Abstract
Although protein replacement therapy provides effective treatment for hemophilia A patients, about a third of severe patients develop neutralizing inhibitor antibodies to factor VIII. Adoptive transfer of regulatory T cells (Tregs) has shown promise in treating unwanted immune responses. In previous studies, transferred polyclonal Tregs ameliorated the anti-factor VIII immune responses in hemophilia A mice. In addition, factor VIII-primed Tregs demonstrated increased suppressive function. However, antigen-specific Tregs are a small fraction of the total lymphocyte population. To generate large numbers of factor VIII-specific Tregs, the more abundant murine primary CD4+ T cells were lentivirally transduced ex vivo to express Foxp3 and a chimeric antigen receptor specific to factor VIII (F8CAR). Transduced cells significantly inhibited the proliferation of factor VIII-specific effector T cells in suppression assays. To monitor the suppressive function of the transduced chimeric antigen receptor expressing T cells in vivo, engineered CD4+CD25+Foxp3+F8CAR-Tregs were sorted and adoptively transferred into hemophilia A mice that are treated with hydrodynamically injected factor VIII plasmid. Mice receiving engineered F8CAR-Tregs showed maintenance of factor VIII clotting activity and did not develop anti-factor VIII inhibitors, while control CD4+T cell or PBS recipient mice developed inhibitors and had a sharp decrease in factor VIII activity. These results show that CD4+ cells lentivirally transduced to express Foxp3 and F8CAR can promote factor VIII tolerance in a murine model. With further development and testing, this approach could potentially be applied to human hemophilia patients.
Introduction
Hemophilia A (HemA) is a sex-linked genetic disorder which causes a deficiency of functional factor VIII (FVIII), resulting in diminished blood clotting ability. Typically, HemA patients receive FVIII protein intravenously to manage their condition1. However, around 30% of severe patients develop an alloimmune response to FVIII protein, and produce anti-FVIII antibodies that inhibit the function of FVIII, typically referred to as inhibitors2. Immune tolerance induction (ITI) is a treatment regimen that attempts to promote tolerance of FVIII, but is costly and does not succeed in all patients3, 4. Thus, alternative methods to overcome the complication of FVIII inhibitors are needed. Regulatory T cells (Tregs) play an important role in mediating the immune response5, 6. In our previous studies, IL-2 complexed with an anti-IL-2 monoclonal antibody was injected into HemA mice to expand Tregs in vivo7, 8. When administered in combination with FVIII gene therapy, experimental mice did not show the development of inhibitors, while control mice quickly developed inhibitors and lost FVIII function. It has also been demonstrated that expanding Tregs ex vivo while priming them with FVIII before adoptive transfer to HemA mice can also promote FVIII tolerance more effectively than the adoptive transfer of naïve Tregs9.
Although FVIII-specific expansion of Tregs showed improved tolerance of FVIII, the number of the FVIII-specific Tregs is a very small percentage of the total Treg population. In recent years, CD19-CAR engineered effector T cells has been demonstrated to be highly effective in the treatment of leukemia10–12. Similar strategies were subsequently adopted to generate antigen-specific Tregs to prevent graft-versus-host disease13, 14, multiple sclerosis15, type-1 diabetes16, and other immune-related disorders17. These redirected antigen-specific Tregs all exerted superior regulation of the immune responses towards disease-associated targets compared to other approaches. In this study, we explored if functional FVIII-specific Tregs can be efficiently generated by the chimeric antigen receptor (CAR) approach to modulate anti-FVIII immune responses.
Recently, human CD4+CD25+CD127low Tregs were isolated and transduced by retroviral vectors carrying a FVIII-specific CAR sequence18. These engineered Tregs were demonstrated to suppress FVIII-specific immune responses more effectively compared with non-specific Tregs. However, long-term tolerance induction to FVIII in HemA mouse models cannot be thoroughly tested due to rapid rejection of human Tregs in immunocompetent HemA mice. In addition, due to the plasticity and transient nature of the adoptively transferred Tregs, we sought to generate more stable CAR-Tregs by transducing murine CD4+ T cells with a lentivirus (LV) carrying a CAR with high affinity to FVIII (F8CAR) linked to a murine Foxp3 sequence. In this way, we also can overcome the significant obstacle by transducing the more abundant CD4+ T cells instead of scarce CD4+CD25+ Tregs for translating this technology to clinical application. We found that these engineered FVIII-specific T cells exhibit characteristics of Tregs. These cells, referred to as F8CAR-Tregs, demonstrated highly suppressive activity towards proliferation of F8CAR transduced responder T cells (F8CAR-Tresps) and inhibitor mouse CD4+ T cells. Furthermore, adoptive transfer of F8CAR-Tregs to HemA mice prevented inhibitor formation and maintained FVIII function when combined with FVIII gene therapy.
Methods
Factor VIII-specific CAR (F8CAR) and F8CAR with murine Foxp3 (F8CAR-mFoxp3) plasmid generation
The F8CAR construct is composed of a FVIII-specific scFv sequence, followed by a murine IgG hinge, the transmembrane and intracellular domain of murine CD28, the cytoplasmic domain of murine 4-1BB, and murine CD3ζ. The F8CAR sequence was fused with the murine Foxp3 gene using a F2A peptide linker to produce the F8CAR-mFoxp3 construct (Figure 1A). The FVIII specific scFv sequence was derived from the peripheral blood mononuclear cells of inhibitor patients and selected using phase display libraries with immunoprecipitation to target the C1 domain of FVIII protein 19. Sequence KM33 was selected for its high affinity to and low disassociation from the C1 domain, as well as its ability to bind to the A3 domain. A human scFv which could potentially be applied to human patients was selected for this project since we delivered a human FVIII gene into mice. Sequences for murine CD28, 4-1BB, CD3ζ, and Foxp3 were obtained on UniProt. F8CAR and F8CAR-mFoxp3 sequences were synthesized by Life Technologies (Carlsbad, CA). F8CAR and F8CAR-mFoxp3 were each cloned into lentiviral vectors directed by an MND promoter or retroviral vectors (pMXs-Neo; Cell Biolabs, San Diego, CA). Larger quantities of F8CAR and F8CAR-mFoxp3 lentiviral plasmids were produced by GenScript (Piscataway, NJ).
Figure 1. Lentiviral transduction in 293T cells with F8CAR and F8CAR-mFoxp3 transgenes.
