Abstract
Nervous system development proceeds via well-orchestrated processes involving a balance between progressive and regressive events including stabilization or elimination of axons, synapses, and even entire neurons. These progressive and regressive events are driven by functionally antagonistic signaling pathways with the dominant pathway eventually determining whether a neural element is retained or removed. Many of these developmental sculpting events are triggered by final target innervation necessitating a long-distance mode of communication. While long-distance progressive signaling has been well characterized, particularly for neurotrophic factors, there remains relatively little known about how regressive events are triggered from a distance. Here we discuss the emergent phenomenon of long-distance regressive signaling pathways. In particular, we will cover (a) progressive and regressive cues known to be employed after target innervation, (b) the mechanisms of long-distance signaling from an endosomal platform, (c) recent evidence that long-distance regressive cues emanate from platforms like death receptors or repulsive axon guidance receptors, and (d) evidence that these pathways are exploited in pathological scenarios.
Keywords: axon transport, degeneration, neurotrophin, p75NTR, signaling
1 |. INTRODUCTION
Assembly of a functional nervous system requires overproduction of every element followed by an organized culling to stabilize or remove components (Box 1). Much like creating a sculpture requires controlled chiseling of a shapeless mound of clay, nervous system development also begins with supernumerary neurons, axons, and synapses and as development proceeds a portion of these components are removed via regressive processes like neuronal death, synapse elimination, and axon pruning. While the last 50 years has defined many of the progressive and regressive pathways that govern nervous system development, we as a community still have much to learn about precisely how these signaling pathways govern nervous system development. Moreover, we now appreciate that, much like cancer inappropriately re-engages developmental programs to promote uncontrolled proliferation, neural development pathways are often re-engaged in adulthood to inappropriately disassemble the nervous system resulting in neurodegenerative diseases.
BOX 1. Known unknowns for long-distance regressive signaling.
What is the nature and mode of transport for regressive signals?
Can a single “flavor” of regressive signaling endosome generate several different signals, depending on its location? Alternatively, is function partitioned amongst heterogeneous pools of regressive endosomes?
Are there common vesicles for opposing long-distance progressive and regressive signals, which may be utilized depending on the context and requirements?
How is the counterbalance of long-distance progressive and regressive signals regulated at different stages of development? Under what conditions is one pathway dominant over the other?
How are local progressive and regressive signals restricted to a given locale or integrated to provide an overall response?
The notion of antagonistic progressive and regressive signals governing nervous system development is a concept that appears universal for many common phenomena including natural pattern formation (Meinhardt & Gierer, 2000; Turing, 1990). This concept can be applied to the formation of sand dunes, dried river beds, spots, and stripes on animals. Beyond what we consider to be classic biological patterns, this principle also applies to processes like specification and survival–death decisions. For example, it is well established that shortly after blastula formation, a balance between activating and inhibitory (e.g., BMP4, WNT, Noggin, Chordin) signaling dictates the formation of neuroectoderm, a tissue that gives rise to the entire nervous system (Linker & Stern, 2004). From there, antagonistic signals continue to govern the relative differentiation, migration, and axon guidance of all the neurons and glia in the central and peripheral nervous system (PNS; Butler & Bronner, 2015; Prasad, Charney, & García-Castro, 2019). In this regard, Notch and Numb are evolutionarily conserved, opposing factors necessary for cell fate specification from flies to vertebrates. The asymmetric localization of Numb in stem cells and its antagonistic actions toward Notch, are required for neuronal fate specification, specifically after asymmetric division (Petersen, Tang, Zou, & Zhong, 2006; Zhong, Feder, Jiang, Jan, & Jan, 1996). Along with neurogenesis, neuronal migration and axon guidance are other key morphogenetic events which rely on the opposing actions of attractive and repulsive cues to ensure that the neuronal cell bodies reach the correct locations before nascent axons advance toward their appropriate targets. The choice of migratory paths is governed by attractants like neurotrophins, whereas diffusible cues such as SLIT1, netrin 1, and ephrin family members act as repellants (Hamasaki, Goto, Nishikawa, & Ushio, 2001; Marín et al., 2003; Zimmer et al., 2008). Of course this type of “patterning” is not unique to the nervous system and can be observed in mesoderm and endoderm derived organs as well.
One feature that distinguishes the nervous system from all other organ systems is that neurons often bear very long axonal processes. These axons find their targets using many of the same principles used in cell migration (Kolodkin & Tessier-Lavigne, 2011). Attractive cues tend to be permissive and instructive for axons to grow toward a target, whereas repulsive cues prevent axons from wandering off course. Once an axon arrives at its target, several additional patterning events must occur, including synapse formation/restriction, axon branching/pruning, survival/death decisions, and even further differentiation decisions (Lewin & Barde, 1996; Luo et al., 2007; Moqrich et al., 2004; Wheeler et al., 2014). As such, the nervous system has acquired novel long-distance antagonistic regulatory mechanisms to govern patterning that is triggered by target innervation.
Long-distance signaling is an emergent property of many progressive and regressive cues. Because the long-distance signaling of progressive cues like neurotrophins have been extensively reviewed (Ascano, Bodmer, & Kuruvilla, 2012; Barford, Deppmann, & Winckler, 2017; Scott-Solomon & Kuruvilla, 2018; Zweifel, Kuruvilla, & Ginty, 2005), this review will be focused on long-distance regressive signaling where much less is known. We will (a) give a brief overview of progressive and regressive cues that are known to be employed after target innervation; (b) discuss known mechanisms of long-distance signaling from an endosomal platform; (c) provide an overview of recent evidence that regressive cues emanating from platforms like death receptors or repulsive axon guidance receptors can promote their signals at a distance; (d) discuss how these pathways might be exploited in pathological scenarios.
2 |. OVERVIEW OF PROGRESSIVE AND REGRESSIVE CUES FROM TARGET TISSUES
2.1 |. Post-target innervation progressive signaling
By virtue of their length and the fact that they must appropriately wire up with inputs and outputs, neurons face unique challenges. For example, how can a neuron change its transcriptional program as a function of whether it has connected with an appropriate or inappropriate target? Once connected with its targets, how does the distal end of the neuron relay signals across the axons to the cell body and dendrites? Answers to these questions can be gleaned from a series of elegant chick embryo transplantation experiments from individuals including Rita-Levi Montalcini, Viktor Hamburger, and Elmer Bueker whereby they first observed that by removing or adding additional limb buds to developing chick embryos they could diminish or exaggerate the growth of peripheral neurons, respectively (Hamburger, 1939; Hamburger & Levi-Montalcini, 1949; Oppenheim, 1991). Next, upon grafting a mouse sarcoma into the body wall of a chick embryo, they observed a clear quantitative relationship between the size of sarcoma implant and amount of neuronal growth (Bueker, 1948). From these experiments, they surmised the existence of a diffusible cue capable of promoting axon outgrowth and neuron survival. This led to the neurotrophic factor hypothesis which posits that neurons, particularly in the PNS, compete for limiting amounts of target-derived trophic factors in order to survive (Cohen, Levi-Montalcini, & Hamburger, 1954; Levi-Montalcini & Hamburger, 1951; Oppenheim, 1991). In collaboration with Stanley Cohen, they later purified the archetypal neurotrophic factor, which they named nerve growth factor (NGF; Cohen, 1960; Cohen & Levi-Montalcini, 1956). We now know that NGF is part of a larger family of homologous, structurally related secreted factors called neurotrophins (Hallböök, Wilson, Thorndyke, & Olinski, 2006). Besides NGF, additional neurotrophins were identified including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5), each of which have been found to have prominent expression in various targets of the peripheral and central nervous system (CNS; Hohn, Leibrock, Bailey, & Barde, 1990; Ip et al., 1992; Jones & Reichardt, 1990; Leibrock et al., 1989; Rosenthal et al., 1990). All neurotrophins can bind to the pan neurotrophin receptor p75NTR and each neurotrophin also binds to a cognate receptor tyrosine kinase; NGF binds TRK-A, BDNF&NT4 bind TRK-B and NT3 binds TRK-C (Chao, 2003; Dechant & Barde, 2002; Gentry, Barker, & Carter, 2004). Similar to neurotrophins, RET receptor ligands are expressed predominantly in intermediate and final targets (e.g., Glial cell line-derived neurotrophic factor [GDNF], Neurturin) and are known to mediate a wide range of developmental events including axon growth and pathfinding in sympathetic (Enomoto et al., 2001), sensory (Honma, Kawano, Kohsaka, & Ogawa, 2010), and motor (Bonanomi et al., 2012) neurons as well as maintenance of dopaminergic neurons in CNS (Ito & Enomoto, 2016; Tomac et al., 1995).
It is now well established that these target derived trophic factors control a range of patterning events during peripheral nervous system development, including synapse formation, axon branch stabilization, survival, and even differentiation (Lewin & Barde, 1996; Luo et al., 2007; Moqrich et al., 2004; Villarroel-Campos, Schiavo, & Lazo, 2018; Wheeler et al., 2014). Furthermore, many of these actions require long-distance retrograde signaling emanating from target organs like eye, heart, and skin (Glebova & Ginty, 2005; Snider, 1994). In this way, the target defines the form and function of the innervating afferent and efferent neurons (i.e., target matching). While these examples provide useful analogies for studying other long-distance developmental cues, it will be necessary to eventually obtain a full accounting of which of the 58 receptor tyrosine kinase (RTKs) and 1,000s of non-RTK receptors are capable of long-distance signaling. While the field has made progress in understanding the molecular basis of long-distance progressive signaling (discussed in Section 3), much less is known about long-distance regressive signaling.
2.2 |. Post-target innervation regressive signaling
In contrast to their role in progressive signaling described above, all the neurotrophins and their immature pro-forms can also mediate regressive events via binding to the pan neurotrophin receptor, p75NTR. The regressive processes mediated by p75NTR include neuronal apoptosis, axon pruning or branch elimination, growth cone collapse, and synapse restriction (Deppmann et al., 2008; Frade, Rodríguez-Tébar, & Barde, 1996; Naska, Lin, Miller, & Kaplan, 2010; Pathak & Carter, 2017; Rabizadeh et al., 1993; Sharma et al., 2010; Singh et al., 2008). Importantly, the function of p75NTR is context dependent (e.g., cell type, subcellular locale, and co-receptor expression). For example, p75NTR promotes regressive signaling in sympathetic neurons but synergizes with TRK signaling in sensory neurons to promote progressive signaling (Cheng, Jin, Rose, & Deppmann, 2018; K. F. Lee, Davies, & Jaenisch, 1994). p75NTR is one of 29 tumor necrosis factor receptor (TNFR) family members, several of which have been shown to mediate regressive signaling in neurons, albeit with much less promiscuous ligand binding (Roux & Barker, 2002; Vilar, 2017).
Axon guidance cues have also been described to continue to function after target innervation. For example, repulsive axon guidance cues like semaphorin 3a (sema3a), Slit-Robo and Eph-Ephrins have been associated with cell death in multiple cell types after target innervation (Dickinson, Fegan, Ren, Hillier, & Duncan, 2011; Gagliardini & Fankhauser, 1999; Kellermeyer, Heydman, Mastick, & Kidd, 2018; H. Lee, Park, Kang, & Park, 2015; Wehner et al., 2016). Presumably, this is a way in which neurons innervating incorrect targets can be rapidly removed from a pool competing for neurotrophic factors derived from correct targets.
While there are several in vitro examples of these regressive cues working locally at dendrites, axons, and/or cell bodies, in physiological settings it remains unclear where these signals originate. One can deduce that repulsive axon guidance cues that induce cell death are present far from the cell body and that the signal originating at the axon must travel long-distance to influence soma death. Likewise, if axons arrive at incorrect targets, having a specific retrograde death signal may sharpen the broader competition for survival as previously modeled and observed empirically (Deppmann et al., 2008; Suo et al., 2014). Below, we describe early evidence that long-distance regressive signaling exists and examine its role in development and pathology. First, we briefly describe the progressive long-distance signaling endosome (SE) as a template to consider regressive long-distance signaling.
3 |. THE SIGNALING ENDOSOME (SE): SPECIALIZED VESICLES FOR LONG-DISTANCE SIGNALING
How does a signal originating at the distal axon travel millimeters (or even up to a meter in some long-projecting neurons such as spinal motor neurons innervating the foot) to influence events at the cell body and dendrites? Currently, several lines of evidence suggest that, along with their receptors, neurotrophins are internalized into membranous vesicles at the plasma membrane and transported retrogradely along the axon (Barford et al., 2017; Grimes et al., 1996; N. Yamashita & Kuruvilla, 2016; Zhang, Moheban, Conway, Bhattacharyya, & Segal, 2000). Mobley and colleagues coined these specialized vesicles, SEs (Barford et al., 2017; Grimes et al., 1996; N. Yamashita & Kuruvilla, 2016; Zhang et al., 2000). Several years of investigation has identified several key steps in long-distance retrograde signaling: (a) Internalization, (b) attachment to dynein motors, (c) deposition in cell bodies and dendrites, and (d) molecular and functional diversification. These findings may provide a template for considering how other types of long-distance signaling might occur, however, it should be noted that it is unlikely that regressive long-distance signaling will follow precisely the same steps. In this section, we will briefly describe the consensus in the field with respect to progressive long-distance signaling by neurotrophic factors, which has been described in several reviews (Barford et al., 2017; Matusica & Coulson, 2014; Wu, Cui, He, Chen, & Mobley, 2009; N. Yamashita & Kuruvilla, 2016; Zahavi, Maimon, & Perlson, 2017; Zweifel et al., 2005).
In the SE model, TRK receptors are activated by ligand binding which leads to autophosphorylation of the TRK receptors and endocytosis of this ligand–receptor complex (Ascano et al., 2012; Ginty & Segal, 2002; Huang & Reichardt, 2003). Endocytosis, specifically of TRK-A, is enhanced by the dephosphorylation of the GTPase dynamin via calcineurin (Bodmer, Ascaño, & Kuruvilla, 2011). While NGF is able to promote internalization and retrograde transport of the TRK-A receptor, NT3, which also binds to TRK-A, is unable to do so (Kuruvilla et al., 2004). Notably, NT3 binds to TRK-A as a lower affinity heterologous ligand compared to TRK-C receptor mentioned above (Ivanisevic, Zheng, Woo, Neet, & Saragovi, 2007). This differential effect of NGF and NT3 to promote long-distance trophic signaling is due to the ability of NGF-TRK-A interaction to withstand the acidic endosomal lumen. The perdurance of the NGF-TRK-A complex in an acidic environment allows for cortical actin severing, which was found to be an obligate step for long-distance trafficking rather than rapid recycling (the fate of the NT-3-TRK-A complex) (Harrington et al., 2011).
