Abstract
Objective:
To evaluate racial/ethnic disparities in follow-up adherence of incidental pulmonary nodules (IPN) utilizing a cascade of care framework, representing the multi-stage pathway from IPN diagnosis to timely follow-up adherence.
Methods:
We retrospectively identified a cohort of 1,562 patients diagnosed with IPN requiring follow-up in a tertiary healthcare system in 2016. We examined racial/ethnic disparities in follow-up adherence by developing a multi-stepped cascade of care model (provider communication, follow-up exam ordering/scheduling, adherence) to identify where patients were most likely to fall off the path towards adherence. We measured racial/ethnic adherence disparities via descriptive statistics and multivariate modeling, controlling for sociodemographic, communication and health characteristics.
Results:
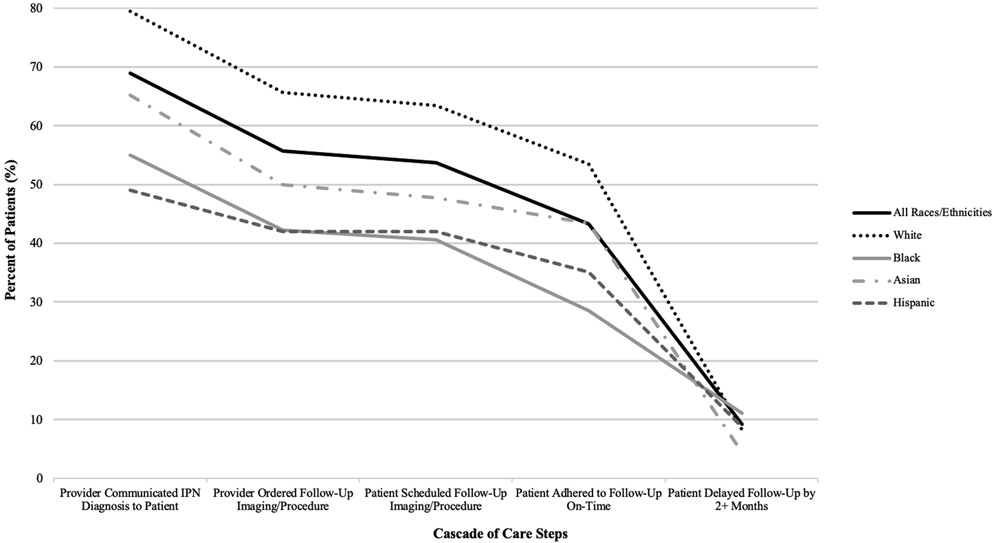
Among 1,562 patients whose IPN required follow-up, unadjusted results show non-White patients are less likely to meet each step on the cascade than White patients: provider-patient IPN communication - 55% Black, 80% White; follow-up ordering/scheduling - 42%, 41% Black; 66%, 64% White; and timely adherence - 29% Black, 54% White. Adjusting for provider communication, sociodemographic and health characteristics, Black patients are at increased odds of never adhering to and delaying follow-up compared to White patients (OR 1.30 [0.90-1.89] and 2.51 [1.54-4.09], respectively).
Discussion:
We demonstrate substantial racial/ethnic disparities in IPN follow-up adherence that persist after adjusting for multiple characteristics. The cascade of care demonstrates where on the adherence pathway patients are at risk of falling off, enabling specific targets for health policy and clinical interventions. Radiologists can play a key role in improving IPN follow-up via increased patient care involvement.
Keywords: health disparities, cascade of care, adherence, incidental pulmonary nodule, computed tomography
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States.1 Early stage lung cancer most often manifests as indeterminate pulmonary nodules (IPNs) on imaging exams, many of which are discovered serendipitously during evaluation for an unrelated medical condition. The annual incidence of IPNs is estimated at 1.6 million and continues to rise due to increased utilization of advanced imaging exams such as CT for a multitude of medical conditions.2
Non-invasive and/or invasive follow-up of IPNs is key for early diagnosis of lung malignancies, and evidence from large lung cancer screening randomized controlled trials (such as the National Lung Screening Trial in the United States and the Nelson Trial in Europe) indicates that finding pulmonary nodules on CTs in otherwise asymptomatic patients leads to improved cancer-related mortality due to early detection, diagnosis and treatment.3, 4, 5, 6 Still, radiology literature suggests follow-up adherence for IPN is as low as 40%. 7 Radiologist provider communication and structured reporting have been found to influence adherence, however other socioeconomic and demographic features have not been thoroughly examined, including racial/ethnic disparities. 7, 8, 9, 10, 11, 12, 13
Substantial literature demonstrates wide gaps in health outcomes across racial/ethnic lines in the United States, with people of color living shorter and less healthy lives as the result of a multitude of structural and interpersonal factors disproportionately and systematically affecting their well-being.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Barriers to healthcare access and utilization affecting medical adherence is one factor related to poorer health outcomes of people of color and low-socioeconomic status (SES). Whereas adherence has often been emphasized to be the result of patient behaviors, research has shown that broader structural, institutional, and provider factors are important contributors to poor adherence.19, 20, 21, 24, 25, 26 Therefore, exploring issues such as provider implicit bias, suboptimal provider-patient communication, and lack of access to regular healthcare, is necessary for understanding racial/ethnic disparities in adherence. 15, 16, 17
Black patients are at higher risk of lung cancer mortality and are often diagnosed at later stages than non-Black patients.14 Therefore, understanding disparities in IPN diagnosis across racial/ethnic lines is critical in order to address disparities in lung cancer morbidity/mortality. As early detection and diagnosis of lung cancer relies on adequate follow-up of IPNs, delaying or not obtaining follow-up can lead to delayed diagnosis of malignancy, contributing to avoidable morbidity and mortality.