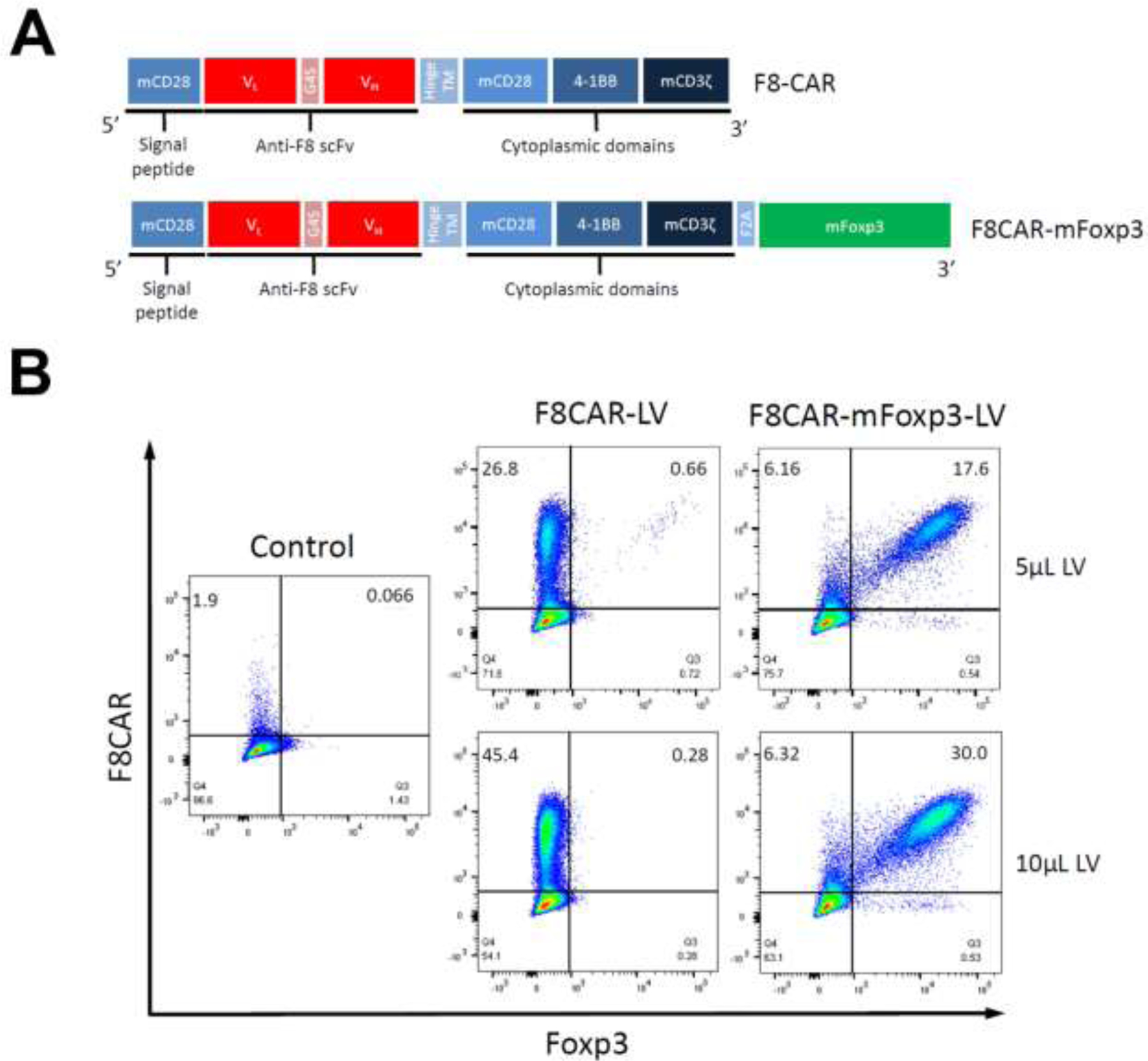
(A) Scheme of the FVIIICAR constructs. The FVIII-CAR constructs were made by in-fusion cloning incorporating an FVIII-specific scFv followed by intracellular portions of signaling moieties of immune receptors into a lentiviral vector. The signaling moieties were composed of CD28, 4-1BB, CD3 signaling domains. For the F8CAR-mFoxp3 construct, murine Foxp3 cDNA was fused at the end of signaling moieties via F2A peptide. (B) Binding of FVIII to FVIII-CAR on lentivirus transduced cells. Lentivirus carrying F8CAR and F8CAR-mFoxp3 transgene were produced to test the transduction efficiency in HEK 293T cells. To detect FVIII-specific scFv, the transduced cells were incubated with human FVIII protein followed by anti-FVIII antibody. Control showed that the un-transduced cells did not bind to FVIII protein and anti-FVIII antibody.
F8CAR and F8CAR-mFoxp3 lentivirus production and titration
VSV-G (Pantropic retorviral packaging system, Cell Biolabs) and ecotropic envelop-pseudotyped (Ecotropic tetroviral packaging system, Cell Biolabs) retrovirus were produced according to the manufacturer’s protocol. The lentivirus were produced by transient transfection of three plasmids using polyethylenimine (PEI) in HEK293T cells20. 1.2x107 cells were seeded in 15-cm plates. Cells were incubated at 37°C for 5 hours with 25mM chloroquine diphosphate21 and then transfected with F8CAR or F8CAR-mFoxp3 plasmid, pPAX2 packaging plasmid (AddGene, Watertown, MA) and VSV-G (pMT2, AddGene) or Cocal envelope plasmids22. Transfection media was incubated for 16 to 18 hours, and then the medium was changed to DMEM without phenol red with 2% FBS and 1% L-glutamine for virus production. After 48 hours, the medium was collected and sterile filtered. Virus was concentrated 100-fold by centrifuging overnight at 9000g and re-suspending in HBSS buffer. Fresh HEK 293T cells were used to titrate concentrated virus. HEK293T cells were plated at 2x105 cells per mL in a 12 well plate with polybrene at 8 µg/mL in DMEM with 10% FBS, 1% HEPES, and 1% L-glutamine. After 5 days, F8CAR and Foxp3 expression were stained and characterized by flow cytometry, as described below. Viral titers were determined by qPCR23.
Animals
All mice were kept in a specific pathogen-free environment at Seattle Children’s Research Institute (SCRI) according to National Institutes of Health guidelines for animal care and the guidelines of SCRI. Protocols were approved by the Institutional Animal Care and Use Committee at SCRI. HemA mice (F8 exon 16 knockout) with C57BL/6 (BL/6) genetic background were generated by crossing the mixed background HemA mice (SV129/BL6) with CD45.1 BL/6 mice and CD45.2 BL/6 mice24 for eight generations, respectively. Both strains of BL/6 mice were purchased from the Jackson Laboratory. Only male HemA mice were used in this study.
Murine CD4+ T cells isolation, transduction, and sorting
Splenic CD4+ T cells were isolated from HemA C57BL/6-CD45.1 mice by magnetic separation using a murine CD4+ T cell isolation kit (Miltenyi Biotec Bergisch Gladbach, Germany). Isolated CD4+ T cells were plated at 1x106 cells per mL in RPMI with 10% FBS, 1% L-glutamine, 1% sodium pyruvate, 1% HEPES, and 100U/mL mIL-2 and stimulated with anti-CD3/CD28 Dynabeads (Thermo Fisher Waltham, MA) at a 1:1 ratio for 48 hours. After stimulation, Dynabeads were removed, and the stimulated T cells were rested in 24-well plates with 1x106 cells per mL per well for 16 hours. 12-well plates were coated with 10µg/cm2 of RetroNectin (Takara Bio Kusatsu, Japan) overnight at 4°C. Plates were then blocked for 30 minutes at room temperature with 1% BSA in PBS. Blocking buffer was rinsed with PBS, and 400µL of 100x virus (~2x108 IFU to achieve ~200 MOI) was added to each well. RetroNectin coated plates with virus were then centrifuged at 1000g for 2 hours at 30°C. 2x106 CD4+ cells were then added to each well with complete RPMI and 8µg/mL polybrene, and centrifuged at 500g for 30 minutes at 30°C without removing the viral supernatant. Plates were then incubated at 37°C for 24 hours. Medium was replaced and another 400µL of 100x virus was added to each well. Plates were centrifuged at 500g for 30 minutes at 30°C and then moved to the incubator. Medium for cells transduced with lentivirus were supplemented with 2000 U/mL recombinant murine IL-2 throughout all transduction steps in order to maintain Treg phenotype and survival. Two days after the second transduction, cell debris was removed via Ficoll centrifugation. 4mL of Ficoll-Paque (GE Healthcare Life Sciences, Marlborough, Massachusetts) was layered under transduced cells in fresh RPMI and then centrifuged for 30 minutes at 400g at 30°C. The layer of cells between RPMI and Ficoll layers was collected and stained for viability and F8CAR expression. Stained cells were then sorted on a FACSAria (BD Biosciences, Franklin Lakes, New Jersey).
Flow cytometry
Flow cytometry was performed to evaluate transduction of T cells by our retroviral and lentiviral vectors. F8CAR expression was characterized by first incubating cells with human FVIII protein. Cells were then stained with FITC conjugated anti-human FVIII antibody (Affinity Biologicals, ON, Canada). Transduced cells were stained with Alexa Fluor 700 anti-mouse CD4 (Thermo Fisher), PE anti-mouse CD25 (BioLegend San Diego, CA), Brilliant Violet 510™ anti-mouse CD45.1 (BioLegend), PE-Cyanine7 anti-mouse CD45.2 (Thermo Fisher), and Alexa Fluor 647 anti-mouse Foxp3 (Thermo Fisher) antibodies. eFluor 450 fixable viability dye (eBioscience San Diego, CA) was used to gate out dead cells. Stained cells were analyzed on an LSRII Flow Cytometer (BD Biosciences) and data compiled on FlowJo software (TreeStar). Stained naive mouse cells were used as compensation controls and also normalization controls at different time points. Cell sorting was performed on the FACSAria (BD Biosciences).
