Abstract
The ability to suppress distractions is essential to successful completion of goal-directed behaviors. Several behavioral studies have recently provided strong evidence that learned suppression may be particularly efficient in reducing distractor interference. Expectations about a distractor’s repeated location, color, or even presence is rapidly learned and used to attenuate interference. In this study, we use a visual search paradigm in which a color singleton, which is known to capture attention, occurs within blocks with high or low frequency. The behavioral results show reduced singleton interference during the high compared to the low frequency block (Won et al., 2019). The fMRI results provide evidence that the attenuation of distractor interference is supported by changes in singleton, target, and non-salient distractor representations within retinotopic visual cortex. These changes in visual cortex are accompanied by findings that singleton-present trials compared to non-singleton trials produce greater activation in bilateral parietal cortex, indicative of attentional capture, in low frequency, but not high frequency blocks. Together, these results suggest that the readout of saliency signals associated with an expected color singleton from visual cortex is suppressed, resulting in less competition for attentional priority in frontoparietal attentional control regions.
Keywords: singleton distraction, visual search, functional magnetic resonance imaging, visual cortex, MVPA
1. Introduction
Imagine taking a walk through a snowy landscape when a red cardinal lands on a nearby tree branch. It is likely that your attention will be captured by the bird because of its visual salience, produced by its unique color against the uniform background. This type of sensory-driven attentional capture has been well documented in human behavior (Egeth & Yantis, 1997; Itti & Koch, 2000; Posner, Snyder, & Davidson, 1980; Theeuwes, 1991; Treisman & Gelade, 1980) and is known to drive attentional and oculomotor systems in the brain (Bisley & Goldberg, 2006; Bogler, Bode, & Haynes, 2011; de Fockert, Rees, Frith, & Lavie, 2001; de Fockert & Theeuwes, 2012; Geng & Mangun, 2009; Sprague, Itthipuripat, Vo, & Serences, 2018; Suzuki & Gottlieb, 2013). While the ability to orient attention towards novel and salient objects is essential for adaptive human behaviors, continuous or frequent attentional capture by salient objects that are task-irrelevant (i.e., distractors) is deleterious for goal-driven behaviors. Thus, the ability to control and reduce distraction, particularly when it recurs and is predictable, is important for human behavior. Yet, the mechanisms that lead to distractor attenuation based on learned ignoring are still poorly understood.
The role of learned expectations in distractor suppression has been the subject of a surge of behavioral studies in recent years (Chelazzi, Marini, Pascucci, & Turatto, 2019; Failing & Theeuwes, 2018; Gaspelin & Luck, 2018; Geng, Won, & Carlisle, 2019; Noonan, Crittenden, Jensen, & Stokes, 2018). This work has led to a consensus that predictable characteristics of recurring distractors are easily learned and used in a highly effective way to reduce interference. For example, a number of studies have shown that spatial locations with a high probability of containing a salient distractor are de-prioritized and this reduces distractor interference. Evidence of spatial de-prioritization comes from the finding that it takes longer to find targets that (infrequently) appear in that same location (Failing & Theeuwes, 2018; Ferrante et al., 2018; Wang & Theeuwes, 2018a, 2018b; Wang, van Driel, Ort, & Theeuwes, 2019; Zhang, Allenmark, Liesefeld, Shi, & Müller, 2019). Likewise, a spatially unpredictable color or luminance singleton distractor that initially captures attention, no longer does so when it recurs (Gaspelin, Gaspar, & Luck, 2019; Geng & Diquattro, 2010; Geyer, Muller, & Krummenacher, 2006; Vatterott & Vecera, 2012). Interestingly, the color of the singleton does not have to be the same as long as the color singleton is predictably a distractor (Gaspelin, Leonard, & Luck, 2017; Stilwell & Vecera, 2019; Vatterott, Mozer, & Vecera, 2018; Won, Kosoyan, & Geng, 2019; Yantis & Egeth, 1999; Zhang et al., 2019). Statistical learning of distractor properties, regardless of whether it is spatial or feature-based, appears to occur relatively fast. The number of repetitions required for attentional capture to be attenuated is relatively few suggesting that learned suppression may build on local repetition suppression (Chetverikov, Campana, & Kristjansson, 2017a, 2017b) or sensory habituation (Bonetti & Turatto, 2019; Chelazzi et al., 2019; Turatto, Bonetti, Pascucci, & Chelazzi, 2018; Won & Geng, 2020).
While learned suppression has emerged within the behavioral literature as a powerful modulator of attentional capture by distractors, there is still sparse evidence for where distractor processing is attenuated in the brain during learned suppression. The few studies of distractor suppression using fMRI have mostly used spatial cues to indicate the upcoming location of distractors. For example, Serences et al. (2004) used a spatial cue that indicated the location of target stimuli and probability of dense distractors. They found that when the cue indicated probable distractors, there was an increase in preparatory activation in visual ROIs (V1, V2, VP, V4v) corresponding to the expected location of distractors. On those trials, behavioral performance was also unimpaired by distractors. In contrast, preparatory activation was absent in ROIs that encoded locations unlikely to contain distractors. When distractors appeared in those locations, performance suffered (Serences, Yantis, Culberson, & Awh, 2004). Others have since reported similar findings that foreknowledge of a distractor location resulted in increased BOLD activation in retinotopic visual cortex accompanied by weaker indices of distractor interference in behavior (Munneke, Heslenfeld, Usrey, Theeuwes, & Mangun, 2011; Ruff & Driver, 2006). These results show that activation increases in visual cortex are an indicator of preparatory distractor suppression.
Fewer studies have used fMRI to look for evidence of learned suppression for features or objects in visual cortex, but of those that have, some have found evidence for the suppression of stimulus-evoked brain responses to distractors in visual cortex (Adam & Serences, 2020; although see, Reeder, Olivers, Hanke, & Pollmann, 2018; Reeder, Olivers, & Pollmann, 2017; Seidl, Peelen, & Kastner, 2012). For example, Reeder et al. (2017) found evidence for a decrease in univariate activation in response to negative color cue compared to a positive cue in large regions of visual cortex. Adam and Serences (2020) more recently reported that target and distractors with consistent, repeating, colors resulted in target enhancement and distractor suppression, respectively, compared to target and distractor colors that randomly switched. Interestingly, suppression only occurred for distractors adjacent to the target, suggesting that competition plays a role in inducing suppression (Alvarez & Cavanagh, 2005; Schwartz et al., 2005; Sereno & Kosslyn, 1991; Stormer, Alvarez, & Cavanagh, 2014; Walter, Quigley, & Mueller, 2014). These results suggest that learned expectations about distractors can lead to suppression of the stimulus-evoked response in early visual cortex compared to unexpected distractors, particularly when distractor competition is strong.
There is also evidence for early visual suppression of stimulus-evoked distractor responses from event-related potential (ERP) studies. In particular, active distractor suppression for a variety of distractor types has been associated with the Pd ERP (Hickey, DiLollo, & McDonald, 2008; Sawaki & Luck, 2010), which has been shown to arise from visual cortex in response to feedback from the frontal eye fields (FEF), an area involved in the control of spatial attention (Cosman, Lowe, Zinke, Woodman, & Schall, 2018). Interestingly, Wang et al., (2019) found a Pd component in response to a salient distractor no matter where it appeared in the visual search display, however, the Pd was earlier for distractors appearing in probable locations (van Moorselaar & Slagter, 2019; Wang et al., 2019).
