Abstract
Three-dimensional (3D) in vitro models are excellent tools for studying complex biological systems because of their physiological similarity to in vivo studies, cost-effectiveness and decreased reliance on animals. The influence of tissue microenvironment on the cells, cell-cell interaction and the cell-matrix interactions can be elucidated in 3D models, which are difficult to mimic in 2D cultures. In order to develop a 3D model, the required cell types are derived from the tissues or stem cells. A 3D tissue/organ model typically includes all the relevant cell types and the microenvironment corresponding to that tissue/organ. For instance, a full corneal 3D model is expected to have epithelial, stromal, endothelial and nerve cells, along with the extracellular matrix and membrane components associated with the cells. Although it is challenging to develop a corneal 3D model, several attempts have been made and various technologies established which closely mimic the in vivo environment. In this review, three major technologies are highlighted: organotypic cultures, organoids and 3D bioprinting. Also, several combinations of organotypic cultures, such as the epithelium and stroma or endothelium and neural cultures are discussed, along with the disease relevance and potential applications of these models. In the future, new biomaterials will likely promote better cell-cell and cell-matrix interactions in organotypic corneal cultures.
Keywords: Cornea, 3D culture, in vitro models, organotypic culture, organoids, 3D bioprinting
1. Introduction
The cornea is a transparent, avascular organ, which aids in focusing the light onto the retina. The human cornea consists of five different layers arranged in order from the anterior to the posterior—epithelium, Bowman’s membrane, stroma, Descemet’s membrane (DM) and endothelium (DelMonte and Kim, 2011). Each of these layers is important in maintaining structural integrity and corneal functions, including corneal transparency. The corneal epithelium is a multi-cellular layer composed of non-keratinized, stratified squamous epithelial cells, along with the epithelial basement membrane composed of laminins, nidogens, perlecan, collagen IV and other heparan sulfate proteoglycans (Maurice, 1956; Torricelli et al., 2013). The superficial epithelial cells are polygonal with microvilli architecture and epithelial barrier function that acts as a permeability barrier for foreign molecules, including growth factors from the tears such as transforming growth factor (TGF)β1 and TGFβ2 (de Oliveira RC and Wilson SE, unpublished data, 2020). The basal epithelial cells are cuboidal and mitotically active to replenish the superficial epithelial layers. Bowman’s membrane, which is present in some species, is an acellular layer composed of randomly-oriented collagen fibrils in the anterior stroma that differs in structure from underlying stroma (Wilson and Hong, 2000; Wilson, 2020a). The stroma is composed of regularly arranged layers of collagen fibers interspaced with keratocytes—which are the cells involved in stromal tissue maintenance, repair and regeneration (Quantock and Young, 2008). Upon wounding of stroma through an infection or injury, superficial to mid-stromal keratocytes undergo apoptosis, depending on the severity of the injury, and keratocytes distal to the wound become motile, mitotically active “corneal fibroblasts” (Wilson, 2002). These fibroblasts show moderately high expression of fibrosis-related genes, which include fibronectin, tenascin and metalloproteinases (Yam et al., 2020). With ongoing delivery TGFβ1 and/or TGFβ2 from the tears and epithelium—due to delayed regeneration of epithelial barrier function and the epithelial basement membrane (de Oliveira RC and Wilson SE, unpublished data, 2020), many of these corneal fibroblasts differentiate into alpha smooth muscle actin-expressing myofibroblasts that produce high levels of disorganized extracellular matrix (ECM) and initiate stromal contraction. This subsequently leads to scarring corneal opacity, that often causes vision loss (Jester et al., 1999; Wilson, 2020b; Rocher et al., 2020). Additionally, the corneal stroma is occupied by the corneal nerves (axons originating from the trigeminal nerve), with high densities present in the anterior stroma, within the subepithelial nerve plexus, and the epithelium (Marfurt et al., 2010; Meek and Knupp, 2015). The posterior of the stroma is lined by the acellular Descemet’s basement membrane, composed of many ECM components, including collagen VIII, collagen IV, laminins, perlecan and nidogens (Timpl and Brown, 1996; de Oliveira et al., 2020). Attached to Descemet’s membrane is a monolayer of specialized homogenous hexagonal endothelial cells, which are closely packed together (Bahn et al., 1984). The presence of tight junction and ionic pumps in the endothelial cells facilitates fluid exchange across the posterior surface of the cornea (Klyce, 2020), thereby playing a critical role in regulating corneal hydration.
A full 3D in vitro model of the cornea would consist of four or five layers (depending on the species). However, major challenges are associated with maintaining culture conditions demanded by each cell type. Furthermore, the biggest challenge lies in mimicking the in vivo environment and structure, and associated intraocular pressure. In spite of these challenges, however, several attempts have been made to develop 3D in vitro models of the cornea.
2. Importance of 3D in vitro models
Three-dimensional models have the potential to provide a better understanding of corneal healing, regeneration, and diseases. Three-dimensional in vitro models uniquely elucidate cellular and matrix interactions mimicking the complex in vivo environments. The influence of the tissue microenvironment on the cell behaviors, cell-cell interactions and cell-matrix communications is best studied in 3D models (Edmondson et al., 2014). Thus, compared to 2D cultures, the presence of ECM in a 3D culture greatly influences cell proliferation, differentiation and survival (Baker and Chen, 2012; Bonnier et al., 2015). Furthermore, cells in a 3D environment have physiological access to nutrients, metabolites, oxygen and signaling molecules (Duval et al., 2017). Most 3D models utilize cells derived from primary cultures and/or stem cells to develop corneal constructs—which makes these models attractive for studying infection, injury, fibrosis and regenerative mechanisms. Also, in vitro studies are typically more cost-effective than animal studies (Kapałczyńska et al., 2016). Finally, these models can be utilized for high-throughput screening of drugs to evaluate their pharmacological effects and possible toxicities (Langhans, 2018).
3. 3D Organotypic cornea models
An organotypic model is generated by utilizing the cells derived directly from the tissues (Dongari-Bagtzoglou and Kashleva, 2006). These cells are typically mixed with a biological or synthetic ECM components and cultured on a porous membrane support (Chioni and Grose, 2008), and in certain cases, the cells are encouraged to secrete their own ECM components by culturing in appropriate media (McKay et al., 2019). The choice of cell types used in a particular study is defined by the query of interest or therapeutic goal. Organotypic models can be used to elucidate cell-cell and cell-matrix interactions occurring in organs. Alternatively, they may be used to study the formation of basement membrane components and how they influence fibrosis and other tissue repair mechanisms. In addition to local cell-cell communications, the indirect cell communications mediated by growth factors, cytokines and extracellular vesicles can also be studied using these 3D organotypic culture models (Acheva et al., 2017). Several combinations of 3D organotypic cornea models have been developed thus far and are described below.