3H-thymidine incorporation assays.
Inhibitor mice were produced by injecting HemA C57BL/6-CD45.2 mice with 2 Units of FVIII protein (Kogenate® FS Antihemophilic Factor, Bayer HealthCare LLC, CA) three times a week for two weeks. Inhibitor titer was measured by Bethesda assay two weeks after the last injection. Natural responder CD4+ T cells (nTresps) were isolated from HemA inhibitor mice by magnetic separation using a murine CD4+ T cell isolation kit (Miltenyi Biotec). Transduced responder CD4+ cells (F8CAR-Tresps) were produced by F8CAR-LV transduction of CD4+ splenocytes. 8x104 F8CAR-Tresps or nTresps were co-cultured with the appropriate ratio of F8CAR-mFoxp3 transduced T cells (F8CAR-Tregs) in RPMI 1640 supplemented with 10 U/mL human FVIII protein and 100 U/mL IL-2. 1.5 x 105 irradiated CD4− cells were added to serve as antigen presenting cells (APCs) in a total culture volume of 200µL. 3H-thymidine was added three days later, and cells were cultured for 18 hours before analyzing 3H-thymidine incorporation with a Betaplate scintillation counter. Percent suppression was calculated using the equation:
CFSE proliferation and suppression assays
F8CAR-Tresps were first stained with CFSE dye (eBioscience) and then cultured alone or co-cultured with unstained F8CAR-Tregs in the presence of 10U/mL human FVIII protein + APCs, FVIII protein only, APCs only, or anti-CD3/CD28 Dynabeads along with 100U/ml IL-2 for 4 days. Proliferation of CFSE stained cells was measured by flow cytometry.
In vivo adoptive transfer
8–12 week old male HemA C57BL/6-CD45.2 mice were used as recipient mice for adoptive transfer. F8CAR-mFoxp3 transduced CD4+ cells were produced as described above. Human FVIII plasmid was hydrodynamically injected into each recipient mice on Day 0. Four hours after hydrodynamic injection, experimental mice received 4x105 F8CAR-mFoxp3 transduced CD4+ cells by retro-orbital injection. Peripheral blood was collected by retro-orbital bleeding. Plasma was separated from blood samples by centrifuging at 500g for 5 minutes. Activated partial thromboplastin time (aPTT) was performed to measure FVIII function, and Bethesda assay was used to measure inhibitor titer. PBMCs were characterized by flow cytometry.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism 7 software. Two-tailed unpaired student’s T-test or one-way analysis of variance (ANOVA) was used to evaluate significance of single time point experiments, while repeated measures ANOVA (two-way ANOVA followed by post hoc Bonferron’s multiple comparison tests) was used to determine statistical significance in experiments involving measurements over an extended time course. P-values < 0.05 were considered statistically significant.
Results
Production of F8CAR and F8CAR-mFoxp3 lentiviral vectors
Previous experiments performed in our lab have shown that polyclonal Tregs undergoing in vitro FVIII-specific expansion have improved efficacy in preventing FVIII inhibitor development9. Here, we pursue the use of a chimeric antigen receptor approach to produce a uniform population of FVIII-specific Tregs. To achieve this, we first produced lentiviral vectors carrying a F8CAR or F8CAR-mFoxp3 transgene in HEK 293T cells. F8CAR expression was characterized with flow cytometry by incubating the cells with human FVIII protein and then staining with a FITC conjugated anti-human FVIII antibody. Significant transduction was detected, demonstrating that the soluble FVIII protein can efficiently bind to F8CAR expressed on the cell surface. Titration of F8CAR and F8CAR-mFoxp3 lentiviruses showed that 100x concentrated virus had titers ≥ 5x108 IFU/mL (Figure 1B). These analyses confirm our ability to generate high-titer F8CAR and F8CAR-mFoxp3 lentiviral vectors for ex vivo transduction.
Lentiviral transduction of murine CD4+ T cells
After establishing the high transduction efficiency of F8CAR-LV and F8CAR-mFoxp3-LV in HEK 293T cells and efficient binding of FVIII to FVIII-CAR, we explored optimal conditions for transducing murine CD4+ T cells to generate F8CAR-Tresp and F8CAR-Tregs. First, CD4+ T cells isolated from HemA C57BL/6 mice were stimulated and transduced by lentiviral vectors pseudotyped with VSVG envelope protein. FVIII-CAR and Foxp3 expression were characterized via flow cytometry as described above. However, it was found that the transduction efficiency (~7%) was not ideal for generating engineered F8CAR-Tregs (Figure 2A). We then tested retroviral vectors pseudotyped with either VSVG or ecotropic envelope proteins, which resulted in even lower transduction efficiency. Lastly, we investigated the transduction efficiency of lentiviral vectors pseudotyped with a Cocal envelope protein. The Cocal envelope protein’s increased ability to transduce CD4+ T cells is believed to be a result of its higher affinity to the LDL receptor, more efficient cell membrane fusion, or higher avidity to entry receptors due to a higher density of envelope molecules per virion22. It has been observed to have broad tropism25 and has over 70% sequence identity with VSVG, although it has not been definitively determined if it enters cells through the same receptors. Flow cytometry showed that lentivirus pseudotyped with the Cocal envelope protein achieved higher transduction efficiency (>21%) in murine CD4+ T cells (Figure 2A). In order to decrease the background fluorescence from dead cells, a fixable viability stain was used to gate on viable CD4+ T cells (Figure 2B).
Figure 2. Transduction efficiency in murine CD4+ T cells by lentivirus and retrovirus pseudotyped with different envelop proteins.