A common feature of organelle long-distance retrograde trafficking is attachment to the molecular motor dynein, which slides along microtubule rails toward cell bodies (Box 2). Signaling derived from post-endocytic NGF-TRK-A or BDNF-TRK-B is required for long-distance retrograde trafficking, and by extension, association with dynein. For example, it has been shown that for TRK-A, PI3K signaling, but not MAPK signaling, is required for retrograde transport (Kuruvilla, Ye, & Ginty, 2000). Alternatively, MAPK signaling enhanced BDNF-TRK-B endosome binding to dynein intermediate chain (DIC) (Mitchell et al., 2012). TRK-B-containing SEs were reported to use the dynein adaptor Snapin, which interacts with DIC and recruits the dynein motor complex, however, the finding that axonal transport of TRK-B is not completely abolished in neurons lacking Snapin indicates that multiple adaptors recruit dynein to these endosomes (Zhou, Cai, Xie, & Sheng, 2012).
BOX 2. The transport machinery in axons and dendrites.
Axonal transport relies on polarized distribution of microtubules. The microtubules are uniformly oriented in axons with their plus-end facing toward distal axon terminals (Rao & Baas, 2018; Yau et al., 2016). Distal dendrites display a similar orientation of microtubule, whereas mixed orientations of microtubules can be found in proximal dendrites (Tas et al., 2017). Molecular motor complexes drive the directional transport of cargoes with kinesins moving in an anterograde direction and dynein in a retrograde direction, toward the cell bodies (Maday, Twelvetrees, Moughamian, & Holzbaur, 2014). The dynein complex is formed by six components comprising heavy, light and intermediate chains and has been extensively investigated for its role in axonal transport (Bhabha, Johnson, Schroeder, & Vale, 2016). Dynein intermediate chain interacts with the mega Dalton dynactin complex which in turn activates dynein motors for retrograde transport (Ayloo et al., 2014; Chowdhury, Ketcham, Schroer, & Lander, 2015; McKenney, Huynh, Tanenbaum, Bhabha, & Vale, 2014; Reck-Peterson, Redwine, Vale, & Carter, 2018). Initial studies on retrograde transport of trophic signals showed that internalized receptors and cognate neurotrophin ligands are rapidly transported from axon terminals by dynein, sliding along the microtubule rails to elicit a survival response in neuronal soma (Ehlers, Kaplan, Price, & Koliatsos, 1995; Heerssen, Pazyra, & Segal, 2004; Riccio, Pierchala, Ciarallo, & Ginty, 1997). Similarly, retrograde degenerative signaling by the p75NTR receptor also depends on dynein and dynactin subunit p150GLUED (Escudero et al., 2019; Pathak et al., 2018).
Upon arrival at the cell body, progressive SEs accumulate in perinuclear regions as well as dendrites (Barford et al., 2018; Sharma et al., 2010). Presumably, these locations hint at function. For example, it has long been speculated that SEs may reside close to (but not inside) the nucleus to facilitate transcriptional changes, which are important for patterning events and neuronal survival after target innervation. In contrast, progressive endosomes in the dendrite have been shown to facilitate synapse formation in a transcription independent manner (Sharma et al., 2010). In this way the target derived cue defines when and where a synaptic connection will form, which may be part of the basis for another fascinating problem in developmental biology: How a circuit’s form and function are matched to the needs of a target (i.e., target/systems matching).
We now appreciate that target derived neurotrophic signaling from an endosomal platform can regulate myriad developmental patterning events (e.g., target innervation, axon growth, and synapse formation). However, it is unclear whether the progressive endosome(s) that mediates all of these functions are molecularly uniform. In other words, does that same “flavor” of endosome mediate both synapse formation and cell survival? We know from classic cell biology studies that endosomes mature going from early endosomes (RAB5+) to late (RAB7+) or recycling (RAB4+ or RAB11+) endosomes, which may describe what an endosome may be doing once it gets back to the cell body (Figure 1 and Table 1).
FIGURE 1.
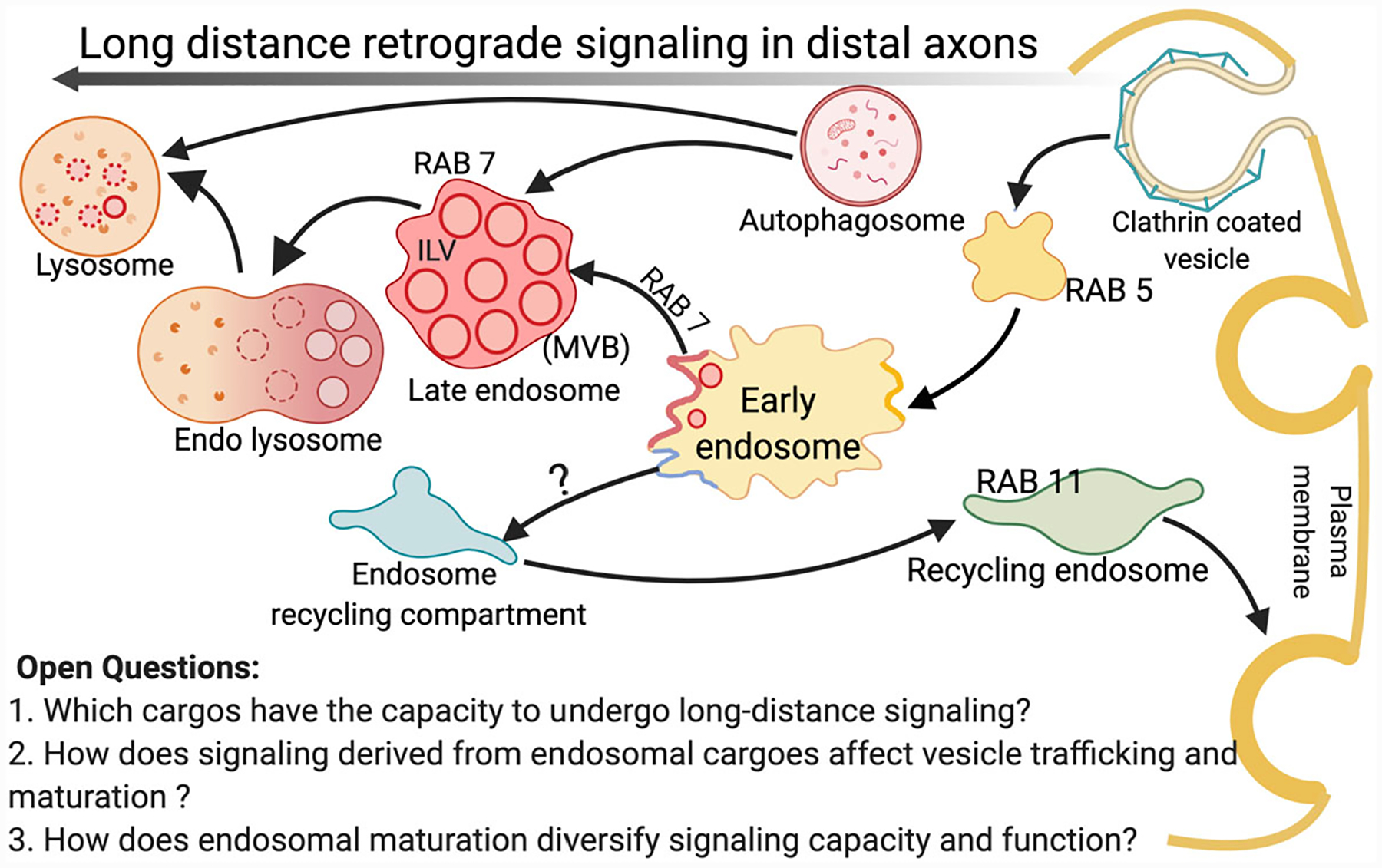
Vesicles involved in retrograde axonal transport and signaling. After ligand binding receptors are typically internalized at the plasma membrane of distal axons. RAB5 or early endosome Antigen1 (EEA1) is a marker for early endosomes where the cargo is sorted into different endocytic compartments, while RAB7 is thought to control the maturation of late endosomes, their retrograde trafficking, and fusion to lysosomes. RAB5 or RAB7 labeled vesicles, lysosomes, and autophagosomes have all been observed to undergo retrograde transport. The intralumenal vesicles (ILVs) accumulate in the lumen of endosomes, which consequently change their composition and morphology to convert into a multivesicular body (MVB), which can also be retrogradely transported
TABLE 1.
Vesicles known to retrogradely transport the cargo in different kinds of neurons
| Organelle (marker) | Cargo | Neuron | References |
|---|---|---|---|
| Autophagosome (LC3) | Cytosolic proteins (S, H), ubiquitin (S, H) | Sensory Hippocampal |
Maday, Wallace, & Holzbaur (2012) Maday et al. (2014) |
| Early endosome (RAB5/EEA1) | NGF (S), p-TRK-A & other TRK signaling proteins(S), TRK-B (M), p75NTR (M), [(P)2X3] (S), TRK-A (Sym), p75NTR (Sym) | Sensory Motor Sympathetic |
Delcroix et al. (2003) Deinhardt et al. (2006) Chen et al. (2012) Barford et al. (2018) Escudero et al. (2019) |
| Late endosomes (RAB7) | P75NTR (M), TRK-B (C), purinergic receptor [(P)2X3] (S), TRK-A(Sym) | Motor Cortical sensory sympathetic |
Deinhardt et al. (2006) Zhou et al. (2012) Chen et al. (2012) Barford et al. (2018) |
| Lysosomes (LAMP1/Cathepsin) | β-Secretase (BACE1), amyloid-β | Cortical | Gowrishankar et al. (2015) |
| Multivesicular bodies (MVB; CD63) | TRK-A (Sym), p75NTR (Sym) | Sympathetic |
Ye et al. (2018) Pathak et al. (2018) |
| Recycling endosomes (RAB11) | TRK-A(Sym) | Sympathetic | Barford et al. (2018) |
Note: Neuron type is indicated in parenthesis next to the cargo. S, sensory; H, hippocampal; M, motor; Sym, sympathetic. Cargoes in bold indicate overexpressed, otherwise endogenous. Cargoes and references in green are for progressive signaling, red is for regressive signaling and black is undetermined.
However, whether there is a division of labor amongst particular pools of early, late, or recycling SEs with respect to mediating post-target innervation patterning events remains unclear. Much is still being learned about where these molecular diversification events occur. Do they move retrogradely down the axon as a uniform population and then undergo maturation and molecular diversification in the cell body; or do they travel down the axon prediversified, primed to mediate distinct functions? The answer likely lies somewhere in the middle (Barford et al., 2017, 2018; Scott-Solomon & Kuruvilla, 2018; Wu et al., 2009; N. Yamashita & Kuruvilla, 2016; M. Ye, Lehigh, & Ginty, 2018).
4 |. RECENT EVIDENCE FOR LONG-DISTANCE REGRESSIVE SIGNALING
Early evidence regarding long-distance regressive signaling came from work in compartmentalized cultures examining c-JUN N-terminal kinase (JNK) and c-JUN (Lindwall & Kanje, 2005; Mok, Lund, & Campenot, 2009; Box 3). It was found that in response to injury or trophic factor withdrawal, phosphorylated-c-JUN (p-c-JUN) accumulated in the nucleus (Estus et al., 1994; Ham et al., 1995). In many scenarios including trophic withdrawal, accumulation of p-c-JUN is required for sympathetic neuron death (Eilers et al., 2001; Whitfield, Neame, Paquet, Bernard, & Ham, 2001). Interestingly, c-JUN phosphorylation was still observed when distal axons were trophically deprived while cell bodies received trophic support, suggesting a regressive signal originating from distal axons (Mok et al., 2009). The origin of these regressive signals at axon terminals was further supported by the observation that c-JUN activation can be prevented if the trophic support was available only on the axons, not the cell bodies. Along with other supporting data from sympathetic neurons, it was demonstrated that the retrogradely transported regressive signal contributed to c-JUN activation in a protein kinase C (PKC) and glycogen synthase kinase 3 (GSK3)-dependent manner (Mok et al., 2009). It is important to note that in conditions where trophic support was provided at the cell body, no cell death was observed despite the accumulation of p-c-JUN in the nucleus indicating cross-talk between progressive and regressive signals downstream of p-c-JUN.
BOX 3. c-Jun regulation for neuronal apoptosis.
c-JUN is a bZIP transcription factor belonging to the AP1 family of dimeric transcription factors (includes c-FOS, FOS-b, FRA1, JUN-B, and JUN-D) which bind to TPA responsive DNA elements (TRE; Deppmann, Alvania, & Taparowsky, 2006). c-JUN transactivation is a two-step process requiring docking of JNK to an NH2-terminal sequence followed by its phosphorylation (Hibi, Lin, Smeal, Minden, & Karin, 1993). Phosphorylated c-JUN alone binds to DNA as a low affinity homodimer or with high affinity as a heterodimer with FOS family members to regulate pro-apoptotic genes like MAPK phosphatase 1 and apoptotic facilitators of the BCL-2 protein family, such as DP5, BIM and PUMA (Ham et al., 1995; Harris & Johnson, 2001; Putcha et al., 2003; Whitfield et al., 2001; Wyttenbach & Tolkovsky, 2006). In addition to regulating the phosphorylation state of c-JUN, NGF withdrawal and the subsequent regressive signaling also induces c-JUN expression (Estus et al., 1994).