3.1. Epithelial-stromal cultures
This model is important in understanding the cell-cell communications between corneal fibroblasts and epithelial cells, and thus it can be used to explore corneal wound healing responses after injuries or infections. The schematic workflow for developing a 3D organotypic model with corneal fibroblasts and epithelial cells is depicted in Fig. 1. McKay et al. (2019) developed a 3D in vitro model by culturing the corneal fibroblasts (CF) in appropriate medium for a period of 4 weeks, so as to enable the deposition of matrix components by the fibroblast cells, and epithelial cells were subsequently cultured on the surface to mimic the 3D environment. Interestingly, these investigators identified the expression of matrix proteins, such as collagen type III, fibronectin and thrombospondin at the CF-epithelial interface—similar to what is observed in vivo. For instance, the expression and assembly of epithelial basement membrane proteins such as laminin alpha5, laminin beta3 and nidogen1 in a 3D organotypic culture of CF and epithelial cells is shown in Fig.2. A fascinating aspect of this study was that no external matrix components were added for cell adhesion, and, therefore, the cells were allowed to mature and produce extracellular matrix themselves under optimized media conditions. A limitation of this model is that the multilayered stratified epithelium—involving apical epithelial cells, wings cells and basal cells—which are observed in vivo—was not reported in this model. Also, since the CF cultures were maintained for a relatively long period of 4 weeks, numerous apoptotic bodies were generated within the stroma. In most in vitro stromal constructs developed thus far, the thickness of ECM that developed did not exceed 120–150 μm—which is only 1/3 the thickness of the normal human corneal stroma—even after stimulation with vitamin C and TGFβ1 for a period of 4 weeks (McKay et al., 2019). To overcome this limitation, a 3D stacked construct was developed, in which multiple self-assembled constructs were generated and stacked upon each other. Interestingly, this model was utilized to elucidate the role of sphingolipids in human corneas (Priyadarsini et al., 2018).
Fig. 1.
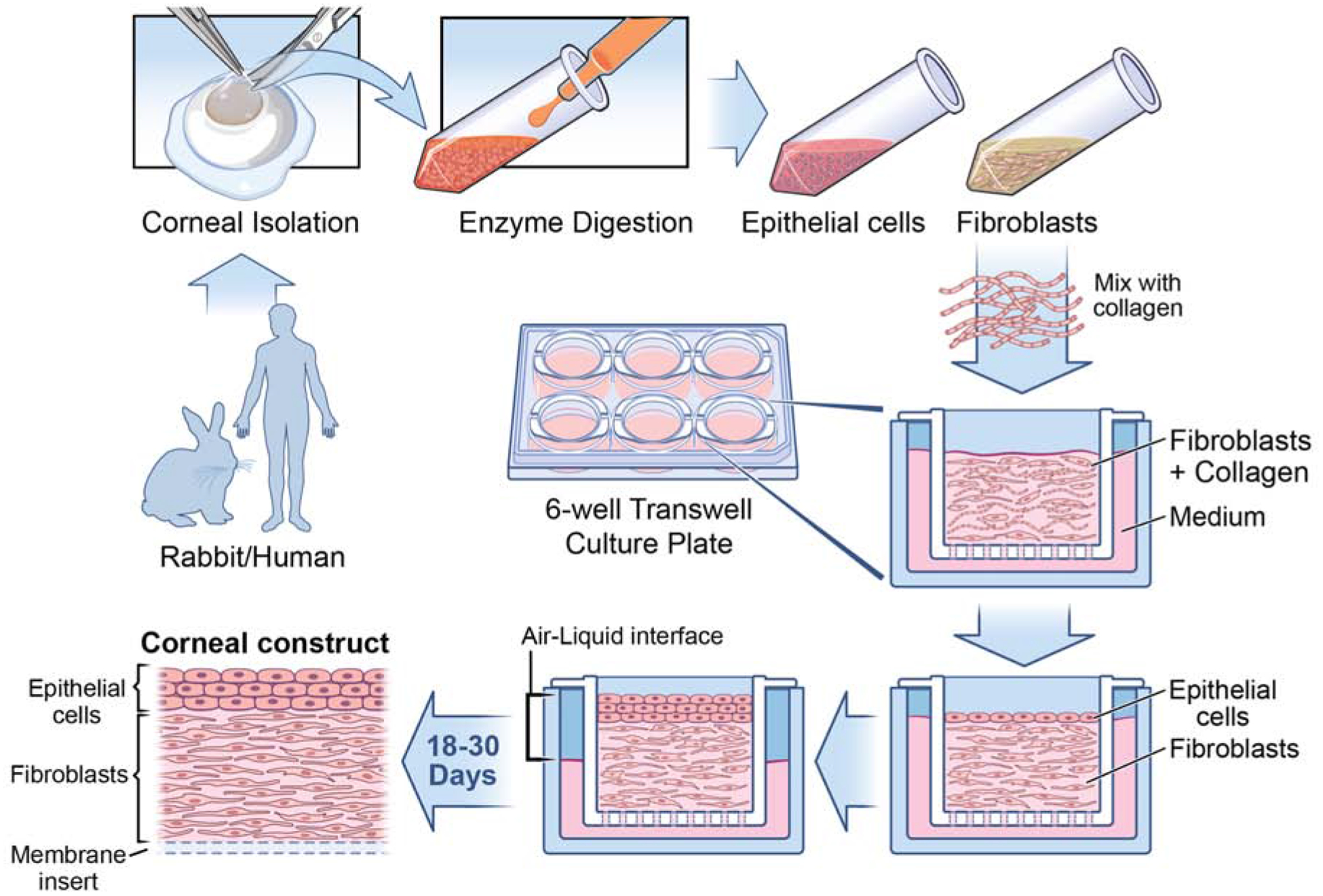
Corneal fibroblast and epithelial 3D organotypic culture workflow. Rabbit/Human corneas subjected to epithelium scrape and descemetorhexis, isolating epithelial cells and stromal layer. Corneal keratocytes are obtained through overnight incubation of stromal layer with collagenase medium (50 mg of collagenase and 25 mg of Hyaluronidase in 50 ml HBSS). The following day, the desired cell type is collected via brief centrifugation and cultured in fibroblast medium containing Fibroblast growth factor-2 (40ng/ml), which promotes fibroblast differentiation. Once the required cell count is reached, fibroblasts are seeded along with collagen onto the insert of a 6 well tissue culture tray and the medium is added to both inside and outside of the insert. After 5 days of culture, epithelial cells are seeded above the collagen layer. From this stage onwards, the medium is added only to the bottom of the insert so as to maintain the cells in both air and liquid phase, which is vital in culturing cells in 3D. The cells are maintained in the air-liquid interface for about 18–30 days to get the 3D corneal construct containing fibroblasts embedded in collagen with epithelial cells on the top. It is interesting to observe the presence of multiple layers of epithelial cells above the newly deposited EBM, similar to what is observed in vivo.