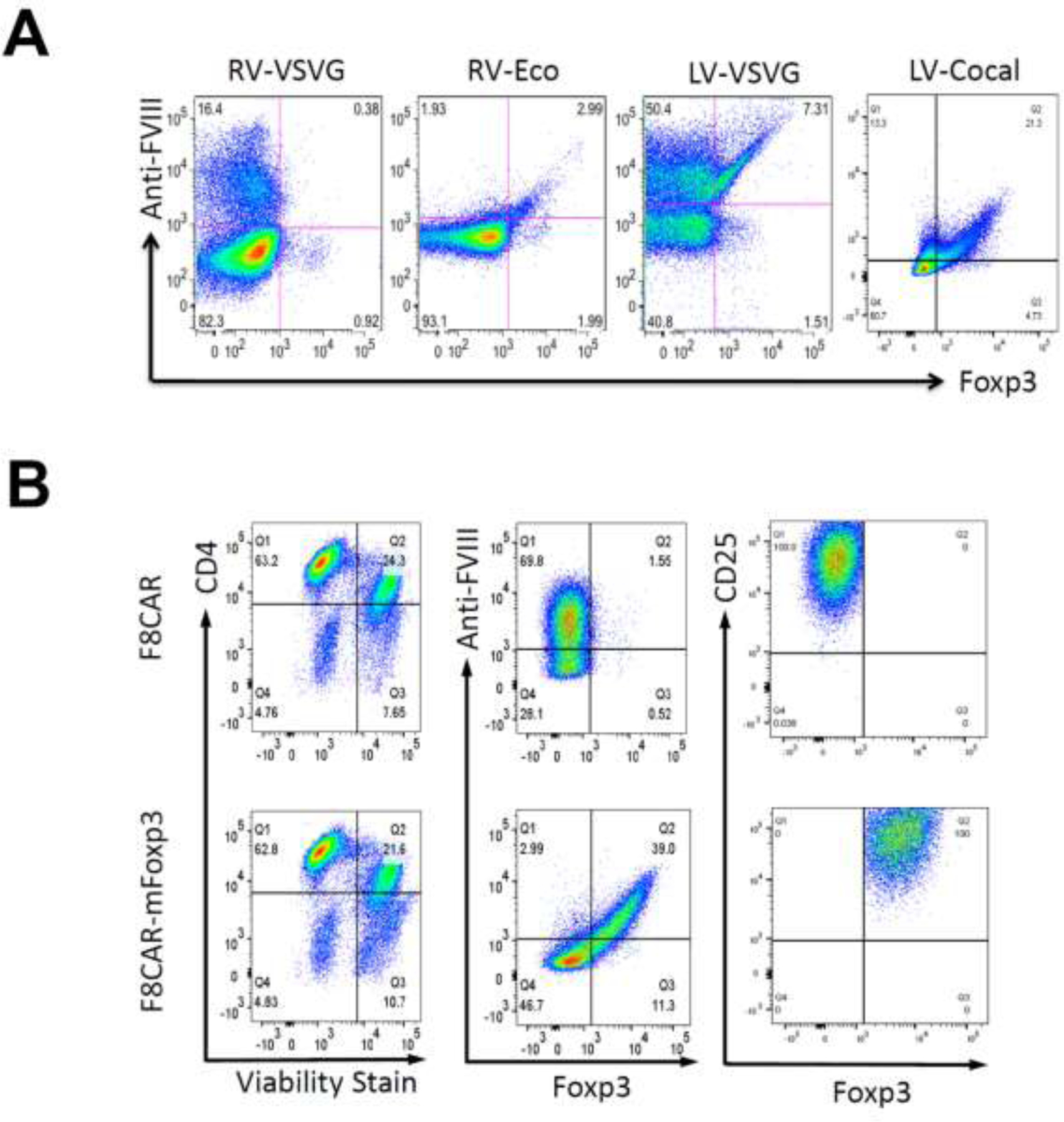
(A) Viral vectors including VSVG and ecotropic envelop-pseudotyped retroviral vectors and VSV-G and Cocal envelop-pseudotyped lentiviral vectors were tested to increase transduction in murine CD4+ T cells. (B) Fixable viability dye eFluor 450 was adopted to decrease anti-FVIII-FITC background produced by dead cells. Anti-FVIII and Foxp3 were gated on CD4+ viable T cells. F8CAR-LV and F8CAR-Foxp3-LV pseudotyped with Cocal envelop protein showed the best transduction efficiency in murine CD4+ T cells. In addition, CD25 were gated on transduced cells to measure T cell activation.
Assessment of F8CARTreg suppressive function in vitro
Transduced cells were collected and sorted using Ficoll centrifugation and cell sorting. Suppressive function of FVIII-CAR expressing T cells was evaluated with the 3H-thymidine incorporation assay as well as the CFSE proliferation assay. Natural responder CD4+ T cells (nTresps) were isolated from inhibitor mice and transduced responder CD4+ cells (F8CAR-Tresps) were produced by F8CAR-LV transduction of CD4+ splenocytes. F8CAR-mFoxp3 transduced T cells (F8CAR-Tregs) were co-cultured with nTresps or F8CAR-Tresps and APCs in a FVIII-specific 3H-thymidine incorporation assay. F8CAR-mFoxp3 transduced T cells demonstrated suppression of nTresp and F8CAR-Tresp cell proliferation with a ratio-dependent response. Because nTresp had a lower percentage of FVIII-specific inhibitor T cells responding to FVIII stimulation, F8CAR-mFoxp3 transduced T cells exerted higher suppression of nTresp proliferation compared to suppression of F8CAR-Tresp proliferation (Figure 3A).
Figure 3. Suppressive function of F8CAR-Tregs were examined in 3H-thymidine incorporation assay and CFSE proliferation assay.
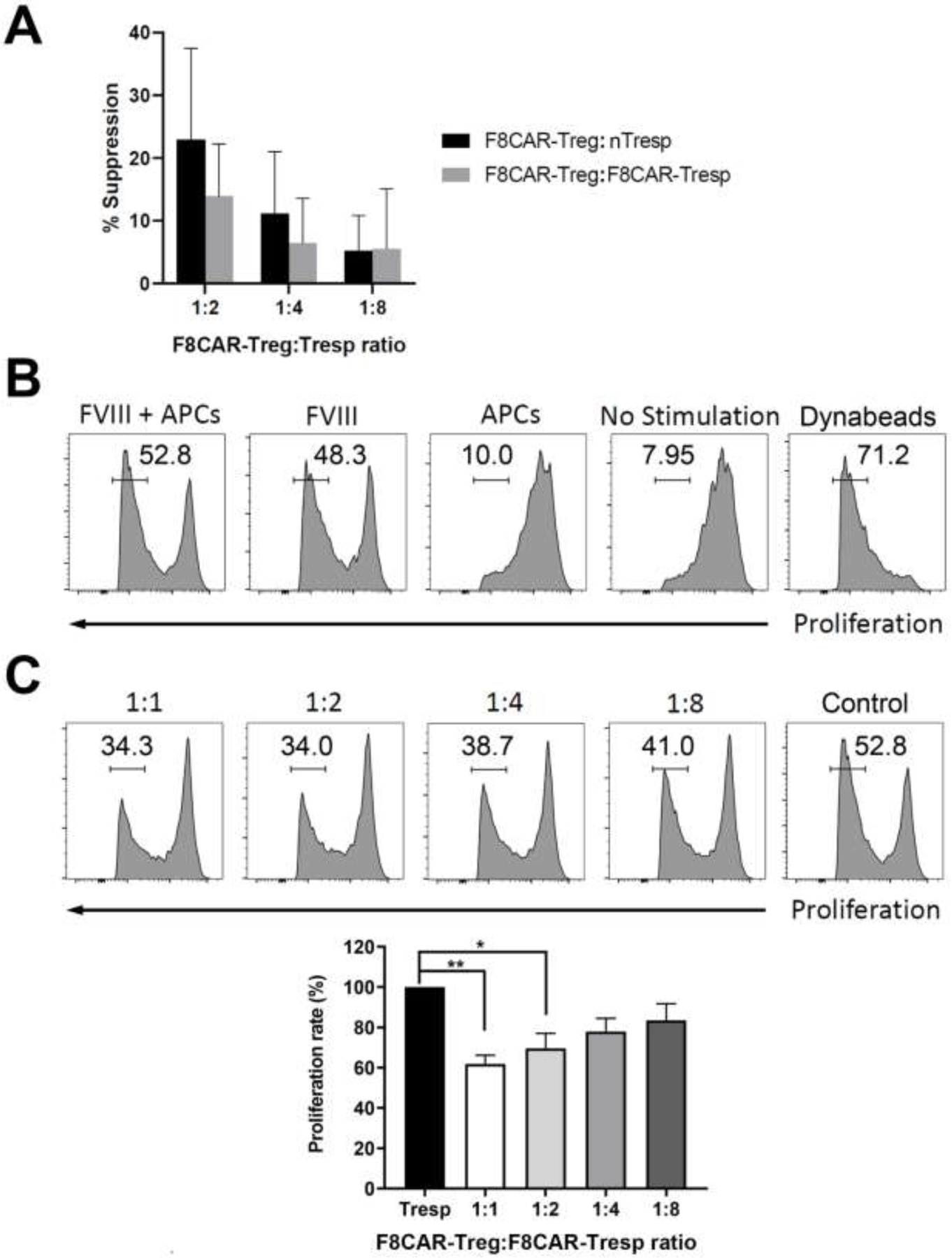
(A) F8CAR-mFoxp3 transduced T cells (F8CAR-Tregs) were cultured at decreasing ratios with either CD4+ T effector cells isolated from FVIII-primed HemA BL6 inhibitor mice (nTresp) or F8CAR transduced T cells (F8CAR-Tresp) in the presence of APCs and 10 U/ml human FVIII protein. 3H-Thymidine was added to the plate after 72 hrs incubation, and the plate was cultured for another 18 hrs. Thymidine incorporation was measured in counts per minute with Betaplate scintillation counter. There is no statistical significance between the nTresp and F8CAR-Tresp groups. However, significant differences were observed between the suppressed cells (Tregs+Tresp) and Tresp only (p < 0.01) for both groups. (B) In the CFSE proliferation assay, F8CAR-Tresps were stained with CFSE dye and cultured with anti-CD3/CD28 Dynabeads, FVIII protein plus APCs, FVIII protein only, or APCs only to stimulate T cells proliferation. (C) The suppressive function of F8CAR-Tregs was measured by co-culturing with F8CAR-Tresps, which were stained with CFSE dye before plating, at decreasing ratios in the presence of APCs and 10 U/ml FVIII protein. F8CAR-Tresps cultured in the presence of APCs and 10 U/ml human FVIII protein were used as controls. Four days later, CFSE expression was measured on flow cytometry. Top panel: representative flow cytometry figures of the FVIII-specific suppressive assay. Bottom panel: Summary of suppressive function of F8CAR-Tregs at decreasing ratios.