Exploring racial/ethnic disparities in adherence and pinpointing where patients may be differentially at risk of dropping off the path towards adherence has meaningful implications for clinicians and can inform policy. A cascade of care framework represents such a conceptual map, illustrating the ordinal sequence of steps patients must undertake in navigating the pathway from diagnosis of a condition to its treatment/resolution. This model has been used in HIV/AIDS, Hepatitis C, tuberculosis, and diabetes adherence research. The cascade is operationalized as a patient’s pathway from testing, diagnosis, linkage to care, retention in care, and adherence. Disruptions in this pathway can have detrimental effects on health outcomes.25, 26, 27, 28, 29
Although diagnosis and management of IPNs entails a similar cascade of care, most studies on IPN adherence have not, to our knowledge, conceptualized adherence as a process, but rather as binary 8, 9, 11, 13 This misses the complexity of adherence, which is actually an ordinal sequence of steps fraught with potential barriers for patients. Patients are initially diagnosed with IPN on an imaging test (chest CT) by a radiologist, whose diagnosis is routed to the ordering provider, who then communicates the diagnosis to their patient and discusses its implications, while emphasizing the need for follow-up imaging/procedures (generally recommended by the radiologist). The recommended follow-up is then ordered, scheduled, and completed, ideally within the recommended timeframe. One barrier is that patients must receive information about their IPNs from their ordering provider. The quality and quantity of information provided to patients of color is often lower than what White patients obtain19, 20 with detrimental effects on the remaining steps of the cascade, limiting patient’s knowledge of their diagnosis and jeopardizing adherence to recommended follow-up.
The goal of our study is to examine the extent to which racial/ethnic disparities in IPN follow-up adherence exist. We dissect these disparities via a cascade of care framework, pinpointing exactly where patients fall off the path to adherence, providing actionable information for health policy interventions aiming to increase adherence.
Methods
Patient Selection, Data Collection, and Organization
This study received local IRB approval with a HIPAA waiver of informed consent. We retrospectively collected longitudinal data from chest CT reports and electronic medical records (EMR) for a cohort of patients diagnosed with IPN in a large tertiary healthcare system in the northeastern United States in 2016, examining their trajectories towards follow-up adherence between the time of their initial CT in 2016 until 2019. Eligible patients were identified via a radiology-centric search engine (Montage, Nuance Corporation), and included anyone who received a chest CT for any medical indication in 2016 in which a previously undiagnosed IPN was detected. This was enabled by searching unique IPN cryptic codes included in CT chest reports by the reading radiologist. These cryptic codes (Supplemental Table 1) were developed by our radiology department and are inserted into the radiology report of any patient determined by a radiologist to have an IPN. Our IPN coding scheme maps with Fleischner Society 2005/2013 guidelines. By design, the scheme excludes patients with a known malignancy, with previously known IPN, or with nodules the interpreting radiologist considers benign, a priori.8, 9 Our radiology records search resulted in 1,846 unique patients, the entire population of CT scans with IPNs in this institution in 2016. We excluded 284 patients who were 1) under 18 at the time of their initial CT scan (n=3), or for whom 2) the radiologist recommended no further follow-up (n=281). Our final analytic sample of 1,562 patients was managed through REDCap.30 Each patient EMR was thoroughly reviewed to obtain sociodemographic, geographic (cross-streets of address), and clinical information from the date of the initial chest CT (2016) until 2019.