Fig. 2.
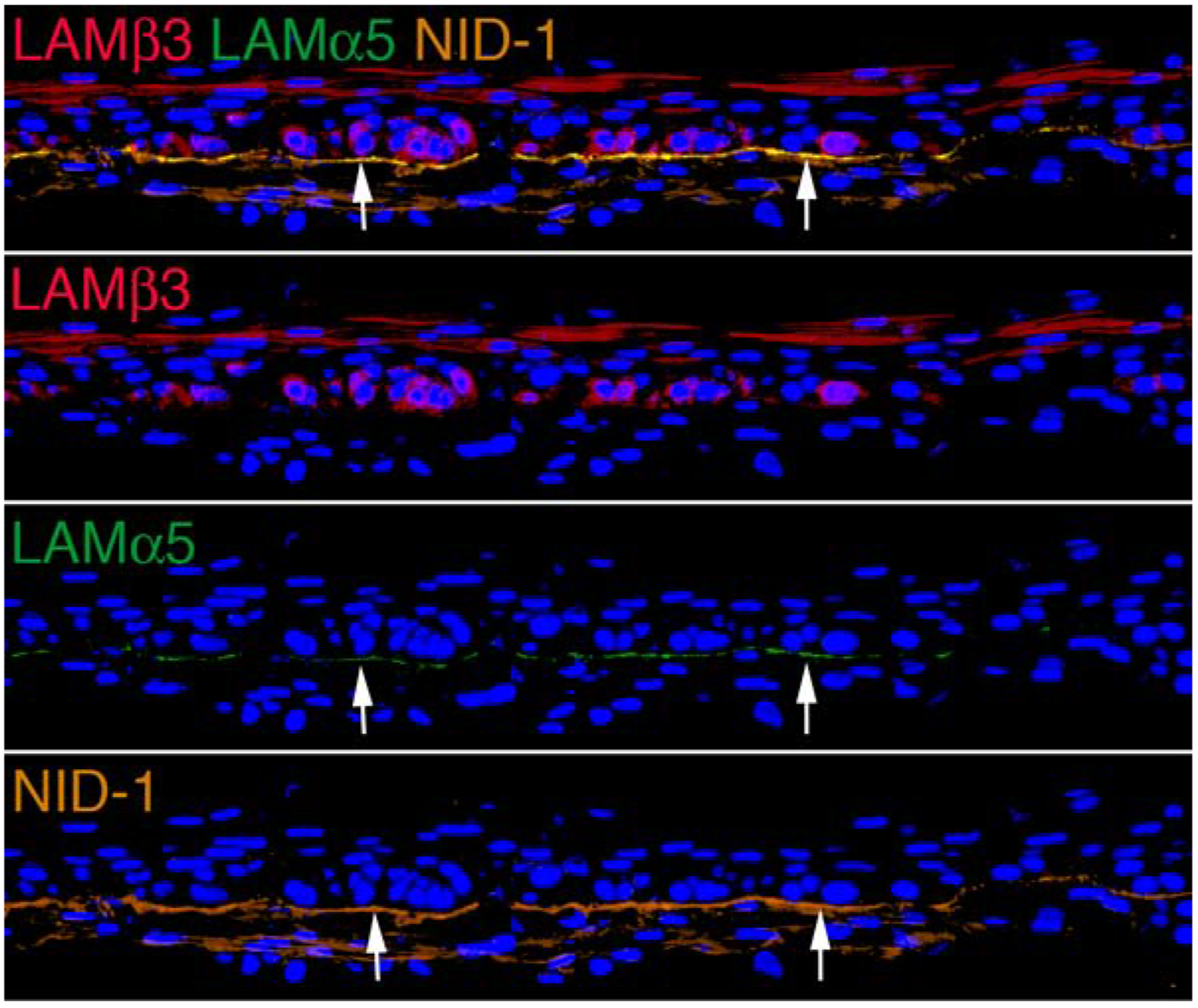
Epithelial basement membrane (EBM) formation in epithelial-corneal fibroblast organotypic cultures. After 18 days of co-culture of rabbit corneal epithelial cells and corneal fibroblasts the tissue was immersed in 4% paraformaldehyde for an hour at room temperature, 50% sucrose for 1–2 hour at 4°C, 2M sucrose for 1–2 hours at 4°C, and finally the samples were stored in optimal cutting temperature (OCT) molds for sectioning. Triplex immunohistochemistry for EBM components laminin beta 3 (usually a component in laminin 332), laminin alpha 5 (usually a component in laminins 511 and 521) and nidogen-1 was performed as previously described (Saikia et al, 2018). It can be seen that EBM (arrows) is assembled between the basal epithelial cells and underlying corneal fibroblasts, with the components arranged as they are in corneas with regenerating EBM after photorefractive keratectomy. Blue is DAPI in all panels. Mag 200X.
The use of keratocytes rather than CFs in these constructs might better reflect the in vivo milieu, but these approaches have been limited by the low proliferative and migratory properties of keratocytes (Zieske et al., 2001). However, if a 3D model with keratocytes could be developed, it might serve as a better model to study corneal physiology and wound healing. It is challenging, however, to maintain keratocytes because they so easily differentiate into corneal fibroblasts that lose expression of keratocan and aldehyde dehydrogenase family 3, member A1 (ALDH3A1) (Kumar et al., 2016). In order to maintain the keratocyte phenotype, alignment and fusiform cell shape, Zhang et al., (2017) developed a corneal biomimetic model by modifying the surface topography of the biomaterial and introducing a mechanical strain, which is thought to resemble that in the native cornea. Silk fibroin with high mechanical strength was utilized in these studies (Hazra et al., 2016). It is well-known that mechanical strain has an important impact on the behavior and properties of the corneal cells, and the cornea itself, with its dome-shaped structure (DelMonte et al., 2011). The cornea has mechanical strain from 0% – 7%, depending on the specific location in the cornea (Hollman et al., 2013). In those experiments, the surface topography was altered by developing silk films with 300 or 600 grooves mm−1 via surface patterning and the Flexcell tension system to develop mechanical strain with different strength from 0% – 6%, and different shapes. Interestingly, silk fibroin films with 600 grooves mm−1 and 3% dome-shaped mechanical strain supported better cell alignment and enhanced retention of keratocyte and ECM markers (Zang et al., 2017). This is likely to be an important advance, not just for the development of 3D models, but also in the development of tissue engineered corneal replacement grafts.