In contrast to distractor suppression, there is substantial evidence from neurophysiology and human fMRI that salient stimuli that capture attention are specifically encoded in the lateral intraparietal sulcus (LIP in monkeys; more generally intraparietal sulcus, i.e. IPS, in humans) of the parietal lobe (Bisley & Goldberg, 2006; Bogler et al., 2011; Buschman & Miller, 2007; de Fockert et al., 2001; de Fockert & Theeuwes, 2012; Geng & Mangun, 2009; Hodsoll, Mevorach, & Humphreys, 2009; Ipata, Gee, Gottlieb, Bisley, & Goldberg, 2006; Mevorach, Humphreys, & Shalev, 2006; Sprague et al., 2018; Suzuki & Gottlieb, 2013). Although distractor signals are also found within other regions of the oculomotor and attentional network including the FEF, pulvinar, and superior colliculus (Bisley & Mirpour, 2019; Corbetta, Patel, & Shulman, 2008; Seidl et al., 2012; Xuan et al., 2016), the parietal cortex appears to play a special role in processing perceptual saliency (Geng & Mangun, 2009 ; Hodsoll et al., 2009; Mevorach, Hodsoll, Allen, Shalev, & Humphreys, 2010; Mevorach et al., 2006). For example, distractor related activity in LIP is attenuated when salient stimuli are successfully ignored (Ipata et al., 2006) suggesting that the response of parietal neurons to salient stimuli reflects the strength of attentional capture. LIP neurons also respond earlier than frontal areas to salient sensory signals (Buschman & Miller, 2007) both when they are relevant and irrelevant (Bisley & Goldberg, 2006). In addition to saliency signals, these regions also incorporate information about a stimulus’ “top-down” relevance. The integration of top-down and bottom-up signals has lead to the characterization of LIP and related regions as priority maps from which attention is allocated based on a winner-take-all computation (Bisley & Goldberg, 2010; Bogler et al., 2011; Gottlieb, Balan, Oristaglio, & Suzuki, 2009; Pollmann et al., 2003).
Taken together, these studies suggest that expectations alter distractor processing in visual cortex, and this change leads to better suppression of attentional capture and behavioral interference; however, when that does not occur (or fails), then information about the salient distractor is carried forward and encoded in parietal cortex within the frontoparietal attentional network, producing greater competition for attention and the need for reactive attentional control (Geng, 2014; Marini, Demeter, Roberts, Chelazzi, & Woldorff, 2016; van Diepen, Miller, Mazaheri, & Geng, 2016). Here we test these predictions directly with a previously used distractor suppression paradigm (Won et al., 2019) that found better behavioral suppression of color singletons when they were frequent and expected compared to rarer and therefore more surprising. We hypothesized that saliency signals from predictable, frequent, distractors would be attenuated in visual cortex, produce weaker activation in parietal cortex, and less attentional capture. Infrequent distractors were expected to show the opposite pattern and produce stronger behavioral costs associated with attentional capture.
2. Materials and Methods
2.1. Participants
Twenty-six subjects ranging in age from 19 to 31 (mean age, 24.7) participated in a 2h session and received monetary compensation. Data from two participants were excluded from analyses due to a poor behavioral performance (lower than 3 standard deviation from mean accuracy) and a scanner malfunction, which resulted in a final group of 24 subjects (12 females). This sample size was chosen based on the results of a power analysis to detect a behavioral attentional capture effect using the data from Won et al. (2019). To achieve a power of 95% and an alpha of 5% with an effect size (dz) of 8.55, a sample size of only 4 participants would be needed. This suggests that the behavioral effect we wished to detect was highly reliable, but we chose sample size of 24, a number well over the sample size of the three most closely related fMRI studies (Munneke et al., 2011; Ruff & Driver, 2006; Serences et al., 2004). All participants had normal or corrected-to-normal vision and no neurological history. Informed consent was obtained according to procedures approved by the Institutional Review Board of the University of California, Davis.
2.2. Stimuli and design
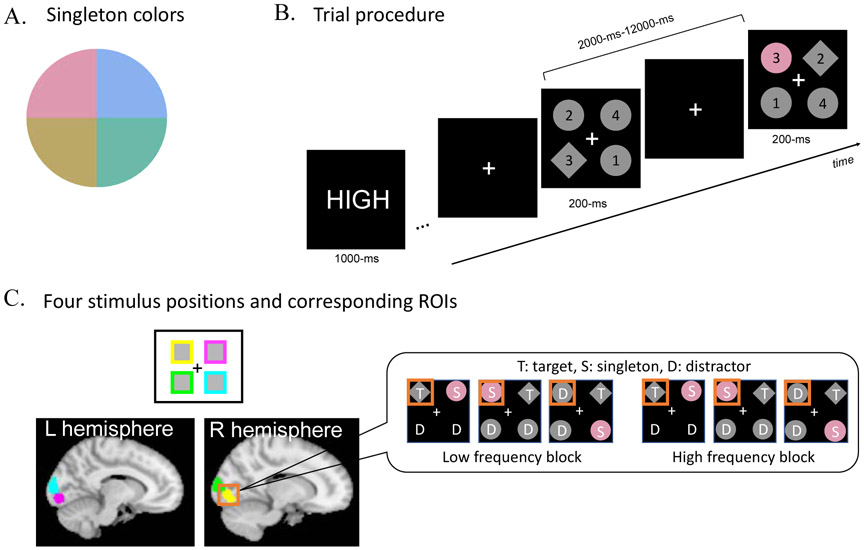
Search displays contained four gray shapes – three diamonds, (2.8° x 2.8°, distractors) and one circle (2.5° in diameter, target), or one diamond (target) and three circles (distractors) – on a black background. The target shape was counterbalanced across participants. The target was always a fixed shape and the non-targets were all another shape. Thus, while we expected that subjects would rely mostly on feature-search mode given the certainty of the target identity (Hout & Goldinger, 2015), it is possible that shape pop-out also contributed to target localization at the time of stimulus onset. The eccentricity (center of each item to the center of screen) was 3°. Each shape was randomly assigned with a number between 1 through 4 (1° x 1°). The target shape appeared randomly but equally often in the four possible locations. Importantly, on some trials, one distractor was drawn in color, i.e., the color singleton. Note that hereafter, “singleton” refers the uniquely colored distractor object. The color of the singleton was drawn from four possible values from a CIE Lab color wheel (radius: 39, luminance: 70, a* = 0, b* = 0, Figure 1A). The colors were chosen to be maximally different from each other in hue but to similar in luminance. Participants were informed that the color singleton is task-irrelevant and were encouraged to ignore it. The singleton location and color were equally, but randomly chosen among the four possible locations and colors. A white fixation cross (1° x 1°) was maintained throughout a scan.
Figure 1.
Singleton colors, trial procedure, and ROIs. A. Singleton colors were chosen from CIELab color space (see text), B. At the beginning of each block, a cue indicated the upcoming block’s singleton frequency (HIGH or LOW) followed by a series of visual search trials on which participants were asked to find the predefined target (in this example, the diamond) and make a button press to indicate the number inside. C. Illustration of the four ROIs for each stimulus location (one in each visual quadrant) defined by the functional localizer and the Juelich probabilistic atlas for V1-V3. Display inset illustrates the six experimental conditions from which data were analyzed within a single ROI.
The experiment was composed of eight experimental scans. Each experimental scan consisted of two blocks, a low frequency block and a high frequency block, which order was counterbalanced across eight scans. In the low frequency block, the singleton appeared on 25% of trials (i.e., 16 trials among 64 trials); in the high frequency block, the singleton appeared on 80% of trials (i.e., 16 trials among 20 trials). Importantly, we kept the absolute number of singleton trials the same across blocks in order to optimize statistical comparisons between singleton trials in the two frequency conditions, as this was the main interest of this study.
2.3. Procedure
Participants were asked to keep fixating on the central fixation cross during the experiment and reminded not to move their head. At the beginning of each block, participants were shown a text cue (i.e., “HIGH” or “LOW”) indicating the singleton frequency of the upcoming block (Figure 1B). A fixation display followed for a randomly jittered duration resulting in an SOA with a mean of 4000-ms, ranging from 2000-12000-ms. The fixation cross very briefly blinked 500-ms before the search display to notify the subject of the upcoming search display. The search display appeared for only 200-ms. The short display duration and unpredictable target location was used to prevent systematic eye-movements in response to the target location. Participants were asked to find the preassigned target (e.g., the gray diamond) and then report the number inside of the target by pressing a corresponding button on a button box within 1300-ms. Participants completed 84 trials in each of the eight experimental scans, which took 6.8 min each.
The retinotopic locations of the visual search stimuli were localized using two functional localizer scans. Each scan consisted of 24 trials, with each trial comprised of a colored square (2.5° x 2.5°) in one of four locations. The location and color of the squares were the same as those used in the visual search displays from the main experiment. On each trial, the square flickered 10 times (500-ms on and 500-ms off). Each color and location were probed once in a four-trial sequence and followed by a 20-sec fixation period. In order to ensure fixation on the central fixation cross, participants were asked to make a button press whenever the fixation briefly (500-ms) turned to gray. This change occurred once during each trial at a randomly chosen time point between the 2nd and 9th square. We used R program (R Core Team, 2013; http://www.R-project.org/) and JASP software (www.jasp.org) for the statistical analysis.