We geocoded patients’ addresses’ cross-streets in order to match patients to their census block groups (the smallest geographic unit for which the U.S. Census reports data), gathering information on respective median household income and education levels from 2016 American Community Survey 5-year estimates.31 We created census block SES indices as surrogates for patient SES, due to individual-level SES information often being incompletely and inconsistently reported in the EMR.32
Variables and Statistical Analysis
Descriptive statistics of our patients’ sociodemographic and health characteristics, for the full sample and stratified by patient race/ethnicity, included the following variables: race/ethnicity, sex, age, health insurance status, smoking status, comorbidities, census block SES, CT context, and IPN cryptic code (Table 1). CT context (where the patient obtained his/her initial CT) and IPN cryptic code were collected from radiology reports; other variables were collected from the EMR. Census block SES was dichotomized as low-SES (>50% of census block group had not attended college, median household income <$50,000) and mid/high-SES (>50% of census block group had attended college, median household income >$50,000). Comorbidities reflect ICD-10 diagnoses recorded in the EMR pertaining to chronic diseases (as opposed to acute conditions or diagnostic hypotheses), categorized by etiologic groups (cardiovascular, immunologic, oncologic, respiratory, environmental, psychiatric, musculoskeletal, gastrointestinal, and genitourinary conditions). Cryptic codes indicate IPN size (in millimeters) and composition (S = solid, ground-glass = G, part-solid = GS). In all tables, codes are ranked by immediacy of recommended follow-up, from least concerning for malignancy and least urgent, to most concerning for malignancy and most urgent.
Table 1:
Patient Demographic, SES, and Health Characteristics
| Provider Characteristics | Full Sample |
…By Race/Ethnicity | |||
|---|---|---|---|---|---|
| All Races | White | Black | Asian | Hispanic | |
| N (% of Total) | 1562 (100.0) | 882 (56.5) | 577 (36.9) | 46 (2.9) | 57 (3.7) |
| Demographic, Social, Economic Characteristics | |||||
| Female (%) | 53.4 | 51.3 | 58.8 | 32.6 | 49.1 |
| Mean Age | 62.7 | 64.3 | 60.5 | 65.5 | 57.8 |
| SD | 14.0 | 13.0 | 14.8 | 13.4 | 15.8 |
| Neighborhood SES (%) | |||||
| Low | 40.0 | 19.1 | 73.7 | 21.7 | 36.8 |
| Middle and High | 58.6 | 79.3 | 25.1 | 78.3 | 63.2 |
| Missing | 1.4 | 1.7 | 1.2 | 0.0 | 0.0 |
| Health Insurance (%) | |||||
| Private | 25.9 | 32.2 | 15.8 | 34.8 | 24.6 |
| Medicaid | 15.0 | 4.7 | 30.5 | 10.9 | 22.8 |
| Medicare | 57.4 | 62.5 | 51.1 | 50.0 | 49.1 |
| None | 1.6 | 0.7 | 2.6 | 4.4 | 3.5 |
| Health Characteristics | |||||
| Smoking status (%) | |||||
| Never smoker | 38.4 | 39.0 | 33.8 | 65.2 | 54.4 |
| Former smoker | 45.1 | 50.0 | 40.0 | 30.4 | 31.6 |
| Current smoker | 16.5 | 11.0 | 26.2 | 4.4 | 14.0 |
| Comorbidities (%) | |||||
| Cardiovascular | 6.2 | 8.3 | 3.3 | 6.5 | 1.8 |
| Immune-mediated | 1.1 | 1.1 | 0.9 | 0.0 | 3.5 |
| Oncologic | 12.3 | 15.8 | 6.2 | 15.2 | 17.5 |
| Environmental | 4.7 | 2.8 | 7.5 | 6.5 | 3.5 |
| Respiratory | 13.6 | 15.8 | 10.6 | 6.5 | 15.8 |
| Endocrine | 10.2 | 9.1 | 11.6 | 19.6 | 5.3 |
| Other (MSK, GI, GU, ) | 35.0 | 33.5 | 38.1 | 26.1 | 33.3 |
| Psychological | 8.8 | 5.4 | 14.2 | 0.0 | 12.3 |
| No comorbidities | 8.3 | 8.3 | 7.6 | 19.6 | 7.0 |
| CT Context (%) | |||||
| Outpatient | 60.4 | 72.3 | 43.0 | 63.0 | 49.1 |
| Inpatient | 17.9 | 17.0 | 18.7 | 19.6 | 22.8 |
| Emergency | 21.7 | 10.7 | 38.3 | 17.4 | 28.1 |
| IPN (Lowest to Highest Concern) (%) | |||||
| S4 | 34.7 | 32.0 | 37.6 | 23.9 | 38.6 |
| S6 | 26.8 | 26.2 | 27.4 | 28.3 | 29.8 |
| G2 | 11.3 | 13.0 | 8.2 | 21.7 | 8.8 |
| S8 | 11.8 | 12.9 | 9.7 | 17.4 | 10.5 |
| GS1 | 2.2 | 2.8 | 1.4 | 4.4 | 0.0 |
| GS2 | 1.9 | 1.8 | 1.7 | 2.2 | 3.5 |
| S10 | 4.6 | 3.7 | 6.2 | 0.0 | 5.3 |
| S12 | 6.7 | 6.8 | 7.1 | 2.2 | 3.5 |
Unadjusted results depicting the cascade of care towards IPN follow-up adherence, stratified by patient race/ethnicity, are shown in Table 2. We conceptualized the cascade of care in six steps (Figure 1). Step 1: radiologist detection and provision of follow-up recommendation; 2: ordering provider receives radiologist report; 3: provider communication regarding the IPN with the patient; 4: provider orders follow-up imaging and/or procedures; 5: patient schedules follow-up; and 6: patient obtains follow-up. A 3-level categorical outcome (e.g. never adherent to follow-up, delayed follow up (> 2 months), and timely adherence (within +/− 2 months of recommended time frame) was chosen based on clinical grounds and in order to capture the nuances between delayed care which may or may not be clinically relevant (depending on the actual finding and the length of the delay), and forgone care, which is more likely to be clinically relevant.