Retinoic acid (RA), which is a metabolite of Vitamin A, was found to maintain the keratocyte phenotype, when culturing them in serum-free conditions (Gouveia and Connon, 2013). Later, RA was found to control the keratocyte phenotype, behaviour and keratocyte-specific gene and protein expression in a 3D corneal stroma model (Abidin et al., 2015). Human keratocytes encapsulated in collagen gels were compressed under load to develop a 3D corneal stroma. It was then cultured in a serum-free medium supplemented with 10 μM retinoic acid for 30 days. Interestingly, RA was found to enhance keratocyte proliferation until 30 days, with increased expression of keratocyte-specific genes and proteins, such as keratocan, lumican and decorin. Furthermore, RA was found to inhibit the contractility of keratocytes, which eventually allowed the proper deposition of ECM. This is considered a promising approach to maintain the keratocyte phenotype in 3D corneal constructs.
Wei et al. (2018) developed a 3D corneal stroma equivalent with keratocytes utilising the properties of amniotic membrane. The ultrathin amniotic membrane (UAM) was laminated and the keratocytes were distributed between the space of the layered ultrathin amniotic membrane. The keratocytes maintained their phenotype and secreted ECM, making the UAM attain almost half the thickness of human cornea over 8 weeks in culture. Moreover, this corneal stromal equivalent was utilized in lamellar keratoplasty, wherein it was well integrated with the recipient cornea and completely epithelialized without myofibroblast differentiation. Recently, patterned substrates used to culture keratocytes were found to have an important role in maintaining keratocyte behaviour and phenotype (Bhattacharjee, 2020). Photolithography and polymer molding techniques were used to fabricate micropatterns consisting of linear aligned and orthogonally aligned microchannels on the surface of polydimethylsiloxane. Alhough the cells on orthogonal substrates showed a spread morphology, cells cultured on both the patterned substrates showed higher expression of keratocyte-specific genes (Bhattacharjee, 2020), indicating the importance of patterned substrates in developing corneal constructs for maintaining the keratocyte phenotype.
In a new study (TM Shiju and SE Wilson, unpublished data, 2020), organotypic cultures were used to demonstrate that cell-cell interactions involving corneal epithelial and corneal fibroblast cells were necessary for the epithelial basement membrane (EBM) to be generated. Neither cell type alone could produce the EBM. Conversely, epithelial cells and myofibroblasts, in organotypic cultures simulating fibrotic corneas, could not regenerate TGFβ-modulating EBM.
3.2. Epithelial-stroma cultures, with nerves
Although the cell-cell interaction between the stromal and epithelial cells could be studied in the previous model, the absence of nerves in corneal tissue models was considered a deficiency for some applications, which was addressed by the in vitro model developed by Mckay et al., (2019). Interestingly, thin silk protein films were used as scaffolds for supporting the growth of stroma and epithelial layers, and a silk porous sponge was utilized to support neuronal growth in this 3D model. The stromal layer is composed of human corneal stromal stem cells on micropatterned silk films with interlaying collagen type 1 (Ghezzi et al., 2017). The silk films were functionalized with RGD (arginine-glycine-aspartate) peptide to support matrix synthesis by stromal cells, providing a proper alignment and facilitating growth (Gil et al., 2010). Unlike collagen, these silk films did not contract and were found to slowly degrade in vitro (Lawrence et al., 2009). Silk sponge rims with outer diameter of 15 mm were cut and coated with poly-D-lysine. Human induced neural stem cells (hiNSC) were seeded onto the silk sponges and were placed at the periphery of the insert where a stacked construct of corneal stromal and epithelial cells was placed at the center. Although the earlier studies used the dorsal root ganglions to mimic the neurons in the 3D models (Wang et al., 2017), the use of hiNSC is considered an advantage in this study. Over 4 weeks of culturing, hiNSC has the ability to transition gradually into differentiated nerves with elongated nerve fibers. Interestingly, neurons were found to innervate both the stromal and epithelial layer and improved the viability of the corneal model. This model could be used as an important tool for understanding the interactions between the nerves and corneal cells, and paves the way to elucidate corneal nerve regeneration after injury and dysfunction/degeneration associated with pathophysiologic conditions such as corneal neuropathies.
In a similar fashion, Sharif et al. (2018) developed a 3D model to study the cellular interaction between the stromal cells and nerve cells. In that model, the stromal cells were allowed to secrete their own extracellular matrix to study the cell-matrix interaction, in combination with the cell-cell interaction between the fibroblasts and neuronal cells. The authors proposed this model to study diabetic keratopathy, keratoconus and Fuchs’ dystrophy (Priyadarsini et al., 2018). Extending these multicellular cultures further, Deardorff et al. (2018) developed a model to consider the influence of the micro-niche environment surrounding cells. Interestingly, that model utilized hiNSC which were generated from human fibroblasts to develop sensory neurons (Cairns et al., 2016). Notably, hiNSC has the ability to grow in co-culture conditions with multiple cell types, and neuronal differentiation from hiNSC was more rapid and robust compared to the neuronal differentiation from induced pluripotent stem cells (Deardorff et al., 2018). In addition to the neural component, a “bioreactor” was used to maintain a dynamic culture condition and an anterior chamber was introduced to mimic intraocular pressure and tear flow. This model may be well-suited to study corneal pain syndromes, dry eye disease and ocular hypertension, in addition to use in toxicology and drug discovery studies. For instance, this model was used to study the pain response while using a nociceptive stimulant capsaicin and the healing response was analyzed using serum treatment (Siran et al., 2018). Alternatively, the drug testing studies can be performed on a microfluidic 3D cornea on a chip assay. Bai et al. (2020) used primary corneal epithelial and endothelial cells isolated from C57BL/6J mice, cultured separately in two peripheral channels to set up a 3 channel microfluidic device. Collagen matrix was used in the central channel to form a 3D model and a condensed collagen layer was formed in the epithelium channel to mimic the Bowman’s layer. This is unlike the other organotypic cultures described above, wherein the basement membrane components were allowed to secrete and assemble by the cells themselves. Although this model is useful in studying the permeability of drugs, the lack of a multi-layered epithelium and stromal cells are limitations.
