun 2019, 10:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel B, Longo P, Miley MJ, Montoya M, Harris E, de Silva AM: Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl Trop Dis 2017, 11:e0005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sapparapu G, Fernandez E, Kose N, Bin Cao, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. : Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016, 540:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diamond MS, Ledgerwood JE, Pierson TC: Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu Rev Med 2019, 70:121–135. [DOI] [PubMed] [Google Scholar]
- 90.Gallichotte EN, Young EF, Baric TJ, Yount BL, Metz SW, Begley MC, de Silva AM, Baric RS: Role of Zika Virus Envelope Protein Domain III as a Target of Human Neutralizing Antibodies. MBio 2019, 10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (*) Transplant of the E domain III of ZIKV into a DENV4 virus does not transfer potent neutralization of ZIKV by anti-ZIKV serum antibodies, suggesting that the potent neutralizing determinant is elsewhere.
- 91.Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, Schaefer-Babajew D, Avila-Rios S, Nogueira L, Patel R, et al. : Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017, 169:597–609.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dussupt V, Sankhala RS, Gromowski GD, Donofrio G, De La Barrera RA, Larocca RA, Zaky W, Mendez-Rivera L, Choe M, Davidson E, et al. : Potent Zika and dengue cross-neutralizing antibodies induced by Zika vaccination in a dengue-experienced donor. Nat Med 2020, 26:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castanha PMS, Erdos G, Watkins SC, Falo LD, Marques ETA, Barratt-Boyes SM: Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI insight 2020, 5:el33653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawiecki AB, Christofferson RC: Zika Virus-Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication in Vitro. J Infect Dis 2016, 214:1357–1360. [DOI] [PubMed] [Google Scholar]
- 95.Watanabe S, Tan NWW, Chan KWK, Vasudevan SG: Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J Infect Dis 2019, 219:223–233. [DOI] [PubMed] [Google Scholar]
- 96.Fowler AM, Tang WW, Young MP, Mamidi A, Viramontes KM, McCauley MD, Carlin AF, Schooley RT, Swanstrom J, Baric RS, et al. : Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 2018, 24:743–750.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (**) LysMCre+Ifnar1fl/fl mice with maternal antibodies to ZIKV have enhanced viral load, morbidity, and mortality following DENV2 challenge, but, in reverse, anti-DENV2 antibodies do not enhance ZIKV viral load or morbidity in a mouse pregnancy model.
- 97.George J, Valiant WG, Mattapallil MJ, Walker M, Huang YJS, Vanlandingham DL, Misamore J, Greenhouse J, Weiss DE, Verthelyi D, et al. : Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci Rep 2017, 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pérez-Guzmán EX, Pantoja P, Serrano-Collazo C, Hassert MA, Ortiz-Rosa A, Rodríguez IV, Giavedoni L, Hodara V, Parodi L, Cruz L, et al. : Time elapsed between Zika and dengue virus infections affects antibody and T cell responses. Nat Commun 2019, 10:4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valiant WG, Huang Y-JS, Vanlandingham DL, Higgs S, Lewis MG, Mattapallil JJ: Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg Microbes Infect 2018, 7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Subramaniam KS, Lant S, Goodwin L, Grifoni A, Weiskopf D, Turtle L: Two Is Better Than One: Evidence for T-Cell Cross-Protection Between Dengue and Zika and Implications on Vaccine Design. Front Immunol 2020, 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elong Ngono A, Shresta S: Immune Response to Dengue and Zika. Annn Rev Immunol 2018, 36:279–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiskopf D, Angelo M a, Sidney J, Peters B, Shresta S, Sette A: Immunodominance Changes as a Function of the Infecting Dengue Virus Serotype and Primary versus Secondary Infection. J Virol 2014, 88:11383–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grifoni A, Pham J, Sidney J, O’Rourke PH, Paul S, Peters B, Martini SR, de Silva AD, Ricciardi MJ, Magnani DM, et al. : Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J Virol 2017, 91:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grifoni A, Costa-Ramos P, Pham J, Tian Y, Rosales SL, Seumois G, Sidney J, de Silva AD, Premkumar L, Collins MH, et al. : Cutting Edge: Transcriptional Profiling Reveals Multifunctional and Cytotoxic Antiviral Responses of Zika Virus-Specific CD8 + T Cells . J Immunol 2018, 201:3487–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reynolds CJ, Watber P, Santos CNO, Ribeiro DR, Alves JC, Fonseca ABL, Bispo AJB, Porto RLS, Bokea K, de Jesus AMR, et al. : Strong CD4 T Cell Responses to Zika Virus Antigens in a Cohort of Dengue Virus Immune Mothers of Congenital Zika Virus Syndrome Infants. Front Immunol 2020, 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reynolds CJ, Suleyman OM, Ortega-Prieto AM, Skelton JK, Bonnesoeur P, Blohm A, Carregaro V, Silva JS, James EA, Maillère B, et al. : T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci Rep 2018, 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kostyuchenko VA, Lim EXY, Zhang S, Fibriansah G, Ng T, Ooi JSG, Shi J, Lok S: Letter Structure of the thermally stable Zika virus. Nature 2016, 533:425–428. [DOI] [PubMed] [Google Scholar]