Subjects practiced the main experiment outside of the scanner to be trained with a search task and familiarized with the frequency and singleton manipulations. They completed eight runs of 84 trials that consisted of 64 low frequency trials (16 singleton trials and 48 non-singleton trials) and 20 high frequency trials (16 singleton trials and 4 non-singleton trials) prior to scanning. The training took around 30 min.
2.4. fMRI Analysis
2.4.1. fMRI acquisition and preprocessing
MRI scanning was performed on a 3-Tesla Siemens Skyra scanner (Siemens Health Care, Erlangen, Germany) with a 32-channel phased-array head coil at the Imaging Research Center at the University of California, Davis. A T2-weighted echoplanar imaging (EPI) sequence was used to acquire whole-brain volumes of 48 axial slices of 3 mm thickness (TR/TE 2100/2.98ms, flip angle 75°, base/phase resolution 70/100, FOV 210 mm). Each scan session acquired 285 volumes (399.2 s) and consisted of 84 experimental trials. A total of 8 scan session were acquired. An MPRAGE T1-weighted structural image was acquired for visualizing the associated anatomy (TR/TE 2100/2.98 ms, flip angle 7°, base/phase resolution 256/100, FOV 256 mm, Sagittal acquisition; acquisition time = 7:28). fMRI data were preprocessed using SPM12 (Wellcome Department of Imaging Neuroscience, University College London, UK) running with Matlab 2019a (MathWorks). The first five functional images of each run were dummy scans to allow for equilibrium effects. The preprocessing included slice-time correction and realignment using a two-pass procedure in order to register the images to the mean of the images after the first realignment. The high-resolution images (MPRAGE) was used to determine parameters for spatial normalization into the standard MNI reference brain and with the parameters, EPI images were normalized and smoothed with an 8 mm (FWHM) isotropic kernel. Head motion was minimal (mean +/− sd across 6 parameters = .117+/−.08mm; .002+/−.001°).
2.4.2. Region of Interest Analysis
A functional localizer was run in order to localize the retinotopic locations of the four visual search stimuli within visual cortex1. The scan parameters were identical to the main experiment except that each scan acquired 280 volumes. The localizer was comprised of 10 second blocks of the four stimulus locations (top-left, top-right, bottom-left, and bottom-right). Each block was modeled by a 10 sec boxcar convolved with a canonical hemodynamic response function. The regions of interest (ROIs) were selected by a statistical contrast of each location condition against the other three conditions. A combined Juelich V1-V3 atlas was used as a mask to select visual cortex ROIs. The threshold was pfwe < .05 for cluster sizes > 10 voxels2. The average number of selected voxels at each of the four locations was 817.6 voxels (Figure 1C).
A general linear model (GLM) was constructed with 24 regressors to fully describe the two frequency conditions (high, low) crossed with the 12 possible spatial configurations of stimuli on different trials (i.e., all combinations of the target in each of four locations crossed with the three possible singleton locations given each target location). For example, one regressor was used for trials with the target in the top-left, the singleton in the top-right, and other distractors in the bottom-left and bottom-right. Thus, this model provided independent estimates of the BOLD response for each stimulus type (target, singleton, distractor) in each of the four visual locations. Two additional regressors were defined for the non-singleton trials in the high frequency and low frequency blocks.
Next, beta values from each of the three stimulus types (target, singleton, distractor) occurring in the same visual location were averaged. For example, all the beta values from trials with a target in the top left visual quadrant were extracted from voxels within the lower right visual cortex ROI, as defined by the functional localizer, and averaged. This resulted in a single averaged beta value for the target in each of the four possible locations, providing separate measures for the target stimulus in each retinotopic region (Figure 1C). The same procedure was used for creating averaged beta values for the singleton and distractor stimuli in each visual location. Note that the distractor appeared in two visual locations in each display, thus, the averaged beta values were composed from more data than the other two stimulus types. This resulted in beta values for each stimulus type extracted from each of the four visual ROIs corresponding to four quadrants of the visual field.
MVPA was implemented by using a binary linear support vector machine (SVM). The beta maps from the ROI analysis (described above, but without averaging across runs) were normalized to remove univariate difference between conditions (Misaki, Kim, Bandettini, & Kriegeskorte, 2010) and entered into a binary linear SVM classifier with leave-one-run-out cross-validation for each pair of stimuli using LIBSVM using a default cost parameter of 1 (Chang & Lin, 2011). We estimated the classification accuracy for the target versus singleton, the target versus distractor, and the singleton versus distractor in each of two frequency blocks separately, for each visual ROI in each person. The final classification accuracy in visual cortex was estimated by averaging the classification accuracies across all four ROIs (one for each visual quadrant) for each person and then across all participants. Group-level significance was established using a nonparametric statistical test of the classification accuracy. The chance-level distribution was created by 10,000 permutations of each classification pair for each individual by randomizing the labels of the training and testing data set. Next, these classification accuracies were averaged across participants and ROIs, yielding a chance-level distribution for the group classification. The permuted p value (one-tailed) was calculated using a formula: (n + 1)/(10,000 + 1). Here, n refers to the counts of the permuted accuracies that were equal or greater than the observed group average accuracy. Family wise error was controlled for all pairs of classification by using Bonferroni correction.
2.4.3. Whole brain analyses
A separate GLM was constructed with four trial types (singleton trials in the high frequency block; non-singleton trials in the high frequency block; singleton trials in the low frequency block; non-singleton trials in the low frequency block). Each trial was modeled by a stick function convolved with a canonical hemodynamic response. Linear contrasts of parameter estimates were estimated for each participant and combined for the group level in a random effects general linear model. We corrected for multiple comparisons using cluster correction at pfwe < .05, with a height cut-off of p < .001 uncorrected for clusters with greater than 10 voxels.
3. Results
3.1. Behavior
We excluded slow (RTs greater than 2.5 standard deviations from the individual mean) and inaccurate trials from behavioral and image data analyses (an average of 4.5% data were excluded per person). The average accuracy was 97.4% (SD = 2.0%).
RT and accuracy data were combined into a single metric of behavior, the inverse efficiency score (IE; RT / accuracy) (Townsend & Ashby ,1978, 1983). IE was entered into a repeated measures ANOVA with trial type (singleton,non-singleton) and frequency (low, high). There was a significant main effect of singleton presence, with worse performance on singleton trials than non-singleton trials, F(1, 23) = 11.972, p = .002, η2p = .342, but no main effect of frequency, F(1, 23) = 3.254, p = .084. Critically, there was a significant interaction between the trial type and the frequency, F(1, 23) = 4.775, p = .039, η2p = .172, which indicates that interference by a singleton was reduced in the high frequency blocks (singleton trial: mean = 7.14, SD = .92 vs. non-singleton: mean = 7.05, SD = 1.15) compared to the low frequency blocks (singleton trial: mean = 7.14, SD = .96 vs. non-singleton trial: mean = 6.83, SD = .93); this pattern replicates our previous behavioral finding (Won et al., 2019; Figure 2).
Figure 2.
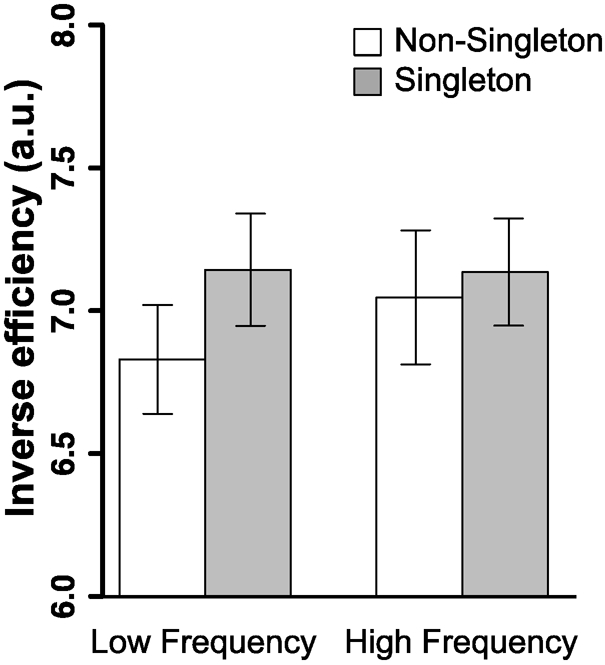
Behavioral performance in the scanner. The inverse efficiency (IE) score was calculated as RT (ms) divided by percent accuracy (%); lower IE indicates better search performance. These results show that the interference by a singleton was reduced in the high frequency condition compared to that in the low frequency condition. All error bars shown here and in subsequent figures are ± 1 standard error of the mean.