3.3. Corneal endothelial-stromal cultures
Three-dimensional organotypic cultures are especially useful in studies of cell-cell and cell-matrix communications—which may not be feasible in in vivo models. For example, the effect of endothelial cell therapy in formation of DM and restoration of pump/barrier function can be best studied using 3D stromal constructs seeded with endothelial cells. Hutcheon et al. (2019) demonstrated that their 3D stromal construct provided a platform for corneal endothelial cells to differentiate and form monolayers and enabled these investigators to study newly synthesized DM. This was further supported by the findings of Bourget et al. (2016) that the self-assembled stromal components aid in enhancing the expression of markers such as Na+/K+ ATPase pumps and Na+/HCO3− in corneal endothelial cells (Bourget and Proulx, 2016). Furthermore, this model can be used to explore potential interactions between corneal endothelial cells and keratocytes, and other cells that may cooperate in maintenance and potential regeneration of DM after injury.
4. 3D models with a single cell type
Organotypic cultures with only epithelial cells were developed to study the effects of topical drugs on epithelial cells by incubating the primary epithelial cells on membrane inserts and exposing the cells to the air-liquid interface. A reconstructed human corneal epithelial model (EpiOcular™) was developed by MatTek Life Sciences and consisted of human corneal epithelial cells in 3D cultures that formed a stratified squamous epithelium (Kaluzhny et al., 2018). This model was used as a screening tool for identification of chemicals with the potential for ocular surface toxicity (Garcia et al., 2019; da Silva et al., 2020). Also, a 3D corneal epithelial tissue model was used to study oxidative stress-related corneal epithelial injuries (Kaluzhny et al., 2020). In this study, oxidative stress was induced with non-toxic UV radiation, hydrogen peroxide, nitrogen mustard and desiccating conditions, which provided insights into the changes associated with dry eye disease. Chacon et al., (2019) developed a human corneal epithelial model (QobuR) utilizing normal limbal tissue. The trans-epithelial electrical resistance of QobuR was found to be around 1500 Ωcm2 on day 7 of cellular differentiation, which is comparable with the native cornea displaying functional markers. This model was useful in examining the ocular irritancy of ophthalmic drugs, barrier disruptions and drug permeability studies (Chacon et al., 2019).
Several attempts have been made to develop tissue-engineered stromal replacements. Scaffolds such as synthetic polymers, gelatin hydrogel, collagen hydrogels, collagen sponges, and collagen films have been utilized in various studies to replace the stromal matrix (Orwin et al., 2003; Crabb and Hubel, 2008). Interestingly, even biomaterials such as decellularized porcine stroma, silk fibroin, and amniotic membranes have been used to replace or support the corneal stroma in 3D models (Yoeruek et al., 2012). Notably, keratocytes cultured on amniotic membranes maintained their phenotype and expressed keratocyte markers, such as keratocan (Espana et al., 2003). Recently, Che et al. (2019) developed an ultrathin amniotic membrane by removing the loose matrix to increase the transparency, strength and elasticity of the tissue. These investigators reported success in developing a corneal stromal construct by culturing keratocytes on amniotic membranes for use in transplantation studies.
5. Corneal organoids
Corneal organoids are important 3D models to probe the corneal development process and to understand diseases associated with abnormal development. Corneal development is initiated by the surface ectoderm, and the inductive interaction between the ectoderm and the lens vesicle drives the neural crest-derived mesenchymal stem cells to form the corneal endothelium (Graw, 2010). Stromal keratocytes develop in the space between the epithelium and the lens vesicle (Lwigale, 2015). In complex diseases with poorly understood etiologies, such as Fuchs’ dystrophy and keratoconus, it’s important to consider the influences of all the relevant corneal cells and their interaction with the ECM that might be involved in the disease (Jurkunas, 2018; Kobashi and Rong, 2017). Research with corneal organoids has played an important role in exploring these types of diseases.
For example, corneal organoids were developed from human fetal fibroblast-derived induced pluripotent stem cells (iPSCs) by Foster et al. (2017). The sequence of events involved in corneal organoid development include anterior neural commitment using Matrigel, inhibition of Wnt signaling, manual dissection of the neural vesicle with retinoic acid exposure, and inhibition of Notch signaling (Foster et al., 2017). Interestingly, the process of differentiation is well-orchestrated in a manner very similar to the in vivo development of eye. Furthermore, these organoids had all three major cell types of cornea, key extracellular matrix proteins and collagen fibrils. Susaimanickam and coworkers (2017) overcame complex cell enrichment protocols involved in tissue-specific cell expansion from iPSCs in a 2D culture system, and developed a 3D culture system from both human embryonic stem cells and iPSC, which they referred to as ‘Minicorneal’ organoids. In the future, these types of organoid models will likely be used in high-throughput drug screening and toxicology studies (Lancaster and Knoblich, 2014).
6. 3D bioprinting of corneal stroma
3D bioprinting is an emerging technology with huge potential for fabrication of human tissues for clinical applications. Thus, bioprinting, which falls under additive manufacturing technology, is expected to play an important role in regenerative medicine to produce synthetic functional prostheses (Papaioannou et al., 2019). The major advantage of bioprinting lies in generating corneas with accurate geometry and optical properties— similar to a native human cornea. Several processing technologies which are based on laser, micro-extrusion and micro-stereolithography have been tried for bio-fabrication (Ahn et al., 2018; Isaacson et al., 2018; Sorkio et al., 2018). With bioprinting, it’s possible to deposit biological materials layer-by-layer in a prescribed format that yields very similar anatomy to organotypic models (Zhang and Zhang, 2015). In general, an “in-silico” model of the tissue is developed and exported to the 3D printing software. A suitable biomaterial is selected based on the tissue to be developed and combined with the cells to form the bio-ink. The compatibility of the biomaterials with the cells and the viability of the cells in the process are studied in advance. The bio-ink is then loaded into the bioprinting cartridge and the tissue of interest is “printed.” In an example application, corneal stromal equivalents were developed using an in-house collagen bio-ink encapsulated with corneal keratocytes, which yielded more than 90% cell viability at 1 day post-printing and 83% cell viability at 7 days post-printing (Isaacson et al., 2018). The post-printing stage is critical since cell-mediated remodeling of the construct occurs under appropriate physiological conditions to develop a tissue with the desired structural and functional properties. A major advantage of this model over other in vitro models is the capacity to develop a curved corneal geometry, which was recently shown to affect cell migration and collagen alignment in the tissue (Gouveia et al., 2019).