Although a role for JNK and c-JUN in neuronal cell death is well established, there remain many open questions regarding the mechanism of activation and the substrates downstream of JNK. Initially, studies from Rubin and colleagues found that expression of a “dominant negative” c-JUN protein (lacking a transactivation domain) could rescue sympathetic neuron death induced by trophic factor deprivation (TFD) (Ham et al., 1995). Corroborating evidence came from analysis of c-Jun knockout neurons or application of a JNK inhibitor, which resulted in resistance to TFD induced death (Eilers et al., 2001; Harding et al., 2001; Maroney et al., 1999; Palmada et al., 2002). Surprisingly, neurons harboring a c-Jun gene that is unable to be phosphorylated by JNK were capable of undergoing apoptosis in response to TFD (Besirli, Wagner, & Johnson, 2005). This finding set up a scenario whereby c-JUN expression but not its activation is required for trophic withdrawal induced cell death. Johnson and colleagues later suggested that this apparent paradox could be resolved in models where JNK mediated phosphorylation of substrates other than c-JUN are also required for cell death after TFD (Besirli et al., 2005; Putcha et al., 2003). Although JNK pathways have been under investigation for more than two decades, its complexity is still perplexing with an increasing number of interacting pathways and proteins which can also be direct substrates of JNK activity as recently reviewed (Zeke, Misheva, Reményi, & Bogoyevitch, 2016).
How does signaling from the distal axons activate c-JUN in the nucleus? The mixed lineage kinase (MLK) family member, Dual leucine zipper kinase, DLK provided an important clue: Lewcock and colleagues demonstrated that DLK activation in axons following TFD induced JNK signaling via its association with the scaffolding protein JNK-interacting protein, JIP3, leading to c-JUN phosphorylation and neuronal apoptosis (Ghosh et al., 2011). DLK is activated in several stress responses (Simon & Watkins, 2018; Siu, Sengupta Ghosh, & Lewcock, 2018) and is regulated by its palmitoylation, phosphorylation, and interactions with other signaling partners prior to ubiquitin-mediated degradation (Huntwork-Rodriguez et al., 2013). Palmitoylation of DLK was shown to modulate its vesicular localization and axonal trafficking, which further affects its catalytic activity and interaction with other signaling proteins in sensory axons (Holland et al., 2016).
Although it is evident that DLK-JNK-c-JUN signaling can result in regressive actions in neurons, such as cell death and axon degeneration, there are several complications to this simple linear degenerative pathway. For example, neurons contain high levels of constitutively activated JNK even in the absence of any stress related stimuli but they some-how have the ability to discriminate the basal JNK activity from pro-apoptotic signaling (Coffey, Hongisto, Dickens, Davis, & Courtney, 2000). Studies using Jnk null mice have demonstrated that each of the three mammalian Jnk genes has specific functions, which explains at least in part how this selectivity is achieved. In particular, JNK3 is critical for mediating neuronal death in most contexts, while JNK1 appears to be constitutively active (Coffey et al., 2002). However, even the subtypes of JNKs cannot fully explain the divergent actions of these kinases. For example, Goldstein and colleagues demonstrated that after nerve injury retrograde signaling to c-JUN by the JNK3-JIP complex promotes sensory axon regeneration (Barnat et al., 2010; Cavalli, Kujala, Klumperman, & Goldstein, 2005; Kenney & Kocsis, 1998; Lindwall, Dahlin, Lundborg, & Kanje, 2004). Some degree of selective signaling is likely to be mediated via the formation of specific combinations of JNKs, JIPs, and their upstream kinases, resulting in highly specific JNK signaling complexes with defined signaling outputs (Waetzig & Herdegen, 2005). Moreover, there is a tendency to view kinase activation as a simple binary signal, on or off. However, modeling of MAP kinase signaling, followed by empirical testing, has revealed that the frequency and intensity of activation as well as positive and negative feedback loops can significantly affect the outcome for the cell (Kochańczyk et al., 2017). A classic example that has been extensively modeled is the differential response of PC12 cells to kinetically distinct ERK signaling: These cells differentiate in response to sustained activation of ERK but proliferate following transient stimulation (Marshall, 1995; Qui & Green, 1992; Traverse, Gomez, Paterson, Marshall, & Cohen, 1992). Therefore, it is likely that factors such as ligand concentration, time of exposure, and local concentration of JNK and its regulators at the axon terminal, in the SEs and back at the cell soma, all have a role in determining the effects of JNK activation.
In addition, the neuronal cell body may serve as the final arbitrator of the response to various retrograde signals, including differential JNK complexes. For example, preventing new transcription or inhibition of GSK3β in cell bodies prevents axon degeneration in response to local TFD (Chen et al., 2012; Gerdts, Summers, Sasaki, DiAntonio, & Milbrandt, 2013). These reports suggested that local activation of pro-degenerative signals only in the axons may not be sufficient to cause cell death or axon degeneration and that cell bodies may also play a critical role. More conclusive evidence for integration of degenerative signaling by the neuronal soma came from a recent report demonstrating that the apoptotic machinery is present in sensory axons, but its ability to induce axon degeneration and ultimately neuron death was dependent on signaling from the cell body. Following TFD of distal axons, there was an increase in the transcription of the pro-apoptotic Bcl-2 family member Puma in the cell bodies (Simon et al., 2016). The increase in PUMA expression required both AKT inhibition and activation of the DLK/JNK/c-JUN pathway (Figure 2). Under pro-survival conditions, trophic factors received from distal axons up regulate the transcription of the pro-survival factor Bcl-w, whose mRNA is then transported anterogradely into axons where it is translated to suppress the apoptotic machinery required for degeneration (Cosker, Pazyra-Murphy, Fenstermacher, & Segal, 2013; Pazyra-Murphy et al., 2009). Thus NGF regulates transcription and subsequent axonal transport of Bcl-w by an RNA-binding protein to protect axons from degeneration (Cosker, Fenstermacher, Pazyra-Murphy, Elliott, & Segal, 2016). Following TFD, the increase in PUMA was sufficient to overcome the effects of axonal BCL-w and its related factor BCL-XL, leading to axon degeneration (Simon et al., 2016). These results indicated the presence of a cell body-derived anterograde degenerative signal; however, this signal was distinct from PUMA itself (PUMA is not trafficked to the axon) and remains to be identified.
FIGURE 2.
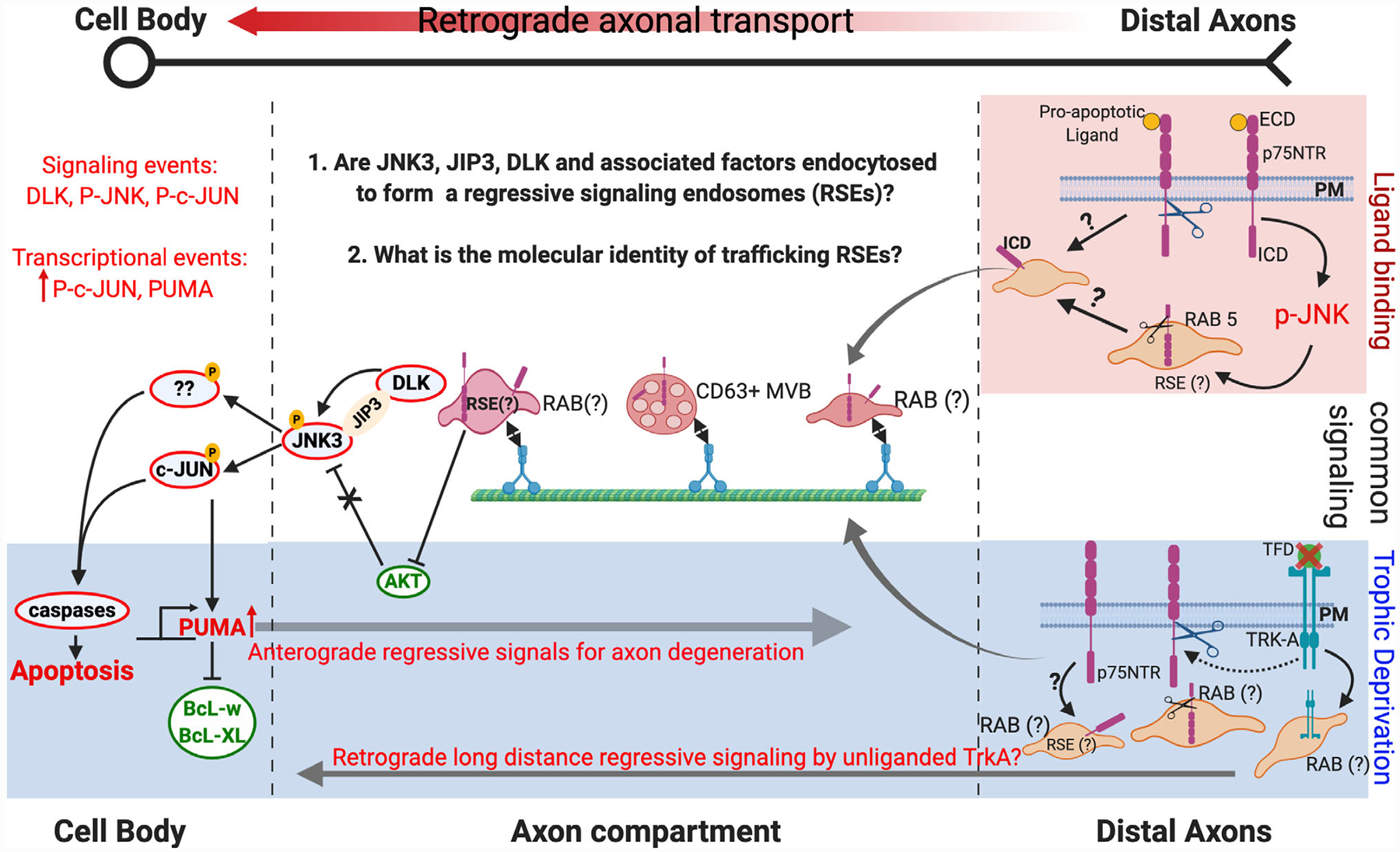
Comparison of regressive signaling under pro-apoptotic ligand binding and trophic factor deprivation (TFD). Top shade (red) depicts signaling after pro-apoptotic ligand binding to p75NTR, white area depicts common signaling while the bottom shade (blue) indicates signals identified under trophic factor deprivation (TFD) due to NGF withdrawal. Scissors indicate cleavage by γ-secretase to release the intracellular domain (ICD) of the receptor. Red outlined circles indicate regressive signaling components and Green outlined circles represent progressive signaling components. PM, plasma membrane; ECD, extracellular domain; ICD, intracellular domain; RSE, regressive signaling endosome. The sequence of events for p75NTR endocytosis and cleavage by γ-secretase in distal axons is not known. p75NTR does not function in a regressive manner in all cellular contexts (e.g., sensory neurons) and unliganded TRK-A has been shown to signal (also see section 4 B.2 on dependence receptors). Whether unliganded TRK-A is a component of RSE or contributes to p75NTR mediated long-distance regressive signaling remains to be tested
In an extensive review article on axonal transcription and translation, Twiss and colleagues recently tabulated the axonal transcriptomes of different neuronal subtypes (Kar, Lee, & Twiss, 2017) which raises another important question whether axonally translated proteins are affected by insults at the distal axon. Does the alteration in axonal translation affect mitochondrial dynamics and transport, which may contribute to energy imbalance within the axon? Does this energy imbalance within axons ultimately result in generation of retrograde regressive signals leading to neuronal apoptosis?
It is still far from clear how retrograde regressive signaling is initiated at the axon terminals and the components of the regressive signaling complex remain to be fully elucidated. The upstream neurotrophin receptors required for activation of long-distance regressive signaling were recently identified. However, it is not yet known whether DLK-JNK signaling complexes also have an analogous requirement for an activated death receptor as established for progressive SE carrying the survival signaling effectors like AKT after TRK receptor activation. Several candidate receptors will be explored below.
4.1 |. P75NTR mediated long-distance regressive signaling
Work from our group and others has shown that the death receptor p75NTR is capable of conveying regressive signals long-distances from distal axons back to the cell body (Escudero et al., 2019; Pathak et al., 2018). Interestingly, the pathways and molecules that p75NTR engages to induce cell death in response to pro-apoptotic ligand binding are distinct from TFD as shown in Figure 2. For example, both p75NTR activation by ligand binding and TFD stimulate phosphorylation of c-JUN by JNK; however, c-JUN is required for TFD but not ligand-mediated p75NTR induced apoptosis (Palmada et al., 2002).
Is the mechanism by which p75NTR conveys long-distance regressive signals from the distal tips of axons to cell bodies similar to what has been observed in progressive SEs? Certainly, we and others have shown that full length p75NTR can be retrogradely transported to cell bodies in a dynein dependent manner similar to what was described above for TRK-A, (Table 1; Escudero et al., 2019; Heerssen et al., 2004; Pathak et al., 2018). However, retrograde transport of full-length p75NTR may not be relevant to its regressive functions, since p75NTR is widely appreciated to undergo regulated proteolysis to impart the majority of its regressive functions (Kenchappa et al., 2006; Skeldal, Matusica, Nykjaer, & Coulson, 2011; Vicario, Kisiswa, Tann, Kelly, & Ibáñez, 2015). Indeed, we found that the liberated intracellular domain (ICD) of p75NTR was required for long range apoptotic signaling in response to TFD or ligand application to distal axons in sympathetic neurons (Pathak et al., 2018). Surprisingly, the nuclear enzyme histone deacetylase HDAC1 was found to be constitutively present in sympathetic axons and upon p75NTR activation, it deacetylated the dynactin subunit p150GLUED, which enhanced its interaction with the intermediate chain (DIC) of the molecular motor dynein. The increased interaction of p150GLUED with dynein promoted retrograde transport of p75NTR’s apoptotic signal (Pathak et al., 2018). In contrast, in CNS neurons HDAC1 was exported into the axons only in response to injury, where it inhibited mitochondrial transport by hindering interaction with the anterograde motor kinesin, thereby promoting axon degeneration (Kim et al., 2010).