Table 2:
Descriptive Statistics of Cascade of Care for IPN Follow Up
| Outcomes | Full Sample | …by Race/Ethnicity | |||
|---|---|---|---|---|---|
| All Races | White | Black | Asian | Hispanic | |
| N | 1562 | 882 | 577 | 46 | 57 |
| Adherence Pathway (%) | |||||
| (1) Provider-Patient Disclosure | |||||
| Provider Communicated Diagnosis to Patient | 69.0 | 79.5 | 55.1(<0.001) | 65.2(<0.001) | 49.1(<0.001) |
| (2) Ordering/Scheduling | |||||
| Provider Ordered Follow-up Imaging/Procedure | 55.7 | 65.7 | 42.3(<0.001) | 50.0(<0.001) | 42.1(<0.001) |
| Patient Scheduled Follow-up Imaging/Procedure | 53.8 | 63.5 | 40.6(<0.001) | 47.8(<0.001) | 42.1(<0.001) |
| (3) Adherence | |||||
| Follow-up Obtained within Recommended Timeframe | 43.3 | 53.5 | 28.6(<0.001) | 43.4(<0.001) | 35.1(<0.001) |
| Follow-up Obtained, but Delayed by 2+ Months | 9.2 | 8.3 | 11.1(<0.001) | 4.4(<0.001) | 8.8(0.489) |
| Follow-up Never Obtained | 47.4 | 38.2 | 60.3(<0.001) | 52.2(<0.001) | 56.1(<0.001) |
Note. P-values shown in parentheses, reflecting two-tailed t-tests between Black/Asian/Hispanic and White patients.
Figure 1:
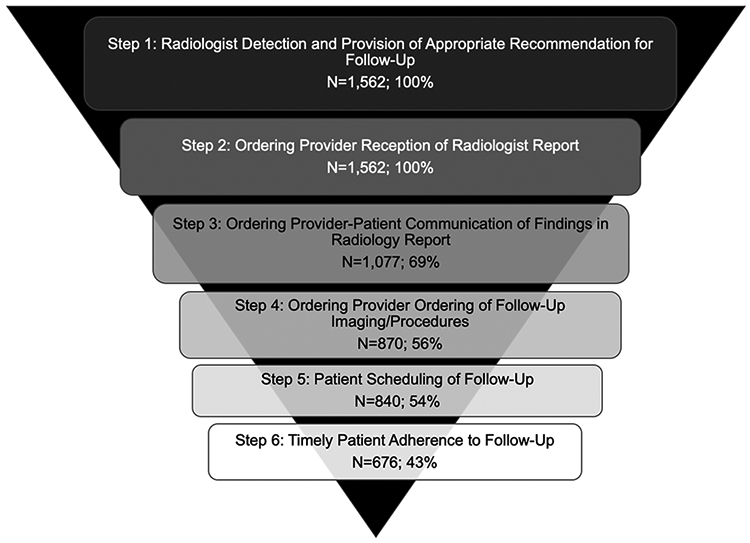
Cascade of Care Model Applied to the Pathway from Detection to Timely Completion of IPN Follow-Up
Graphic depiction of the cascade of care framework for IPN follow up, breaking the process from IPN detection to timely adherence to follow up in 6 consecutive steps, with absolute number and percentage of patients retained at each step
Adherence was assessed through EMR review, which also allowed assessment of when providers ordered follow-up and if/when patients succeed in scheduling follow-up, (intermediate steps of the cascade). We determined if follow-up recommendations were adhered to outside of the healthcare system evaluated using a mechanism called Care Everywhere, which allows for medical record sharing across participating hospitals and practices in the United States.33
Finally, we performed four multinomial logistic regressions using adherence as our outcome variable, race/ethnicity as our main predictor variable, and controls including provider-patient communication of IPN, sociodemographic (age, sex, census block SES, health insurance), and health characteristics (smoking status, comorbidities, specific IPN cryptic code, CT context). We chose to model adherence as the outcome for our multinomial models in lieu of other intermediate steps in the cascade of care, which are necessary (but not sufficient) steps towards the outcome we hypothesize to be clinically meaningful: adherence.