Laser-assisted printing was also employed to generate corneal tissue constructs using human stem cells. Epithelial cells were obtained from limbal epithelial stem cells that were derived from human embryonic stem cells, and stromal cells were generated from human adipose-derived stem cells. A mixture of recombinant human collagen type 1 and laminin was used as the bioink. Interestingly, this corneal construct had a stratified epithelium with apical expression of cytokeratin 3 (CK3) and basal expression of progenitor markers, in addition to key proliferation markers (Sorkio et al., 2018). More recently, Duarte-Campos et al. (2019), have developed a corneal construct using “drop-on demand technology,” which employed a controlled shear stress at the printing nozzle, and thereby improved the cell-viability in the printed tissue. Furthermore, in order to improve the stability of corneal construct and precision in printing, agarose hydrogel mixed with collagen type 1 was used as the bioink. The most encouraging aspect of this model was the presence of keratocytes expressing phenotypic markers such as keratocan and lumican, even after the bioprinting process.
There are several advantages for 3D bioprinting over other methods in generating corneal constructs that can be utilized in regenerative medicine. This method has precise control over the shape and physical properties of the corneal constructs that include the thickness and curvature of the cornea. It is also possible to bio-print a personalized artificial cornea based on the corneal geometric data and anatomy of the individual eye. Because of advances in biomaterials and novel bioinks, this method is expected to have better control over the surface quality and mechanical control in the future (Zhang et al., 2019). However, there remain several challenges that need to be resolved. Probably the most difficult challenges are to provide the corneal transparency and refractive power needed for optimal vision, and optimal microporosity—which is important for the diffusion of oxygen, carbon dioxide and the nutrients required for the corneal cells. The next challenge is to maintain the microarchitecture within the construct with defined fibrillar size, space and cell alignment and phenotype needed for normal corneal transparency. A corneal construct that is targeted towards regenerative medicine should also have stable biomechanical properties, so as to withstand suturing, clipping and other procedures involved in transplant surgery. The in vivo evaluation of the bioprinted corneas in suitable animal models, determination of cell-fate post-implantation, demonstration of stromal regenerative ability, such as scar-free healing and new collagen production and organization, and control of optimal tissue stiffness still need to be addressed (Fuest et al., 2020).
7. Processing of 3D in vitro cultured samples
The samples collected from 3D models can be processed for quantitative PCR (qPCR), Western blotting, ELISA, immunohistochemistry, transmission electron microscopy (TEM) and scanning electron microscopy. In an organotypic culture, the epithelial cells can be separated from the stroma by treating with ethylenediaminetetraacetic acid (EDTA), and, therefore, the cell layers can be analyzed separately. Many commercial kits are available for isolation of DNA, RNA, and protein from 3D cultures—which can be used further for gene or protein expression analyses using qPCR and western blotting. For immunohistochemistry, samples are typically treated with 4% paraformaldehyde for an hour at room temperature, 50% sucrose for 1–2 hour at 4°C, 2 M sucrose for 1–2 hours at 4°C, and finally the samples are stored in optimal cutting temperature (OCT) molds for sectioning. For TEM, samples are commonly fixed in 4% paraformaldehyde, 2.5% glutaraldehyde in 0.2 M cacodylate buffer, pH 7.4.
8. Implications of 3D in vitro models
Each of the aforementioned models can be utilized for high-throughput drug screening and toxicity studies (Ravi et al., 2015). Since most of these models are generated using human cells, they may better reflect the native human corneal physiology, and to some extent, corneal architecture. This could make these models a useful alternative to animal research in identifying novel therapeutics. These models are also more suitable for identifying personalized medicines (Perkhofer et al., 2018; Dutta et al., 2017). These models can also be used to study cell-biomaterial interactions, which can be integrated in a microfluidic chip for diagnostic device development (Nguyen et al., 2013). Furthermore, using cell-encapsulating hydrogels, these 3D in vitro models can also be used as biosensors (Zhou et al., 2011). The 3D organotypic cultures can also be used to study wound healing and corneal fibrosis, where the interaction between an epithelial or endothelial cell and adjacent stromal cells can be elucidated in detail. Also, the role of basement membranes (BMs) in pathophysiology and how BMs are regenerated during repair processes can be studied using these models. Corneal organoids will likely play an important role in understanding the development biology of cornea and to study diseases associated with development and degeneration of cornea.
9. Conclusion
Three-dimensional corneal in vitro models are highly useful modalities to study cell-cell interactions, development, and disease pathophysiology. Improvements will lead to less reliance on live animal models for corneal research and are likely to provide future treatments for corneal disorders and diseases.
Three-dimensional (3D) in vitro models are excellent tools for studying complex biological systems
3D organ models typically include relevant cell types and the microenvironment corresponding to that organ
Organotypic cultures, organoids and 3D bioprinting are three major technologies commonly used for 3D in vitro models
Funding
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30- EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Department of Defense grant VR180066 and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement
The authors do not have any commercial or proprietary interests in this study.