Interestingly, axonal translation of dynein regulators can modify retrograde transport (Sahoo, Smith, Perrone-Bizzozero, & Twiss, 2018). For example, NGF induced retrograde transport of progressive SEs are regulated by locally translated mRNAs encoding Lis1 and p150Glued (Pafah1b1 and Dctn1, respectively), while only locally synthesized Lis1 is necessary for the retrograde transport of a pro-apoptotic signal generated in response to TFD (Villarin, McCurdy, Martínez, & Hengst, 2016). Both LIS1 and p150GLUED stimulate dynein (Baumbach et al., 2017; Gutierrez, Ackermann, Vershinin, & McKenney, 2017; King & Schroer, 2000; Pandey & Smith, 2011) and are involved in the initiation of retrograde transport toward cell bodies (Jha, Roostalu, Cade, Trokter, & Surrey, 2017; Moughamian & Holzbaur, 2012). These locally synthesized dynein regulators in distal axons may constitute an “on demand” system to alter retrograde transport of pro-survival to pro-death signals comprising p75NTR. Understanding the mechanism of p75NTR internalization in axons and signals governing the association between p75NTR and dynein represents an important molecular handle that will enable the field to define the precise mechanism by which regressive signals couple to the retrograde motor and execute their effects in cell bodies.
It is intriguing that the mechanism of p75NTR internalization depends on the neuronal type and the receptor’s spatial localization. For example, p75NTR follows different internalization pathways in the cell bodies compared to the axons of motor neurons. In the axons, clathrin dependent and independent pathways have been reported. The internalization path directs the trafficking of the internalized receptor, with clathrin dependent mechanisms targeting p75NTR for retrograde transport (Deinhardt, Reversi, Berninghausen, Hopkins, & Schiavo, 2007). In sympathetic neuron cell bodies, p75NTR partitions into lipid-raft membrane domains upon ligand binding and undergoes internalization in both a clathrin dependent and independent manner (Claudia A Escudero et al., 2014; Hibbert, Kramer, Miller, & Kaplan, 2006). In the absence of trophic signaling, BDNF treatment led to p75NTR internalization into RAB5-positive endosomes, and then rapid sorting to RAB11-recycling endosomes or CD63 positive multivesicular bodies avoiding the degrative route (Bronfman, Lazo, Flores, & Escudero, 2014; Escudero et al., 2014; Pathak et al., 2018). It is important to note here, BDNF can only bind to p75NTR in sympathetic neurons due to lack of Trk-B in these neurons. The sorting of p75NTR to RAB5-positive endosomes was shown to require axonal JNK activation after BDNF treatment exclusively on distal axons (Escudero et al., 2019). JNK activation was also shown to be a prerequisite for proteolytic processing of p75NTR (Kenchappa et al., 2010). This raises the possibility that p75NTR is cleaved in the endosomes to release the p75ICD (Figure 2). Indeed, the C-terminal fragment of p75NTR is enriched in the endosomal membranes and cleaved in a γ-secretase dependent manner (Urra et al., 2007). Together, these findings also raise several other interesting questions: (a) whether secretases, JNK, DLK and p75NTR itself are part and parcel of the same complex? (b) Whether JIP3 facilitates the transport of this complex in endocytic vesicles? (c) What is the molecular identity and nature of these vesicles and is there heterogeneity, both in the vesicle types and the signals they generate?
Although the above discussion focused on p75NTR binding to neurotrophins, it is important to point out that this receptor binds to many ligands and modifies several different receptors through direct interaction, leading to a variety of signals, beyond JNK activation. For example, in addition to neuronal apoptosis, p75NTR has also been shown to inhibit axonal growth by mediating the negative effects of myelin-associated glycoprotein (Yamashita, Higuchi, & Tohyama, 2002) and Nogo (Wang et al., 2002) through interaction with the Nogo receptor, NG-R (Yu, Guo, & Feng, 2004). A role for p75NTR in growth cone retraction has also been reported (Deinhardt et al., 2011) and recently this effect was reported to depend on p75NTR surface accumulation after pro-NGF interaction (Marchetti et al., 2019). The p75NTR-mediated growth cone retraction involved association of p75NTR with the VPS10-domain containing receptor SorCS2, leading to a decrease in the activity of the actin polymerization regulators FASCIN and RAC1 (Deinhardt et al., 2011). Additional regressive pathways associated with p75NTR include ceramide production, nuclear accumulation of NRIF and NF-kB translocation (Kraemer, Yoon, & Carter, 2014; Pathak & Carter, 2017). However, it remains an open question how distinct ligands and/or co-receptors influence p75NTR long-distance regressive signaling. During normal periods of developmental cell death, p75NTR is shown to promote survival of TRK expressing sensory neurons (Cheng et al., 2018). Similarly in the developing cerebellum, p75NTR signaling regulate granule neuron survival through decrease in its interaction with TNFR-associated factor 6 (TRAF6; Kisiswa, Fernández-Suárez, Sergaki, & Ibáñez, 2018). Thus, understanding how p75NTR switches its preferences for different co-receptors resulting in diverse signaling outputs, remains a long standing open question.
4.2 |. Long-distance signaling of regressive axon guidance cues
Long-distance regressive signaling has also been observed or suggested with axon guidance cue-receptor pairs including semaphorin-Neuropilin/Plexin, Slit-Robo, Netrin-deleted in colorectal carcinoma (DCC)/UNC-5, and Eph-Ephrin (Hamasaki et al., 2001; Kellermeyer et al., 2018; Marín et al., 2003; Wehner et al., 2016; Yue et al., 1999). Neurons are exposed to these repulsive cues at intermediate and final targets. This positioning allows proper pathfinding, which is required to build a functional nervous system. At cell biological level, these factors often promote growth cone collapse, and some have been implicated in suppression of neurite extension (Miyazaki et al., 1999; Niclou, Franssen, Ehlert, Taniguchi, & Verhaagen, 2003). There is growing evidence that receptors associated with growth cone attraction or repulsion are coupled to anti-caspase or pro-caspase activity. This has been proposed to be a mechanism to some axon guidance cue inputs resulting in attraction or repulsion (Kellermeyer et al., 2018). Caspase activity at distal axons is thought to be sub-lethal, and repulsive cues have largely been thought to act locally at the site of ligand receptor engagement. However, evidence is emerging that several repulsive cues may also act at a distance to regulate other regressive events such as cell death and synapse remodeling (Wehner et al., 2016).
4.2.1 |. Semaphorin3a
A hint that semaphorins may be able to undergo retrograde transport came from the observation that that Sema3a is endocytosed and trafficked from distal axons to cell bodies in compartmentalized sympathetic neuron cultures (Fournier et al., 2000). In sensory neurons it has been shown that Sema3a can promote death in NGF but not NT3 dependent neurons, which underscores the importance of cellular context for this signaling event (Gagliardini & Fankhauser, 1999). Using compartmentalized cultures, it was found that application of Sema3a only to the axonal chambers resulted in a decrease in cell number by more than half (Ben-Zvi et al., 2008). On the contrary, mice lacking Sema3a-neuropilin1 (NPN-1) signaling display normal developmental apoptosis in sensory and motor neurons (Haupt, Kloos, Faus-Kessler, & Huber, 2010). Pierchala and colleagues followed up on these studies using sympathetic neurons and found that, in vitro, long-distance Sema3a death signaling required microtubules (implicating dynein), which is similar to the other examples of long-distance regressive signaling from above (Figure 3). However, in contrast to the other mechanisms, Sema3a induces death via caspase 8 activation and does not require new transcription. This study went on to show that the receptors, NPN-1 and PlexinA3, are required for this effect and knockout of PlexinA3 and Npn-1 resulted in a significant reduction of apoptosis during early SCG development, in vivo (Wehner et al., 2016).
FIGURE 3.
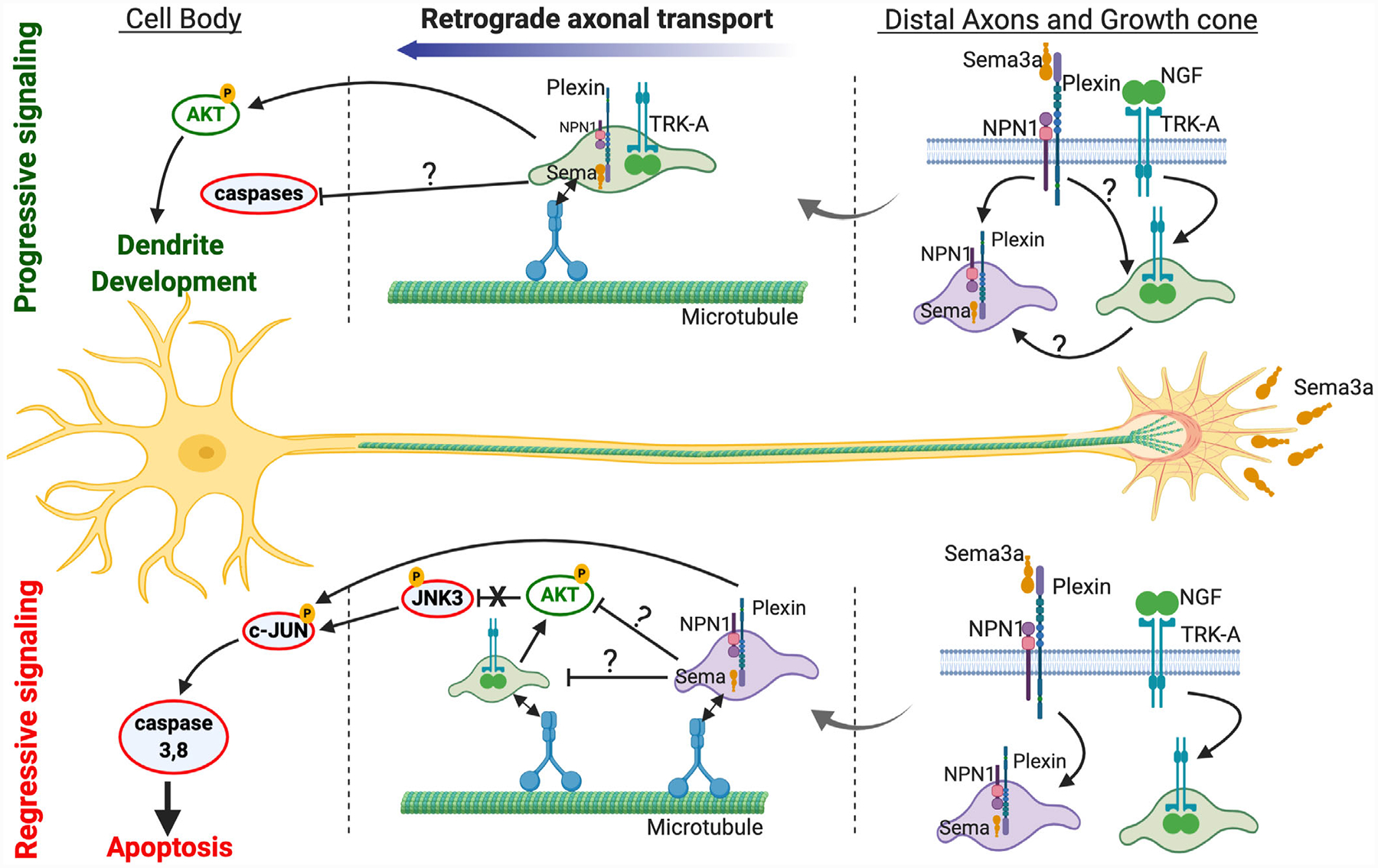
Long-distance signaling by Semaphorin 3a. Semaphorin 3A (Sema3A) exerts its biological actions through receptors, neuropilin-1 (Npn1) and plexin family members. For dendrite development, Sema3a are shown to induce PlexA4 colocalization and interaction with TRK-A receptor in growth cones and along axons (N. Yamashita et al., 2016). For regressive signaling, Sema3a signals retrograde apoptosis in developing sympathetic neurons via PlexinA3-NPN-1 which requires caspase 3 and 8 but does not depend on the availability of NGF on distal axons (Wehner et al., 2016). The untested hypothetical segments of retrograde signaling by Sema3a are indicated by question marks. Sema3a is depicted as sema due to space limitation
Sema3a has also been implicated in long-distance progressive signaling. Goshima and colleagues found that similar to peripheral neurons, hippocampal neurons are capable of transporting Sema3a from distal axons to the somatodendritic compartment (N. Yamashita et al., 2014). However, in contrast to peripheral neurons where sema3a-PlexinA3 long-distance complexes were found to induce death, the authors found that Sema3a-PlexinA4 long-distance complexes induced dendrite elaboration by promoting localization of AMPA receptor subunit, GLU-A2 to distal ends of hippocampal dendrites. This group later showed that propagation of this long-distance signaling relies on TRK-A and its downstream signaling pathway PI3K but surprisingly did not require TRK-A’s cognate ligand, NGF (N. Yamashita, Yamane, Suto, & Goshima, 2016). This implies that the Sema3a-PlexA4 complex may “hijack” the long-distance transport mechanism of classic progressive signals, an enticing molecular mechanism for long-distance regulation of dendrite development.
4.2.2 |. Dependence receptors
Beyond Sema3a signaling, there are observations that several guidance cues also act as dependence receptors. That is, in the presence of ligand, they can promote progressive events like differentiation, proliferation and survival but in the absence of ligand, they actively promote regressive signaling (Bredesen, Mehlen, & Rabizadeh, 2005). For example, in Drosophila, Netrin binding to DCC can result in attractive axon guidance, however in the unliganded condition DCC activates caspase cascades resulting in cell death (Finci, Zhang, Meijers, & Wang, 2015; Mehlen et al., 1998). Several other receptors including c-KIT, EPH-B3, UNC5, Patched-1, and RET have also been identified as dependence receptors (Negulescu & Mehlen, 2018). Additionally, several of the receptors that are discussed above have been identified as dependence receptors including p75NTR, TRK-A, and TRK-C (Bredesen et al., 2005; Nikoletopoulou et al., 2010; Rabizadeh et al., 1993). It stands to reason that these unliganded receptors may activate regressive processes in a long-distance manner by virtue of the fact that they are often trafficked to distal axons and the switch from liganded to unliganded would likely be detected most prominently at the distal tips of axons residing in intermediate and final targets. However, the mechanism of action for the long-distance transmission of such a signal remains unexamined. A common feature of most dependence receptors is the presence of a caspase cleavage site on the intracellular domain (Negulescu & Mehlen, 2018). This raises the intriguing possibility that the liberated ICD of these receptors may induce death from a distance in a manner similar to p75NTR. While the field has only scratched the surface for the role of dependence receptors in neural development, the notion of active and opposing signaling in liganded and unliganded states has profound implications and suggests that long-distance progressive and regressive signaling may be two sides of the same coin or in this case two sides of the same receptor. We will next explore this notion in the context of neurodegenerative diseases where it has been observed in a handful of examples that loss of long-distance progressive signaling is accompanied by an increase in long-distance regressive signaling.