Controls were added in a stepwise fashion for each of the four models, with race/ethnicity as the main predictor in each model. Model 1 controls for patient sociodemographic characteristics. Model 2 adds provider-patient IPN communication controls to Model 1. Model 3 adds health characteristics controls to Model 1. Model 4 includes all predictor and control variables. Model results are reported as odds ratios (OR) with 95% confidence intervals. Controls are marked with an “X” when included in a model. Models with all estimates are presented as Supplemental Table 2. All statistical analyses were performed on Stata (v15, Stata Corporation).
Results
Table 1 presents descriptive statistics for our cohort, presented with all patients combined and stratified by race/ethnicity. Most patients were either White or Black. Black patients were most likely among all patient racial/ethnic groups to have Medicaid insurance, to live in low-SES census blocks, and to have had their initial CT scan in the ER.
Table 2 and Figures 1 and 2 display the unadjusted adherence cascade of patients diagnosed with IPN, for all patients and stratified by race/ethnicity. Black and Hispanic patients are least likely to receive communication of an IPN from their provider. They also have the lowest rates of scheduling, ordering, and adherence to follow-up exams/procedures.
Figure 2:
Racial/Ethnic Disparities in the Cascade of Care towards IPN Follow-Up Adherence
All patients start at 100% at receiving IPN diagnosis on CT report (not shown). Graph shows patient drop-off between provider communication of IPN diagnosis (Step 1 in Table 2) to ultimate patient (non)-adherence (Step 3).
Table 3 presents multinomial logistic modeling of follow-up adherence. Model 1 shows the odds of Black patients never obtaining and delaying follow-up, compared to adhering on-time, were 2.1 times those of White patients, controlling for sociodemographic characteristics. Similarly, the odds of Hispanic patients never adhering to follow-up recommendations were 1.9 times those of White patients, and the odds of delaying follow-up were 1.5 times those of White patients, although this latter finding was not statistically significant.
Table 3:
Multinomial Logistic Regression Models of Patient Adherence for Recommended IPN Follow-Up
| Demographics, SES (M1) |
M1 + Notification | M1 + Health | M1 + All Characteristics |
|||||
|---|---|---|---|---|---|---|---|---|
| VARIABLES | Never Obtained |
Delayed 2+ Months |
N | D | N | D | N | D |
| Race (ref = White) | ||||||||
| Black | 2.16[1.65 - 2.84] | 2.13[1.36 - 3.33] | 1.54[1.11 - 2.13] | 2.08[1.33 - 3.25] | 1.52[1.09 - 2.13] | 2.52[1.55 - 4.10] | 1.30[0.90 - 1.89] | 2.51[1.54 - 4.09] |
| Asian | 1.60[0.86 - 2.98] | 0.62[0.14 - 2.72] | 1.26[0.58 - 2.71] | 0.62[0.14 - 2.73] | 1.34[0.64 - 2.79] | 0.57[0.13 - 2.56] | 1.06[0.46 - 2.47] | 0.56[0.12 - 2.53] |
| Hispanic | 1.92[1.06 - 3.46] | 1.47[0.53 - 4.06] | 0.87[0.40 - 1.89] | 1.30[0.46 - 3.64] | 1.57[0.76 - 3.22] | 1.63[0.57 - 4.63] | 1.01[0.43 - 2.39] | 1.46[0.51 -4.18] |
| Age | 0.99[0.98 - 1.00] | 0.99[0.98 - 1.00] | 1.00[0.99 - 1.01] | 0.99[0.98 - 1.00] | 1.01[1.00 - 1.02] | 1.00[0.98 - 1.02] | 1.02[1.01 - 1.03] | 1.00[0.98 - 1.02] |
| Sex (ref = Male) | ||||||||
| Female | 1.42[1.14 - 1.177] | 1.26[0.88 - 1.82] | 1.24[0.95 - 1.61] | 1.25[0.87 - 1.81] | 1.25[0.96 - 1.63] | 1.31[0.89 - 1.93] | 1.19[0.89 - 1.59] | 1.30[0.88 - 1.91] |
| Census Block SES (ref = Low) | ||||||||
| Mid/High | 0.56[0.43 - 0.73] | 0.75[0.48 - 1.15] | 0.68[0.50 - 0.93] | 0.78[0.50 - 1.20] | 0.73[0.53 - 1.00] | 0.74[0.47 - 1.17] | 0.79[0.56 - 1.11] | 0.76[0.48 - 1.20] |
| Missing | 0.92[0.37 - 2.27] | 0.44[0.05 - 3.61] | 0.89[0.29 - 2.67] | 0.43[0.05 - 1.20] | 0.97[0.36 - 2.62] | 0.38[0.05 - 3.19] | 0.86[0.28 - 2.65] | 0.39[0.05 - 3.29] |
| Provider Communicated Diagnosis to Patient (ref = Did Not Communicate) | X | X | X | X | ||||
| X | X | X | X | |||||
| Health Insurance (ref = Private) | X | X | X | X | ||||
| Smoking Status (ref = Never) | X | X | X | X | ||||
| Comorbidities (ref = None) | X | X | X | X | ||||
| TPN (ref = S4) | X | X | X | X | ||||
| CT Context (ref = Outpatient) | X | X | X | X | ||||
| Constant | 2.15 [1.21 - 3.81] | 0.32 [0.13 - 0.84] | 22.28 [9.95 - 49.91] | 0.97[0.17 - 0.64] | 0.60[0.25 - 1.42] | 0.33[0.09 - 1.22] | 5.75[2.02 - 16.37] | 0.95[0.22 - 4.10] |
| Observations | 1,562 | 1,562 | 1,562 | 1,562 | 1,562 | 1,562 | 1,562 | 1,562 |
Note. Results presented in odds ratios. 95% confidence intervals presented in brackets.