References
- Abidin FZ, Gouveia RM, Connon CJ, 2015. Application of retinoic acid improves form and function of tissue engineered corneal construct. Organogenesis 11, 122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheva A, Schettino G, Prise KM, 2017. Pro-inflammatory signaling in a 3D organotypic skin model after low LET irradiation-NF-κB, COX-2 activation, and impact on cell differentiation. Front. Immunol 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CB, Kim Y, Park SJ, Hwang Y, Lee JW, 2018. Development of arginine- glycine-aspartate-immobilized 3D printed poly(propylene fumarate) scaffolds for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed 29, 917–931. [DOI] [PubMed] [Google Scholar]
- Bahn CF, Falls HF, Varley GA, Meyer RF, Edelhauser HF, Bourne WM, 1984. Classification of Corneal Endothelial Disorders Based on Neural Crest Origin. Ophthalmology 91, 558–563. [DOI] [PubMed] [Google Scholar]
- Bai J, Fu H, Bazinet L, Birsner AE, D’Amato RJ, 2020. A method for developing novel 3D cornea-on-a-chip using primary murine corneal epithelial and endothelial. Cells. Front. Pharmacol 11, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Chen CS, 2012. Deconstructing the third dimension-how 3D culture microenvironments alter cellular cues. J. Cell Sci 125, 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee P, Cavanagh BL, Ahearne M, 2020. Influence of micropatterned substrates on keratocyte phenotype. Sci. Rep 10, 6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnier F, Keating ME, Wrobel TP, Majzner K, Baranska M, Garcia-Munoz A, Blanco A, Byrne HJ, 2015. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. In Vitro. 29, 124–131. [DOI] [PubMed] [Google Scholar]
- Bourget JM, Proulx S, 2016. Characterization of a corneal endothelium engineered on a self-assembled stromal substitute. Exp. Eye Res 145, 125–129. [DOI] [PubMed] [Google Scholar]
- Cairns DM, Chwalek K, Moore YE, Kelley MR, Abbott RD, Moss S, Kaplan DL, 2016. Expandable and rapidly differentiating human induced neural stem cell lines for multiple tissue engineering applications. Stem Cell Reports 7, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón M, Vázquez N, Berisa S, Persinal M, Sanchez M, Baamonde B, Alfonso JF, Cueto LFV, Merayo-Lloves J, Meana A, 2019. QobuR - A new in vitro human corneal epithelial model for preclinical drug screening. Eur. J. Pharm. Biopharm 136, 164–173. [DOI] [PubMed] [Google Scholar]
- Che X, Wu H, Jia C, Sun H, Ou S, Wang J, Jeyalatha MV, He X, Yu J, Zuo C, Liu Z, Li W, 2019. A novel tissue-engineered corneal stromal equivalent based on amniotic membrane and keratocytes. Invest. Ophthalmol. Vis. Sci 60, 517–527. [DOI] [PubMed] [Google Scholar]
- Chioni AM, Grose R, 2008. Organotypic modelling as a means of investigating epithelial-stromal interactions during tumourigenesis. Fibrogenesis Tissue Repair 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb RAB, Hubel A, 2008. Influence of matrix processing on the optical and biomechanical properties of a corneal stroma equivalent. Tissue Engineering 14, 173–182. [DOI] [PubMed] [Google Scholar]
- da Silva ACG, Chialchia AR, de Castro EG, Silva MRLE, Arantes DAC, Batista AC, Kitten GT, Valadares MC, 2020. A new corneal epithelial biomimetic 3D model for in vitro eye toxicity assessment: Development, characterization and applicability. Toxicol. In Vitro 62, 104666. [DOI] [PubMed] [Google Scholar]
- Deardorff PM, McKay TB, Wang S, Ghezzi CE, Cairns DM, Abbott RD, Funderburgh JL, Kenyon KR, Kaplan DL, 2018. Modeling Diabetic Corneal Neuropathy in a 3D In Vitro Cornea System. Sci. Rep 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelMonte DW, Kim T, 2011. Anatomy and physiology of the cornea. J. Cataract Refract. Surg 37, 588–598. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Kashleva H, 2006. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat. Protoc 1, 2012–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte Campos DF, Rohde M, Ross M, 2019. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. A 107, 1945–1953. [DOI] [PubMed] [Google Scholar]
- Dutta D, Heo I, Clevers H, 2017. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med 23, 393–410. [DOI] [PubMed] [Google Scholar]
- Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z, 2017. Modeling physiological events in 2D vs. 3D cell culture. Physiology 32, 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Edmondson R, Broglie JJ, Adcock AF, Yang L, 2014. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol 12, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, He H, Kawakita T, Di Pascuale MA, Raju VK, Liu CY, Tseng SCG, 2003. Human Keratocytes Cultured on Amniotic Membrane Stroma Preserve Morphology and Express Keratocan. Invest. Ophthalmol. Vis. Sci 44, 5136–5141. [DOI] [PubMed] [Google Scholar]
- Foster JW, Wahlin K, Adams SM, Birk DE, Zack DJ, Chakravarti S, 2017. Cornea organoids from human induced pluripotent stem cells. Sci. Rep 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuest M, Yam GH, Mehta JS, Duarte-Campos DF, 2020. Prospects and challenges of translational corneal bioprinting. Bioengineering (Basel). 7, E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Capallere C, Arcioni M, Brulas M, Plaza C, Meyrignas C, Bauza E, Botto JM, 2019. Establishment and performance assessment of an in-house 3D Reconstructed Human Cornea-Like Epithelium (RhCE) as a screening tool for the identification of liquid chemicals with potential eye hazard. Toxicol. In Vitro 61, 104604. [DOI] [PubMed] [Google Scholar]
- Ghezzi CE, Marelli B, Omenetto FG, Funderburgh JL, Kaplan DL, 2017. 3D functional corneal stromal tissue equivalent based on corneal stromal stem cells and multi-layered silk film architecture. PLoS One. 12, e0169504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil ES, Mandal BB, Park SH, Marchant JK, Omenetto FG, Kaplan DL, 2010. Helicoidal multi-lamellar features of RGD-functionalised silk biomaterials for corneal tissue engineering. Biomaterial 31, 8953–8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia RM, Connon CJ, 2013. The effects of retinoic acid on human corneal stromal keratocytes cultured in vitro under serum-free conditions. Invest. Ophthalmol. Vis. Sci 54, 7483–7491. [DOI] [PubMed] [Google Scholar]
- Gouveia RM, Lepert G, Gupta S, Mohan RR, Paterson C, Connon CJ, 2019. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J, 2010. Eye development. Curr. Top. Dev. Biol 90, 343–386. [DOI] [PubMed] [Google Scholar]
- Hazra S, Nandi S, Naskar D, Guha R, Chowdhury S, Pradhan N, Kundu SC, Konar A, 2016. Non-mulberry silk fibroin biomaterial for corneal regeneration. Sci. Rep 6, 21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman KW, Shtein RM, Tripathy S, Kim K, 2013. Using an ultrasound elasticity microscope to map three-dimensional strain in a porcine cornea. Ultrasound Med. Biol 39, 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon AEK, Zieske JD, Guo X, 2019. 3D in vitro model for human corneal endothelial cell maturation. Exp. Eye Res 184, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson A, Swioklo S, Connon CJ, 2018. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res 173, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavanagh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J. Cell. Sci 112, 613–22. [DOI] [PubMed] [Google Scholar]