5 |. LONG-DISTANCE REGRESSIVE SIGNALING IN NEURAL PATHOLOGY
Alterations in axonal trafficking are now being reported as possible mechanisms driving pathology underlying nerve injury and several neurodegenerative disorders including Amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD) (Guadagno & Progida, 2019; Perlson, Maday, Fu, Moughamian, & Holzbaur, 2010). While loss of axonal transport of progressive signaling appears to be a hallmark of various neurological diseases (Maday et al., 2014; Sleigh, Rossor, Fellows, Tosolini, & Schiavo, 2019) a theme is emerging that this is accompanied by increases in long-distance regressive signaling.
5.1 |. Motor neuron degeneration
Two major forms of motor neuron pathology, ALS and spinal muscular dystrophy are characterized by neuromuscular junction (NMJ) disruption, axon degeneration, and eventual death of motor neurons. Axonal transport defects are considered amongst the earliest disease phenotypes in ALS with eventual diminution of long-distance progressive neurotrophic signaling being implicated in the degeneration of these neurons (De Vos & Hafezparast, 2017). Beyond defects in long-distance progressive signaling (e.g., p-TRK, ERK1/2, and ERK5) an increase in long-distance regressive signaling (e.g., p-JNK, p75ICD, and cleaved caspase8) has also been observed in the SOD1G93A mouse model of ALS (Perlson et al., 2009). The retrograde transport of pro-degenerative signals was also supported by another study utilizing these mice, wherein chronic impairment of dynein/dynactin function and axonal transport by overexpression of dynein adaptor, bicaudal D2-N terminus demonstrated a delay in disease onset and increased life span (Teuling et al., 2008). The mechanism for switching from long-distance prosurvival to apoptotic signaling is not known and seems to be context dependent and subject to combinatorial regulation by several factors (Bothwell, 2019; Fu, Hardy, & Duff, 2018; Perlson et al., 2010). Nevertheless, delineation of this molecular switch would represent an attractive therapeutic target.
5.2 |. Alzheimer’s disease
AD has also been associated with disrupted long-distance trafficking of progressive signals, however it is still not clear whether the axonal transport defects are the cause, a facilitating factor, or the result of regressive signaling initiated in axons (X.-Q. Chen & Mobley, 2019; X.-Q. Chen, Sawa, & Mobley, 2018). A useful model of AD has been to study individuals with Down syndrome (DS) who develop AD-like neuropathology by the age 40 with 100% penetrance (Ballard, Mobley, Hardy, Williams, & Corbett, 2016). DS is a congenital defect with trisomy of chromosome 21, triplicating the dosage of Amyloid precursor protein (APP) (Doran et al., 2017; Prasher et al., 1998; Wiseman et al., 2015). In a mouse model of DS (Ts65Dn), Mobley and colleagues showed that NGF transport was compromised, which promoted degeneration of basal forebrain cholinergic neurons (BFCNs) (Salehi et al., 2006). However, disrupted retrograde transport due to increased APP dosage affected only a subset of endosomal cargoes, without any detectable change in components of the dynein–dynactin complex in the Ts65Dn hippocampus (Salehi et al., 2006). It is tempting to speculate an involvement of long-distance regressive signaling, which may lead to cholinergic neuron degeneration in DS, with implications in AD. Indeed, several studies hint at active long-distance regressive signaling in AD. For example, axonal exposure to Aβ42 (Amyloid β 1–42) has been shown to be sufficient to induce long-distance neurodegeneration in AD brains (Liu et al., 2008), reasoning that alterations within axons are the primary events resulting in classical pathological changes in AD (Krstic & Knuesel, 2013). In support of this, exposure of axon terminals to pathogenic levels of Aβ42 initiated axonal synthesis of proteins, including the transcription factor ATF4 which retrogradely transmitted neurodegenerative signals sufficient to induce cholinergic neuron apoptosis (Baleriola et al., 2014). Along the same line, it has been shown that amyloid fibrils are retrogradely transported in axons (Brahic, Bousset, Bieri, Melki, & Gitler, 2016; Song et al., 2014). Interestingly, p75NTR and Sortilin are able to bind and internalize the amyloid peptide, mediating neuritic dystrophy, and neuronal death (Hu et al., 2013; Ovsepian et al., 2014; Takamura et al., 2012). Consistent with this, reducing p75NTR from the brain or basal forebrain cholinergic neurons, reduced amyloidosis and the cognitive impairment observed in AD mouse models (Jian et al., 2016; Qian et al., 2019). Considering all the evidence, an attractive hypothesis is that amyloid binding to p75NTR in axons contributes to retrograde transport of regressive signaling and axonal pathology in AD brain.
6 |. CONCLUSION
Long-distance regressive signaling is a relatively new field and the approaches to study this type of signaling remain underdeveloped. As mentioned above, the more mature field of long-distance progressive signaling, provides somewhat of a playbook for examining other forms of long-distance signaling. As a first level analysis, compartmentalized culture approaches (e.g., Campenot chambers, microfluidic devices) are used to determine whether a ligand added to distal axon compartments can induce signaling events, transcriptional responses, or developmental consequences (e.g., synapse restriction, cell death) in the soma. While this type of analysis asks whether long-distance signaling occurs in vitro, it does not address how it occurs. This remained a point of contention for many years with respect to long-distance NGF progressive signaling in the PNS (Ginty & Segal, 2002) as there were several mechanisms proposed including ligand dependent or independent transport of TRK-A+ endosomes, and a “domino model” or “wave propagation model” whereby long-distance signaling occurs independent of an endosomal platform (Miller & Kaplan, 2001; Senger & Campenot, 1997). Using specialized compartmentalized culture systems (3 chamber), pharmacology, and cell permeable ligand neutralizing antibodies Ginty and colleagues challenged each of these models and concluded that the NGF-TRK-A SE is the primary mode of long-distance progressive signaling in sympathetic neurons (H. Ye, Kuruvilla, Zweifel, & Ginty, 2003). This same level of experimental scrutiny has not yet been applied to determine how long-distance regressive signals are carried.
Beyond the playbook used for long-distance progressive signaling, long-distance regressive signals present several unique challenges that will require approach development beyond what is described above. For example, many regressive signaling events require receptor cleavage, which necessitates the tracking of full-length receptors and their cleavage products. Moreover, if a liberated intracellular domain is the primary carrier of long-distance regressive signal, by what mechanism does it associate with the endosomal carrier? Another challenge that the more mature field of long-distance progressive signaling has historically faced is, whether in vitro observations are relevant in vivo. Approaches whereby an epitope tag is knocked in frame to a receptor extracellular domain have provided a foothold for examining long-distance trafficking in vivo (Barford et al., 2018; Lehigh, West, & Ginty, 2017; M. Ye et al., 2018). Similar tools must be developed for regressive receptors, which will allow antibody feeding in target tissues and in vivo imaging in somas and dendrites, while also accounting for cleavage events.
The pathways that we have described above as mediators of long-distance regressive signaling are by no means exhaustive and will almost certainly expand with our understanding of this emergent principle. The vast majority of growth factors and axon guidance cues have not been tested for their ability to signal long distances. However, clues have emerged for other pathways like EphrinA5-EPH-A, indicating that they promote long-distance regressive signaling (Yue et al., 1999). There are several other pathways that also represent excellent candidates to mediate regressive signaling at a distance including SMAD, Slit-Robo, TNFR family members, GPCRs, and so on. However, the challenge going forward will be to test the capacities of individual ligand-receptor pairs or downstream signaling pathways using approaches like compartmentalized culture systems and further examine them in vivo. In this way, we can begin to ask the who, what, when, where, why, and how of long-distance regressive signaling.
ACKNOWLEDGMENTS
We thank Dr Bettina Winckler, Dr Austin Keeler, Dr Anthony Spano, and Ashley Mason for helpful discussions and comments on the manuscript. We are grateful to Dr Brian Pierchala (Indiana University) and Dr Thomas Kidd (University of Nevada, Reno) for insightful conversations during the preparation of this manuscript. We also acknowledge the helpful discussions from Brandon Podyma, Sushanth Kumar, Yu Yong, and Dr Vitally Zimyanin. Our apologies to authors whose work could not be cited due to space considerations. The development of ideas described in this article were supported by NIH-NINDS grant R01NS091617 and the Owens family foundation awarded to C. D. D.; NIH grant R01NS102365 and R01NS107456 to B. D. C.; Fondecyt 1171137/PIA BASAL AFB170005 to F. C. B.
Funding information
Fondo Nacional de Desarrollo Científico y Tecnológico, Grant/Award Number: 1171137/PIA BASAL AFB170005; National Institute of Neurological Disorders and Stroke, Grant/Award Numbers: R01NS091617, R01NS102365, R01NS107456; Owens family foundation
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
REFERENCES
- Ascano M, Bodmer D, & Kuruvilla R (2012). Endocytic trafficking of neurotrophins in neural development. Trends in Cell Biology, 22(5), 266–273. 10.1016/j.tcb.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayloo S, Lazarus JE, Dodda A, Tokito M, Ostap EM, & Holzbaur ELF (2014). Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nature Communications, 5, 4807 10.1038/ncomms5807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleriola J, Walker CA, Jean YY, Crary JF, Troy CM, Nagy PL, & Hengst U (2014). Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell, 158(5), 1159–1172. 10.1016/j.cell.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Mobley W, Hardy J, Williams G, & Corbett A (2016). Dementia in Down’s syndrome. Lancet Neurology, 15(6), 622–636. 10.1016/S1474-4422(16)00063-6 [DOI] [PubMed] [Google Scholar]
- Barford K, Deppmann C, & Winckler B (2017). The neurotrophin receptor signaling endosome: Where trafficking meets signaling. Developmental Neurobiology, 77(4), 405–418. 10.1002/dneu.22427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford K, Keeler A, McMahon L, McDaniel K, Yap CC, Deppmann CD, & Winckler B (2018). Transcytosis of TrkA leads to diversification of dendritic signaling endosomes. Scientific Reports, 8(1), 4715 10.1038/s41598-018-23036-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnat M, Enslen H, Propst F, Davis RJ, Soares S, & Nothias F (2010). Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. The Journal of Neuroscience, 30(23), 7804–7816. 10.1523/JNEUROSCI.0372-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach J, Murthy A, McClintock MA, Dix CI, Zalyte R, Hoang HT, & Bullock SL (2017). Lissencephaly-1 is a context-dependent regulator of the human dynein complex. eLife, 6, e21768 10.7554/eLife.21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Manor O, Schachner M, Yaron A, Tessier-Lavigne M, & Behar O (2008). The Semaphorin receptor PlexinA3 mediates neuronal apoptosis during dorsal root ganglia development. The Journal of Neuroscience, 28(47), 12427–12432. 10.1523/JNEUROSCI.3573-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besirli CG, Wagner EF, & Johnson EM (2005). The limited role of NH2-terminal c-Jun phosphorylation in neuronal apoptosis: Identification of the nuclear pore complex as a potential target of the JNK pathway. The Journal of Cell Biology, 170(3), 401–411. 10.1083/jcb.200501138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabha G, Johnson GT, Schroeder CM, & Vale RD (2016). How dynein moves along microtubules. Trends in Biochemical Sciences, 41(1), 94–105. 10.1016/j.tibs.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer D, Ascaño M, & Kuruvilla R (2011). Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth. Neuron, 70(6), 1085–1099. 10.1016/j.neuron.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi D, Chivatakarn O, Bai G, Abdesselem H, Lettieri K, Marquardt T, … Pfaff SL (2012). Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell, 148(3), 568–582. 10.1016/j.cell.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M (2019). Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function. [version 1; peer review: 3 approved]. F1000Research, 8, 1658 10.12688/f1000research.19174.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M, Bousset L, Bieri G, Melki R, & Gitler AD (2016). Axonal transport and secretion of fibrillar forms of α-synuclein, Aβ42 peptide and HTTExon 1. Acta Neuropathologica, 131(4), 539–548. 10.1007/s00401-016-1538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE, Mehlen P, & Rabizadeh S (2005). Receptors that mediate cellular dependence. Cell Death and Differentiation, 12(8), 1031–1043. 10.1038/sj.cdd.4401680 [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Lazo OM, Flores C, & Escudero CA (2014). Spatiotemporal intracellular dynamics of neurotrophin and its receptors. Implications for neurotrophin signaling and neuronal function. Handbook of Experimental Pharmacology, 220, 33–65. 10.1007/978-3-642-45106-5_3 [DOI] [PubMed] [Google Scholar]
- Bueker ED (1948). Implantation of tumors in the hind limb field of the embryonic chick and the developmental response of the lumbosacral nervous system. The Anatomical Record, 102(3), 369–389. 10.1002/ar.1091020309 [DOI] [PubMed] [Google Scholar]
- Butler SJ, & Bronner ME (2015). From classical to current: Analyzing peripheral nervous system and spinal cord lineage and fate. Developmental Biology, 398(2), 135–146. 10.1016/j.ydbio.2014.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, & Goldstein LSB (2005). Sunday driver links axonal transport to damage signaling. The Journal of Cell Biology, 168(5), 775–787. 10.1083/jcb.200410136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV (2003). Neurotrophins and their receptors: A convergence point for many signalling pathways. Nature Reviews. Neuroscience, 4 (4), 299–309. 10.1038/nrn1078 [DOI] [PubMed] [Google Scholar]