Controlling for provider-patient communication of IPN diagnosis in Model 2 reduced the odds ratio of never adhering from 2.2 to 1.5, such that racial/ethnic disparities between Black-White disparities in never adhering were diminished after adjusting for provider-patient communication. Gaps in delayed follow-up were not much reduced after controlling for provider-patient communication. In Model 3, we controlled for health characteristics to determine if differences in comorbidities, smoking status, IPN features, and context in which the initial CT was obtained could explain disparities in adherence. Nevertheless, this model shows disparities in never adhering still existed between Black and White patients after including these controls, and delayed adherence disparities actually increased, such that the odds of Black patients delaying follow-up were 2.5 those of White patients. In Model 4, controlling for all demographic, socioeconomic, and health characteristics, Black-White disparities in delayed adherence remained statistically significant, such that the odds of Black patients delaying follow-up were 2.5 times those of White patients. Black-White gaps in never adherence remained, and the odds a Black patient never adhering were 1.3 those of White patients. However, this last finding was not statistically significant.
Discussion
Our main finding is that the pathway towards IPN follow-up is racially stratified, whereby non-White patients are less likely than White patients to complete each step on the cascade of care towards timely follow-up adherence. In particular, racial/ethnic disparities in provider-patient communication are starkly apparent, with Black and Hispanic patients least likely to have their IPN diagnosis communicated to them by a provider compared to White patients. This leads to diminished provider ordering/scheduling of follow-up and increased patient non-adherence, which has potentially negative implications for ultimate patient outcomes. Although we cannot ascertain here why provider-patient communication of IPN is lower for non-White compared to White patients, one reason may be implicit bias, whereby providers are less likely to communicate incidental findings to those whom they believe will not act on follow-up recommendations. For Asian and Hispanic patients, language may be a factor, and providers may be less likely to communicate all the nuances of a radiology report to patients with whom they cannot adequately communicate, or whom they perceive to have a lower level of English ability.
Our adjusted results controlling for all sociodemographic and health characteristics (including IPN size/attenuation) demonstrate that Black patients are more likely than White patients to never adhere to and to delay follow-up, although the former finding was not statistically significant in final models. Model 2 is particularly salient for informing intervention, as controlling for provider-patient communication of IPN reduces the odds of never adhering significantly, specifically for Black patients. These findings indicate that the provider communication can meaningfully impact never adherence, as one might expect. Nevertheless, Model 4, including all controls, still does not resolve racial/ethnic disparities in adherence, specifically for Black patients, although this finding was not statistically significant. Other unmeasured characteristics are likely responsible for remaining disparities. Regarding delayed follow-up, few characteristics diminish the Black-White gap, and in fact, controlling for health characteristics actually widens the gap. Ultimately, few findings in multinomial models for Hispanic and Asian were statistically significant, likely reflecting the small sub-sample sizes of these groups.
These disparities as evidenced by the cascade show the potential value of such a model for examining IPN follow-up adherence, as opposed to a binary approach, which does not allow one to adequately pinpoint where exactly patients fall off the path to adherence. We show adherence depends on a sequential process with multiple discrete steps. Our results demonstrate patients of color are at higher risk of not being notified of potentially important information, which impacts subsequent steps to adherence. Even still, provider-patient communication cannot account for all racial/ethnic gaps in adherence, as shown in our final multivariate logistic regression model. Therefore, other factors not accounted for in this study, such as the quality of provider/patient communication, patient level of health literacy, or perceived importance of follow-up on the part of provider/patient, may further explain racial/ethnic gaps in adherence. Furthermore, no variable fully explains delayed adherence, and controlling for health characteristics seems to magnify racial/ethnic gaps for this outcome.
Our study has implications for informing interventions from a medical and health policy standpoint. Specifically, in showing poor adherence is partly attributable to suboptimal provider-patient communication with patients of color, with detrimental effects on adherence. Furthermore, gaps between ordering/scheduling of follow-up and adherence indicates that there is opportunity for provider/practice intervention to remind patients to complete their follow-up. Radiologists may also play a role in this by involving themselves to a greater extent in patient care and communication, through developing systems to remind ordering providers to discuss incidental findings, or through influencing health system policy by taking on a more central role in parallel with the ordering provider in communicating important findings and follow-up options directly to patients.