- Jurkunas UV, 2018. Fuchs Endothelial Corneal Dystrophy Through the Prism of Oxidative Stress. Cornea 37, S50–S54. [DOI] [PubMed] [Google Scholar]
- Kaluzhny Y, Kinuthia MW, Lapointe AM, Truong T, Klausner M, Hayden P, 2020. Oxidative stress in corneal injuries of different origin: Utilization of 3D human corneal epithelial tissue model. Exp. Eye Res 190, 107867. [DOI] [PubMed] [Google Scholar]
- Kaluzhny Y, Kinuthia MW, Truong T, Lapointe AM, Hayden P, Klausner M, 2018. New human organotypic corneal tissue model for ophthalmic drug delivery studies. Invest. Ophthalmol. Vis. Sci 59, 2880–2898. [DOI] [PubMed] [Google Scholar]
- Kapałczyńska M, Kolendo T, Przybyla W, 2018. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch. Med. Sci 14, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyce SD, 2020. Endothelial pump and barrier function. Exp. Eye Res, in press [DOI] [PubMed] [Google Scholar]
- Kobashi H, Rong SS, 2017. Corneal Collagen Cross-Linking for Keratoconus: Systematic Review. Biomed. Res. Int 2017, 8145651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Pandit A, Zeugolis DI, 2016. Progress in Corneal Stromal Repair: From Tissue Grafts and Biomaterials to Modular Supramolecular Tissue-Like Assemblies. Adv. Mater 28, 5381–5399. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA, 2014. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Langhans SA, 2018. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL, 2009. Silk film biomaterials for cornea tissue engineering. Biomaterials 30, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY, 2015. Corneal Development: Different Cells from a Common Progenitor. Prog. Mol. Biol. Transl. Sci 134, 43–59. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L, 2010. Anatomy of the human corneal innervation. Exp. Eye. Res 90, 478–492. [DOI] [PubMed] [Google Scholar]
- Maurice DM, 1956. The structure and transparency of the cornea. J. Physiol I36, 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Ford A, Wang S, 2019. Assembly and Application of a Three-Dimensional Human Corneal Tissue Model. Curr. Protoc. Toxicol 81, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Karamichos D, Hutcheon AEK, Guo X, Zieske JD, 2019. Corneal epithelial–stromal fibroblast constructs to study cell–cell communication in vitro. Bioengineering (Basel) 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Knupp C, 2015. Corneal structure and transparency. Prog. Retin. Eye Res 49, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TA, Yin TI, Reyes D, Urban GA, 2013. Microfluidic chip with integrated electrical cell-impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal. Chem 85, 11068–11076. [DOI] [PubMed] [Google Scholar]
- Orwin EJ, Borene ML, Hubel A, 2003. Biomechanical and optical characteristics of a corneal stromal equivalent. J. Biomech. Eng 125, 439–444. [DOI] [PubMed] [Google Scholar]
- Papaioannou TG, Manolesou D, Dimakakos E, Tsoucalas G, Vavuranaski M, Tousoulis D, 2019. 3D bioprinting methods and techniques: Applications on artificial blood vessel fabrication. Acta Cardiol. Sin 35, 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkhofer L, Frappart PO, Muller M, Kleger A, 2018. Importance of organoids for personalized medicine. Per. Med 15, 461–465. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, Rowsey TG, Ma JX, Karamichos D, 2018. Unravelling the stromal-nerve interactions in the human diabetic cornea. Exp. Eye Res 164, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantock AJ, Young RD, 2008. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev. Dyn 237, 2607–2621. [DOI] [PubMed] [Google Scholar]
- Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD, 2015. 3D cell culture systems: Advantages and applications. J. Cell. Physiol 230, 16–26. [DOI] [PubMed] [Google Scholar]
- Rocher M, Robert PY, Desmoulière A, 2020. The myofibroblast, biological activities and roles in eye repair and fibrosis. A focus on healing mechanisms in avascular cornea. Eye (Lond). 34, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia P, Thangavadivel S, Medeiros CS, Lassance L, de Oliveira RC, Wilson SE, 2018. IL-1 and TGF-β modulation of epithelial basement membrane components perlecan and nidogen production by corneal stromal cells. Invest. Ophthalmol. Vis. Sci 59, 5589–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Priyadarshini S, Rowsey TG, Ma JX, Karamichos D, 2018. Corneal tissue engineering: An in vitro model of the stromal-nerve interactions of the human cornea. J. Vis. Exp 131, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siran W, Ghezzi CE, Cairns DM, 2018. Human Corneal Tissue Model for Nociceptive Assessments. Adv. Healthc. Mater 7, e1800488. [DOI] [PubMed] [Google Scholar]
- Sorkio A, Koch L, Koivusalo L, Deiwick A, Miettinem S, Chichkov B, Skottman H, 2018. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 171, 57–71. [DOI] [PubMed] [Google Scholar]
- Susaimanickam PJ, Maddileti S, Pulimamidid VK, Boyinpally SR, Naik RR, Naik MN, Reddy GB, Sangwan VS, Mariappan I, 2017. Generating minicorneal organoids from human induced pluripotent stem cells. Development 144, 2338–2351. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC, 1996. Supramolecular assembly of basement membranes. Bioessays, 18, 123–132. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE, 2013. The corneal epithelial basement membrane: structure, function, and disease. Invest. Ophthalmol. Vis. Sci 54, 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ghezzi CE, Gomes R, Pollard RE, Funderburgh JL, Kaplan DL, 2017. In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 112, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020a. Bowman’s layer in the cornea–structure and function and regeneration. Exp. Eye Res, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020b. Corneal wound healing. Exp. Eye Res, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Hong JW, 2000. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea 19, 417–20. [DOI] [PubMed] [Google Scholar]
- Yam GHF, Riau AK, Funderburgh ML, Mehta JS, Jhanji V, 2020. Keratocyte biology. Exp. Eye Res 196, 108062. [DOI] [PubMed] [Google Scholar]
- Yoeruek E, Bayyoud T, Maurus C, 2012. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 90, 125–131. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xue Q, Li J, Ma L, Yao Y, Ye H, Cui Z, Yang H, 2019. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys 71, 68–78. [DOI] [PubMed] [Google Scholar]
- Zhang C, Du L, Sun P, Shen L, Zhu J, Pamg K, Wu X, 2017. Construction of tissue-engineered full-thickness cornea substitute using limbal epithelial cell-like and corneal endothelial cell-like cells derived from human embryonic stem cells. Biomaterials 124, 180–194. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen J, Backman LJ, Malm AD, Danielson P, 2017. Surface topography and mechanical strain promote keratocyte phenotype and extracellular matrix formation in a biomimetic 3D corneal model. Adv. Healthc. Mater 6, 10. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, 2015. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem. Biophys 72, 777–782. [DOI] [PubMed] [Google Scholar]
- Zhou L, Huang G, Wang S, 2011. Advances in cell-based biosensors using three-dimensional cell-encapsulating hydrogels. Biotechnol. J 6, 1466–1476. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Guimarães SR, Hutcheon AE, 2001. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp. Eye Res 72, 33–39. [DOI] [PubMed] [Google Scholar]