3.2. Visual ROIs
3.2.1. MVPA
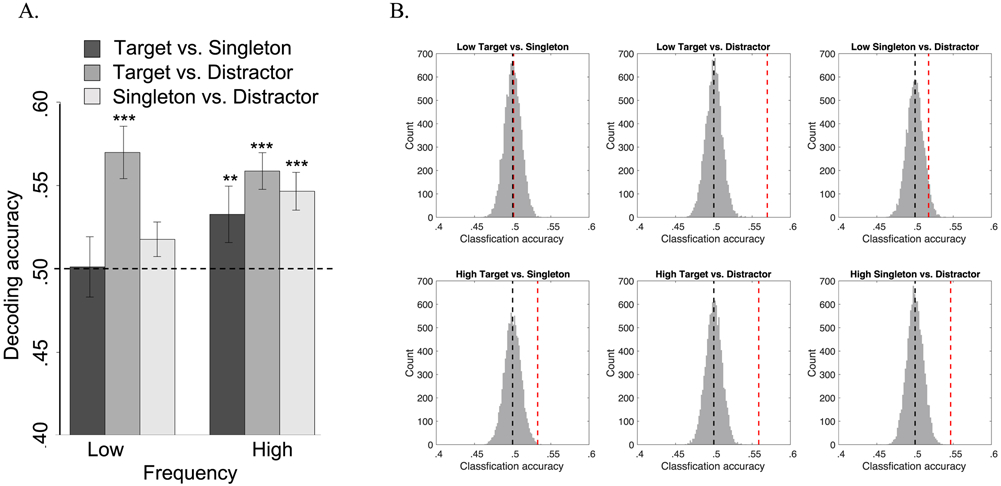
The primary hypothesis to test was that visual information regarding the singleton would differ as a function of the frequency block in which it appeared. We used MVPA that is sensitive to distributed spatial information to test if the singleton’s identity could be better distinguished from targets and distractors from the same trials in the high compared to low frequency block. Distinct information encoding of the singleton compared to other simultaneously competing stimuli should allow the singleton to be better identified as a non-target and more efficiently suppressed. In order to maximize the competition necessary to select the target, we limited our analysis to only trials with singleton distractors that were adjacent (vertically or horizontally aligned) to the target and excluded trials with diagonal singletons (Adam and Serences, 2020 VSS poster presentation; also supplemental materials S1-S2).
Next, we extracted the beta values from the singleton, target, and distractor stimuli from singleton trials in the low and high frequency blocks. The data from each stimulus type and for each frequency condition were compared using separate binary linear SVM classifiers and significance was established using a non-parametric permutation test (see Methods). Most importantly, targets and singletons were classified with greater than chance accuracy in the high frequency block (mean = .53, SD = .08, pBonf corr < .005) but not the low frequency block (mean = .50, SD = .09, pBonf corr > 1). Similarly, singletons and distractors (mean = .55, SD = .06, pBonf corr < .001) were also classified with above chance accuracy in the high frequency but not in the low frequency block (mean = .52, SD = .05, pBonf corr = .31). However, classification of targets and distractors from both blocks was significant (mean = .56, SD = .05 for the high frequency; mean = .57, SD = .08 for the low frequency, both pBonf corr < .001) (Figure 3, see also S1). Together these results clearly show that information about singletons, targets, and distractors had more distinct sensory representations during the high frequency block; perhaps this distinctiveness gives rise to better discrimination of targets from singletons, which leads to more efficient target selection and singleton suppression.
Figure 3.
MVPA results. A. Classification accuracy for each stimulus pair in each frequency block. The dotted line indicates chance level decoding (50%), *** indicates p < .001, ** indicates p < .01. B. Illustration of the permuted null distributions (vertical black dotted line indicates mean) and actual classification accuracy (vertical red dotted line) between stimulus types in each frequency block.
3.2.2. Univariate analyses
The preceding analyses demonstrated more distinctive information encoding of stimuli in the high frequency block compared to the low frequency block. Next we used complementary univariate analyses to test for evidence of differences in global visual processing of the stimulus types. Beta values for each stimulus type were averaged across runs and the four visual cortical quadrants. The data were entered into a repeated measures ANOVA with stimulus type (singleton, target, distractor) and frequency block (low, high). We found a significant main effect of stimulus type, F(2, 46) = 14.353, p < .001, η2p = .384, but no main effect of frequency block, F < 1. Most importantly, the interaction was significant, F(2, 46) = 4.673, p = .014, η2p = .169.
The interaction between stimulus type and frequency block was due to different patterns of singletons and distractors activations between two frequencies. In the low frequency block, there was greater activation in response to singletons than distractors, t(23) = 3.710, p = .001, Cohen’s d = .757, BF10 = 30.9483 whereas in the high frequency block, there was no difference in activation between singletons and distractors, t(23) = .298, p = .768, BF01 = 4.473. Consistent with the main effect of stimulus type, there was greater activation for targets compared to the other two stimuli in both blocks, low frequency block target vs. singleton: t(23) = 2.430, p = .023, Cohen’s d = .496, target vs. distractor: BF10 = 2.401; t(23) = 6.452, p < .001, Cohen’s d = 1.317, BF10 = 13239; high frequency block, target vs. singleton: t(23) = 2.535, p = .019, Cohen’s d = .517, BF10 = 2.899, target vs. distractor: t(23) = 3.163, p = .004, Cohen’s d = .646, BF10 = 9.808. Targets are processed with greater intensity than singletons and distractors in both frequency blocks, but singletons evoke greater activation than distractors only in the low frequency block. This suggests that processing of the singleton is suppressed in the high frequency block (Figure 4; see Supplemental Figure S2 for full results from all spatial configurations of the target and singleton).
Figure 4.
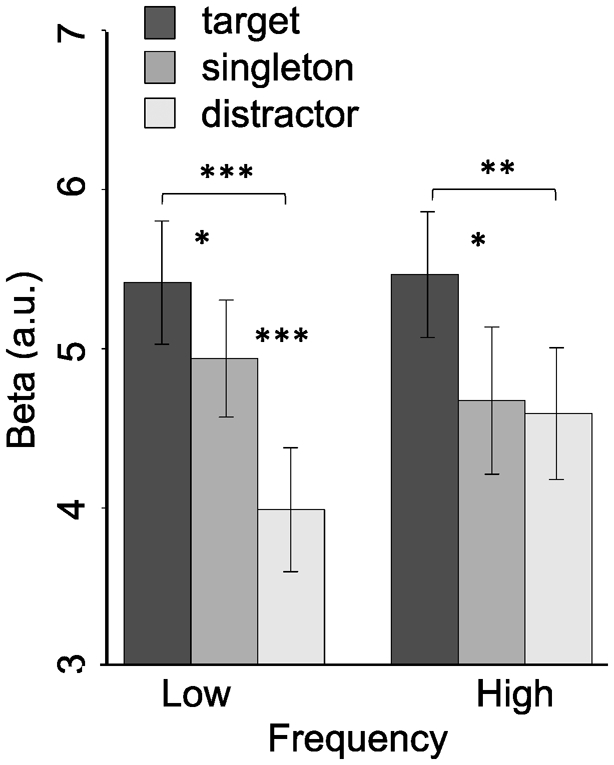
Univariate beta values for each stimulus type collapsed across the four visual ROIs. *** indicates p < .001, ** indicates p < .01, * indicates p < .05.