This study had several limitations. First, we evaluated a single health system, not necessarily representative at the national level. Second, its retrospective nature coupled with data extraction from the EMR means the data may have limited external validity to other health systems. Third, SES data (income, educational attainment) approximate each patient’s true SES since we had to rely on census blocks (averaging a geographic area of 600-3,000 people).34 Nonetheless, this is likely the best quality approximation one can attain with publicly available U.S. census data, noting SES data are typically not available in any EMR. Fourth, we developed specific cut-points of adherence (never, delayed by 2+ months, on time) in our multinomial models, rather than measuring it as a continuous variable (days to follow-up). Finally, patients who obtained follow up imaging at another institution that does not participate in Care Everywhere would not have been captured in this analysis as adherent, however we do not believe this accounts for a substantial proportion of our cohort.
Future research must examine other factors impacting non-adherence and delayed follow-up, such as the quantity/quality of communication between patients and providers and patient health literacy. Our work emphasizes how race/ethnicity plays a substantial role in independently explaining patient adherence to follow up of IPN, and this has important clinical, public health, and policy implications. Given communication and adherence disparities are patterned by race/ethnicity, health policy should focus on reframing the training of medical students and practicing physicians to foster better understanding of structural reasons for health inequalities, which can help them deliver more effective and higher quality care. Radiology practices can also play a substantial role in assuring IPNs are relayed to patients, for example through developing systems by which radiologists actively participate in communicating IPNs to patients, while ensuring ordering providers are aware of it, leveraging in person and electronic means of communication. Health care policies can also help incentivize desirable patient behavior, particularly focusing resources on those most at risk of non-adherence or delayed adherence, in order to maintain these patients on the pathway to adherence and ultimately obtain better health outcomes for the most vulnerable populations. A cascade of care framework for patients diagnosed with IPN can bolster our understanding of non-adherence to follow up recommendations, specifically across patient racial/ethnic groups.
Supplementary Material
Summary Sentence.
We demonstrate racial/ethnic disparities on each step of the cascade of care towards adherence for incidental pulmonary nodules (IPN), particularly between provider communication of IPN and follow-up ordering/scheduling, and between ordering/scheduling and timely follow-up completion.
Take-Home Points.
We conceptualize a multi-step pathway in order to understand disparities in adherence to follow-up for incidental pulmonary nodules (IPN) across patient racial/ethnic groups
We find patients are most at risk of being lost to follow-up between imaging diagnosis and ordering provider/patient communication of findings, and between ordering/scheduling of follow-up examinations and timely completion of follow up.
Black patients are most at risk of dropping off the path to adherence compared to White patients, controlling for provider/patient communication, demographic, and health characteristics.
Acknowledgments
Funding: The first author received support from the Population Research Training Grant (NIH T32 HD007242) awarded to the Population Studies Center at the University of Pennsylvania by the National Institutes of Health’s (NIH)’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
RS: None
EB: Siemens research grants, not related to this study
Statement of Data Integrity: The author(s) declare(s) that they had full access to all of the data in this study and the author(s) take(s) complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Thakur MK, Ruterbusch JJ, Schwartz AG, Gadgeel SM, Beebe-Dimmer JL, & Wozniak AJ (2018). Risk of second lung cancer in patients with previously treated lung cancer: analysis of surveillance, epidemiology, and end results (SEER) data. Journal of Thoracic Oncology, 13(1), 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner N, Porter A, Gould M, Li X, Vachani A, & Silvestri G (2017). Physician assessment of pretest probability of malignancy and adherence with guidelines for pulmonary nodule evaluation. Chest, 152(2), 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team. (2013). Results of initial low-dose computed tomographic screening for lung cancer. New England Journal of Medicine, 368(21), 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alshora S, McKee BJ, Regis SM, Kitts AKB, Bolus CC, McKee AB, & Wald C (2018). Adherence to radiology recommendations in a clinical CT Lung Screening program. Journal of the American College of Radiology, 15(2), 282–286. [DOI] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team. (2019). Lung cancer incidence and mortality with extended follow-up in the national lung screening trial. Journal of Thoracic Oncology, 14(10), 1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson Q (2020). NELSON trial: reduced lung-cancer mortality with volume CT screening. The Lancet Respiratory Medicine, 8(3), 236. [DOI] [PubMed] [Google Scholar]