In order to confirm the suppressive capability of FVIII-specific F8CAR-Tregs, a suppressive assay was also performed to measure the proliferation of CFSE labeled F8CAR-Tresp co-cultured with F8CAR-Tregs in the presence of FVIII. Proliferation resulting from FVIII-specific stimulation of the FVIII-CAR on F8CAR-Tresps was assessed by flow cytometry (Figure 3B). F8CAR-Tresps were stained with CFSE dye and their proliferation was monitored by incubating with FVIII + APCs, FVIII only, APC only, or anti-CD3/CD28 Dynabeads for four days. F8CAR-Tresps expanded efficiently with Dynabeads, while they did not expand well without stimulus, in the presence of APCs only, or with a non-specific factor IX antigen (data not shown). Most importantly, F8CAR-Tresps responded to human FVIII protein robustly with or without APCs. These results indicate that F8CAR can respond to soluble FVIII protein to induce proliferation of F8CAR engineered T cells without FVIII being processed and presented by APCs. Thus, we can infer that the binding of FVIII to F8CAR activated F8CAR engineered T cells to efficiently proliferate in an antigen-specific manner.
After we confirmed the function of FVIII-CAR, different ratios of F8CAR-Tregs were co-cultured with F8CAR-Tresps stained with CFSE dye in the presence of FVIII protein and APCs to monitor suppressive potential (Figure 3C). After a four-day incubation, CFSE analysis showed that F8CAR-Tresp proliferation was inhibited by F8CAR-Tregs. As the ratio of F8CAR-Tregs increased, F8CAR-Tresp proliferation decreased, indicating that F8CAR engineered Tregs are highly suppressive and antigen-specific.
Adoptive transfer of F8CAR-Tregs into HemA BL6 mice
After assessing the suppressive capability of F8CARTregs in vitro, we then investigated if F8CAR-Tregs could prevent anti-FVIII inhibitor formation in vivo by adoptively transferring the engineered T cells into HemA mice. FVIII plasmids driven by a hepatocyte-specific promoter were delivered into the liver via hydrodynamic tail vein injection. It has previously been shown that high levels of FVIII expression (100–200% of normal FVIII) in hepatocytes can be achieved in the first two weeks following this method of gene therapy26. In immunodeficient mice, therapeutic levels of FVIII expression persisted for the duration of the experiment (>6 months; data not shown). However, in immunocompetent HemA mice, initial high levels of FVIII expression dropped to undetectable levels after 2 weeks due to the generation of anti-FVIII inhibitors. Therefore, this mouse model is well suited to evaluate the ability of F8CAR-Tregs to suppress anti-FVIII inhibitor responses and induce long-term tolerance to FVIII. The engineered cells were produced by transducing CD4+ T cells isolated from HemA CD45.1BL/6 mice using F8CAR-mFoxp3-LV as described above. After sorting by flow cytometry, 4x105 F8CAR+ T cells were retro-orbitally injected into HemA CD45.2BL/6 mice (n=2–4/group) which had received hydrodynamically injected FVIII plasmid (Figure 4A). Control groups of mice were injected with naïve CD45.1+CD4+ T cells (n=2–4/group) or PBS (n=3/group). Peripheral blood samples were collected by retro-orbital bleeding to monitor exogenous T cell population and endogenous Treg population. Plasma samples were separated for APTT analysis of human FVIII protein expression and anti-FVIII inhibitor production.
Figure 4. Adoptive transfer of F8CAR-Tregs into CD45.2 HemA BL6 mice and tracking on exogenous and endogenous Treg populations.
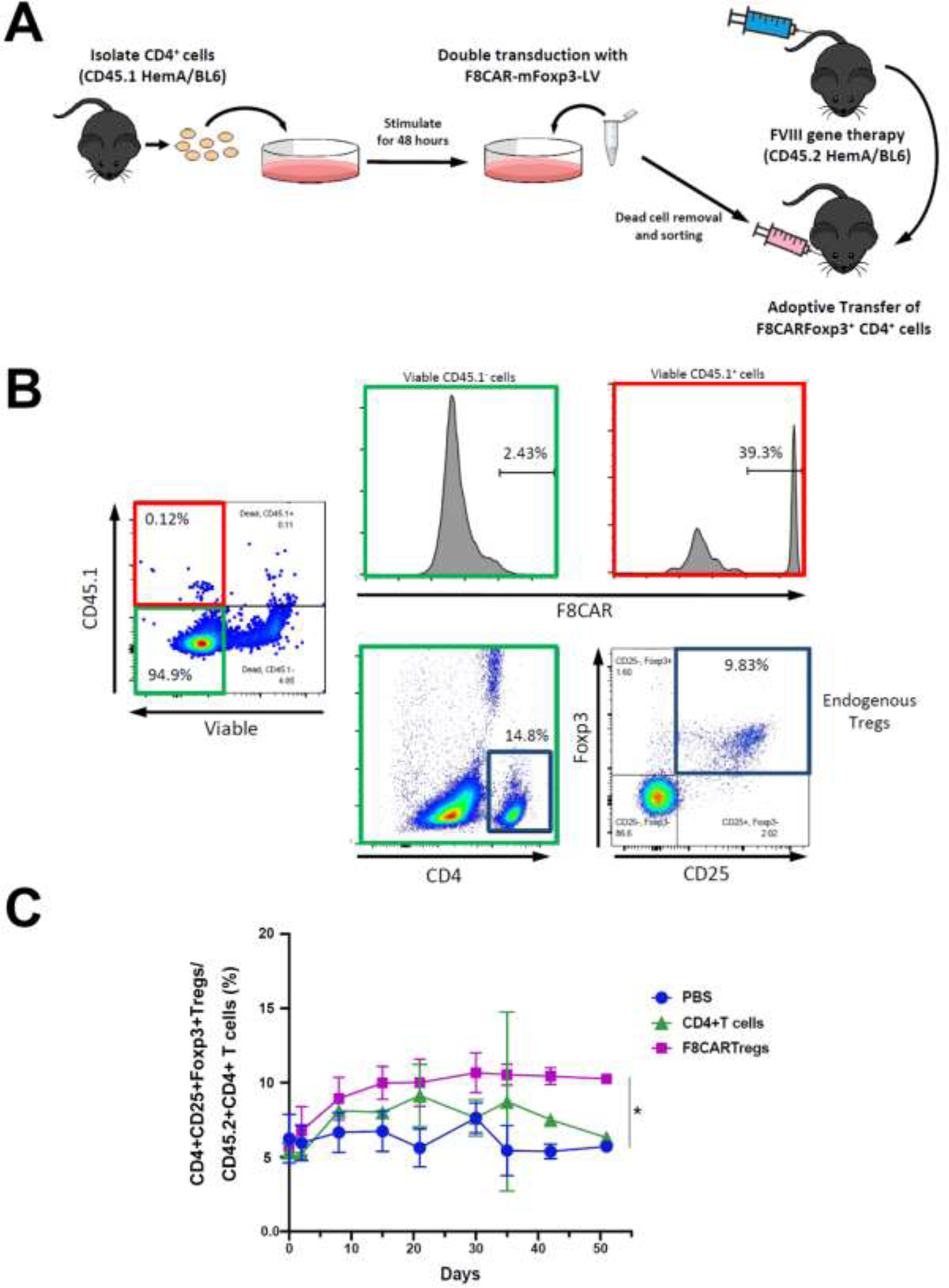
(A) FVIII plasmid was hydrodynamically injected into experimental and control mice. Experimental CD45.2 HemA BL6 mice (n=2–4/group) were retro-orbitally injected with F8CAR-mFoxp3-LV transduced CD45.1+CD4+T cells. Control mice were injected with untransduced CD45.1+CD4+T cells (n=2–4/group) or PBS (n=3/group). Blood samples were collected by retro-orbital bleeding for staining on exogenous and endogenous Treg populations. (B) Exogenous and endogenous Treg populations were analyzed by flow cytometry. Viable transduced CD45.1+T cells (left panel, boxed in red) were gated to examine FVIII-CAR expression (right top panel) and are compared to viable CD45.1− T cells (middle top panel). Endogenous Tregs, defined as CD4+CD25+Foxp3+ cells (middle and right bottom panel, boxed in blue), were gated among CD45.1− T cells (left panel, boxed in green). (C) Endogenous Treg population was monitored over time. Data shown were expressed as a mean with standard deviation. *p < 0.05. These experiments were performed repeatedly in three sets of experiments without significant variance between experiments.