The interaction was due to a null difference between singletons and distractors in the high frequency block, but inspection of the data reveals that this was due in part to an increase in distractor activation, not just a reduction of singleton activation (similar to the behavioral result). One possible explanation for the increase in distractor activation is that it reflects a strategy of global sensory suppression in the high frequency block. Global suppression would reduce sensory sensitivity overall (leading to increased distractor activation) but also have the effect of attenuating the “attend-to-me” signal of the expected, salient, singleton in an unknown location and of an unknown color. Pre-stimulus increases in univariate activation in anticipation of the need to suppress sensory processing associated with a distractor has been found in spatial cuing paradigms of the distractor location (Munneke et al., 2011; Ruff & Driver, 2006; Serences et al., 2004).
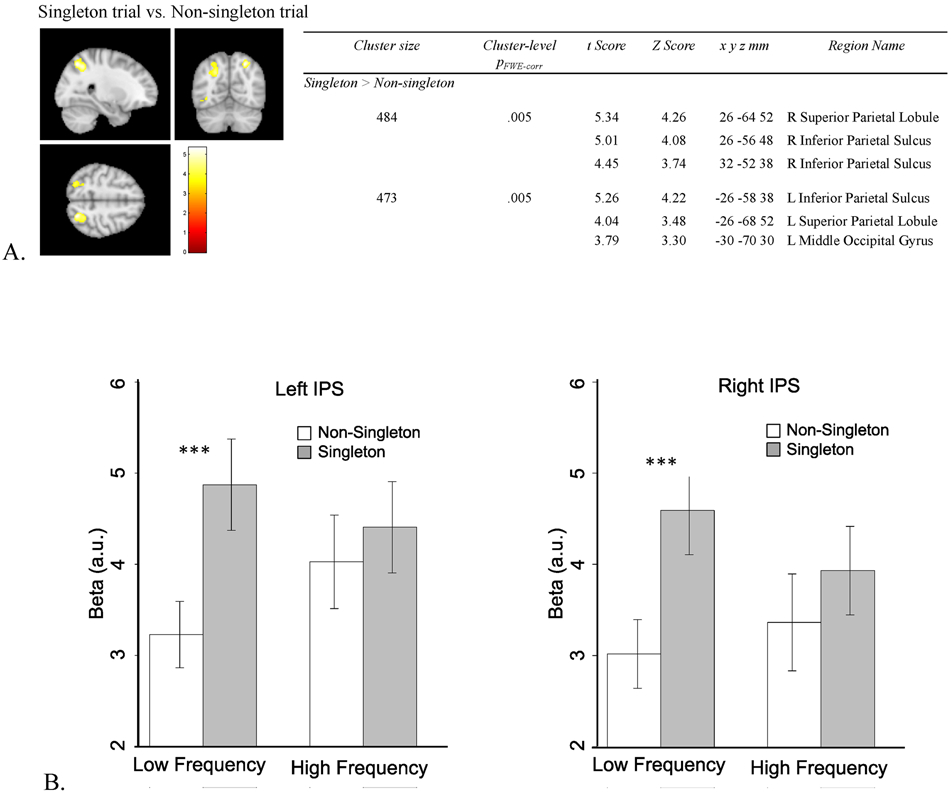
3.3. Whole brain analysis to identify regions that encode singleton salience
If the differences in results for the two frequency blocks in visual cortex are related to differences in singleton suppression, as we hypothesize, then we should see weaker evidence of singleton capture in parietal and frontal cortex in the high frequency block compared to the low frequency block. In order to be as inclusive as possible in searching for regions of the brain that might respond to singleton presence overall, we created a univariate whole brain contrast for regions showing greater activation for singleton trials compared to non-singleton trials4 (Figure 5A). This resulted in only two significant clusters corresponding to left and right IPS, consistent with the literature on attentional capture (see Introduction). To determine if the profile of attentional capture was modulated by singleton frequency as we hypothesized, we extracted the beta values for singleton and non-singleton trials from both frequency blocks from the two IPS clusters (Figure 5B). Extraction of these data do not constitute “double dipping” because the main effect of singleton presence used to select the parietal ROIs is orthogonal to the comparison of interest for the extracted betas, which involves the relative difference between singleton and non-singleton trials as a function of frequency block (i.e., an interaction between trial type and frequency block).
Figure 5.
Whole brain analysis. A. Brain regions that show the higher BOLD response in the singleton trials than that in non-singleton trials. B. Beta values extracted from bilateral IPS. *** indicates p < .001.
The extracted betas for left and right IPS were entered into a trial type (singleton, non-singleton), and frequency (low, high) repeated measures ANOVA. Because the main effect of trial type is based on the contrast from which IPS was selected, we do not report main effects to avoid circularity. The interaction between singleton presence and frequency was significant in both left and right IPS, for left IPS, F(1, 23) = 7.924, p = .010, η2p = .256; for right IPS, F(1, 23) = 4.212, p = .052, η2p = .155 (Figure 5B). Both interactions were due to a greater difference in activation for singleton compared to non-singleton trials in the low frequency condition than in the high frequency condition. Paired t-tests revealed significant differences between the singleton and non-singleton trials in the low frequency block, lIPS: t(23) = 6.691, p < .001, Cohen's d = 1.366, BF10 = 22075.48, rIPS: t(23) = 6.622, p < .001, Cohen's d = 1.352, BF10 = 19068.26, but not in the high frequency block, lIPS: t(23) = 1.047, p = .306, BF01 = 2.851, rIPS: t(23) = 1.440, p = .163, BF01 = 1.875. The pattern mimics the behavioral results (cf. Figure 2). The univariate contrast of the simple effect of singleton minus non-singleton trials from only the low frequency block are in supplementary results (S3); the results illustrate wider scale activation of the frontoparietal network when the contrast is limited to the low frequency block.
4. Discussion
The ability to suppress distraction is essential to efficient goal-oriented behaviors. Recent work from behavioral studies suggest that learned expectations are a powerful way to reduce distractor interference. Seeing distractors repeatedly in predictable locations, configurations, or features leads to a decrease in interference from those distractors (Chelazzi et al., 2019; Chetverikov et al., 2017a, 2017b; Failing & Theeuwes, 2018; Gaspelin & Luck, 2018; Geng et al., 2019; Noonan et al., 2018). For example, the study on which the current experiment is based, we showed that colored singleton distractors produced less interference when they occurred frequently and could be predicted, compared to when they occurred infrequently. This suggests that learned expectations are highly efficient and can occur for any number of distractor properties. This mimics the literature on target selection, which has also found statistical regularities to be a powerful source of information that guide attention towards targets without requiring awareness (Anderson, 2016; Awh, Belopolsky, & Theeuwes, 2012; Druker & Anderson, 2010; Geng & Behrmann, 2005; Hansmann-Roth, Chetverikov, & Kristjansson, 2019; Hoffman & Knude, 1999; Jiang, 2018; Shaw & Shaw, 1977).
In our current study, we tested the brain systems involved in the suppression of a perceptually salient singleton based on its frequency of occurrence. Importantly, visuospatial properties such as its location or color could not be predicted. Nevertheless, this expectation was sufficient to alter processing of the singleton in visual cortex and reduce its sensory readout to attentional control regions in parietal and frontal cortex.
In visual cortex, the MVPA results revealed that singletons, targets, and distractors all had distinct representations in visual cortex on singleton trials in the high frequency block but singletons could not be distinguished readily from targets or distractors in the low frequency block. We speculate that this greater distinctiveness in the high frequency block reflected better information separation of each stimulus, which in turn, attenuated readout of the “attend-to-me” saliency signal associated with the singleton to subsequent parietal and frontal regions. The finding that singleton distractors of unknown color and location can be effectively suppressed in visual cortex simply based on an expectancy of their presence is a novel finding that has not been shown previously.
Interestingly, however, the pattern of univariate activation in the two frequency blocks suggested that the suppressive effects of the singleton were not due only to a reduction in activation of the singleton but also an increase in activation for distractors. This global increase indicates that participants may have employed an effortful strategy in high frequency blocks that attenuate sensory processing overall. This increase may not be obvious in response to targets, which are selected and processed to a greater extent, leading to saturation effects. Global univariate increases in sensory activation related to anticipatory suppression of sensory information is consistent with previous findings that increases in retinotopic visual cortex lead to better distractor suppression following spatial cues (Munneke et al., 2011; Ruff & Driver, 2006; Serences et al., 2004). These studies used experimental designs that separated signals associated with preparatory distractor suppression from target enhancement leading to the conclusion that increases in univariate BOLD activity related to attentional expectations are not only related to gain enhancement of relevant task-relevant sensory representations (Hopfinger, Buonocore, & Mangun, 2000; McMains, Fehd, Emmanouil, & Kastner, 2007; Sylvester, Shulman, Jack, & Corbetta, 2009), but also reflect suppression of expected distractors.