- Chen M, Maloney JA, Kallop DY, Atwal JK, Tam SJ, Baer K, … Watts RJ (2012). Spatially coordinated kinase signaling regulates local axon degeneration. The Journal of Neuroscience, 32(39), 13439–13453. 10.1523/JNEUROSCI.2039-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-Q, & Mobley WC (2019). Alzheimer disease pathogenesis: Insights from molecular and cellular biology studies of oligomeric aβ and tau species. Frontiers in Neuroscience, 13, 659 10.3389/fnins.2019.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-Q, Sawa M, & Mobley WC (2018). Dysregulation of neurotrophin signaling in the pathogenesis of Alzheimer disease and of Alzheimer disease in down syndrome. Free Radical Biology & Medicine, 114, 52–61. 10.1016/j.freeradbiomed.2017.10.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng I, Jin L, Rose LC, & Deppmann CD (2018). Temporally restricted death and the role of p75NTR as a survival receptor in the developing sensory nervous system. Developmental Neurobiology, 78(7), 701–717. 10.1002/dneu.22591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Ketcham SA, Schroer TA, & Lander GC (2015). Structural organization of the dynein-dynactin complex bound to microtubules. Nature Structural & Molecular Biology, 22(4), 345–347. 10.1038/nsmb.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey ET, Hongisto V, Dickens M, Davis RJ, & Courtney MJ (2000). Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. The Journal of Neuroscience, 20(20), 7602–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey ET, Smiciene G, Hongisto V, Cao J, Brecht S, Herdegen T, & Courtney MJ (2002). C-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. The Journal of Neuroscience, 22(11), 4335–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S (1960). Purification of a nerve-growth promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum. Proceedings of the National Academy of Sciences of the United States of America, 46(3), 302–311. 10.1073/pnas.46.3.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Levi-Montalcini R (1956). A nerve growth-stimulating factor isolated from snake venom. Proceedings of the National Academy of Sciences of the United States of America, 42(9), 571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Levi-Montalcini R, & Hamburger V (1954). A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proceedings of the National Academy of Sciences of the United States of America, 40(10), 1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Pazyra-Murphy MF, Fenstermacher SJ, & Segal RA (2013). Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. The Journal of Neuroscience, 33(12), 5195–5207. 10.1523/JNEUROSCI.3862-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Fenstermacher SJ, Pazyra-Murphy MF, Elliott HL, & Segal RA (2016). The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nature Neuroscience, 19(5), 690–696. 10.1038/nn.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, & Hafezparast M (2017). Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiology of Disease, 105, 283–299. 10.1016/j.nbd.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, & Barde Y-A (2002). The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nature Neuroscience, 5(11), 1131–1136. 10.1038/nn1102-1131 [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, … Hempstead BL (2011). Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Science Signaling, 4(202), ra82 10.1126/scisignal.2002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, … Schiavo G (2006). Rab5 and Rab7 Control endocytic sorting along the axonal retrograde transport pathway. Neuron, 52(2), 293–305. 10.1016/j.neuron.2006.08.018 [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Reversi A, Berninghausen O, Hopkins CR, & Schiavo G (2007). Neurotrophins redirect p75NTR from a clathrin-independent to a clathrin-dependent endocytic pathway coupled to axonal transport. Traffic, 8(12), 1736–1749. 10.1111/j.1600-0854.2007.00645.x [DOI] [PubMed] [Google Scholar]
- Delcroix J-D, Valletta JS, Wu C, Hunt SJ, Kowal AS, & Mobley WC (2003). NGF signaling in sensory neurons. Neuron, 39(1), 69–84. 10.1016/s0896-6273(03)00397-0 [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Alvania RS, & Taparowsky EJ (2006). Cross-species annotation of basic leucine zipper factor interactions: Insight into the evolution of closed interaction networks. Molecular Biology and Evolution, 23(8), 1480–1492. 10.1093/molbev/msl022 [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, & Ginty DD (2008). A model for neuronal competition during development. Science, 320(5874), 369–373. 10.1126/science.1152677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Fegan KS, Ren X, Hillier SG, & Duncan WC (2011). Glucocorticoid regulation of SLIT/ROBO tumour suppressor genes in the ovarian surface epithelium and ovarian cancer cells. PLoS One, 6(11), e27792 10.1371/journal.pone.0027792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran E, Keator D, Head E, Phelan MJ, Kim R, Totoiu M, … Lott IT (2017). Down syndrome, partial trisomy 21, and absence of Alzheimer’s disease: The role of APP. Journal of Alzheimer’s Disease, 56(2), 459–470. 10.3233/JAD-160836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Kaplan DR, Price DL, & Koliatsos VE (1995). NGF-stimulated retrograde transport of trkA in the mammalian nervous system. The Journal of Cell Biology, 130(1), 149–156. 10.1083/jcb.130.1.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers A, Whitfield J, Shah B, Spadoni C, Desmond H, & Ham J (2001). Direct inhibition of c-Jun N-terminal kinase in sympathetic neurones prevents c-Jun promoter activation and NGF withdrawal-induced death. Journal of Neurochemistry, 76(5), 1439–1454. 10.1046/j.1471-4159.2001.00150.x [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, & Milbrandt J (2001). RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development, 128(20), 3963–3974. [DOI] [PubMed] [Google Scholar]
- Escudero CA, Cabeza C, Moya-Alvarado G, Maloney MT, Flores CM, Wu C, … Bronfman FC (2019). C-Jun N-terminal kinase (JNK)-dependent internalization and Rab5-dependent endocytic sorting mediate long-distance retrograde neuronal death induced by axonal BDNF-p75 signaling. Scientific Reports, 9(1), 6070 10.1038/s41598-019-42420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero CA, Lazo OM, Galleguillos C, Parraguez JI, Lopez-Verrilli MA, Cabeza C, … Bronfman FC (2014). The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. Journal of Cell Science, 127(Pt 9), 1966–1979. 10.1242/jcs.141754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, & Johnson EM (1994). Altered gene expression in neurons during programmed cell death: Identification of c-Jun as necessary for neuronal apoptosis. The Journal of Cell Biology, 127(6 Pt 1), 1717–1727. 10.1083/jcb.127.6.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finci L, Zhang Y, Meijers R, & Wang JH (2015). Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Progress in Biophysics and Molecular Biology, 118(3), 153–160. 10.1016/j.pbiomolbio.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, & Strittmatter SM (2000). Semaphorin3A Enhances endocytosis at sites of receptor-F-Actin colocalization during growth cone collapse. The Journal of Cell Biology, 149(2), 411–422. 10.1083/jcb.149.2.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Rodríguez-Tébar A, & Barde YA (1996). Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature, 383(6596), 166–168. 10.1038/383166a0 [DOI] [PubMed] [Google Scholar]
- Fu H, Hardy J, & Duff KE (2018). Selective vulnerability in neurodegenerative diseases. Nature Neuroscience, 21(10), 1350–1358. 10.1038/s41593-018-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardini V, & Fankhauser C (1999). Semaphorin III can induce death in sensory neurons. Molecular and Cellular Neurosciences, 14 (4–5), 301–316. 10.1006/mcne.1999.0787 [DOI] [PubMed] [Google Scholar]
- Gentry JJ, Barker PA, & Carter BD (2004). The p75 neurotrophin receptor: Multiple interactors and numerous functions. Progress in Brain Research, 146, 25–39. 10.1016/S0079-6123(03)46002-0 [DOI] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Sasaki Y, DiAntonio A, & Milbrandt J (2013). Sarm1-mediated axon degeneration requires both SAM and TIR interactions. The Journal of Neuroscience, 33(33), 13569–13580. 10.1523/JNEUROSCI.1197-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, & Lewcock JW (2011). DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. The Journal of Cell Biology, 194(5), 751–764. 10.1083/jcb.201103153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty DD, & Segal RA (2002). Retrograde neurotrophin signaling: Trk-ing along the axon. Current Opinion in Neurobiology, 12(3), 268–274. 10.1016/S0959-4388(02)00326-4 [DOI] [PubMed] [Google Scholar]
- Glebova NO, & Ginty DD (2005). Growth and survival signals controlling sympathetic nervous system development. Annual Review of Neuroscience, 28, 191–222. 10.1146/annurev.neuro.28.061604.135659 [DOI] [PubMed] [Google Scholar]
- Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, … Mobley WC (1996). Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. The Journal of Neuroscience, 16(24), 7950–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno NA, & Progida C (2019). Rab gtpases: Switching to human diseases. Cell, 8(8), E909 10.3390/cells8080909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez PA, Ackermann BE, Vershinin M, & McKenney RJ (2017). Differential effects of the dynein-regulatory factor Lissencephaly-1 on processive dynein-dynactin motility. The Journal of Biological Chemistry, 292(29), 12245–12255. 10.1074/jbc.M117.790048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, … Ferguson SM (2015). Massive accumulation of luminal pro-tease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proceedings of the National Academy of Sciences, 112(28), E3699–E3708. 10.1073/pnas.1510329112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallböök F, Wilson K, Thorndyke M, & Olinski RP (2006). Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain, Behavior and Evolution, 68(3), 133–144. 10.1159/000094083 [DOI] [PubMed] [Google Scholar]
- Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, & Rubin LL (1995). A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron, 14(5), 927–939. 10.1016/0896-6273(95)90331-3 [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Goto S, Nishikawa S, & Ushio Y (2001). A role of netrin-1 in the formation of the subcortical structure striatum: Repulsive action on the migration of late-born striatal neurons. The Journal of Neuroscience, 21(12), 4272–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V (1939). Motor and sensory hyperplasia following limb-bud transplantations in Chick embryos. Physiological Zoology, 12(3), 268–284. 10.1086/physzool.12.3.30151503 [DOI] [Google Scholar]
- Hamburger V, & Levi-Montalcini R (1949). Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. The Journal of Experimental Zoology, 111(3), 457–501. 10.1002/jez.1401110308 [DOI] [PubMed] [Google Scholar]
- Harding TC, Xue L, Bienemann A, Haywood D, Dickens M, Tolkovsky AM, & Uney JB (2001). Inhibition of JNK by overexpression of the JNL binding domain of JIP-1 prevents apoptosis in sympathetic neurons. The Journal of Biological Chemistry, 276(7), 4531–4534. 10.1074/jbc.C000815200 [DOI] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, & Ginty DD (2011). Recruitment of Actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell, 146(3), 421–434. 10.1016/j.cell.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, & Johnson EM (2001). BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. The Journal of Biological Chemistry, 276(41), 37754–37760. 10.1074/jbc.M104073200 [DOI] [PubMed] [Google Scholar]
- Haupt C, Kloos K, Faus-Kessler T, & Huber AB (2010). Semaphorin 3A-Neuropilin-1 signaling regulates peripheral axon fasciculation and pathfinding but not developmental cell death patterns. The European Journal of Neuroscience, 31(7), 1164–1172. 10.1111/j.1460-9568.2010.07154.x [DOI] [PubMed] [Google Scholar]
- Heerssen HM, Pazyra MF, & Segal RA (2004). Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nature Neuroscience, 7(6), 596–604. 10.1038/nn1242 [DOI] [PubMed] [Google Scholar]
- Hibbert AP, Kramer BMR, Miller FD, & Kaplan DR (2006). The localization, trafficking and retrograde transport of BDNF bound to p75NTR in sympathetic neurons. Molecular and Cellular Neurosciences, 32(4), 387–402. 10.1016/j.mcn.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, & Karin M (1993). Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes & Development, 7(11), 2135–2148. 10.1101/gad.7.11.2135 [DOI] [PubMed] [Google Scholar]
- Hohn A, Leibrock J, Bailey K, & Barde YA (1990). Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature, 344(6264), 339–341. 10.1038/344339a0 [DOI] [PubMed] [Google Scholar]
- Holland SM, Collura KM, Ketschek A, Noma K, Ferguson TA, Jin Y, … Thomas GM (2016). Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proceedings of the National Academy of Sciences of the United States of America, 113(3), 763–768. 10.1073/pnas.1514123113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y, Kawano M, Kohsaka S, & Ogawa M (2010). Axonal projections of mechanoreceptive dorsal root ganglion neurons depend on ret. Development, 137(14), 2319–2328. 10.1242/dev.046995 [DOI] [PubMed] [Google Scholar]
- Hu Y, Lee X, Shao Z, Apicco D, Huang G, Gong BJ, … Mi S (2013). A DR6/p75(NTR) complex is responsible for β-amyloid-induced cortical neuron death. Cell Death & Disease, 4, e579 10.1038/cddis.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, & Reichardt LF (2003). Trk receptors: Roles in neuronal signal transduction. Annual Review of Biochemistry, 72, 609–642. 10.1146/annurev.biochem.72.121801.161629 [DOI] [PubMed] [Google Scholar]
- Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, … Lewcock JW (2013). JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. The Journal of Cell Biology, 202(5), 747–763. 10.1083/jcb.201303066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Ibáñez CF, Nye SH, McClain J, Jones PF, Gies DR, … Squinto SP (1992). Mammalian neurotrophin-4: Structure, chro-mosomal localization, tissue distribution, and receptor specificity. Proceedings of the National Academy of Sciences of the United States of America, 89(7), 3060–3064. 10.1073/pnas.89.7.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, & Enomoto H (2016). Retrograde transport of neurotrophic factor signaling: Implications in neuronal development and pathogenesis. Journal of Biochemistry, 160(2), 77–85. 10.1093/jb/mvw037 [DOI] [PubMed] [Google Scholar]
- Ivanisevic L, Zheng W, Woo SB, Neet KE, & Saragovi HU (2007). TrkA receptor “hot spots” for binding of NT-3 as a heterologous ligand. The Journal of Biological Chemistry, 282(23), 16754–16763. 10.1074/jbc.M701996200 [DOI] [PubMed] [Google Scholar]