- 108.Goo L, Dowd KA, Smith ARY, Pelc RS, DeMaso CR, Pierson TC: Zika Virus Is Not Uniquely Stable at Physiological Temperatures Compared to Other Flaviviruses. MBio 2016, 7:e01396–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goo L, VanBlargan LA, Dowd KA, Diamond MS, Pierson TC: A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLOS Pathog 2017, 13:e1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao H, Xu L, Bombardi R, Nargi R, Deng Z, Errico JM, Nelson CA, Dowd KA, Pierson TC, Crowe JE, et al. : Mechanism of differential Zika and dengue virus neutralization by a public antibody lineage targeting the DIII lateral ridge. J Exp Med 2020, 217:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gallichotte EN, Dinnon KH, Lim X-N, Ng T-S, Lim EXY, Menachery VD, Lok S-M, Baric RS: CD-loop Extension in Zika Virus Envelope Protein Key for Stability and Pathogenesis. J Infect Dis 2017, 216:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prasad VM, Miller AS, Klose T, Sirohi D, Buda G, Jiang W, Kuhn RJ, Rossmann MG: Structure of the immature Zika virus at 9 A resolution. Nat Struct Mol Biol 2017, 24:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goo L, DeMaso CR, Pelc RS, Ledgerwood JE, Graham BS, Kuhn RJ, Pierson TC: The Zika virus envelope protein glycan loop regulates virion antigenicity. Virology 2018, 515:191–202. [DOI] [PubMed] [Google Scholar]; (*) Truncation of the ZIKV E glycan loop at N154 to resemble DENV renders ZIKV susceptible to anti-FL antibodies and antibodies targeting other cryptic epitopes, suggesting the glycan loop modulates ZIKV susceptibility to enhancing antibodies.
- 114.Bos S, Viranaicken W, Frumence E, Li G, Desprès P, Zhao RY, Gadea G: The Envelope Residues E152/156/158 of Zika Virus Influence the Early Stages of Virus Infection in Human Cells. Cells 2019, 8:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carbaugh DL, Baric RS, Lazear HM: Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis. J Virol 2019, 93:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khoo US, Chan KYK, Chan VSF, Lin CLS: DC-SIGN and L-SIGN: The SIGNs for infection. J Mol Med 2008, 86:861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mondotte JA, Lozach P-Y, Amara A, Gamarnik AV: Essential Role of Dengue Virus Envelope Protein N Glycosylation at Asparagine-67 during Viral Propagation. J Virol 2007, 81:7136–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alen MMF, Dallmeier K, Balzarini J, Neyts J, Schols D: Crucial role of the N-glycans on the viral E-envelope glycoprotein in DC-SIGN-mediated dengue virus infection. Antiviral Res 2012, 96:280–287. [DOI] [PubMed] [Google Scholar]
- 119.Davis CW, Mattel LM, Nguyen HY, Ansarah-Sobrinho C, Dorns RW, Pierson TC: The location of asparagine-linked glycans on west nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin). J Biol Chem 2006, 281:37183–37194. [DOI] [PubMed] [Google Scholar]
- 120.Rodenhuis-Zybert IA, Moesker B, da Silva Voorham JM, van der Ende-Metselaar H, Diamond MS, Wilschut J, Smit JM: A Fusion-Loop Antibody Enhances the Infectious Properties of Immature Flavivirus Particles. J Virol 2011, 85:11800–11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raut R, Corbett KS, Tennekoon RN, Premawansa S, Wijewickrama A, Premawansa G, Mieczkowski P, Rückert C, Ebel GD, De Silva AD, et al. : Dengue type 1 viruses circulating in humans are highly infectious and poorly neutralized by human antibodies. Proc Natl Acad Sci 2019, 116:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]; (**) DENV1 virions in blood of primary dengue patients appear to be mature, whereas even a single passage in cell culture produces partially immature virions.
- 122.Junjhon J, Lausumpao M, Supasa S, Noisakran S, Songjaeng A, Saraithong P, Chaichoun K, Utaipat U, Keelapang P, Kanjanahaluethai A, et al. : Differential Modulation of prM Cleavage, Extracellular Particle Distribution, and Virus Infectivity by Conserved Residues at Nonfurin Consensus Positions of the Dengue Virus pr-M Junction. J Virol 2008, 82:10776–10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. : Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 2018, 379:327–340. [DOI] [PubMed] [Google Scholar]
- 124.World Health Organization: Weekly epidemiological record 7th September 2018. 2018, 93:457–476. [Google Scholar]
- 125.Biswal S, Reynales H, Llorens XS, Lopez P, Tabora CB, Kosalaraksa P, Sirivichayakul C, Watanaveeradej V, Rivera L, Espinoza F, et al. : Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med 2019, 381:2009–2019. [DOI] [PubMed] [Google Scholar]
- 126.Maciejewski S, Ruckwardt TJ, Morabito KM, Foreman BM, Burgomaster KE, Gordon DN, Pelc RS, DeMaso CR, Ko S, Fisher BE, et al. : Distinct neutralizing antibody correlates of protection among related Zika virus vaccines identify a role for antibody quality. Sci Transi Med 2020, 12:eaaw9066. [DOI] [PubMed] [Google Scholar]; (*) While different DNA ZIKV vaccine candidates produced the same neutralizing antibody titers in non-human primates, they were not equivalently protective, and neutralizing antibodies to mature ZIKV virions and those that were maturation-insensitive were better predictors of in vivo protection.