Given that the BOLD signal reflects a combination of pre- and post-synaptic neural activity and other vascular and metabolic processes (Attwell & Iadecola, 2002; Ekstrom, 2010; Logothetis, 2008; Logothetis & Wandell, 2004), an increase in BOLD that reflects a change in sensory processing is agnostic to the underlying neuronal mechanisms. Increased univariate activation in preparatory suppression may reflect predictive coding or imagery of upcoming distracting stimuli, or neural inhibition (Ruff & Driver, 2006; Serences et al., 2004). However, we note that while the suppression effects we observed were in visual cortex, it remains unclear whether these effects arise from local processing within visual cortex or through feedback from frontoparietal regions. Nevertheless, these previous studies and our current one, provide clear evidence that mechanisms of distractor suppression operate to attenuate distractor processing in visual cortex in order to reduce cognitive and behavioral interference.
Finally, consistent with the idea that singleton salience is suppressed in visual cortex, we found no evidence of attentional capture in frontoparietal attentional control regions by singletons relative to distractors in the high frequency block. In contrast, singleton trials in the low frequency condition produced robust increases in activation in parietal cortex compared to their non-singleton counterparts. Activation in IPS is consistent with a wide literature showing that IPS encodes the capture of bottom-up salience driven attentional signals (Bisley & Goldberg, 2006; Bogler et al., 2011; Buschman & Miller, 2007; de Fockert et al., 2001; de Fockert & Theeuwes, 2012; Geng & Mangun, 2009; Mevorach et al., 2010; Mevorach et al., 2006; Sprague et al., 2018; Suzuki & Gottlieb, 2013). Although more inferior portions of the parietal cortex have also been associated with attentional reorienting, it is important to remember that TPJ and the ventral attentional network are involved in reorienting, but specifically towards unexpected but potentially relevant stimuli (Corbetta et al., 2008; Geng & Vossel, 2013; Igelstrom & Graziano, 2017). For example, TPJ is associated with invalidly cued targets in a spatial cueing paradigm (Doricchi, Macci, Silvetti, & Macaluso, 2010; Dugue, Merriam, Heeger, & Carrasco, 2018; Vossel, Thiel, & Fink, 2006) and selection of target-colored distractors (DiQuattro, Sawaki, & Geng, 2014; Painter, Dux, & Mattingley, 2015). The visually salient singletons in our study were completely task-irrelevant and therefore do not carry any signals associated with task-relevance.
The most critical result here is the finding that low frequency singleton trials produced large changes in parietal regions and the entire frontoparietal network (see Supplemental materials) compared to non-singleton trials in the classic profile of attentional capture; in contrast, high frequency singleton trials could not be distinguished from non-singleton trials in any of these regions indicating that the saliency signals from the singleton were already attenuated by the time information about the visual search display reached parietal cortex, affording a reduction in attentional capture.
5. Conclusion
Together, our results provide clear evidence that the mechanisms necessary to suppress saliency signals associated with an expected color singleton operate in visual cortex. This was true despite the fact that the expectation was only for the frequency of the singleton occurring, not of its specific location or color. When singletons were frequent and expected, the information encoded about the singleton was more distinct from targets and non-salient distractors, but the univariate activation was more similar to non-salient distractors. This suggests that expectations improved sensory discrimination of the singleton while reducing global levels of stimulus-evoked BOLD activation, allowing the readout of saliency signals associated with the color singleton to be attenuated. The success of this attenuation was evident in the activation patterns in parietal and frontal regions in response to singleton present vs. singleton absent trials: trials with singletons only showed increased activation compared to non-singleton trials in the low frequency block, as would be expected by a trials with stimuli that capture attention, but not in the high frequency block. Together our results provide a novel demonstration of how learned expectations produce sensory suppression for singleton distractors during visual search.
Supplementary Material
Acknowledgements:
We thank Sahana Deo for behavioral data collection and Phil Witkowski and Xinger Yu for helpful comments and discussions. This work supported by NSF and BCS-201502778 and NIH-RO1-MH113855-01 to JJG.
Footnotes
We report how we determined our sample size, all data exclusions (if any), all data inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
All the presentation code, fMRI data, behavioral data, and summary data are available at https://osf.io/mpc46/
No part of the study procedures was pre-registered prior to the research being conducted. No part of the study analysis procedures was pre-registered prior to the research being conducted.
We excluded one out of two functional localizer scans from one subject in the ROI analyses due to scanner malfunction during the functional localizer scan.
For one subject, there were no voxels that survived pfwe < .05, # of voxel > 10 for the upper right quadrant stimulus, so the threshold was lowered to uncorrected p < .0001, # of voxel > 10 to create that one subject’s lower left hemisphere ROI.
We provide the Bayes factor (BF) corresponding to the t-tests (JASP Team, 2019; Rouder, Speckman, Sun, Morey, &Iverson, 2009). BFs quantify the relative likelihood of obtaining the observed data under the null hypothesis compared to the alternative hypothesis and have the advantage of being equally well suited to quantify the evidence for and against the null hypothesis. Evidence in favor of the null hypothesis is denoted as BF01 and in favor of the alternative hypothesis as BF10.
This analysis is based on a GLM with unequal number of trials in the non-singleton trial condition between the low- and high-frequency blocks. To be sure that that these results are not a spurious consequence of unequal numbers in this one condition, we conducted the same whole brain contrast from a separate GLM in which non-singleton trials from the low-frequency condition were divided across two regressors: one included a numerically matched number of non-singleton trials per run (four) as the high-frequency condition and the other modeled the remaining trials. The whole brain contrast of singleton minus non-singleton trials using only the numerically matched regressor for non-singleton low-frequency trials resulted in the same pattern of results: bilateral IPS were still the only two significant clusters at pFWEcorr < .05.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam KCS, & Serences JT (2020). Evidence for suppression of distractors in early visual cortex. Paper presented at the Vision Sciences, Virtual VSS. [Google Scholar]
- Alvarez GA, & Cavanagh P (2005). Independent resources for attentional trackign in the left and right visual hemifields. Psychological Science, 6(8), 637–643. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2016). The attention habit: how reward learning shapes attentional selection. Ann N Y Acad Sci, 1369(1), 24–39. doi: 10.1111/nyas.12957 [DOI] [PubMed] [Google Scholar]