- Jha R, Roostalu J, Cade NI, Trokter M, & Surrey T (2017). Combinatorial regulation of the balance between dynein microtubule end accumulation and initiation of directed motility. The EMBO Journal, 36(22), 3387–3404. 10.15252/embj.201797077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian C, Zou D, Luo C, Liu X, Meng L, Huang J, … Wu Y (2016). Cognitive deficits are ameliorated by reduction in amyloid β accumulation in Tg2576/p75(NTR+/−) mice. Life Sciences, 155, 167–173. 10.1016/j.lfs.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Jones KR, & Reichardt LF (1990). Molecular cloning of a human gene that is a member of the nerve growth factor family. Proceedings of the National Academy of Sciences of the United States of America, 87(20), 8060–8064. 10.1073/pnas.87.20.8060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AN, Lee SJ, & Twiss JL (2017). Expanding axonal transcriptome brings new functions for axonally synthesized proteins in health and disease. The Neuroscientist, 24(2), 111–129. 10.1177/1073858417712668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermeyer R, Heydman LM, Mastick GS, & Kidd T (2018). The role of apoptotic signaling in axon guidance. Journal of Developmental Biology, 6(4), 24 10.3390/jdb6040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Tep C, Korade Z, Urra S, Bronfman FC, Yoon SO, & Carter BD (2010). p75 neurotrophin receptor-mediated apoptosis in sympathetic neurons involves a biphasic activation of JNK and up-regulation of tumor necrosis factor-alpha-converting enzyme/ADAM17. The Journal of Biological Chemistry, 285(26), 20358–20368. 10.1074/jbc.M109.082834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, & Carter BD (2006). Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron, 50(2), 219–232. 10.1016/j.neuron.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Kenney AM, & Kocsis JD (1998). Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. The Journal of Neuroscience, 18(4), 1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, & Casaccia P (2010). HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nature Neuroscience, 13(2), 180–189. 10.1038/nn.2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, & Schroer TA (2000). Dynactin increases the processivity of the cytoplasmic dynein motor. Nature Cell Biology, 2(1), 20–24. 10.1038/71338 [DOI] [PubMed] [Google Scholar]
- Kisiswa L, Fernández-Suárez D, Sergaki MC, & Ibáñez CF (2018). RIP2 gates TRAF6 interaction with death receptor p75NTR to regulate cerebellar granule neuron survival. Cell Reports, 24(4), 1013–1024. 10.1016/j.celrep.2018.06.098 [DOI] [PubMed] [Google Scholar]
- Kochańczyk M, Kocieniewski P, Kozłowska E, Jaruszewicz-Błońska J, Sparta B, Pargett M, … Lipniacki T (2017). Relaxation oscillations and hierarchy of feedbacks in MAPK signaling. Scientific Reports, 7, 38244 10.1038/srep38244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, & Tessier-Lavigne M (2011). Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harbor Perspectives in Biology, 3(6), a001727 10.1101/cshperspect.a001727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer BR, Yoon SO, & Carter BD (2014). The biological functions and signaling mechanisms of the p75 neurotrophin receptor. Handbook of Experimental Pharmacology, 220, 121–164. 10.1007/978-3-642-45106-5_6 [DOI] [PubMed] [Google Scholar]
- Krstic D, & Knuesel I (2013). Deciphering the mechanism underlying late-onset Alzheimer disease. Nature Reviews. Neurology, 9(1), 25–34. 10.1038/nrneurol.2012.236 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Ye H, & Ginty DD (2000). Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron, 27(3), 499–512. 10.1016/S0896-6273(00)00061-1 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, & Ginty DD (2004). A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell, 118(2), 243–255. 10.1016/j.cell.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Lee H, Park S, Kang Y-S, & Park S (2015). EphA receptors form a complex with caspase-8 to induce apoptotic cell death. Molecules and Cells, 38(4), 349–355. 10.14348/molcells.2015.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Davies AM, & Jaenisch R (1994). p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development, 120(4), 1027–1033. [DOI] [PubMed] [Google Scholar]
- Lehigh KM, West KM, & Ginty DD (2017). Retrogradely transported TrkA endosomes signal locally within dendrites to maintain sympathetic neuron synapses. Cell Reports, 19(1), 86–100. 10.1016/j.celrep.2017.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, … Barde YA (1989). Molecular cloning and expression of brain-derived neurotrophic factor. Nature, 341(6238), 149–152. 10.1038/341149a0 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, & Hamburger V (1951). Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. The Journal of Experimental Zoology, 116(2), 321–361. 10.1002/jez.1401160206 [DOI] [PubMed] [Google Scholar]
- Lewin GR, & Barde YA (1996). Physiology of the neurotrophins. Annual Review of Neuroscience, 19, 289–317. 10.1146/annurev.ne.19.030196.001445 [DOI] [PubMed] [Google Scholar]
- Lindwall C, Dahlin L, Lundborg G, & Kanje M (2004). Inhibition of c-Jun phosphorylation reduces axonal outgrowth of adult rat nodose ganglia and dorsal root ganglia sensory neurons. Molecular and Cellular Neurosciences, 27(3), 267–279. 10.1016/j.mcn.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Lindwall C, & Kanje M (2005). Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Molecular and Cellular Neurosciences, 29(2), 269–282. 10.1016/j.mcn.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Linker C, & Stern CD (2004). Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development, 131(22), 5671–5681. 10.1242/dev.01445 [DOI] [PubMed] [Google Scholar]
- Liu L, Orozco IJ, Planel E, Wen Y, Bretteville A, Krishnamurthy P, … K. (2008). A transgenic rat that develops Alzheimer’s disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiology of Disease, 31(1), 46–57. 10.1016/j.nbd.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, & Ginty DD (2007). A hierarchical NGF signaling cascade controls ret-dependent and ret-independent events during development of nonpeptidergic DRG neurons. Neuron, 54(5), 739–754. 10.1016/j.neuron.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Maday S, Wallace KE, & Holzbaur ELF (2012). Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. Journal of Cell Biology, 196(4), 407–417. 10.1083/jcb.201106120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, & Holzbaur ELF (2014). Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron, 84(2), 292–309. 10.1016/j.neuron.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti L, Bonsignore F, Gobbo F, Amodeo R, Calvello M, Jacob A, … A. (2019). Fast-diffusing p75NTR monomers support apoptosis and growth cone collapse by neurotrophin ligands. Proceedings of the National Academy of Sciences of the United States of America, 116(43), 21563–21572. 10.1073/pnas.1902790116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Plump AS, Flames N, Sánchez-Camacho C, Tessier-Lavigne M, & Rubenstein JLR (2003). Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development, 130(9), 1889–1901. 10.1242/dev.00417 [DOI] [PubMed] [Google Scholar]
- Maroney AC, Finn JP, Bozyczko-Coyne D, O’Kane TM, Neff NT, Tolkovsky AM, … Greene LA (1999). CEP-1347 (KT7515), an inhibitor of JNK activation, rescues sympathetic neurons and neuronally differentiated PC12 cells from death evoked by three distinct insults. Journal of Neurochemistry, 73(5), 1901–1912. 10.1046/j.1471-4159.1999.01901.x [DOI] [PubMed] [Google Scholar]
- Marshall CJ (1995). Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell, 80(2), 179–185. 10.1016/0092-8674(95)90401-8 [DOI] [PubMed] [Google Scholar]
- Matusica D, & Coulson EJ (2014). Local versus long-range neurotrophin receptor signalling: Endosomes are not just carriers for axonal transport. Seminars in Cell & Developmental Biology, 31, 57–63. 10.1016/j.semcdb.2014.03.032 [DOI] [PubMed] [Google Scholar]
- McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, & Vale RD (2014). Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science, 345(6194), 337–341. 10.1126/science.1254198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, & Bredesen DE (1998). The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature, 395(6704), 801–804. 10.1038/27441 [DOI] [PubMed] [Google Scholar]
- Meinhardt H, & Gierer A (2000). Pattern formation by local self-activation and lateral inhibition. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 22(8), 753–760. [DOI] [PubMed] [Google Scholar]
- Miller FD, & Kaplan DR (2001). On Trk for retrograde signaling. Neuron, 32(5), 767–770. 10.1016/s0896-6273(01)00529-3 [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, Blasier KR, Jeffery ED, Ross MW, Pullikuth AK, Suo D, … Pfister KK (2012). Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport. The Journal of Neuroscience, 32(44), 15495–15510. 10.1523/JNEUROSCI.5599-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Sakai T, Fujioka S, Mori T, Ohoka Y, … S. (1999). Developmental localization of semaphorin H messenger RNA acting as a collapsing factor on sensory axons in the mouse brain. Neuroscience, 93(1), 401–408. 10.1016/s0306-4522(99)00134-7 [DOI] [PubMed] [Google Scholar]
- Mok S-A, Lund K, & Campenot RB (2009). A retrograde apoptotic signal originating in NGF-deprived distal axons of rat sympathetic neurons in compartmented cultures. Cell Research, 19(5), 546–560. 10.1038/cr.2009.11 [DOI] [PubMed] [Google Scholar]
- Moqrich A, Earley TJ, Watson J, Andahazy M, Backus C, Martin-Zanca D, … Patapoutian A (2004). Expressing TrkC from the TrkA locus causes a subset of dorsal root ganglia neurons to switch fate. Nature Neuroscience, 7(8), 812–818. 10.1038/nn1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughamian AJ, & Holzbaur ELF (2012). Dynactin is required for transport initiation from the distal axon. Neuron, 74(2), 331–343. 10.1016/j.neuron.2012.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naska S, Lin DC, Miller FD, & Kaplan DR (2010). p75NTR is an obligate signaling receptor required for cues that cause sympathetic neuron growth cone collapse. Molecular and Cellular Neurosciences, 45(2), 108–120. 10.1016/j.mcn.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Negulescu A-M, & Mehlen P (2018). Dependence receptors—The dark side awakens. The FEBS Journal, 285(21), 3909–3924. 10.1111/febs.14507 [DOI] [PubMed] [Google Scholar]
- Niclou SP, Franssen EHP, Ehlert EME, Taniguchi M, & Verhaagen J (2003). Meningeal cell-derived semaphorin 3A inhibits neurite outgrowth. Molecular and Cellular Neurosciences, 24(4), 902–912. 10.1016/s1044-7431(03)00243-4 [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L, … Y.-A. (2010). Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature, 467(7311), 59–63. 10.1038/nature09336 [DOI] [PubMed] [Google Scholar]
- Oppenheim RW (1991). Cell death during development of the nervous system. Annual Review of Neuroscience, 14, 453–501. 10.1146/annurev.ne.14.030191.002321 [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Antyborzec I, O’Leary VB, Zaborszky L, Herms J, & Oliver Dolly J (2014). Neurotrophin receptor p75 mediates the uptake of the amyloid beta (Aβ) peptide, guiding it to lysosomes for degradation in basal forebrain cholinergic neurons. Brain Structure & Function, 219(5), 1527–1541. 10.1007/s00429-013-0583-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmada M, Kanwal S, Rutkoski NJ, Gustafson-Brown C, Johnson RS, Wisdom R, & Carter BD (2002). C-Jun is essential for sympathetic neuronal death induced by NGF withdrawal but not by p75 activation. The Journal of Cell Biology, 158(3), 453–461. 10.1083/jcb.200112129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey JP, & Smith DS (2011). A Cdk5-dependent switch regulates Lis1/Ndel1/dynein-driven organelle transport in adult axons. The Journal of Neuroscience, 31(47), 17207–17219. 10.1523/JNEUROSCI.4108-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, & Carter BD (2017). Retrograde apoptotic signaling by the p75 neurotrophin receptor. Neuronal Signaling, 1(1), NS20160007 10.1042/NS20160007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, Stanley EM, Hickman FE, Wallace N, Brewer B, Li D, … Carter BD (2018). Retrograde degenerative signaling mediated by the p75 neurotrophin receptor requires p150Glued deacetylation by axonal HDAC1. Developmental Cell, 46(3), 376–387.e7. 10.1016/j.devcel.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazyra-Murphy MF, Hans A, Courchesne SL, Karch C, Cosker KE, Heerssen HM, … Segal RA (2009). A retrograde neuronal survival response: Target-derived neurotrophins regulate MEF2D and bcl-w. The Journal of Neuroscience, 29(20), 6700–6709. 10.1523/JNEUROSCI.0233-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Jeong G-B, Ross JL, Dixit R, Wallace KE, Kalb RG, & Holzbaur ELF (2009). A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. The Journal of Neuroscience, 29(31), 9903–9917. 10.1523/JNEUROSCI.0813-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu M-M, Moughamian AJ, & Holzbaur ELF (2010). Retrograde axonal transport: Pathways to cell death? Trends in Neurosciences, 33(7), 335–344. 10.1016/j.tins.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PH, Tang H, Zou K, & Zhong W (2006). The enigma of the numb-notch relationship during mammalian embryogenesis. Developmental Neuroscience, 28(1–2), 156–168. 10.1159/000090761 [DOI] [PubMed] [Google Scholar]
- Prasad MS, Charney RM, & García-Castro MI (2019). Specification and formation of the neural crest: Perspectives on lineage segregation. Genesis, 57(1), e23276 10.1002/dvg.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, & Butler AC (1998). Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Annals of Neurology, 43(3), 380–383. 10.1002/ana.410430316 [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, … Johnson EM (2003). JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron, 38(6), 899–914. 10.1016/s0896-6273(03)00355-6 [DOI] [PubMed] [Google Scholar]
- Qian L, Milne MR, Shepheard S, Rogers M-L, Medeiros R, & Coulson EJ (2019). Removal of p75 Neurotrophin receptor expression from cholinergic basal forebrain neurons reduces amyloid-β plaque deposition and cognitive impairment in aged APP/PS1 mice. Molecular Neurobiology, 56(7), 4639–4652. 10.1007/s12035-018-1404-2 [DOI] [PubMed] [Google Scholar]
- Qui MS, & Green SH (1992). PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron, 9(4), 705–717. 10.1016/0896-6273(92)90033-a [DOI] [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, & Bredesen DE (1993). Induction of apoptosis by the low-affinity NGF receptor. Science, 261(5119), 345–348. 10.1126/science.8332899 [DOI] [PubMed] [Google Scholar]