Exogenous CD45.1+ T cells were detected in the recipient mice following adoptive transfer (Figure 4B, left panel). Viable CD45.1+ T cells (Figure 4B, right top panel) were gated from endogenous CD45.1− T cells (Figure 4B, middle top panel) to confirm FVIII-CAR expression. Endogenous CD4+CD25+Foxp3+ Tregs (Figure 4B, middle and right bottom panels) were also gated and examined for any changes over time. Interestingly, we observed an increase of endogenous Tregs in F8CAR-Tregs (p < 0.05) and CD4+ T cell (statistically not significant) treated mice 10 days following FVIII plasmid treatment and the Tregs remained at higher levels in F8CAR-Treg recipient mice for at least 50 days (Figure 4C). These data indicated that adoptively transferred F8CAR-Tregs have the potential to activate and induce proliferation of endogenous Tregs to facilitate FVIII-specific tolerance induction.
Following adoptive transfer, the mice receiving F8CAR-Tregs had persistent FVIII expression, whereas the mice receiving control CD4+ T cells or PBS showed transient FVIII expression with FVIII levels dropping to undetectable levels at 4–7 weeks (Figure 5A). Significant titers of anti-FVIII inhibitory antibodies were observed in control CD4+ T cells and PBS treated mice, whereas no inhibitory antibodies were detected in F8CAR-Treg recipient mice (Figure 5B). These results indicate that adoptive transfer of F8CAR-Tregs was able to prevent anti-FVIII inhibitory antibody formation and facilitate antigen-specific tolerance induction.
Figure 5. Evaluation of FVIII gene expression levels and anti-FVIII inhibitor antibody titers following adoptive transfer of F8CAR-Tregs in HemA mice.
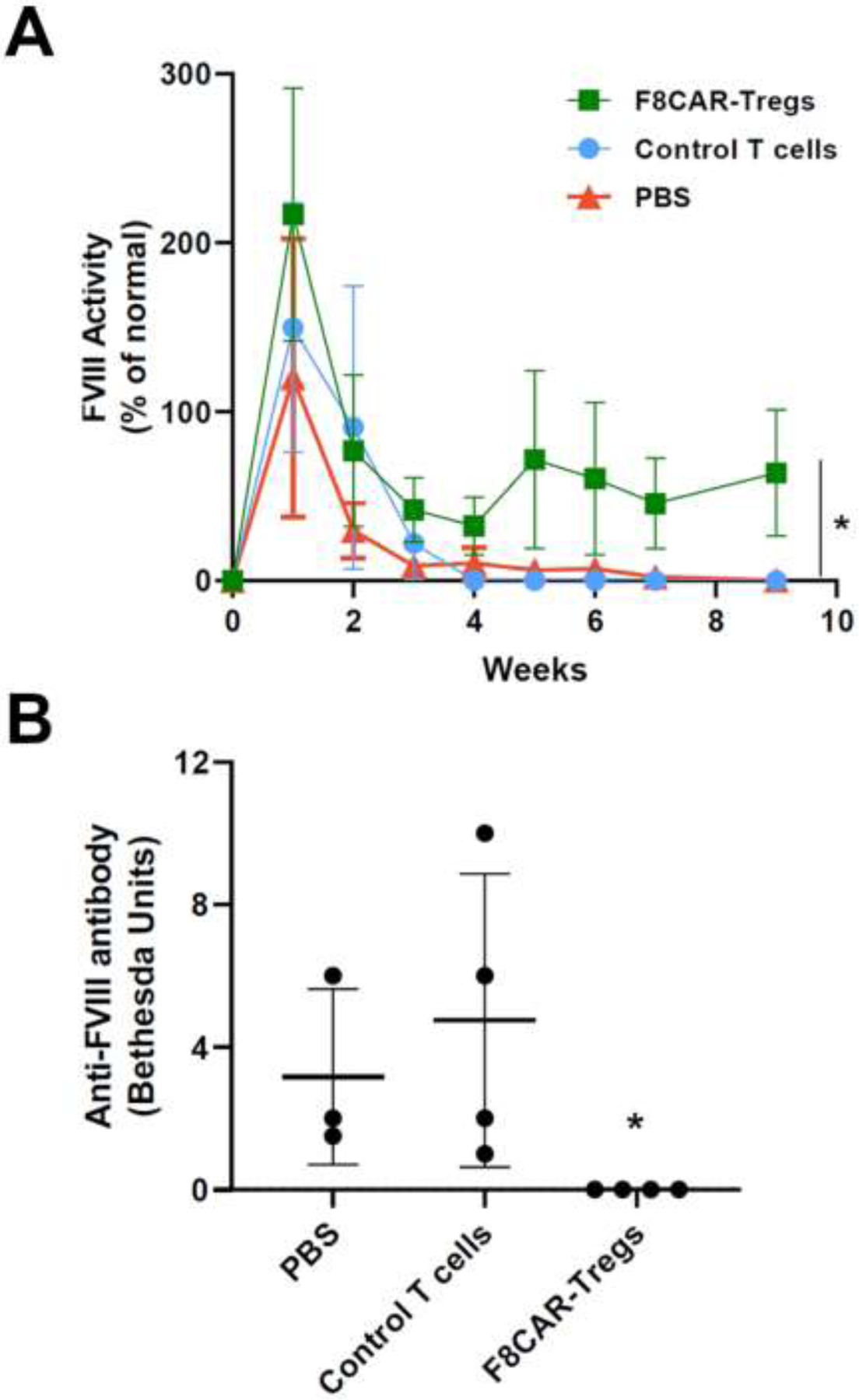
Mouse experiments were the same as those described in Figure 4. Plasma samples were separated from retro-orbitally collected blood. (A) A FVIII-specific aPTT assay was used to examine FVIII activity over time. Data are expressed as mean with standard deviation. (B) Bethesda assays were used to evaluate the anti-FVIII inhibitory antibody titers in the plasma at 9 weeks post adoptive transfer of F8CAR-Tregs. Data shown were expressed as a mean with standard deviation. *p < 0.05.
Discussion
One of the significant obstacles to successfully treat HemA patients is the formation of inhibitory antibodies against FVIII following protein replacement therapy. Current gene therapy trials exclude patients who have a history of inhibitor formation. It is expected that this problem may occur in patients with inhibitor history or at high risk of inhibitor formation. Our group and others have developed successful strategies to prevent FVIII-specific immune responses in HemA animal models including immunomodulation using monoclonal antibody (mAb) therapies27, an IL-2 mutein28, and others2, 29. Interestingly, most successful protocols involve the induction of activated regulatory T cells to create a regulatory immune environment during tolerance induction30. Previously, we have demonstrated that adoptive transfer of transgenic CD4+Foxp3+ Tregs significantly reduced anti-FVIII antibody titers in FVIII plasmid-treated recipient HemA mice31. We found that the transferred Tregs activated endogenous Tregs in the recipient mice via an infectious tolerance mechanism, leading to long-term tolerance and limited recall responses following a second challenge. In the current study, we further developed a clinically feasible adoptive Treg cell therapy aiming at modulation of FVIII-specific immune responses.