- Attwell D, & Iadecola C (2002). The neural basis of functional brain imaging signals. TRENDS in Neurosciences, 25(12), 621–625. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci, 16(8), 437–443. doi: 10.1016/j.tics.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, & Goldberg ME (2006). Neural correlates of attention and distractibility in the lateral intraparietal area. J Neurophysiol, 95(3), 1696–1717. doi: 10.1152/jn.00848.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, & Goldberg ME (2010). Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci, 33, 1–21. doi: 10.1146/annurev-neuro-060909-152823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, & Mirpour K (2019). The neural instantiation of a priority map. Curr Opin Psychol, 29, 108–112. doi: 10.1016/j.copsyc.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler C, Bode S, & Haynes JD (2011). Decoding successive computational stages of saliency processing. Curr Biol, 21(19), 1667–1671. doi: 10.1016/j.cub.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Bonetti F, & Turatto M (2019). Habituation of oculomotor capture by sudden onsets: Stimulus specificity, spontaneous recovery and dishabituation. J Exp Psychol Hum Percept Perform, 45(2), 264–284. doi: 10.1037/xhp0000605 [DOI] [PubMed] [Google Scholar]
- Buschman TJ, & Miller EK (2007). Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science, 315(5820), 1860–1862. doi: 10.1126/science.1138071 [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Marini F, Pascucci D, & Turatto M (2019). Getting Rid of Visual Distractors: The Why, When, How and Where. Current Opinion in Psychology. doi: 10.1016/j.copsyc.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Chetverikov A, Campana G, & Kristjansson A (2017a). Rapid learning of visual ensembles. J Vis, 17(2), 21. doi: 10.1167/17.2.21 [DOI] [PubMed] [Google Scholar]
- Chetverikov A, Campana G, & Kristjansson A (2017b). Representing color ensembles. Psychological Science. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58(3), 306–324. doi: 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman JD, Lowe KA, Zinke W, Woodman GF, & Schall JD (2018). Prefrontal Control of Visual Distraction. Curr Biol, 28(3), 414–420 e413. doi: 10.1016/j.cub.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, & Lavie N (2001). The role of working memory in visual selective attention. Science, 291, 1803–1806. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, & Theeuwes J (2012). Role of frontal cortex in attentional capture by singleton distractors. Brain Cogn, 80(3), 367–373. doi: 10.1016/j.bandc.2012.07.006 [DOI] [PubMed] [Google Scholar]
- DiQuattro NE, Sawaki R, & Geng JJ (2014). Effective connectivity during feature-based attentional capture: evidence against the attentional reorienting hypothesis of TPJ. Cereb Cortex, 24(12), 3131–3141. doi: 10.1093/cercor/bht172 [DOI] [PubMed] [Google Scholar]
- Doricchi F, Macci E, Silvetti M, & Macaluso E (2010). Neural correlates of the spatial and expectancy components of endogenous and stimulus-driven orienting of attention in the Posner task. Cereb Cortex, 20(7), 1574–1585. doi: 10.1093/cercor/bhp215 [DOI] [PubMed] [Google Scholar]
- Druker M, & Anderson B (2010). Spatial probability AIDS visual stimulus discrimination. Front Hum Neurosci, 4. doi: 10.3389/fnhum.2010.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue L, Merriam EP, Heeger DJ, & Carrasco M (2018). Specific Visual Subregions of TPJ Mediate Reorienting of Spatial Attention. Cereb Cortex, 28(7), 2375–2390. doi: 10.1093/cercor/bhx140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeth HE, & Yantis S (1997). Visual attention: control, representation, and time course. Annu Rev Psychol, 48, 269–297. doi: 10.1146/annurev.psych.48.1.269 [DOI] [PubMed] [Google Scholar]
- Ekstrom A (2010). How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev, 62(2), 233–244. doi: 10.1016/j.brainresrev.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failing M, & Theeuwes J (2018). Selection history: How reward modulates selectivity of visual attention. Psychon Bull Rev, 25(2), 514–538. doi: 10.3758/S23423-017-1380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante O, Patacca A, Di Caro V, Della Libera C, Santandrea E, & Chelazzi L (2018). Altering spatial priority maps via statistical learning of target selection and distractor filtering. Cortex, 102, 67–95. doi: 10.1016/j.cortex.2017.09.027 [DOI] [PubMed] [Google Scholar]
- Gaspelin N, Gaspar JM, & Luck SJ (2019). Oculomotor Inhibition of Salient Distractors: Voluntary Inhibition Cannot Override Selection History. Vis cogn, 27(3-4), 227–246. doi: 10.1080/13506285.2019.1600090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2017). Suppression of overt attentional capture by salient-but-irrelevant color singletons. Atten PerceptPsychophys, 79(1), 45–62. doi: 10.3758/S23414-016-1209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, & Luck SJ (2018). The Role of Inhibition in Avoiding Distraction by Salient Stimuli. Trends Cogn Sci, 22(1), 79–92. doi: 10.1016/j.tics.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ (2014). Attentional Mechanisms of Distractor Suppression. Current Directions in Psychological Science, 23(2), 147–153. doi: 10.1177/0963721414525780 [DOI] [Google Scholar]
- Geng JJ, & Behrmann M (2005). Spatial probability as an attentional cue in visual search. Perception & Psychophysics, 67(7), 1252–1262. [DOI] [PubMed] [Google Scholar]
- Geng JJ, & Diquattro NE (2010). Attentional capture by a perceptually salient non-target facilitates target processing through inhibition and rapid rejection. J Vis, 10(6), 5. doi: 10.1167/10.6.5 [DOI] [PubMed] [Google Scholar]
- Geng JJ, & Mangun GR (2009). Anterior intraparietal sulcus is senstivie to bottom-up attention driven by stimulus salience. Journal of Cognitive Neuroscience, 21(8), 1584–1601. [DOI] [PubMed] [Google Scholar]
- Geng JJ, & Vossel S (2013). Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev, 37(10 Pt 2), 2608–2620. doi: 10.1016/j.neubiorev.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Won B-Y, & Carlisle NB (2019). Distractor ignoring: Strategies, learning, and passive filtering. Current Directions in Psychological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer T, Muller HJ, & Krummenacher J (2006). Cross-trial priming in visual search for singleton conjunction targets: Role of repeated target and distractor features. Perception & Psychophysics, 68(5), 736–749. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Balan P, Oristaglio J, & Suzuki M (2009). Parietal control of attentional guidance: the significance of sensory, motivational and motor factors. Neurobiol Learn Mem, 91(2), 121–128. doi: 10.1016/j.nlm.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansmann-Roth S, Chetverikov A, & Kristjansson A (2019). Representing color and orientation ensembles: Can observers learn multiple feature distributions? J Vis, 19(9), 2. doi: 10.1167/19.9.2 [DOI] [PubMed] [Google Scholar]
- Hickey C, DiLollo V, & McDonald JJ (2008). Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience, 21(4), 760–775. [DOI] [PubMed] [Google Scholar]
- Hodsoll J, Mevorach C, & Humphreys GW (2009). Driven to less distraction: rTMS of the right parietal cortex reduces attentional capture in visual search. Cereb Cortex, 19(1), 106–114. doi: 10.1093/cercor/bhn070 [DOI] [PubMed] [Google Scholar]
- Hoffman J, & Knude W (1999). Location-specific target expectancies in visual search. Journal of Experimental Psychology: Human Perception and Performance, 25, 1127–1141. [Google Scholar]
- Hopfinger JB, Buonocore MH, & Mangun GR (2000). THe neural mechanisms of top-down attentional control. Nature Neuroscience, 3(3), 284–291. [DOI] [PubMed] [Google Scholar]
- Hout MC, & Goldinger SD (2015). Target templates: the precision of mental representations affects attentional guidance and decision-making in visual search. Atten Percept Psychophys, 77(1), 128–149. doi: 10.3758/S23414-014-0764-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom KM, & Graziano MSA (2017). The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia, 105, 70–83. doi: 10.1016/j.neuropsychologia.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Gottlieb J, Bisley JW, & Goldberg ME (2006). LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat Neurosci, 9(8), 1071–1076. doi: 10.1038/nn1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, & Koch C (2000). A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research, 40, 1489–1506. [DOI] [PubMed] [Google Scholar]
- JASP Team (2019). JASP (Version 0.11.1) [Computer software].