- Rao AN, & Baas PW (2018). Polarity sorting of microtubules in the axon. Trends in Neurosciences, 41(2), 77–88. 10.1016/j.tins.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Redwine WB, Vale RD, & Carter AP (2018). The cytoplasmic dynein transport machinery and its many cargoes. Nature Reviews. Molecular Cell Biology, 19(6), 382–398. 10.1038/s41580-018-0004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, & Ginty DD (1997). An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science, 277(5329), 1097–1100. 10.1126/science.277.5329.1097 [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, … J. W (1990). Primary structure and biological activity of a novel human neurotrophic factor. Neuron, 4(5), 767–773. [DOI] [PubMed] [Google Scholar]
- Roux PP, & Barker PA (2002). Neurotrophin signaling through the p75 neurotrophin receptor. Progress in Neurobiology, 67(3), 203–233. 10.1016/S0301-0082(02)00016-3 [DOI] [PubMed] [Google Scholar]
- Sahoo PK, Smith DS, Perrone-Bizzozero N, & Twiss JL (2018). Axonal mRNA transport and translation at a glance. Journal of Cell Science, 131(8), jcs196808 10.1242/jcs.196808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Delcroix J-D, Belichenko PV, Zhan K, Wu C, Valletta JS, … Mobley WC (2006). Increased app expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron, 51(1), 29–42. 10.1016/j.neuron.2006.05.022 [DOI] [PubMed] [Google Scholar]
- Scott-Solomon E, & Kuruvilla R (2018). Mechanisms of neurotrophin trafficking via Trk receptors. Molecular and Cellular Neurosciences, 91, 25–33. 10.1016/j.mcn.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DL, & Campenot RB (1997). Rapid retrograde tyrosine phosphorylation of trkA and other proteins in rat sympathetic neurons in compartmented cultures. The Journal of Cell Biology, 138(2), 411–421. 10.1083/jcb.138.2.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Deppmann CD, Harrington AW, St Hillaire C, Chen Z-Y, Lee FS, & Ginty DD (2010). Long-distance control of synapse assembly by target-derived NGF. Neuron, 67(3), 422–434. 10.1016/j.neuron.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, … Tessier-Lavigne M (2016). Axon degeneration gated by retrograde activation of somatic pro-apoptotic signaling. Cell, 164(5), 1031–1045. 10.1016/j.cell.2016.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, & Watkins TA (2018). Therapeutic opportunities and pitfalls in the treatment of axon degeneration. Current Opinion in Neurology, 31(6), 693–701. 10.1097/WCO.0000000000000621 [DOI] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, & Miller FD (2008). Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nature Neuroscience, 11(6), 649–658. 10.1038/nn.2114 [DOI] [PubMed] [Google Scholar]
- Siu M, Sengupta Ghosh A, & Lewcock JW (2018). Dual leucine zipper kinase inhibitors for the treatment of neurodegeneration. Journal of Medicinal Chemistry, 61(18), 8078–8087. 10.1021/acs.jmedchem.8b00370 [DOI] [PubMed] [Google Scholar]
- Skeldal S, Matusica D, Nykjaer A, & Coulson EJ (2011). Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling? Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR). BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 33(8), 614–625. 10.1002/bies.201100036 [DOI] [PubMed] [Google Scholar]
- Sleigh JN, Rossor AM, Fellows AD, Tosolini AP, & Schiavo G (2019). Axonal transport and neurological disease. Nature Reviews. Neurology, 15(12), 691–703. 10.1038/s41582-019-0257-2 [DOI] [PubMed] [Google Scholar]
- Snider WD (1994). Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell, 77(5), 627–638. 10.1016/0092-8674(94)90048-5 [DOI] [PubMed] [Google Scholar]
- Song H-L, Shim S, Kim D-H, Won S-H, Joo S, Kim S, … Yoon S-Y (2014). β-Amyloid is transmitted via neuronal connections along axonal membranes. Annals of Neurology, 75(1), 88–97. 10.1002/ana.24029 [DOI] [PubMed] [Google Scholar]
- Suo D, Park J, Harrington AW, Zweifel LS, Mihalas S, & Deppmann CD (2014). Coronin-1 is a neurotrophin endosomal effector that is required for developmental competition for survival. Nature Neuroscience, 17(1), 36–45. 10.1038/nn.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura A, Sato Y, Watabe D, Okamoto Y, Nakata T, Kawarabayashi T, … E. (2012). Sortilin is required for toxic action of Aβ oligomers (AβOs): Extracellular AβOs trigger apoptosis, and intraneuronal AβOs impair degradation pathways. Life Sciences, 91(23–24), 1177–1186. 10.1016/j.lfs.2012.04.038 [DOI] [PubMed] [Google Scholar]
- Tas RP, Chazeau A, Cloin BMC, Lambers MLA, Hoogenraad CC, & Kapitein LC (2017). Differentiation between oppositely oriented microtubules controls polarized neuronal transport. Neuron, 96(6), 1264–1271.e5. 10.1016/j.neuron.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuling E, van Dis V, Wulf PS, Haasdijk ED, Akhmanova A, Hoogenraad CC, & Jaarsma D (2008). A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Human Molecular Genetics, 17(18), 2849–2862. 10.1093/hmg/ddn182 [DOI] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, & Olson L (1995). Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proceedings of the National Academy of Sciences of the United States of America, 92(18), 8274–8278. 10.1073/pnas.92.18.8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, & Cohen P (1992). Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. The Biochemical Journal, 288(Pt 2), 351–355. 10.1042/bj2880351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM (1990). The chemical basis of morphogenesis. Bulletin of Mathematical Biology, 52(1–2), 153–197 discussion 119. [DOI] [PubMed] [Google Scholar]
- Urra S, Escudero CA, Ramos P, Lisbona F, Allende E, Covarrubias P, … F. C (2007). TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. The Journal of Biological Chemistry, 282(10), 7606–7615. 10.1074/jbc.M610458200 [DOI] [PubMed] [Google Scholar]
- Vicario A, Kisiswa L, Tann JY, Kelly CE, & Ibáñez CF (2015). Neuron-type-specific signaling by the p75NTR death receptor is regulated by differential proteolytic cleavage. Journal of Cell Science, 128(8), 1507–1517. 10.1242/jcs.161745 [DOI] [PubMed] [Google Scholar]
- Vilar M (2017). Structural characterization of the p75 neurotrophin receptor: A stranger in the TNFR superfamily. Vitamins and Hormones, 104, 57–87. 10.1016/bs.vh.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Villarin JM, McCurdy EP, Martínez JC, & Hengst U (2016). Local synthesis of dynein cofactors matches retrograde transport to acutely changing demands. Nature Communications, 7, 13865 10.1038/ncomms13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel-Campos D, Schiavo G, & Lazo OM (2018). The many disguises of the signalling endosome. FEBS Letters, 592(21), 3615–3632. 10.1002/1873-3468.13235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig V, & Herdegen T (2005). Context-specific inhibition of JNKs: Overcoming the dilemma of protection and damage. Trends in Pharmacological Sciences, 26(9), 455–461. 10.1016/j.tips.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, & He Z (2002). Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature, 417(6892), 941–944. 10.1038/nature00867 [DOI] [PubMed] [Google Scholar]
- Wehner AB, Abdesselem H, Dickendesher TL, Imai F, Yoshida Y, Giger RJ, & Pierchala BA (2016). Semaphorin 3A is a retrograde cell death signal in developing sympathetic neurons. Development, 143(9), 1560–1570. 10.1242/dev.134627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Heffner DL, Kim S, Espy SM, Spano AJ, Cleland CL, & Deppmann CD (2014). TNF-α/TNFR1 signaling is required for the development and function of primary nociceptors. Neuron, 82(3), 587–602. 10.1016/j.neuron.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, & Ham J (2001). Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron, 29(3), 629–643. 10.1016/s0896-6273(01)00239-2 [DOI] [PubMed] [Google Scholar]
- Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VLJ, … Strydom A (2015). A genetic cause of Alzheimer disease: Mechanistic insights from Down syndrome. Nature Reviews. Neuroscience, 16(9), 564–574. 10.1038/nrn3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Cui B, He L, Chen L, & Mobley WC (2009). The coming of age of axonal neurotrophin signaling endosomes. Journal of Proteomics, 72(1), 46–55. 10.1016/j.jprot.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach A, & Tolkovsky AM (2006). The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. Journal of Neurochemistry, 96(5), 1213–1226. 10.1111/j.1471-4159.2005.03676.x [DOI] [PubMed] [Google Scholar]
- Yamashita N, & Kuruvilla R (2016). Neurotrophin signaling endosomes: Biogenesis, regulation, and functions. Current Opinion in Neurobiology, 39, 139–145. 10.1016/j.conb.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Usui H, Nakamura F, Chen S, Sasaki Y, Hida T, … Y. (2014). Plexin-A4-dependent retrograde semaphorin 3A signalling regulates the dendritic localization of GluA2-containing AMPA receptors. Nature Communications, 5, 3424 10.1038/ncomms4424 [DOI] [PubMed] [Google Scholar]
- Yamashita N, Yamane M, Suto F, & Goshima Y (2016). TrkA mediates retrograde semaphorin 3A signaling through plexin A4 to regulate dendritic branching. Journal of Cell Science, 129(9), 1802–1814. 10.1242/jcs.184580 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, & Tohyama M (2002). The p75 receptor transduces the signal from myelin-associated glycoprotein to rho. The Journal of Cell Biology, 157(4), 565–570. 10.1083/jcb.200202010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Schätzle P, Tortosa E, Pagès S, Holtmaat A, Kapitein LC, & Hoogenraad CC (2016). Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. The Journal of Neuroscience, 36(4), 1071–1085. 10.1523/JNEUROSCI.2430-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Kuruvilla R, Zweifel LS, & Ginty DD (2003). Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron, 39(1), 57–68. 10.1016/s0896-6273(03)00266-6 [DOI] [PubMed] [Google Scholar]
- Ye M, Lehigh KM, & Ginty DD (2018). Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling. eLife, 7, e33012 10.7554/eLife.33012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Guo W, & Feng L (2004). Segregation of Nogo66 receptors into lipid rafts in rat brain and inhibition of Nogo66 signaling by cholesterol depletion. FEBS Letters, 577(1–2), 87–92. 10.1016/j.febslet.2004.09.068 [DOI] [PubMed] [Google Scholar]
- Yue Y, Su J, Cerretti DP, Fox GM, Jing S, & Zhou R (1999). Selective inhibition of spinal cord neurite outgrowth and cell survival by the Eph family ligand ephrin-A5. The Journal of Neuroscience, 19(22), 10026–10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi EE, Maimon R, & Perlson E (2017). Spatial-specific functions in retrograde neuronal signalling. Traffic, 18(7), 415–424. 10.1111/tra.12487 [DOI] [PubMed] [Google Scholar]
- Zeke A, Misheva M, Reményi A, & Bogoyevitch MA (2016). JNK signaling: Regulation and functions based on complex protein-protein partnerships. Microbiology and Molecular Biology Reviews, 80(3), 793–835. 10.1128/MMBR.00043-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, & Segal RA (2000). Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. The Journal of Neuroscience, 20(15), 5671–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, & Jan YN (1996). Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron, 17(1), 43–53. 10.1016/s0896-6273(00)80279-2 [DOI] [PubMed] [Google Scholar]
- Zhou B, Cai Q, Xie Y, & Sheng Z-H (2012). Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell Reports, 2(1), 42–51. 10.1016/j.celrep.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer G, Garcez P, Rudolph J, Niehage R, Weth F, Lent R, & Bolz J (2008). Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. The European Journal of Neuroscience, 28(1), 62–73. 10.1111/j.1460-9568.2008.06320.x [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, & Ginty DD (2005). Functions and mechanisms of retrograde neurotroph in signalling. Nature Reviews. Neuroscience, 6(8), 615–625. 10.1038/nrn1727 [DOI] [PubMed] [Google Scholar]
FURTHER READING
- Aravamudhan P, Raghunathan K, Konopka-Anstadt J, Pathak A, Sutherland DM, Carter BD, & Dermody TS (2020). Reovirus uses macropinocytosis-mediated entry and fast axonal transport to infect neurons. PLOS Pathogens, 16(2), e1008380 10.1371/journal.ppat.1008380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzo JA, Hart RP, Zahn JD, & Pang ZP (2019). Compartmentalized devices as tools for investigation of human brain network dynamics. Developmental Dynamics, 248, 65–77. https://doi.org/10.1002/dvdy.24665 Retrieved from https://doi.org/10.1002/dvdy.24665https://anatomypubs.onlinelibrary.wiley.com/doi/pdf/10.1002/dvdy.24665 Retrieved from https://anatomypubs.onlinelibrary.wiley.com/doi/pdf/10.1002/dvdy.24665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langemeyer L, Fröhlich F, & Ungermann C (2018). Rab GTPase function in endosome and lysosome biogenesis. Trends in Cell Biology, 28(11), 957–970. https://doi.org/10.1016/j.tcb.2018.06.007 Retrieved from https://doi.org/10.1016/j.tcb.2018.06.007https://www.sciencedirect.com/science/article/pii/S0962892418301089?via%3Dihub Retrieved from https://www.sciencedirect.com/science/article/pii/S0962892418301089?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Lawrence RE, & Zoncu R (2019). The lysosome as a cellular centre for signalling, metabolism and quality control. Nature Cell Biology, 21, 133–142. https://doi.org/10.1038/s41556-018-0244-7 Retrieved from https://doi.org/10.1038/s41556-018-0244-7https://www.nature.com/articles/s41556-018-0244-7 Retrieved from https://www.nature.com/articles/s41556-018-0244-7 [DOI] [PubMed] [Google Scholar]
- Martin N, Gonzalo R, Stephanie M, Anne S, Raimund S, Kyoohyun K, … Jochen G (2018). Axonal transport, phase-separated compartments, and neuron mechanics—A new approach to investigate neurodegenerative diseases. Frontiers in Cellular Neuroscience, 12, 358 https://doi.org/10.3389/fncel.2018.00358 Retrieved from https://doi.org/10.3389/fncel.2018.00358https://www.frontiersin.org/articles/10.3389/fncel.2018.00358/full Retrieved from https://www.frontiersin.org/articles/10.3389/fncel.2018.00358/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh JN, Vagnoni A, Twelvetrees AE, & Schiavo G (2017). Methodological advances in imaging intravital axonal transport [version 1; peer review: 3 approved]. F1000Research, 6, 200 https://doi.org/10.12688/f1000research.10433.1 Retrieved from https://doi.org/10.12688/f1000research.10433.1https://f1000research.com/articles/6-200/v1 Retrieved from https://f1000research.com/articles/6-200/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]