Treg cell therapy has many attractive features compared to conventional treatments. It is a personalized therapy targeting FVIII-specific immune responses. The treatment is transient, but can achieve the goal of long-lasting regulation of anti-FVIII immune responses in vivo. Compared to immune tolerance induction (ITI) techniques currently used for hemophilia patients, it will be a much more convenient, less costly, and safer method for treating inhibitory responses. There have been clinical trials using ex vivo expanded polyclonal Tregs to modulate immune responses for diabetes and transplant recipients32–35(Treg trial NCT01624077, THRIL trial NCT02166177 and TRACT Trial NCT02145325). In animal models, however, it was shown that antigen-specific Tregs are more effective than polyclonal cells in achieving therapeutic effects 36–39. We have also shown that Tregs enriched by FVIII specific expansion had better suppressive function towards anti-FVIII immune responses both in vitro and in vivo9. In addition, Tregs have been engineered with T-cell receptors (TCRs)40–42 or chimeric antigen receptors (CARs)18, 36, 43 to generate antigen-specific Tregs. In particular, human CD4+CD25+CD127low Tregs were isolated and transduced by retroviral vectors carrying a FVIII-specific CAR sequence18. These engineered Tregs were demonstrated to suppress FVIII-specific immune responses more effectively when compared to non-specific Tregs. Although these engineered human Tregs were shown to reduce inhibitor formation, the full suppressive function cannot be thoroughly studied due to rapid rejection of human Tregs in immunocompetent HemA mice. Furthermore, Tregs are scarce within the immune system, and current therapies to engineer Tregs isolated from the blood stream can be costly and cumbersome.
In this study, we engineered the more abundant murine CD4+ cells with a third generation lentiviral-CAR vector carrying a FVIII-specific CAR (F8CAR) element composed of a single-chain variable domain antibody fragment (scFv) with high affinity to the C1 domain of human FVIII protein19 and signaling moieties of CD28, 4-1BB, and CD3ζ in hope to increase the potency and persistence of Treg function in vivo. However, recent findings44–46 found that 4-1BB signaling is suboptimal for human Treg function. Therefore, for translation into human applications, engineered human Treg function may be further improved using an optimal CAR construct incorporating CD28 and other signaling domains, not including 4-1BB45. It has been shown that Tregs are highly plastic in vivo; in particular, adoptively transferred exogenous Tregs can be easily converted to other phenotypes47. Thus, a murine Foxp3 cDNA (F8CAR-mFoxp3) was included in the F8CAR construct to generate more stable and effective F8CAR-Tregs. Compared to generating human CAR-Tregs, it is much more difficult to engineer murine CAR-Tregs due to low efficiency of retroviral and lentiviral transduction of murine T cells. Among the four vector systems we tested, Cocal envelope pseudotyped lentiviral vector produced highest transduction efficiency in murine CD4+ T cells. As mentioned earlier, the Cocal envelope is believed to have broad tropism, high affinity to LDL receptors, increased membrane fusion efficiency, and high avidity to hematopoietic cells. We also implemented the use of RetroNectin, spinoculation, and double transduction in order to increase the percentage of successfully transduced murine T cells. RetroNectin has previously been shown to increase lentiviral transduction efficiency by facilitating colocalization of virus particles and their target cells48, 49. Spinoculation of the lentivirus before addition of cells is believed to force virus particles to adhere to the RetroNectin coated culture surface50. A second spinoculation of virus after addition of cells is hypothesized to bring virus particles in contact with the top of the cultured cells. Following transduction of HEK 293T cells or murine CD4+ T cells using either F8CAR-LV or F8CAR-mFoxp3-LV, it was found that soluble FVIII can bind efficiently to the specific FVIII scFv expressed on the transduced cell surface. Furthermore, the F8CAR and F8CAR-mFoxp3 transgene transformed CD4+ T cells to confer characteristics of FVIII-specific CD4+CD25+ F8CAR-Tresps and CD25+Foxp3+ F8CAR-Tregs, respectively.
In the 3H-thymidine incorporation assay, we found that F8CAR-Tresps can respond to FVIII stimulation in the presence of APCs similarly to nTresps isolated from FVIII primed HemA inhibitor mice. In corroboration with our hypotheses, the CFSE proliferation assay showed that proliferation of F8CAR-Tresps can be induced by FVIII with or without the presence of APCs. In addition, we showed that the engineered F8CAR-Tregs highly suppressed the proliferation of engineered F8CAR-Tresps and natural FVIII-specific Tresps. Adoptive transfer of the engineered F8CAR-Tregs to HemA mice prevented inhibitor formation and maintained FVIII function when combined with FVIII gene therapy. Since FVIII stimulation of F8CAR engineered cells can occur in the presence or absence of APCs, it is postulated that the mechanism that leads to antigen-specific tolerance induction may be different compared to other engineered CAR-Tregs that rely on cell-cell interaction36, 42. We found that although there is only a small percentage of exogenous F8CAR-Tregs circulating in mice initially, their presence may have the potential to induce the activation and proliferation of antigen-specific endogenous Tregs to promote infectious tolerance induction. There is a significant elevation of endogenous Tregs during the 8 weeks following adoptive transfer of F8CAR-Tregs and FVIII plasmid challenge, which we hypothesize promoted the generation of endogenous FVIII-specific Tregs and created an environment for induction of FVIII-specific tolerance.
In this study, we have demonstrated that the engineered F8CAR-Tregs were effective in preventing inhibitor formation and can potentially promote long-term FVIII-specific tolerance in HemA mice. Importantly, our method enables the generation of engineered FVIII-specific Tregs from the more abundant CD4+ T cell population instead of anergic and scarce Tregs, addressing a critical manufacturing obstacle for the development of Treg therapy. This strategy could be tested for efficacy in other models, as well as in treatment of human hemophilia patients after further development.
Highlights.
Antigen-specific regulatory T cells were produced by transducing CD4+ T cells with lentivirus carrying factor VIII-specific CAR and Foxp3 cDNA
The gene-modified T cells suppressed anti-FVIII immune responses in vitro and prevented anti-factor VIII antibody production in hemophilia A mice.
Transduced cells were analyzed for suppressive function and demonstrated regulation on anti-factor VIII immune responses
Acknowledgement
This work was supported by a pilot project from NIH-NHLBI grant 5 U01 AI101990 and a NIH-NHLBI grant U54 HL142019. We would also like to thank Amber Vander Kooi and Cameron Rementer for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
The authors declare no conflict of interest.