- Jiang YV (2018). Habitual versus goal-driven attention. Cortex, 102, 107–120. doi: 10.1016/j.cortex.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869–878. doi: 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, & Wandell BA (2004). Interpreting the BOLD signal. Annu Rev Physiol, 66, 735–769. doi: 10.1146/annurev.physiol.66.082602.092845 [DOI] [PubMed] [Google Scholar]
- Marini F, Demeter E, Roberts KC, Chelazzi L, & Woldorff MG (2016). Orchestrating Proactive and Reactive Mechanisms for Filtering Distracting Information: Brain-Behavior Relationships Revealed by a Mixed-Design fMRI Study. J Neurosci, 36(3), 988–1000. doi: 10.1523/JNEUROSCI.2966-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMains SA, Fehd HM, Emmanouil TA, & Kastner S (2007). Mechanisms of feature- and space-based attention: response modulation and baseline increases. J Neurophysiol, 98(4), 2110–2121. doi: 10.1152/jn.00538.2007 [DOI] [PubMed] [Google Scholar]
- Mevorach C, Hodsoll J, Allen H, Shalev L, & Humphreys G (2010). Ignoring the elephant in the room: a neural circuit to downregulate salience. J Neurosci, 30(17), 6072–6079. doi: 10.1523/JNEUROSCI.0241-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach C, Humphreys GW, & Shalev L (2006). Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nat Neurosci, 9(6), 740–742. doi: 10.1038/nn1709 [DOI] [PubMed] [Google Scholar]
- Munneke J, Heslenfeld DJ, Usrey WM, Theeuwes J, & Mangun GR (2011). Preparatory effects of distractor suppression: evidence from visual cortex. PLoS One, 6(12), e27700. doi: 10.1371/journal.pone.0027700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Crittenden BM, Jensen O, & Stokes MG (2018). Selective inhibition of distracting input. Behav Brain Res, 355, 36–47. doi: 10.1016/j.bbr.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Painter DR, Dux PE, & Mattingley JB (2015). Distinct roles of the intraparietal sulcus and temporoparietal junction in attentional capture from distractor features: An individual differences approach. Neuropsychologia, 74, 50–62. doi: 10.1016/j.neuropsychologia.2015.02.029 [DOI] [PubMed] [Google Scholar]
- Pollmann S, Weidner R, Humphreys GW, Olivers CNL, Müller K, Lohmann G, … Watson DG (2003). Separating distractor rejection and target detection in posterior parietal cortex—an event-related fMRI study of visual marking. NeuroImage, 18(2), 310–323. doi: 10.1016/S2053-8119(02)00036-8 [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, & Davidson BJ (1980). Attention and the detection of signals. Journal of Experimental Psychology: General, 109(2). [PubMed] [Google Scholar]
- Reeder RR, Olivers CNL, Hanke M, & Pollmann S (2018). No evidence for enhanced distractor template representation in early visual cortex. Cortex, 108, 279–282. doi: 10.1016/j.cortex.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Reeder RR, Olivers CNL, & Pollmann S (2017). Cortical evidence for negative search templates. Visual Cognition, 25(1-3), 278–290. doi: 10.1080/13506285.2017.1339755 [DOI] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, & Iverson G (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16, 225–237. 10.3758/PBR.16.2.225 [DOI] [PubMed] [Google Scholar]
- Ruff C, & Driver J (2006). Attentional preparation for a lateralized visual distractor: Behavioral and fMRI evidence. Journal of Cognitive Neuroscience, 184, 522–538. [DOI] [PubMed] [Google Scholar]
- Sawaki R, & Luck SJ (2010). Capture versus suppression of attention by salient singletons: electrophysiological evidence for an automatic attend-to-me signal. Atten Percept Psychophys, 72(6), 1455–1470. doi: 10.3758/APP.72.6.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, & Driver J (2005). Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex, 15(6), 770–786. doi: 10.1093/cercor/bhh178 [DOI] [PubMed] [Google Scholar]
- Seidl KN, Peelen MV, & Kastner S (2012). Neural evidence for distracter suppression during visual search in real-world scenes. J Neurosci, 52(34), 11812–11819. doi: 10.1523/JNEUROSCI.1693-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, & Awh E (2004). Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol, 92(6), 3538–3545. doi: 10.1152/jn.00435.2004 [DOI] [PubMed] [Google Scholar]
- Sereno MI, & Kosslyn SM (1991). Discrimination within and between hemifields: a new contraint on theories of attention. Neuropsychologia, 29(7), 659–675. [DOI] [PubMed] [Google Scholar]
- Shaw ML, & Shaw P (1977). Optimal allocation of cognitive resources to spatial locations. Journal of Experimental Psychology: Human Perception and Performance(3), 201–211. [DOI] [PubMed] [Google Scholar]
- Sprague TC, Itthipuripat S, Vo VA, & Serences JT (2018). Dissociable signatures of visual salience and behavioral relevance across attentional priority maps in human cortex. J Neurophysiol, 119(6), 2153–2165. doi: 10.1152/jn.00059.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilwell BT, & Vecera SP (2019). Learned and cued distractor rejection for multiple features in visual search. Atten Percept Psychophys, 81(2), 359–376. doi: 10.3758/S23414-018-1622-8 [DOI] [PubMed] [Google Scholar]
- Stormer VS, Alvarez GA, & Cavanagh P (2014). Within-hemifield competition in early visual areas limits the ability to track multiple objects with attention. J Neurosci, 34(35), 11526–11533. doi: 10.1523/JNEUROSCI.0980-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, & Gottlieb J (2013). Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci, 16(1), 98–104. doi: 10.1038/nn.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, & Corbetta M (2009). Anticipatory and stimulus-evoked blood oxygenation level-dependent modulations related to spatial attention reflect a common additive signal. J Neurosci, 29(34), 10671–10682. doi: 10.1523/JNEUROSCI.1141-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J (1991). Exogenous and endogenous control of attention: the effect of visual onsets and offsets. Percept Psychophys, 49(1), 83–90. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2011456 [DOI] [PubMed] [Google Scholar]
- Treisman AM, & Gelade G (1980). A feature-integration theory of attention. Cogn Psychol, 12(1), 97–136. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7351125 [DOI] [PubMed] [Google Scholar]
- Turatto M, Bonetti F, Pascucci D, & Chelazzi L (2018). Desensitizing the attention system to distraction while idling: A new latent learning phenomenon in the visual attention domain. Journal of Experimental Psychology: General, 147(12), 1827–1850. doi: 10.1037/xge0000503 [DOI] [PubMed] [Google Scholar]
- van Diepen RM, Miller LM, Mazaheri A, & Geng JJ (2016). The Role of Alpha Activity in Spatial and Feature-Based Attention. eNeuro, 3(5). doi: 10.1523/ENEURO.0204-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Moorselaar D, & Slagter HA (2019). Learning What Is Irrelevant or Relevant: Expectations Facilitate Distractor Inhibition and Target Facilitation through Distinct Neural Mechanisms. J Neurosci, 39(35), 6953–6967. doi: 10.1523/JNEUROSCI.0593-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatterott DB, Mozer MC, & Vecera SP (2018). Rejecting salient distractors: Generalization from experience. Atten Percept Psychophys, 80(2), 485–499. doi: 10.3758/S23414-017-1465-8 [DOI] [PubMed] [Google Scholar]
- Vatterott DB, & Vecera SP (2012). Experience-dependent attentional tuning of distractor rejection. Psychon Bull Rev, 19(5), 871–878. doi: 10.3758/S23423-012-0280-4 [DOI] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, & Fink GR (2006). Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage, 32(3), 1257–1264. doi : 10.1016/j.neuroimage.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Walter S, Quigley C, & Mueller MM (2014). Competitive interactions of attentional resources in early visual cortex during sustained visuospatial attention within or between visual hemifields: evidence for the different-hemifield advantage. J Cogn Neurosci, 26(5), 938–954. doi: 10.1162/jocn_a_00547 [DOI] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018a). Statistical regularities modulate attentional capture. J Exp Psychol Hum Percept Perform, 44(1), 13–17. doi: 10.1037/xhp0000472 [DOI] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018b). Statistical regularities modulate attentional capture independent of search strategy. Attention, Perception, & Psychophysics, 80(7), 1763–1774. doi: 10.3758/S23414-018-1562-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, van Driel J, Ort E, & Theeuwes J (2019). Anticipatory Distractor Suppression Elicited by Statistical Regularities in Visual Search. J Cogn Neurosci, 31(10), 1535–1548. doi: 10.1162/jocn_a_01433 [DOI] [PubMed] [Google Scholar]
- Won B-Y, & Geng JJ (2020). Passive exposure attenuates distraction during visual search. J Exp Psychol Gen. doi: 10.1037/xge0000760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won B-Y, Kosoyan M, & Geng JJ (2019). Evidence for second-order singleton suppression based on probabilistic expectations. J Exp Psychol Hum Percept Perform, 45(1), 125–138. doi: 10.1037/xhp0000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B, Mackie MA, Spagna A, Wu T, Tian Y, Hof PR, & Fan J (2016). The activation of interactive attentional networks. Neuroimage, 129, 308–319. doi: 10.1016/j.neuroimage.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, & Egeth HE (1999). On the dissociation between visual salience and stimulus-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance. [DOI] [PubMed] [Google Scholar]
- Zhang B, Allenmark F, Liesefeld HR, Shi Z, & Müller HJ (2019). Probability cueing of singleton-distractor locations in visual search: Priority-map- versus dimension-based inhibition? Journal of Experimental Psychology: Human Perception and Performance, 45(9), 1146–1163. doi: 10.1037/xhp0000652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.