- 7.McDonald J, Koo C, White D, Hartman T, Bender C, & Sykes AG (2017). Addition of the Fleischner Society guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Academic radiology, 24(3), 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa EJM Jr, & Kelly K (2020). Statistical modeling can determine what factors are predictive of appropriate follow-up in patients presenting with incidental pulmonary nodules on CT. European Journal of Radiology, 109062. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa E, & Osuntokun O (2019). Incidental findings in thoracic CTs performed in trauma patients: an underestimated problem. European radiology, 29(12), 6772–6779. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon H, Naidich D, Goo J, Lee K, Leung A, Mayo J, & Rubin G (2017). Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology, 284(1), 228–243. [DOI] [PubMed] [Google Scholar]
- 11.Moseson EM, Wiener RS, Golden SE, Au DH, Gorman JD, Laing AD, & Slatore CG (2016). Patient and clinician characteristics associated with adherence. A cohort study of veterans with incidental pulmonary nodules. Annals of the American Thoracic Society, 13(5), 651–659. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa EJM Jr, Lynch MC, Langlotz CP, & Gefter WB (2016). Optimization of radiology reports for intensive care unit portable chest radiographs: perceptions and preferences of radiologists and ICU practitioners. Journal of Thoracic Imaging, 31(1), 43–48. [DOI] [PubMed] [Google Scholar]
- 13.Ridge CA, Hobbs BD, Bukoye BA, Aronson MD, Boiselle PM, Leffler DA, … & Roberts DH (2014). Incidentally detected lung nodules: clinical predictors of adherence to Fleischner Society surveillance guidelines. Journal of computer assisted tomography, 35(1), 89–95. [DOI] [PubMed] [Google Scholar]
- 14.Japuntich SJ, Krieger NH, Salvas AL, & Carey MP (2018). Racial disparities in lung cancer screening: an exploratory investigation. Journal of the National Medical Association, 110(5), 424–427. [DOI] [PubMed] [Google Scholar]
- 15.Castañeda H, Holmes SM, Madrigal DS, Young MED, Beyeler N, & Quesada J (2015). Immigration as a social determinant of health. Annual review of public health, 36, 375–392. [DOI] [PubMed] [Google Scholar]
- 16.Reskin B (2012). The race discrimination system. Annual Review of Sociology, 35, 17–35. [Google Scholar]
- 17.Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, & Coyne-Beasley T (2015). Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. American journal of public health, 105(12), e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelan J & Link B (2015). Is racism a fundamental cause of inequalities in health? Annual Review of Sociology, 41, 311–330. [Google Scholar]
- 19.Williams DR, & Wyatt R (2015). Racial bias in health care and health: challenges and opportunities. Jama, 314(6), 555–556. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RL, Roter D, Powe NR, & Cooper LA (2004). Patient race/ethnicity and quality of patient–physician communication during medical visits. American journal of public health, 94(12), 2084–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feagin J, & Bennefield Z (2014). Systemic racism and US health care. Social science & medicine, 103, 7–14 [DOI] [PubMed] [Google Scholar]
- 22.Phelan JC, Link BG, & Tehranifar P (2010). Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. Journal of health and social behavior, 51(1_suppl), S28–S40. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney C, Zinner D, Rust G, & Fryer G (2016). Race/ethnicity and health care communication. Medical care, 54(11), 1005–1009. [DOI] [PubMed] [Google Scholar]
- 24.Cuffee YL, Hargraves JL, Rosal M, Briesacher BA, Schoenthaler A, Person S, & Allison J (2013). Reported racial discrimination, trust in physicians, and medication adherence among inner-city African Americans with hypertension. American journal ofpublic health, 103(11), e55–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsdurf H, Hill P, Matteelli A, Getahun H, & Menzies D (2016). The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. The Lancet Infectious Diseases, 16(11), 1269–1278. [DOI] [PubMed] [Google Scholar]
- 26.Kay ES, Batey DS, & Mugavero MJ (2016). The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS research and therapy, 13(1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer M, Ross D, Chartier M, Belperio P, & Backus L (2016). Cascade of care for hepatitis C virus infection within the US Veterans Health Administration. American journal ofpublic health, 106(2), 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanoni B, & Mayer K (2014). The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS patient care and STDs, 28(3), 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali M, Bullard K, Gregg E, & del Rio C (2014). A cascade of care for diabetes in the United States: visualizing the gaps. Annals of internal medicine, 161(10), 681–689. [DOI] [PubMed] [Google Scholar]
- 30.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde J (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson Steven, Schroeder Jonathan, Van Riper David, and Ruggles Steven. IPUMS National Historical Geographic Information System: Version 14.0 [Database]. Minneapolis, MN: IPUMS; 2019. 10.18128/D050.V14.0 [DOI] [Google Scholar]
- 32.Krieger N (1992). Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health, 82(5), 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Healthcare Information and Management Systems Society. (2016). Care Everywhere. Retrieved from https://www.himss.org/resource-environmental-scan/care-everywhere.
- 34.U.S. Census Bureau. (2019). Glossary. Retrieved from http://www.census.gov/programssurveys/geography/about/glossary.html#par_textimage_4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.