References
- 1.Mannucci PM, Mancuso ME, and Santagostino E, How we choose factor VIII to treat hemophilia. Blood, 2012. 119(18): p. 4108–14. [DOI] [PubMed] [Google Scholar]
- 2.Waters B and Lillicrap D, The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost, 2009. 7(9): p. 1446–56. [DOI] [PubMed] [Google Scholar]
- 3.Meeks SL, Chapman RL, Kempton C, and Dunn AL, Late immune tolerance induction in haemophilia A patients. Haemophilia, 2013. 19(3): p. 445–8. [DOI] [PubMed] [Google Scholar]
- 4.Rivard GE, Rothschild C, Toll T, and Achilles K, Immune tolerance induction in haemophilia A patients with inhibitors by treatment with recombinant factor VIII: a retrospective non-interventional study. Haemophilia, 2013. 19(3): p. 449–55. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, and Yamaguchi T, Regulatory T cells: how do they suppress immune responses? Int Immunol, 2009. 21(10): p. 1105–11. [DOI] [PubMed] [Google Scholar]
- 6.Safinia N, Leech J, Hernandez-Fuentes M, Lechler R, and Lombardi G, Promoting transplantation tolerance; adoptive regulatory T cell therapy. Clin Exp Immunol, 2013. 172(2): p. 158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CL, Ye P, Yen BC, and Miao CH, In vivo expansion of regulatory T cells with IL-2/IL-2 mAb complexes prevents anti-factor VIII immune responses in hemophilia A mice treated with factor VIII plasmid-mediated gene therapy. Mol Ther, 2011. 19(8): p. 1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CL, Ye P, Lin J, Djukovic D, and Miao CH, Long-term tolerance to factor VIII is achieved by administration of interleukin-2/interleukin-2 monoclonal antibody complexes and low dosages of factor VIII. J Thromb Haemost, 2014. 12(6): p. 921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BM, Lyle MJ, Chen AC, and Miao CH, Antigen-specific in vitro expansion of factor VIII-specific regulatory T cells induces tolerance in hemophilia A mice. J Thromb Haemost, 2020. 18(2): p. 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett DM, Singh N, Porter DL, Grupp SA, and June CH, Chimeric antigen receptor therapy for cancer. Annu Rev Med, 2014. 65: p. 333–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenderian SS, Ruella M, Shestova O, et al. , CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia, 2015. 29(8): p. 1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tasian SK and Gardner RA, CD19-redirected chimeric antigen receptor-modified T cells: a promising immunotherapy for children and adults with B-cell acute lymphoblastic leukemia (ALL). Ther Adv Hematol, 2015. 6(5): p. 228–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifant CL, Jackson HJ, Brentjens RJ, and Curran KJ, Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics, 2016. 3: p. 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh A, Smith M, James SE, et al. , Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med, 2017. 23(2): p. 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkins O, Keeler AM, and Flotte TR, CAR T-Cell Therapy: Progress and Prospects. Hum Gene Ther Methods, 2017. 28(2): p. 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Sosinowski T, Cox AR, et al. , Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes. J Autoimmun, 2019. 96: p. 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellebrecht CT, Bhoj VG, Nace A, et al. , Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science, 2016. 353(6295): p. 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon J, Schmidt A, Zhang AH, et al. , FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood, 2017. 129(2): p. 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Brink EN, Turenhout EA, Bovenschen N, et al. , Multiple VH genes are used to assemble human antibodies directed toward the A3-C1 domains of factor VIII. Blood, 2001. 97(4): p. 966–72. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Zhou X, Li R, Fu X, and Sun P, Optimized PEI-based Transfection Method for Transient Transfection and Lentiviral Production. Curr Protoc Chem Biol, 2017. 9(3): p. 147–157. [DOI] [PubMed] [Google Scholar]
- 21.Schambach A, Swaney WP, and van der Loo JC, Design and production of retro- and lentiviral vectors for gene expression in hematopoietic cells. Methods Mol Biol, 2009. 506: p. 191–205. [DOI] [PubMed] [Google Scholar]
- 22.Humbert O, Gisch DW, Wohlfahrt ME, et al. , Development of Third-generation Cocal Envelope Producer Cell Lines for Robust Lentiviral Gene Transfer into Hematopoietic Stem Cells and T-cells. Mol Ther, 2016. 24(7): p. 1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerns HM, Ryu BY, Stirling BV, et al. , B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia. Blood, 2010. 115(11): p. 2146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao CH, Harmeling BR, Ziegler SF, et al. , CD4(+)FOXP3(+) regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice. Blood, 2009. 114(19): p. 4034–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trobridge GD, Wu RA, Hansen M, et al. , Cocal-pseudotyped lentiviral vectors resist inactivation by human serum and efficiently transduce primate hematopoietic repopulating cells. Mol Ther, 2010. 18(4): p. 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye P, Thompson AR, Sarkar R, et al. , Naked DNA transfer of Factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice. Mol Ther, 2004. 10(1): p. 117–26. [DOI] [PubMed] [Google Scholar]
- 27.Miao CH, Immunomodulation for inhibitors in hemophilia A: the important role of Treg cells. Expert Rev Hematol, 2010. 3(4): p. 469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen AC, Cai X, Li C, et al. , A Treg-Selective IL-2 Mutein Prevents the Formation of Factor VIII Inhibitors in Hemophilia Mice Treated With Factor VIII Gene Therapy. Front Immunol, 2020. 11: p. 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang AH, Skupsky J, and Scott DW, Factor VIII inhibitors: risk factors and methods for prevention and immune modulation. Clin Rev Allergy Immunol, 2009. 37(2): p. 114–24. [DOI] [PubMed] [Google Scholar]
- 30.Miao CH, Advances in Overcoming Immune Responses following Hemophilia Gene Therapy. J Genet Syndr Gene Ther, 2011. S1. [PMC free article] [PubMed] [Google Scholar]
- 31.Miao CH, Harmeling BR, Ziegler SF, et al. , CD4+FOXP3+ regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice. Blood, 2009. 114(19): p. 4034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marek-Trzonkowska N, Mysliwec M, Siebert J, and Trzonkowski P, Clinical application of regulatory T cells in type 1 diabetes. Pediatr Diabetes, 2013. 14(5): p. 322–32. [DOI] [PubMed] [Google Scholar]
- 33.Bluestone JA, Buckner JH, Fitch M, et al. , Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med, 2015. 7(315): p. 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. , First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol, 2009. 133(1): p. 22–6. [DOI] [PubMed] [Google Scholar]
- 35.Martelli MF, Di Ianni M, Ruggeri L, et al. , HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood, 2014. 124(4): p. 638–44. [DOI] [PubMed] [Google Scholar]
- 36.Dawson NAJ and Levings MK, Antigen-specific regulatory T cells: are police CARs the answer? Transl Res, 2017. 187: p. 53–58. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura E, Sakihama T, Setoguchi R, Tanaka K, and Sakaguchi S, Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol, 2004. 16(8): p. 1189–201. [DOI] [PubMed] [Google Scholar]
- 38.Tarbell KV, Petit L, Zuo X, et al. , Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med, 2007. 204(1): p. 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsang JY, Tanriver Y, Jiang S, et al. , Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest, 2008. 118(11): p. 3619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brusko TM, Koya RC, Zhu S, et al. , Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One, 2010. 5(7): p. e11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YC, Zhang AH, Su Y, et al. , Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood, 2015. 125(7): p. 1107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoeppli RE, MacDonald KG, Levings MK, and Cook L, How antigen specificity directs regulatory T-cell function: self, foreign and engineered specificity. HLA, 2016. 88(1–2): p. 3–13. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald KG, Hoeppli RE, Huang Q, et al. , Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest, 2016. 126(4): p. 1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boroughs AC, Larson RC, Choi BD, et al. , Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight, 2019. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson NAJ, Rosado-Sanchez I, Novakovsky GE, et al. , Functional effects of chimeric antigen receptor co-receptor signaling domains in human Tregs. BioRxiv, 2019: p. Doi: 10.1101/749721. [DOI] [PubMed] [Google Scholar]
- 46.Imura Y, Ando M, Kondo T, Ito M, and Yoshimura A, CD19-targeted CAR regulatory T cells suppress B cell pathology without GvHD. JCI Insight, 2020. 5(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, and Waldmann H, The plasticity and stability of regulatory T cells. Nat Rev Immunol, 2013. 13(6): p. 461–7. [DOI] [PubMed] [Google Scholar]
- 48.Lee HJ, Lee YS, Kim HS, et al. , Retronectin enhances lentivirus-mediated gene delivery into hematopoietic progenitor cells. Biologicals, 2009. 37(4): p. 203–9. [DOI] [PubMed] [Google Scholar]
- 49.Millington M, Arndt A, Boyd M, Applegate T, and Shen S, Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS One, 2009. 4(7): p. e6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anastasov N, Hofig I, Mall S, Krackhardt AM, and Thirion C, Optimized Lentiviral Transduction Protocols by Use of a Poloxamer Enhancer, Spinoculation, and scFv-Antibody Fusions to VSV-G. Methods Mol Biol, 2016. 1448: p. 49–61. [DOI] [PubMed] [Google Scholar]


